Abstract
Objectives
Neuropathic pain is a consequence of many chronic conditions. This study aimed to develop a unidimensional neuropathic pain scale whose scores represent levels of neuropathic pain and distinguish between individuals with neuropathic and non-neuropathic pain conditions.
Methods
A candidate item pool of 42 pain quality descriptors was administered to participants with osteoarthritis, rheumatoid arthritis, diabetic neuropathy, and cancer chemotherapy-induced peripheral neuropathy. A subset of pain quality descriptors (items) that best distinguished between participants with and those without neuropathic pain conditions were identified. Dimensionality of pain descriptors was evaluated in a development sample and cross-validated in a hold-out sample. Item responses were calibrated using an item response theory model, and scores were generated on a T-score metric. Neuropathic pain scale scores were evaluated in terms of reliability, validity, and the ability to distinguish between participants with and without conditions typically associated with neuropathic pain.
Results
Of the 42 initial items, 5 were identified for the Patient Reported Outcome Measurement Information System (PROMIS) Neuropathic Pain Quality scale (PROMIS-PQ-Neuro). The IRT-generated T-scores exhibited good discriminatory ability based on receiver operator characteristic analysis. Score thresholds were identified that optimize sensitivity and specificity. Construct, criterion, and discriminant validity, and reliability of scale scores were supported.
Conclusions
The 5-item PROMIS PQ-Neuro is a short and practical measure that can be used to identify patients more likely to have neuropathic pain and to distinguish levels of neuropathic pain. The data collected will support future research that targets other unidimensional pain quality domains (e.g., nociceptive pain).
Keywords: pain; neuropathy; patient reported outcomes; measurement, PROMIS
INTRODUCTION
Neuropathic pain (NP) can be a consequence of many chronic conditions that share a common mechanism: a lesion or disease of the somatosensory nervous system.(1) These conditions include diabetic neuropathy, post-herpetic and trigeminal neuralgias, HIV-related peripheral neuropathy, and treatment-related neuropathies such as phantom-limb pain or cancer-related chemotherapy neuropathy, among others. Estimates of the prevalence of NP vary considerably by condition (e.g. 3% in Carpal Tunnel Syndrome; 50% in Phantom Limb Pain).(2) In the general population, prevalence estimates of pain with neuropathic characteristics range from 7-10%.(3) The clinical manifestation of NP is distinct, often accompanied by positive (e.g., abnormal or unpleasant sensations) and negative (e.g., decreased sensitivity) signs and symptoms that reflect underlying neural damage.(4) A definitive diagnosis of NP entails identification of the underlying somatosensory dysfunction, but accurate diagnosis can be complicated by varying degrees of neuropathic involvement in pain generation and comorbid nociceptive or idiopathic pain.(4)
Individuals with chronic NP often describe their pain in ways that are distinct from other painful conditions. Systematic differences in pain qualities potentially provide useful diagnostic information to a clinician or researcher, as they may indicate underlying neurological involvement in pain generation.(4) A variety of NP measures have been developed that capitalize on these systematic differences in pain qualities, but they differ in their intended use and application. Some (e.g., PainDETECT, IDpain) were developed primarily as screeners for neuropathic pain symptoms.(5, 6) Others (e.g., Neuropathic Pain Scale, Pain Quality Assessment Scale) have been used to document and evaluate changes in individual symptoms and sensations in response to treatment.(7, 8)
For NP and other chronic pain conditions, accurate and comprehensive assessment remains a critical public health challenge.(9) The Patient-Reported Outcome Measurement Information System (PROMIS) was charged with creating a comprehensive battery of valid and precise health status measures appropriate for use in clinical and research settings.(10) Pain-related PROMIS measures have been developed for Pain Intensity, Pain Interference, and Pain Behavior.(11-13) These and other PROMIS measures can be administered through a variety of modes (e.g. fixed-length pen-and-paper short form surveys, customized paper or electronic surveys, and computer adaptive testing) and when administered together they present an expanded profile of a patient's pain experience. Several recent reviews have detailed the use of available NP measures to create profiles of patients based on patterns of reported symptoms and sensory abnormalities.(14, 15) This approach enables assessment of profile-specific treatment efficacy and may pave the way to more personalized pain treatments. Expanding the battery of available PROMIS pain measures to include NP quality would facilitate more detailed investigations in profile-specific treatment effects and promote comprehensive assessment of patient pain experiences.
Patient responses to items measuring pain qualities were collected during the initial phase of PROMIS data collection, but the response data had substantial multidimensionality making them unsuitable for item banking using a unidimensional item response theory (IRT) model.(16) The objectives of the current study were to: (a) identify a new set of candidate items for measuring pain quality, (b) collect responses to these items in several pain-associated conditions, and (c) derive a subset of pain quality items that could be scaled using IRT and whose scores distinguish among individuals with neuropathic and non-neuropathic pain conditions. This new scale was named the PROMIS Neuropathic Pain Quality scale (PROMIS PQ-Neuro).
METHODS
This study is part of an ongoing project aimed at identifying factors (i.e. clusters of items) within pain quality item banks that are associated with different types of chronic pain.(16) In particular, we hypothesized that items with pain quality descriptors that are used by patients to describe neuropathic pain are more likely to be endorsed by patients with diabetic and cancer chemotherapy related neuropathy.
Item Development
Substantial prior work developed, revised, and tested PROMIS pain quality items for adult (phase 1 of PROMIS 2005-2009) and pediatric populations (phase 2 of PROMIS 2009-2014). This work employed the PROMIS qualitative review methodology that included item identification, classification, selection, review and revision.(17) Clinicians with substantial experience treating chronic pain in these and other patient populations participated in each phase of the study, including final item selection. Focus groups were convened to evaluate domain coverage. Cognitive interviews for each item were conducted, and additional revisions were made as needed prior to field testing. Clinical and psychometric experts then examined item response characteristics and the factor structure of the first generation adult pain quality items following standardized protocols for the development of PROMIS measures. (11, 13, 18, 19) A coherent factor structure was identified, (16) but some item-stems were identified as problematic and potentially confusing. More specifically, multiple item stems included language that assumed the presence of each pain descriptor (e.g. how intense was your sharp pain?). When selecting and revising items for this follow-up study of pain quality, we sought item wording with greater directness and simplicity that would be appropriate for use in both adult and pediatric populations. This approach would enable measurement across the life-course and subsequent linkage studies of adult and pediatric item banks. To that end, draft pediatric items served as a model for item syntax. The final candidate pool for adults was comprised of 42 pain quality items.
Participants
Participants were recruited from two clinical conditions associated with neuropathic pain (diabetic neuropathy [DN] and cancer chemotherapy-induced peripheral neuropathy [CN]) and two conditions not associated with neuropathic pain (rheumatoid arthritis [RA] and osteoarthritis [OA]). Recruiting methods included direct approach in clinical settings, letters of invitation, contacts through participant registries (National Data Bank for Rheumatic Diseases, http://www.arthritis-research.org/) and targeted advertising on advocacy websites. Clinic recruitment sites included Northwestern Medicine Hospitals and Clinics, the Rehabilitation Institute of Chicago's Center for Pain Management, and the University of Washington Diabetes Care Center. Participants provided informed consent and gave authorization to confirm clinical diagnosis with treating physicians. The human subjects review boards of participating institutions approved study procedures, and participants were compensated $20 to $30 in cash or gift cards according to site-specific institutional review board guidelines.
All participants were required to meet the following inclusion criteria: willingness and ability to provide informed consent, ability to read and speak English, age of at least 18 years, and ability to see and interact with a computer screen, mouse, and keyboard. For the cohort with diabetes, additional inclusion criteria included having clinically-confirmed diabetes and self-reported symptoms of painful peripheral neuropathy that began after onset of diabetes and were present for at least three months prior to starting the study; potential participants were excluded when referring physicians suspected that pain might not be attributable to neuropathic dysfunction or when participants had comorbid fibromyalgia, rheumatoid arthritis or limb amputation. For the cohort with chemotherapy-induced peripheral neuropathy, additional inclusion criteria included confirmation of cancer diagnosis, past or current treatment with a chemotherapy agent known to cause peripheral neuropathy, self-reported symptoms of peripheral neuropathy that: (a) began after administration of chemotherapy and (b) were present within a three month period prior to starting the study. Potential participants were excluded when referring physicians suspected that pain might not be attributable to neuropathic dysfunction or if there was a history of comorbid fibromyalgia, rheumatoid arthritis, or stem cell transplant. For the cohorts with osteoarthritis and rheumatoid arthritis, additional inclusion criteria included clinical confirmation of diagnosis; there were no subgroup-specific exclusion criteria.
Measures
All participants completed demographic and clinical information, the 42 candidate pain quality items, 26 affective pain items, (16) and additional PROMIS measures. Participants either responded to measures using paper forms or electronically through the Assessment Center, an internet-based survey system (https://www.assessmentcenter.net). Prior research established the comparability of paper and online versions of PROMIS instruments. (20) Participants who accessed the measures through the Assessment Center were administered PROMIS measures using computer adaptive testing (CAT). Those who used paper measures were administered PROMIS short-form (SF) surveys.
Candidate Pain Quality Items
Development of the 42 candidate PROMIS-NPQ items has been described above. The item-stem for 39 of these items was, “In the past 7 days, did your pain feel...” The three exceptions were: “In the past 7 days, did your pain move to a different part of your body?”; “In the past 7 days, did your pain shoot to a different part of your body?”; and “In the past 7 days, did your pain come and go?” All response options were 5-point Likert scales (1=”not at all”; 5=”very much”).
Other PROMIS measures
The following PROMIS measures were administered using CAT or the specified short forms: Global Physical and Mental Health (SF-version 1.1), Pain Intensity (SF-version 3a), Pain Interference (SF-version 6b), Pain Behavior (SF-version 7a), and PROMIS-29 Profile (version 1.0) measures of Physical Function, Fatigue, Anxiety, Depression, Sleep Disturbance, and Satisfaction with Social Roles. PROMIS measures are reported on a T-score metric (mean = 50; SD = 10) centered on the US general population 2000 census statistics. (10) Two exceptions are the PROMIS Sleep Disturbance and Satisfaction with Social Roles item banks, which are centered on the mean of a calibration sample that was generally more enriched for chronic illness. As a result, a score of 50 on these measures likely represents average levels for somewhat sicker people than the general population.
Analysis
The analytic cohort was randomly split into a development dataset (n=367) and a validation dataset (i.e. holdout sample, n=368). This enabled the specification of the underlying factor structure of patient responses and it's validation to be carried out in separate datasets.(21) The randomization was stratified by diagnostic condition to ensure equivalent proportions of patients with each condition across datasets. Splitting the dataset in this way represented a practical means of specifying and validating the factor structure without having to employ additional waves of data collection that compound response burden to patients, (22, 23) although additional approaches to validation are available.(24-26)
Identification of Potential Items
To identify items more likely to be endorsed by individuals with neuropathic pain conditions, Cramer's V statistic (a sample size adjusted measure of effect size) was calculated to estimate the strength of relationship between diagnostic category and item responses.(27) An exploratory factor analysis (EFA) of the development dataset also was conducted to aid in identification of a potential set of items indicating neuropathic pain (i.e., common factor analysis of the polychoric correlation matrix with Geomin rotation).
Scaling PROMIS PQ-Neuro
To ensure the unidimensionality of responses to the PROMIS-NPQ scale, a CFA was conducted using the polychoric correlation matrix from the validation sample only (i.e., did not include the development dataset). The model posited was that all items loaded on a single dimension. Traditional fit indices guided evaluations of model fit, (CFI, TLI > 0.95), (28) and all factor loadings and residual covariances were evaluated in terms of magnitude and statistical significance (29) Item responses were then calibrated using Samejima's graded response model for polytomous response formats.(30) The calibration used the combined development and validation datasets (n=735) to maximize estimation precision and to offset the cost of estimating additional parameters.(31, 32) Item fit was evaluated with S-X2 (p>0.01), and local dependency was evaluated with standardized LD X2 indices (<10). (33, 34) T-scores representing neuropathic pain quality were derived for all study participants with the mean centered at the mean of the combined sample.
Sensitivity and Specificity
Using the development dataset only, sensitivity and specificity analyses were carried out to identify optimal T score thresholds that maximized discrimination between participants with neuropathic and non-neuropathic pain conditions.(35) For investigators interested in employing a multi-stage screening protocol (e.g., a self-report measure followed by clinical exam), we selected three distinct score thresholds: one to optimize sensitivity, one to optimize specificity, and one representing a balance of both characteristics. The sensitivity and specificity of these three thresholds were re-calculated in the validation sample. Receiver-operator characteristic (ROC) analysis was also carried out by plotting the true positive rate (i.e. sensitivity) against the false positive rate (i.e. 1 – specificity) across all possible threshold values, with the area under the curve serving as an overall indicator of diagnostic utility.
Validity and Reliability Analyses
The sensitivity and specificity analyses served as one evaluation of the validity of PROMIS PQ-Neuro T-scores. Known-groups validity of T-scores was evaluated using ANOVA to compare mean scores across diagnostic categories. Correlations between T-scores of neuropathic pain quality and other health domains also were calculated. Reliability was assessed with Cronbach's alpha, item-total correlations, polychoric correlations (i.e., internal consistency reliability), and inspection of an IRT-based information plot.
All dimensionality assessments were conducted using Mplus 6.12 (Copyright 1998-2011, Muthen & Muthen), including exploratory and confirmatory factor analysis (CFA). IRT calibration was carried with IRTPRO 2.1 (Copyright 2011, Scientific Software International, Inc), which included estimation of item difficulty and discrimination parameters and test information. All other data analyses including the ROC sensitivity/specificity analyses were carried out with Stata/IC 12.1 (Copyright 1985-2011, StataCorp LP).
RESULTS
Participants
A total of 735 participants with CN (n=134), DN (n=181), OA (n=106), and RA (n=314) comprised the analytic cohort. Table 1 reports demographics by diagnostic category. Participants with OA and RA reported having experienced painful symptoms for nearly 20 years (mean=19.4, SD=12.2) and averaged 18.4 years (SD=12.0) since diagnosis. Participants with CN experienced symptoms of neuropathy for an average of 2.2 years (SD=2), while those with DN experienced symptoms of neuropathy for nearly 10 years (mean=9.8, sd=9.2). Substantial deficits in self-reported physical and mental health were observed across all subgroups, with the largest departures from the general population mean observed for physical function (mean T-score of 39.5) and pain interference (mean T-score of 59.2).
Table 1.
Demographic and clinical profile of study participants
| Neuropathic Pain | Non-Neuropathic Pain | All Pain Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | Diabetes | Osteoarthritis | Rheumatoid Arthritis | All Diagnoses | ||||||
| n=134 | % | n=181 | % | n=106 | % | n=314 | % | n=735 | % | |
| Female | 94 | 70.2 | 73 | 40.3 | 93 | 88.6 | 268 | 85.6 | 528 | 71.9 |
| Missing | 1 | - | 1 | - | ||||||
| Race/Ethnicity | ||||||||||
| White | 99 | 73.9 | 149 | 82.8 | 100 | 94.3 | 300 | 96.8 | 648 | 88.8 |
| Black | 28 | 20.9 | 10 | 5.6 | 3 | 2.8 | 3 | 1.0 | 44 | 6.0 |
| Asian | 5 | 3.7 | 2 | 1.1 | 1 | 0.9 | 1 | 0.3 | 9 | 1.2 |
| Multiple/Other | 2 | 1.6 | 19 | 10.6 | 2 | 1.9 | 6 | 2.0 | 29 | 4.0 |
| Missing | 1 | - | 4 | - | 5 | - | ||||
| Education | ||||||||||
| Some high school | 5 | 3.7 | 10 | 5.6 | 1 | 0.9 | 2 | 0.6 | 18 | 2.4 |
| High school / GED | 27 | 20.2 | 20 | 11.2 | 10 | 9.4 | 51 | 16.3 | 108 | 14.8 |
| Some college, technical degree, AA | 33 | 24.6 | 79 | 44.4 | 32 | 30.2 | 97 | 31.0 | 241 | 33.0 |
| College degree (BA/BS) | 43 | 32.1 | 31 | 17.4 | 32 | 30.2 | 94 | 30.0 | 200 | 27.4 |
| Advanced degree (MA,PhD,MD) | 26 | 19.4 | 38 | 21.4 | 31 | 29.3 | 69 | 22.0 | 164 | 22.4 |
| Missing | 3 | - | 1 | - | 4 | - | ||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| Age in years | 52.5 | 13.4 | 58.8 | 13.1 | 64.5 | 9.6 | 58.2 | 11.9 | 58.2 | 12.6 |
| Years since diagnosis | 20.4 | 13.8 | 18.0 | 11.4 | 18.6 | 12.2 | ||||
| Years with symptoms of neuropathy | 2.2 | 2.2 | 9.8 | 9.2 | ||||||
| Years with symptoms of arthritis | 19.2 | 12.5 | 19.5 | 12.1 | ||||||
| PROMIS-Global | ||||||||||
| Physical Health | 41.5 | 10.1 | 40.2 | 8.1 | 41.3 | 7.8 | 41.3 | 8.5 | 41.1 | 8.6 |
| Mental Health | 45.4 | 10.9 | 44.5 | 9.3 | 47.0 | 8.6 | 46.7 | 9.1 | 45.9 | 9.5 |
| PROMIS-29 Profile | ||||||||||
| Physical Function | 39.7 | 7.9 | 39.9 | 8.4 | 37.4 | 6.4 | 39.8 | 7.5 | 39.5 | 7.7 |
| Fatigue | 56.6 | 10.3 | 55.5 | 9.3 | 55.6 | 9.3 | 56.1 | 10.2 | 56.0 | 9.9 |
| Pain Intensity | 50.3 | 10.4 | 50.2 | 7.4 | 53.0 | 6.9 | 51.2 | 6.4 | 51.0 | 7.6 |
| Pain Interference | 57.9 | 10.1 | 58.2 | 9.1 | 60.5 | 7.7 | 59.8 | 7.9 | 59.2 | 8.7 |
| Pain Behavior | 56.4 | 8.2 | 57.0 | 6.5 | 58.2 | 4.3 | 57.5 | 4.9 | 57.3 | 6.0 |
| Sleep Disturbance | 53.7 | 9.3 | 54.6 | 8.2 | 52.9 | 9.5 | 52.8 | 8.6 | 53.4 | 8.8 |
| Depression | 54.6 | 12.1 | 52.9 | 9.8 | 49.9 | 8.7 | 50.4 | 9.3 | 51.7 | 10.0 |
| Anxiety | 56.1 | 12.0 | 52.7 | 9.6 | 50.7 | 9.3 | 50.0 | 8.9 | 51.9 | 10.0 |
| Satisfaction with Social Roles | 44.1 | 9.6 | 42.5 | 8.2 | 42.8 | 9.1 | 43.8 | 9.3 | 43.4 | 9.1 |
Identification of Potential Items
Of the 42 candidate pain quality items, five were more likely to be endorsed by participants with neuropathic pain with moderate to large effect sizes (Cramer's V: 031 - 0.57, all p's < 0.001). These five items characterized pain as: numb, tingly, like pins and needles, stinging, and electrical. In EFA of the development dataset, these five items loaded together on a unique factor with loadings greater than 0.50 for all factor solutions in which 2 through 7 factors were extracted.
Scaling PROMIS PQ-Neuro
Confirmatory factor analysis in the validation dataset supported the unidimensionality of responses to the 5-items with excellent indications of fit (CFI = 0.98 / TLI = 0.96); all factor loadings were statistically significant and greater than 0.75. The highest residual correlation was 0.13; all others were less than 0.10 further supporting unidimensionality of the data.
Responses to the five items were calibrated using IRT, with the metric centered on the mean of the total sample. All items demonstrated adequate fit [p>0.01, S-X2) and were free from problematic local dependency (all LD-X2 indices <10). Discrimination parameters (α) ranged from 1.98 from 4.05 indicating moderate to strong item-level reliability, and mean item difficulty parameters (β) ranged from −0.37 to 2.09 indicating broad coverage of the trait (Table 2). IRT calibration places scores on a logit metric (θ). Scores were linearly transformed to have a mean of 50 and standard deviation of 10 (T-score = θ*10 + 50).
Table 2.
Item level endorsement rates and discrimination (a) and difficulty (b) parameters of 5 items representing neuropathic pain quality
| a | SE | b1 | SE | b2 | SE | b3 | SE | b4 | SE | % endorsed* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | DN | OA | RA | |||||||||||
| In the past 7 days, did your pain feel ... | ||||||||||||||
| Numb | 1.98 | 0.14 | 0.27 | 0.06 | 0.30 | 0.06 | 0.95 | 0.07 | 1.73 | 0.11 | 91.79 | 76.54 | 44.23 | 36.54 |
| Tingly | 3.09 | 0.23 | 0.37 | 0.06 | 0.24 | 0.05 | 0.83 | 0.06 | 1.58 | 0.08 | 86.57 | 80.11 | 51.89 | 45.51 |
| Like pins and needles | 4.05 | 0.37 | 0.31 | 0.05 | 0.19 | 0.05 | 0.70 | 0.05 | 1.22 | 0.06 | 79.10 | 83.98 | 49.06 | 43.77 |
| Stinging | 2.13 | 0.16 | 0.09 | 0.06 | 0.63 | 0.06 | 1.28 | 0.08 | 1.97 | 0.12 | 62.69 | 65.00 | 38.10 | 33.12 |
| Electrical | 2.02 | 0.17 | 0.48 | 0.06 | 0.95 | 0.07 | 1.48 | 0.09 | 2.09 | 0.13 | 49.25 | 54.70 | 29.25 | 20.70 |
endorsed defined as response indicating “a little bit”, “somewhat”, “quite a bit”, or “very much” as opposed to “not at all”.
Sensitivity and Specificity
The sensitivity and specificity of PROMIS PQ-Neuro scores in discriminating between neuropathic and non-neuropathic pain conditions was evaluated using ROC analyses (Table 3). A score threshold of 50 was selected as a cut-point that maximized sensitivity (0.77) and specificity (0.70) across all datasets (Table 3). A score threshold of 43 consistently optimized sensitivity (≥ 0.90), and a threshold of 57 consistently optimized specificity (≥ 0.90).
Table 3.
Sensitivity and specificity of T-score thresholds in predicting neuropathic and non-neuropathic pain conditions
| Dataset | T-score Threshold (≥) | Sensitivity | Specificity | Correctly Classified |
|---|---|---|---|---|
| Development | 43 | 0.90 | 0.38 | 0.60 |
| 50 | 0.76 | 0.73 | 0.74 | |
| 57 | 0.40 | 0.90 | 0.69 | |
| Validation | 43 | 0.93 | 0.42 | 0.64 |
| 50 | 0.77 | 0.67 | 0.71 | |
| 57 | 0.44 | 0.90 | 0.70 | |
| Combined | 43 | 0.92 | 0.40 | 0.62 |
| 50 | 0.77 | 0.70 | 0.73 | |
| 57 | 0.42 | 0.90 | 0.70 |
Validity and Reliability Analyses
Criterion validity was supported by ROC analyses with the area under the curve estimated at 0.80 indicating good predictive ability of T-scores. Known groups validity was supported by the ANOVA comparison of mean T-scores for participants with neuropathic (mean=55.38, 95%CI: 54.50 - 56.26) and non-neuropathic (mean=45.96, 95%CI: 45.20 - 46.72) pain conditions (F=251.74, p<0.0001). No statistically significant differences were observed between those with CN (mean=55.50, 95%CI: 54.13 – 56.88) and DN (mean=55.29, 95%CI: 54.10 - 56.47) nor between those with OA (mean=46.93, 95%CI: 45.43 – 48.42) and RA (mean=45.64, 95%CI: 44.77 – 46.50). Discriminant validity was supported by weak correlations with other health-related domains (r< 0.40) (Table 4).
Table 4.
Correlations* between T-scores of neuropathic pain quality and other health domains
| Neuropathic Pain Quality | 95% Confidence Intervals | ||
|---|---|---|---|
| LB | UB | ||
| PROMIS Pain Measures | |||
| Pain Interference | 0.347 | 0.282 | 0.409 |
| Pain Behavior | 0.367 | 0.303 | 0.428 |
| Pain Intensity | 0.394 | 0.331 | 0.453 |
| PROMIS Global | |||
| Physical Health | −0.380 | −0.441 | −0.316 |
| Mental Health | −0.325 | −0.390 | −0.256 |
| PROMIS-29 Profile (1.0) | |||
| Physical Function | −0.319 | −0.383 | −0.252 |
| Anxiety | 0.383 | 0.319 | 0.443 |
| Depression | 0.342 | 0.276 | 0.404 |
| Fatigue | 0.291 | 0.223 | 0.356 |
| Sleep Disturbance | 0.291 | 0.223 | 0.356 |
| Satisfaction with Social Roles | −0.298 | −0.362 | −0.230 |
all correlations statistically significant with α = 0.05; LB=Lower Bound; UB=Upper Bound
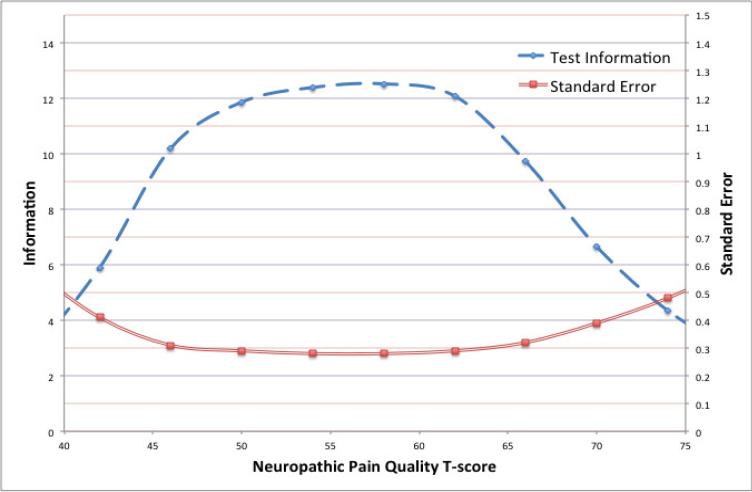
Good internal consistency was indicated by results from the factor analysis and multiple indices of interdependence (Cronbach's alpha = 0.87, item-total correlations corrected for overlap [0.62-0.79], polychoric correlations [0.50 - 0.79]). An expanded evaluation of reliability from the IRT-calibration indicated that item information was maximized (>10) for T-scores between 45 to 65 (Figure 1).
Figure 1.
Reliability of T-scores
CONCLUSIONS
In this study, we used a multistage psychometric approach that included functional evaluations of individual items, factor analytic models, and IRT-based scale calibration to develop the PROMIS PQ-Neuro scale. This 5-item measure discriminates between individuals with neuropathic and non-neuropathic pain conditions and among levels of neuropathic pain quality. The conceptualization and measurement of levels of neuropathic pain were supported by multiple forms of validity evidence (i.e., construct, discriminant, and criterion validity). Moreover, this 5-item measure appears to be reliable and adequately effective at identifying patients with neuropathic involvement in pain generation.
The reliability of PROMIS PQ-Neuro scores was consistent with or higher than the reliability of currently available self-report measures of neuropathic pain including the revised Short form McGill Pain Questionnaire (SF-MPQ-2, neuropathic pain subscale),(36, 37) the Neuropathic Pain Symptom Inventory (NPSI),(38, 39) the Self-report Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS),(40) and the IDpain.(6, 41) More importantly, the PROMIS PQ-Neuro scores demonstrated good sensitivity and specificity in distinguishing neuropathic and non-neuropathic pain. Notably, the results were comparable to other neuropathic measures, but were achieved with fewer items.(5, 39-49) This preliminary validity and reliability evidence supports the use of this new measure in clinical and research applications as an aide to diagnosis and case identification, for monitoring effects of treatment on neuropathic pain quality, and for estimating the prevalence of neuropathic pain in epidemiological investigations.
A self-report measure of NP cannot replace clinical judgment and is not intended as a diagnostic test for NP. Rather, it is intended to be a useful component of a thorough diagnostic or treatment outcome evaluation with regard to pain quality and severity. Standardized assessment of neuropathic pain quality can provide information that is useful and diagnostically relevant when attempting to locate underlying mechanisms of pain generation in a clinical setting.
Limitations & Future Research
In this study, response patterns of participants from four patient populations were evaluated. It is possible that participants from populations with other chronic neuropathic and nociceptive pain conditions describe their pain in systematically different ways. However, more similarities than differences in pain quality descriptors have been reported among individuals with many different NP conditions, suggesting that the clinical expression of NP is likely “transetiological”.(50) Nevertheless, evaluating how PROMIS PQ-Neuro scores function in other pain populations would add to the body of evidence regarding the utility and validity of this new scale. Of particular interest would be evaluations in conditions in which the extent of neuropathic involvement is less clear such as fibromyalgia.(51, 52)
A related limitation pertains to the similarity pain quality descriptors identified in this study to those represented on other self-report measures of NP. The PROMIS PQ-Neuro was not developed to replace currently available measures of NP. To our knowledge, this is the only pain quality scale that was developed using item response theory, which confers several unique benefits, including compatibility with other PROMIS measures, computer adaptive administration through Assessment Center, and a unified approach to score interpretation. The availability of PROMIS item banks assessing Pain-Intensity, -Interference, -Behavior, -Quality and other pain-domains currently under development will collectively provide a unified pain-assessment framework to clinicians and investigators.
An additional limitation relates to pain classification based on disease status and the potentially confounding effects of other known or unknown comorbid conditions on the sensory experience of pain. This is less of a concern for participants in our study with CN and DN who were excluded if physicians suspected pain might not be attributable to neuropathic dysfunction. Those with arthritis however were largely recruited from a national patient registry, which presents separate challenges in identifying the principal underlying source of patient pain. For example, diagnosis of OA or RA for participants recruited from the registry was clinically confirmed, however several participants also had comorbid fibromyalgia. While it was unfeasible to obtain clinical confirmation that arthritis was the primary source of pain for these eight participants, this number is relatively small compared to the full analytic sample and is unlikely to have biased the results in a meaningful way. Nevertheless, future investigations into the diagnostic utility of this measure are warranted in these and other chronic pain populations that carefully establish pain etiology and the physiological processes that lead to distinct sensory profiles of pain quality. Longitudinal evaluations are also warranted to examine potential changes in neuropathic pain quality over time and to evaluate test-retest reliability of this new measure.
Patient-reported pain, like other symptoms, likely represents the aggregate expression of complex biological, cognitive, affective, behavioral, and contextual processes. Moreover, the experience of pain is multi-faceted, and clinical experts have acknowledged the need for more comprehensive pain assessment in clinical research.(53) Valid and reliable measures that are sensitive to systematic differences in pain qualities will likely provide a more informative profile of patient experiences in clinical settings. They also may take on a larger role in advancing our understanding of the mechanisms underlying pain generation and palliation. It is our hope that more robust instrumentation will serve both of these ends and accelerate the development of more effective and personalized clinical treatments of NP.
Acknowledgements
The authors would also like to acknowledge valuable contributions to this project of James Witter, MD, PhD.
Disclosures: This project was funded by the National Institutes of Health under a supplement to the PROMIS Statistical Center, grant number 3U01AR052177-06S1 (PI: David Cella, PhD).
The contents of this manuscript were also developed under a grant from the Department of Education, NIDRR grant number H133P080006 (PI: Allen Heinemann, PhD). However the contents do not necessarily reflect the policy of the Department of Education, and you should not assume endorsement by the Federal Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary results from this study were reported in part at the 19th Annual Meeting of the International Society for Quality of Life Research (ISOQOL), Budapest, Hungary, 24-27 October 2012: Amtmann D, Nowinski C, Cella D, Salem R, Revicki D, Wolfe F, Michaud K, Askew RL. PROMIS Version 2 pain quality item bank discriminates between neuropathic and other types of pain.
Contributor Information
Robert L. Askew, Stetson University.
Karon F. Cook, Northwestern University.
Francis J. Keefe, Duke University.
Cindy J Nowinski, Northwestern University.
David Cella, Northwestern University.
Dennis A. Revicki, Evidera.
Esi M. Morgan DeWitt, Cincinnati Children's Hospital.
Kaleb Michaud, University of Nebraska.
Dace L. Trence, University of Washington.
Dagmar Amtmann, University of Washington.
REFERENCES
- 1.International Association for the Study of Pain Task Force on Taxonomy . IASP Taxonomy. IASP Press; Seattle, WA: 2012. [Google Scholar]
- 2.Sadosky A, McDermott AM, Brandenburg NA, et al. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain practice : the official journal of World Institute of Pain. 2008;8:45–56. doi: 10.1111/j.1533-2500.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 3.van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–62. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Haanpää M, Treede R-D. Diagnosis and classification of neuropathic pain. Pain: Clinical updates. 2010;18:1–6. [Google Scholar]
- 5.Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current medical research and opinion. 2006;22:1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 6.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Current medical research and opinion. 2006;22:1555–65. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MP, Dworkin RH, Gammaitoni AR, et al. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with the Neuropathic Pain Scale. The journal of pain : official journal of the American Pain Society. 2005;6:98–106. doi: 10.1016/j.jpain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MP, Gammaitoni AR, Bolognese JA, et al. The pain quality response profile of pregabalin in the treatment of neuropathic pain. Clin J Pain. 2012;28:683–6. doi: 10.1097/AJP.0b013e31823f9e64. [DOI] [PubMed] [Google Scholar]
- 9.IOM (Institute of Medicine) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington (DC): 2011. [PubMed] [Google Scholar]
- 10.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) Progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–82. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W-H, Revicki DA, Amtmann D, et al. 18th Annual Conference of the International Society for Quality of Life Research. Quality of Life Research; Denver, CO: 2012. Development and Analysis of PROMIS Pain Intensity Scale. [Google Scholar]
- 13.Revicki DA, Chen WH, Harnam N, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–69. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RC, 3rd, Backonja MM. Review of neuropathic pain screening and assessment tools. Curr Pain Headache Rep. 2013;17:363. doi: 10.1007/s11916-013-0363-6. [DOI] [PubMed] [Google Scholar]
- 15.Mulvey MR, Bennett MI, Liwowsky I, et al. The role of screening tools in diagnosing neuropathic pain. Pain management. 2014;4:233–43. doi: 10.2217/pmt.14.8. [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Cook KF, Amtmann D, et al. Exploratory and confirmatory factor analysis of the PROMIS pain quality item bank. Qual Life Res. 2014;23:245–55. doi: 10.1007/s11136-013-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWalt DA, Rothrock N, Yount S, et al. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45:S12–21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks - Plans for the patient-reported outcomes measurement information system (PROMIS). Med Care. 2007;45:S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 20.Bjorner JB, Rose M, Gandek B, et al. Method of administration of PROMIS scales did not significantly impact score level, reliability, or validity. J Clin Epidemiol. 2014;67:108–13. doi: 10.1016/j.jclinepi.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assessment. 1995;7:286–89. [Google Scholar]
- 22.de Vet HC, Ader HJ, Terwee CB, et al. Are factor analytical techniques used appropriately in the validation of health status questionnaires? A systematic review on the quality of factor analysis of the SF-36. Qual Life Res. 2005;14:1203–18. doi: 10.1007/s11136-004-5742-3. dicussion 19-21, 23-4. [DOI] [PubMed] [Google Scholar]
- 23.Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assessment. 1995;7:286–99. [Google Scholar]
- 24.Knafl GJ, Grey M. Factor analysis model evaluation through likelihood cross-validation. Statistical methods in medical research. 2007;16:77–102. doi: 10.1177/0962280206070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood ND, Akloubou Gnonhosou DC, Bowling J. Combining Parallel and Exploratory Factor Analysis in Identifying Relationship Scales in Secondary Data. Marriage & family review. 2015;51:385–95. doi: 10.1080/01494929.2015.1059785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinno A. Exploring the Sensitivity of Horn's Parallel Analysis to the Distributional Form of Random Data. Multivariate Behav Res. 2009;44:362–88. doi: 10.1080/00273170902938969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. Journal of experimental psychology General. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 28.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- 29.Kline RB. Principles and practice of structural equation modeling. 3rd ed. Guilford Press; New York: 2011. [Google Scholar]
- 30.Samejima F. Estimation of Latent Ability Using a Response Pattern of Graded Scores. William Byrd Press; Richmond, VA: 1969. [Google Scholar]
- 31.Embretson SE, Reise SP. Item response theory for psychologists. L. Erlbaum Associates; Mahwah, N.J.: 2000. [Google Scholar]
- 32.Reeve B, Fayers P. Applying item response theory modeling for evaluating questionnaire item and scale properties. In: Fayers P, Hays R, editors. Assessing quality of life in clinical trials: Methods of practice. 2nd ed Oxford University Press; New York: 2005. [Google Scholar]
- 33.Chen WH, Thissen D. Local dependence indexes for item pairs: Using item response theory. J Educ Behav Stat. 1997;22:265–89. [Google Scholar]
- 34.Orlando M, Thissen D. Further investigation of the performance of S-X-2: An item fit index for use with dichotomous item response theory models. Appl Psych Meas. 2003;27:289–98. [Google Scholar]
- 35.Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. Continuing Education in Anaesthesia, Critical Care & Pain. 2008;8:221–23. [Google Scholar]
- 36.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SFMPQ-2). Pain. 2009;144:35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire. J Pain. 2012;13:1250–7. doi: 10.1016/j.jpain.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–57. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Sommer C, Richter H, Rogausch JP, et al. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI). BMC Neurol. 2011;11:104. doi: 10.1186/1471-2377-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett MI, Smith BH, Torrance N, et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. The journal of pain : official journal of the American Pain Society. 2005;6:149–58. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Galvez R, Pardo A, Ceron JM, et al. Linguistic adaptation into Spanish and psychometric validation of the ID-Pain© questionnaire for the screening of neuropathic pain. Med Clin (Barc) 2008;131:572–78. doi: 10.1157/13128018. [DOI] [PubMed] [Google Scholar]
- 42.Backonja MM, Krause SJ. Neuropathic pain questionnaire--short form. Clin J Pain. 2003;19:315–6. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Chan A, Wong S, Chen PP, et al. Validation study of the Chinese Identification Pain Questionnaire for neuropathic pain. Hong Kong Med J. 2011;17:297–300. [PubMed] [Google Scholar]
- 44.De Andres J, Perez-Cajaraville J, Lopez-Alarcon MD, et al. Cultural adaptation and validation of the painDETECT scale into Spanish. Clin J Pain. 2012;28:243–53. doi: 10.1097/AJP.0b013e31822bb35b. [DOI] [PubMed] [Google Scholar]
- 45.Hallstrom H, Norrbrink C. Screening tools for neuropathic pain: can they be of use in individuals with spinal cord injury? Pain. 2011;152:772–9. doi: 10.1016/j.pain.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Kitisomprayoonkul W. Validation study of the Thai ID Pain Scale. J Med Assoc Thai. 2011;94:610–5. [PubMed] [Google Scholar]
- 47.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Padua L, Briani C, Truini A, et al. Consistence and discrepancy of neuropathic pain screening tools DN4 and ID-Pain. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34:373–7. doi: 10.1007/s10072-012-1011-3. [DOI] [PubMed] [Google Scholar]
- 49.Weingarten TN, Watson JC, Hooten WM, et al. Validation of the S-LANSS in the community setting. Pain. 2007;132:189–94. doi: 10.1016/j.pain.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Attal N, Fermanian C, Fermanian J, et al. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138:343–53. doi: 10.1016/j.pain.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Clauw DJ. Fibromyalgia: an overview. The American journal of medicine. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Rowbotham MC. Is fibromyalgia a neuropathic pain syndrome? The Journal of rheumatology Supplement. 2005;75:38–40. [PubMed] [Google Scholar]
- 53.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]