Abstract
There is increasing interest in how exposure to environmental substances can contribute to the onset of Type II diabetes mellitus (T2DM). Impaired insulin release is a hallmark of type I diabetes mellitus and is involved in the progression of T2DM. Both epidemiological and experimental studies show that exposure to the environmental pollutant cadmium (Cd), is associated with hyperglycemia, T2DM and reduced serum insulin. The goal of this review is to examine likely mechanisms of action of Cd-induced dysglycemia based on experimental studies in the literature and from the most recent findings in the Edwards lab. The primary focus of this review will examine how Cd may cause islet dysfunction and subsequent impaired insulin release. Recent findings in the Edwards lab indicate that Cd causes time-dependent and statistically significant changes in fasting leptin, Glucose-dependent Insulinotropic Polypeptide (GIP) and pancreas polypeptide hormone levels in a subchronic animal model of Cd-induced hyperglycemia. This review summarizes the most likely cellular mechanisms by which the ubiquitous environmental contaminant Cd disrupts glucose homeostasis. While individual cellular effects of Cd are reviewed it is likely that no one single mechanism is involved, rather multiple mechanisms exist and work synergistically resulting in islet dysfunction and ultimately dysglycemia.
Keywords: Cadmium, diabetes, GIP, insulin and leptin
1. Introduction
Type II diabetes mellitus (T2DM) is primarily characterized by initial insulin resistance followed by a progressive decline in the function of insulin secreting β-cells. Importantly, only a subset of persons with insulin resistance will develop impaired β-cell function and diabetes, while others will maintain normal glucose levels through a compensatory increase in insulin secretion. The factors leading to β-cell decompensation in some but not in others is the subject of intense research efforts. While genetic factors are likely, diet and other environmental factors are thought to contribute to the development of T2DM [1-3]. Exploring environmental factors contributing towards a reduced function of insulin producing β-cells is therefore highly relevant.
Cadmium (Cd) is an ubiquitous industrial and naturally occurring environmental contaminant that is a group 1 carcinogen with toxic effects in lung, liver, testicular, kidney and bone tissues [4]. The kidney is considered the primary target organ of Cd toxicity with concentrations reaching the highest levels in the renal cortex over time. The biological half-life of Cd is 10 – 30 years and individuals with non-occupational exposure to Cd have the highest levels reached at approximately 50 years of age [5].
While Cd is primarily considered a nephrotoxicant there is a body of literature showing significant correlations between exposure to Cd and the prevalence of prediabetes and/or T2DM [6-8]. Numerous short-term and long-term in vivo Cd exposure models have shown Cd to cause hyperglycemia and disrupt glucose homeostasis in experimental animals [9, 10]. The exact mechanism of action of Cd-induced disruption of glucose homeostasis is unknown. However, overall pancreatic β cell dysfunction and more specifically, impaired glucose stimulated insulin release are likely factors. Cd accumulates within human pancreatic β cells and alters glucose stimulated insulin release in vitro [11]. For individuals with occupational exposure to Cd, fasting serum insulin was shown to be significantly lower in individuals with elevated urinary Cd levels [12]. Additional mechanisms by which Cd may alter glucose homeostasis involve changes in glucose transporter expression in adipocytes [13] and increases in renal and hepatic gluconeogenesis [14, 15]. Taken together, this would indicate that Cd likely has multiple effects in multiple tissues to cause alterations in glucose homeostasis. This review will examine these possible mechanisms and show recent data from the Edwards lab indicating that Cd alters important mediators of metabolism: leptin, glucose-dependent insulinotropic polypeptie (GIP) and pancreas polypeptide.
1.1. Sources of Cd Exposure
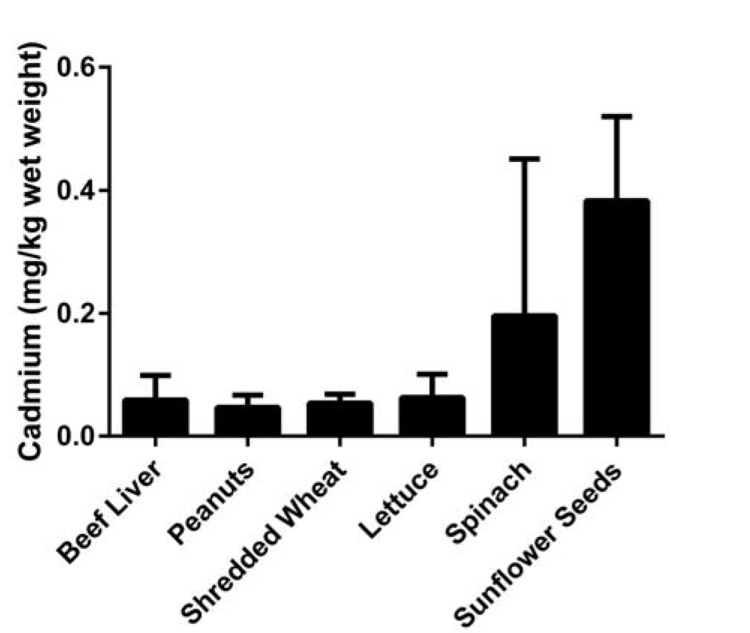
Cd is a widespread environmental and industrial pollutant that currently ranks seventh on the US Environmental Protection Agency (EPA) Priority List of Hazardous Substances. Exposure to Cd can result in damage to the lung, liver, kidney, bone and testes depending upon the dose, route and duration of exposure [16]. Tobacco smoke is the primary source of non-occupational exposure to Cd with each cigarette containing an average of 1.1 µg Cd; 70% of which is present in inhaled smoke [17]. Second to smoking, food is a major source of Cd exposure [18]. Common foods contain amounts of Cd equal to or greater than those found in store-bought beef liver, an organ known to contain elevated Cd, (see Fig. 1). The minimal risk level, which is the estimate of the daily human exposure to a hazardous substance that is likely to be without appreciable risk of adverse non-cancer health effects, is 0.1 µg/kg/day, with chronic oral ingestion of Cd [Agency for Toxic Substances and Disease Registry (ATSDR)]. The EPA analogous measure of toxicity, the reference dose, is 1µg/kg/day for chronic oral Cd ingestion. The World Health Organization (WHO) level is slightly lower with the provisional tolerable monthly intake of 25 µg/kg/month by ingestion. However, the Panel on Contaminants in the Food Chain of the European Food Safety Authority differs significantly with a tolerable weekly intake of 2.5 µg/kg/week or 10.7 µg/kg/month. All of the above organizations established safe levels of Cd exposure based on urinary biomarkers of renal function and urinary Cd data.
Fig. (1).
FDA Total Diet Study (2006-2011) mean ± SD, n=24.
In a 2001 study that used toxicokinetic modeling, the estimated daily dietary intake of Cd for non-smokers was 0.35 ± 0.17 µg/kg/day or 27 ± 12.3 µg/day (mean ± SD) for males living in the US ages 20 – 39 [19]. Slightly lower estimated levels of Cd exposure were reported in females. In a similar 2013 study using data from Thailand, the estimated average dietary Cd intake was 56 µg/day for never-smoker males ages 20 – 39 living in a low exposure area and 224 µg/day in a heavily contaminated area [20]. Therefore Cd has a very narrow safety margin when considering dietary intake levels, especially for cigarette smokers.
1.2. Bioaccumulation of Cd
With long-term Cd exposure, the highest Cd concentration is found in the kidney cortex at concentrations of 5 to 20 fold higher than any other tissue with the order of accumulation being: kidney cortex >> liver > thyroid > pancreas > bone [18, 21, 22]. In all of these studies, Cd content was measured from whole pancreatic tissue samples. Recent studies examining human tissue samples indicate that within the pancreas, Cd may preferentially accumulate in islets [11]. Additional experiments showed model cell lines of pancreatic β cells (MIN6 and 3T3) accumulate Cd in a time-dependent manner [11]. Furthermore, the magnitude of Cd accumulation in functioning, viable islets isolated from Cd-treated animals is similar to the renal cortex (within the same order of magnitude) based on a recent collaborative project between the Edwards and El Muayed labs (manuscript in preparation). Interestingly, isolated islets that were incubated in high glucose (3mg/ml) had lower levels of Cd as compared to islets incubated in low or basal glucose (0.5 mg/ml) solutions.
2. CADMIUM AND HYPERGLYCEMIA / T2DM
Not all studies show Cd to be associated with fasting blood glucose at or above 126 mg/dl. However, since prediabetes is a growing health concern and precursor to T2DM epidemiological and experimental studies showing Cd exposure or effects associated with hyperglycemia are included in the discussion.
2.1. Epidemiological Evidence
There is a growing body of literature showing significant correlations between exposure to Cd, and prevalence of hyperglycemia/T2DM in countries such as: Pakistan [6, 23]; China [24]; USA [7, 25] and Australia [26]. One study has shown no statistically significant association between Cd exposure and diabetes in a heavily Cd-contaminated area of Thailand [27]. However, in a study from the same group the prevalence of diabetes was significantly increased in a 5 year period in a subpopulation with continued high levels of Cd exposure; no change in the prevalence of diabetes was detected in a subpopulation with reduced Cd exposure [28]. Using NHANES data, Wallia et al., showed a significant increase in the odds ratio for prediabetes for individuals with elevated urinary Cd levels while there was a decrease in the estimated insulin resistance and pancreatic β cell function using the Homeostatic Model Assessment, HOMA-IR and HOMA-β, respectively [25].
The most widely used biomarker for Cd exposure in epidemiological studies is urinary Cd. However, the utilization of urinary or blood Cd levels as a measure of Cd exposure has limitations. The internal or absorbed dose of Cd in the renal cortex is superior to blood or urine Cd levels as a measure of low-level, long-term Cd exposure because the concentration of Cd in the renal cortex more accurately represents lifelong Cd exposure [29-31]. Blood Cd concentration only represents recent exposure and does not provide information on total body burden of Cd. Similarly urinary Cd concentration is not straightforward, with low or moderate Cd exposure Cd is almost completely reabsorbed in the proximal tubules. Urinary Cd will not increase significantly until the body burden of Cd is large or kidney damage occurs [29]. This makes interpreting epidemiological Cd data and drawing conclusions from these studies difficult.
2.2. Experimental Studies
Experimental studies using animal models of Cd exposure have shown Cd to have diabetogenic effects in both acute [10] and sub-chronic exposure models [13, 21, 32, 33]. Our own preliminary studies using a subchronic model of Cd exposure in the rat show that Cd caused a gradual elevation in fasting blood glucose levels with significant increases at a time point several weeks prior to any overt signs of Cd-induced renal dysfunction [9]. These effects were associated with a 50% decrease in fasting serum insulin levels and morphological changes within the pancreas.
Similar to epidemiological studies examining Cd, there are limitations or caveats to experimental Cd studies involving animals. Specifically, Cd has a biological half-life measured in decades while the rodents used in most of these studies have a life expectancy of 2 years. As a result, a common and accepted practice is to accelerate or enhance the Cd exposure process by administering Cd at higher daily doses than that to which humans would be exposed. This may make it difficult to directly translate findings from animal studies to human health. However, it is one of the inherent limitations of animal studies that examine the long term effects of Cd. One exception of experimental studies using relatively short term duration of exposure is Kurata et al., (2003) where ovariectomized cynomolgus monkeys were administered two different Cd doses for 13 – 15 months. In that study, animals given the highest Cd dose lost bodyweight at the 6 month experimental time point with fasting blood glucose increasing and fasting insulin decreasing both in a time-dependent manner [34]. Histological examination of pancreatic tissue showed islets that were atrophic with vacuolization and decreased insulin immunolabelling [34].
3. MECHANIMS OF CADMIUM-MEDIATED HYPERGLYCEMIA / T2DM
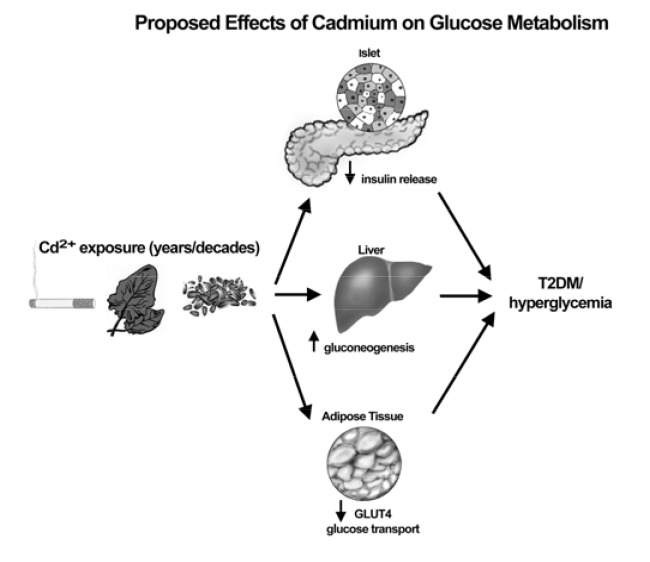
It is clear from the experimental literature that Cd has at the very least a modest diabetogenic effect. This has been shown in variety of short-term and long-term in vivo Cd exposure models [9, 10, 13, 35]. However, there are only a small number of experimental studies that examine the cellular mechanisms of Cd-induced hyperglycemia or diabetes. The few potential mechanisms that have been identified in these studies can be separated into three main categories; increased gluconeogenesis, altered glucose transport and disruption of pancreatic islet function (see Fig. 2). The second half of this paper will review the relevant experimental and mechanistic studies then show novel data from the co-author’s (J. Edwards) lab that reinforces the importance of pancreatic islet function in the etiology of Cd-induced hyperglycemia or diabetes. The literature reviewed here will emphasize studies that involve long term in vivo Cd dosing. However, pertinent in vitro studies with Cd dosing at very low and physiologically relevant concentrations will be included.
Fig. (2).
Summary of general mechanisms of Cd-induced dysglycemia.
3.1. Gluconeogenesis
Gluconeogenesis is an essential process for maintaining glucose homeostasis that accounts for 47% of glucose present in blood after a 15 hour fast in lean, non-diabetic individuals. In T2DM patients, the contribution of gluconeogenesis to total blood glucose is significantly greater [36]. As such, any substance that disrupts gluconeogenesis may be an important aspect in the development of T2DM. In animal studies where rodents were given low-level sub-chronic exposure to Cd, there was a resulting increase in the activity of all four of the key enzymes responsible for gluconeogenesis in both kidney and liver tissues [32]. The increase in activity of the gluconeogenic enzymes was approximately two-fold following Cd exposure and occurred in a dose- and time-dependent manner that was associated with significant increases in blood glucose [15, 35]. Given the importance of gluconeogenesis, further effort is needed to determine by what mechanism Cd alters the activity of gluconeogenic enzymes.
3.2. Glucose Transporters
A hallmark of type-II diabetes is decreased insulin sensitivity that ultimately translates into decreased glucose transport from blood to tissue of various types. Although the literature is limited, there is evidence that Cd may cause a reduction in glucose transport in both adipose and renal tissues. In one study adipocytes isolated from rats previously exposed to Cd had reduced glucose transport activity that was associated with decreased expression of the glucose transporter (GLUT4) [13]. In another study, primary mouse renal cortical cells exposed to Cd in vitro showed a decrease in both glucose uptake and expression of SGLT1, a Na+-dependent glucose symporter [37]. Further studies are needed to sort out the roles of these various organs and their interrelationships in mediating the actions of Cd on glucose transport.
3.3. Pancreatic Islet Dysfunction
Many studies show that Cd decreases serum insulin in animals [9, 33, 34, 38, 39] and humans [12]. However, the mechanism responsible for this effect is unknown. Several likely cellular mechanisms exist that include alterations in: 1) energy metabolism, 2) oxidative stress, 3) Ca channel function and 4) cell-cell adhesion.
Energy Metabolism. β-cell function is very sensitive to alterations in the ratio of ADP/ATP, for review see [40]. The production of ATP, ATPase activity and cytochrome c oxidase expression were decreased in a time-dependent manner in kidney tissue in a study examining long term exposure of Cd in rats [41]. Any substance, including Cd that may alter this ratio would have profound effects on insulin release.
Oxidative Stress. Cd is a well-known inducer of oxidative stress in a variety of tissues and cell types however, few publications exist that examine Cd effects on islets or cell lines that model islets. One study from the El Muayed lab, showed that Cd caused decreases in the reduced form of glutathione in the mouse β cell line, MIN6, and decreased the ratio of oxidized to reduced glutathione in dispersed mouse islets [11]. In addition, the expression of oxidative stress marker genes heme oxygenase 1 and Glutamate-Cysteine Ligase, Modifier Subunit (GCLM) increased significantly in MIN6 cells [11]. In another study, Cd was used to induce the expression of heme oxygenase 1 in the mouse islet cell line, BTC-3 and in the acinar cell line AR42J [42]. Arsenic is another environmental toxicant known to cause oxidative stress that impairs glucose stimulated insulin release with the formation of reactive oxygen species acting as important mediators [43]. With so few publications examining the oxidative effects of Cd on islets there is a need for further study and to address this gap in the literature.
Calcium Channel Function. Electrophysiologists have used Cd for decades as a research tool to investigate Ca channel function by inhibiting Ca conductance in a competitive manner. Using the insulinoma cell line, HIT-T15, Cd accumulation was significantly greater when cells were depolarized in a 20 mM KCl solution. In the presence of the L-type Ca channel inhibitor, nimodipine, Cd accumulation was significantly less [44]. In the same study, nimodipine reduced apoptotsis in HIT-T15 cells exposed to Cd. These are promising findings that support further effort to examine Ca channel blocking effects of Cd as related to insulin release.
Cell-cell Adhesion. Maintenance of cell-cell adhesion is essential for a variety of physiological processes including insulin secretion. The cadherins are a group of transmembrane proteins located at the adherens junction that are responsible for cell-cell adhesion. Recent studies show the essential role cell-cell adhesion and cell adhesion proteins have in islet function with adherens junction proteins E-cadherin [45-47] and β-catenin [48] specifically being essential in glucose-stimulated insulin secretion. Cd alters cell-cell adhesion and the localization of cell adhesion proteins including N- and E-cadherins and β-catenin in kidney tissue [49-51] and E-cadherin and VE-cadherin in the lung [52]. Results of our morphological studies suggest that Cd causes retraction and separation of cells in pancreatic islets [9]. In the canonical Wnt signaling pathway, ligands activate cell surface receptors resulting in a complex cell signaling cascade that leads to the accumulation of β-catenin in the cytosol. From the cytosol, β-catenin can translocate to the nucleus where β-catenin acts as a transcription co-factor and binds with other transcription cofactors such as T cell factor/lymphoid enhancer factor (TCF/LEF) to alter gene expression [53, 54]. Some of the genes that are up-regulated in this process are the proto-oncogenes: c-jun, c-myc and cyclin D1.
4. CHANGES IN METABOLIC BIOMARKER LEVELS DURING LONG-TERM EXPERIMENTAL CADMIUM EXPOSURE
For 12-week long exposure studies, rats were given daily subcutaneous injections of Cd 5 days per week for up to 12 weeks at the dose of 0.6 mg/kg of Cd in the form CdCl2. Animals were purchased from Harlan Laboratories Indianapolis, IN. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Midwestern University, Downers Grove, IL. Animals were housed in an AAALAC accredited facility with 14:10 hour light cycle and access to standard rodent chow and water ad libitum, except that food was withheld 5 hours prior to blood sampling for later analysis for metabolic markers. Rats were anesthetized with ketamine/xylazine (67/7 mg/kg, i.p.) then the abdominal cavity was opened. Blood samples were collected from the exposed vena cava and allowed to coagulate for 45 min at room temperature in microcentrifuge tubes in the presence of dipeptidyl peptidase IV protease inhibitor 1:100 final concentration (catalog #DPP4, Millipore, Billerica, MA) and protease inhibitor cocktail 1:100 final concentration (catalog #P8340, Sigma, St. Louis, MO). After the blood was coagulated the tubes were stored on ice then later centrifuged at 2000g for 15 min at 4°C. The top layer of clear serum was carefully removed and placed in clean microcentrifuge tubes and stored at -80°C until analyzed. The MilliPlex xMAP Kit (Millipore, Billerica, MA) methods were followed according to the manufacturer’s recommended protocol (catalog #RMHMAG-84K-08). MagPix Analyte software package was used to quantify data. The GIP data set had a high degree of variability which is the likely reason no statistically significant changes were detected by the post-tests. Additional markers of metabolism and pancreatic function that were examined but did not show statistically significant changes were: insulin, glucagon, GLP-1, amylin (active) and IL-6 (data not shown).
4.1. Cadmium, Leptin and Islet Function
The relationships between leptin, bodyfat and insulin release are complex and outside the scope of this manuscript, for excellent reviews on this topic please see [55, 56]. In one of the few studies that examined Cd effects on leptin levels, normal and metallothionein-null mice had reduced plasma leptin levels and reduced leptin expression from white adipose tissue following Cd exposure [57]. Furthermore, the metallothionein-null mice had decreased bodyweight and decreased white adipose tissue weight [57]. Previous studies using the same Cd dosing (0.6 mg/kg/day) as the animals used to collect data in Figure 3 showed that Cd exposure resulted in significantly lower body weight over time [58]. Furthermore time to onset of the Cd-induced decrease in weight gain is early and occurs before overt signs of renal dysfunction such as polyuria and proteinuria [49]. Lastly, in a study in which Cd exposure occurred via the oral route (rats were given Cd-containing food pellets), there was a dose- and time-dependent decrease in body weight gain [14]. Interestingly, a study using NHANES data revealed significant inverse relationships between elevated urinary Cd and body mass index or waist circumference in the US population [59]. Experimental studies shown here indicate that during Cd administration, Cd may decrease leptin release by inhibiting adipocyte leptin secretion or cause an overall decrease in adipose tissue. In contrast, other experimental studies examining the effects of Cd following cessation of the administration of Cd, there was a significant increase in individual weight gain and increased fat pad weight (data not shown). Therefore, the effects of Cd on body weight and fat content may be biphasic in nature with in initial loss of bodyweight and fat content during exposure then resulting increase in bodyweight and fat following cessation of exposure.
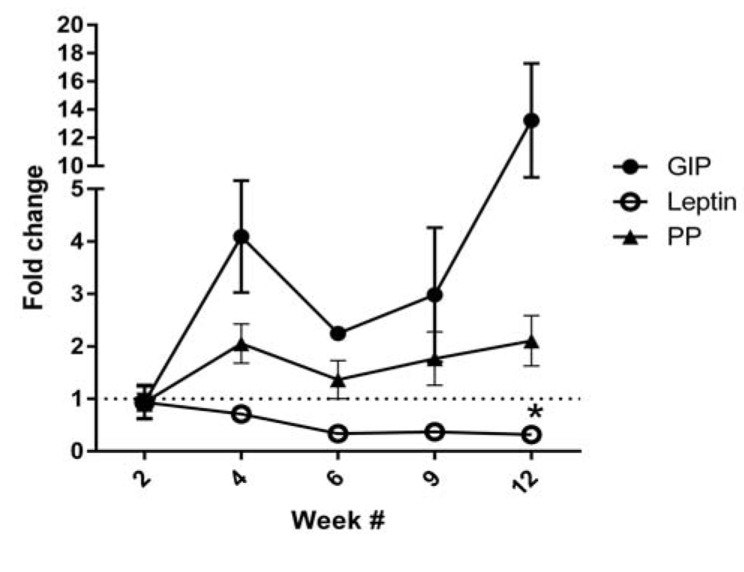
Fig. (3).
Serum samples from 5 hour fasted rats that were given Cd by subcutaneous injection at a dose of 0.6 mg/kg/day for 5 days per week for up to 12 weeks showed statistically significant (2-way ANOVA; p ≤ 0.05) changes in: glucose-dependent insulinotropic polypeptide (GIP), pancreas polypeptide (PP) and leptin. N = 2 for week 6 data, n = 3 for week 9 and n ≥ 4 for weeks 2, 4 and 12. At week 12 only leptin levels were significantly different in Cd vs. control groups as determined by Bonferroni post-tests as indicated by asterisk (*).
4.2. GIP and Cadmium
GIP is a hormone that is released in response to food ingestion from K cells in the duodenum or α-cells in islets and acts directly on β-cells to increase insulin release. The magnitude of increased insulin release was over 6 fold in isolated islets incubated in sub-nano molar concentrations [60]. Our lab has previously reported that Cd causes decreased serum insulin after 12 weeks of Cd exposure at 0.6 mg/kg/day [9]. That there was no change in insulin despite a 15 fold increase in GIP in 12 week Cd treated animals is significant given that GIP increases both basal and glucose-stimulated insulin release [60]. Pancreatic tissue was co-immuno-labelled for GIP and insulin to examine if the change in GIP was due to alterations in islet GIP release. There were no apparent changes in GIP labelling intensity or magnitude in islets (data not shown). As such, the most likely source of serum GIP in the Cd treated animals are the K cells located in the small intestine.
4.3. Pancreas Polypeptide
The source of pancreas polypeptide (PP) are the PP cells within islets and cells scattered throughout the exocrine pancreas and pancreatic duct epithelium. Like GIP, PP is released in response to food ingestion. However, unlike GIP, PP acts to decrease insulin release [61].
5. CONCLUSION
In the last few years, data have emerged that islets accumulate Cd at higher levels compared to other tissues. Since Cd is present at elevated levels it is logical to assume Cd exerts direct effects on islets and likely impairs insulin release. Several studies outlined above support this view. However, an additional confounding factor to the potential direct effects of Cd in islets is that Cd also causes renal dysfunction, which can result in altered islet function and dysglycemia. Considering this, the argument could be made for increased use of in vitro models that specifically examine the effects of Cd on islet function. One consideration for in vitro Cd studies is to not only examine the effects of the ionic form of Cd but to study the effects of Cd-conjugates (e.g. Cd-glutathione, Cd-metallothionein) that are formed after Cd biotransformation in vivo.
It is unclear what role Cd may have in altering body weight or body fat content. Since use of tobacco products is the number one non-occupational source of Cd exposure, additional insights may be gained by examining never-smokers, smokers and ex-smokers. Of these groups, ex-smokers are most at risk for being overweight and obese [62]. Considering the effects of Cd from experimental studies, it is possible that Cd has effects to decrease bodyweight and obesity during smoking exposure then cause an increase in bodyweight and obesity during smoking cessation.
How important the exposure to environmental substances is in determining the likelihood that a person develops either T2DM or prediabetes remains to be determined. However, data shown here demonstrate that long term Cd exposure resulted in disruption of several markers of metabolism and pancreatic function as indicated by changes in serum GIP, PP and leptin. Based on these initial observations, further experiments are warranted to investigate the diabetgenic effects of Cd.
ACKNOWLEDGEMENTS
We would like to thank Victoria Sears and Peter Lamar for their effort in formatting and overall preparation of this manuscript.
LIST OF ABBREVIATIONS
- Cd
Cadmium
- GIP
Glucose-dependent Insulinotropic Polypeptide
- PP
Pancreas Polypeptide
- T2DM
Type II Diabetes Mellitus
CONFLICT OF INTEREST
Funding for this study was provided by Midwestern University as part of Dr. Edwards’s startup funds. Both authors have no conflicts of interest.
REFERENCES
- 1.LeRoith D., Olefsky J., Taylor S., editors. 2003. [Google Scholar]
- 2.Longnecker M.P., Daniels J.L. Environmental contaminants as etiologic factors for diabetes. Environ. Health Perspect. 2001;109(Suppl. 6):871–876. doi: 10.1289/ehp.01109s6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajchenberg B.L. beta-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 2007;28(2):187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 4.Järup L., Berglund M., Elinder C.G., Nordberg G., Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;24(Suppl. 1):1–51. [PubMed] [Google Scholar]
- 5.Friberg L. Cadmium and the kidney. Environ. Health Perspect. 1984;54:1–11. doi: 10.1289/ehp.84541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolachi N.F., Kazi T.G., Afridi H.I., Kazi N., Khan S., Kandhro G.A., Shah A.Q., Baig J.A., Wadhwa S.K., Shah F., Jamali M.K., Arain M.B. Status of toxic metals in biological samples of diabetic mothers and their neonates. Biol. Trace Elem. Res. 2011;143(1):196–212. doi: 10.1007/s12011-010-8879-7. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz G.G., Il’yasova D., Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26(2):468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 8.Satarug S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr. Drug Metab. 2012;13(3):257–271. doi: 10.2174/138920012799320446. [DOI] [PubMed] [Google Scholar]
- 9.Edwards J.R., Prozialeck W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009;238(3):289–293. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell R.R., Early J.L., Nonavinakere V.K., Mallory Z. Effect of cadmium on blood glucose level in the rat. Toxicol. Lett. 1990;54(2-3):199–205. doi: 10.1016/0378-4274(90)90184-N. [DOI] [PubMed] [Google Scholar]
- 11.El Muayed M., Raja M.R., Zhang X., MacRenaris K.W., Bhatt S., Chen X., Urbanek M., O’Halloran T.V., Lowe W.L., Jr Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4(6):405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei L.J., Chen L., Jin T.Y., Nordberg M., Chang X.L. Estimation of benchmark dose for pancreatic damage in cadmium-exposed smelters. Toxicol. Sci. 2007;97(1):189–195. doi: 10.1093/toxsci/kfm016. [DOI] [PubMed] [Google Scholar]
- 13.Han J.C., Park S.Y., Hah B.G., Choi G.H., Kim Y.K., Kwon T.H., Kim E.K., Lachaal M., Jung C.Y., Lee W. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch. Biochem. Biophys. 2003;413(2):213–220. doi: 10.1016/S0003-9861(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 14.Rajanna B., Hobson M., Reese J., Sample E., Chapatwala K.D. Chronic hepatic and renal toxicity by cadmium in rats. Drug Chem. Toxicol. 1984;7(3):229–241. doi: 10.3109/01480548409035105. [DOI] [PubMed] [Google Scholar]
- 15.Chapatwala K.D., Boykin M., Butts A., Rajanna B. Effect of intraperitoneally injected cadmium on renal and hepatic gluconeogenic enzymes in rats. Drug Chem. Toxicol. 1982;5(3):305–317. doi: 10.3109/01480548209041060. [DOI] [PubMed] [Google Scholar]
- 16.Toxicological profile for cadmium. http://www.atsdr.cdc.gov/toxprofiles/ Last accessed 3/11/2015. 2015.
- 17.Nandi M., Slone D., Jick H., Shapiro S., Lewis G.P. Cadmium content of cigarettes. Lancet. 1969;2(7634):1329–1330. doi: 10.1016/S0140-6736(69)90865-4. [DOI] [PubMed] [Google Scholar]
- 18.Satarug S., Garrett S.H., Sens M.A., Sens D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhury H., Harvey T., Thayer W.C., Lockwood T.F., Stiteler W.M., Goodrum P.E., Hassett J.M., Diamond G.L. Urinary cadmium elimination as a biomarker of exposure for evaluating a cadmium dietary exposure--biokinetics model. J. Toxicol. Environ. Health A. 2001;63(5):321–350. doi: 10.1080/15287390152103643. [DOI] [PubMed] [Google Scholar]
- 20.Satarug S., Swaddiwudhipong W., Ruangyuttikarn W., Nishijo M., Ruiz P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013;121(5):531–536. doi: 10.1289/ehp.1104769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei L.J., Jin T.Y., Zhou Y.F. Insulin expression in rats exposed to cadmium. Biomed. Environ. Sci. 2007;20(4):295–301. [PubMed] [Google Scholar]
- 22.Uetani M., Kobayashi E., Suwazono Y., Honda R., Nishijo M., Nakagawa H., Kido T., Nogawa K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals. 2006;19(5):521–525. doi: 10.1007/s10534-005-5619-0. [DOI] [PubMed] [Google Scholar]
- 23.Afridi H.I., Kazi T.G., Kazi N., Jamali M.K., Arain M.B., Jalbani N., Baig J.A., Sarfraz R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008;80(2):280–288. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Lei L., Jin T., Nordberg M., Nordberg G.F. Plasma metallothionein antibody, urinary cadmium, and renal dysfunction in a Chinese type 2 diabetic population. Diabetes Care. 2006;29(12):2682–2687. doi: 10.2337/dc06-1003. [DOI] [PubMed] [Google Scholar]
- 25.Wallia A., Allen N.B., Badon S., El Muayed M. Association between urinary cadmium levels and prediabetes in the NHANES 2005-2010 population. Int. J. Hyg. Environ. Health. 2014;217(8):854–860. doi: 10.1016/j.ijheh.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haswell-Elkins M., Satarug S., O’Rourke P., Moore M., Ng J., McGrath V., Walmby M. Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ. Res. 2008;106(3):379–383. doi: 10.1016/j.envres.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Swaddiwudhipong W., Mahasakpan P., Limpatanachote P., Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ. Res. 2010;110(6):612–616. doi: 10.1016/j.envres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Swaddiwudhipong W., Limpatanachote P., Mahasakpan P., Krintratun S., Punta B., Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environ. Res. 2012;112:194–198. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Prozialeck W.C., Edwards J.R. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals. 2010;23(5):793–809. doi: 10.1007/s10534-010-9288-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17(5):519–523. doi: 10.1023/B:BIOM.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- 31.Fowler B.A. Monitoring of human populations for early markers of cadmium toxicity: a review. Toxicol. Appl. Pharmacol. 2009;238(3):294–300. doi: 10.1016/j.taap.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Merali Z., Singhal R.L. Protective effect of selenium on certain hepatotoxic and pancreotoxic manifestations of subacute cadmium administration. J. Pharmacol. Exp. Ther. 1975;195(1):58–66. [PubMed] [Google Scholar]
- 33.Merali Z., Singhal R.L. Diabetogenic effects of chronic oral cadmium adminstration to neonatal rats. Br. J. Pharmacol. 1980;69(1):151–157. doi: 10.1111/j.1476-5381.1980.tb10895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurata Y., Katsuta O., Doi T., Kawasuso T., Hiratsuka H., Tsuchitani M., Umemura T. Chronic cadmium treatment induces islet B cell injury in ovariectomized cynomolgus monkeys. Jpn. J. Vet. Res. 2003;50(4):175–183. [PubMed] [Google Scholar]
- 35.Rajanna B., Hobson M., Reese J., Sample E., Chapatwala K.D. Chronic hepatic and renal toxicity by cadmium in rats. Drug Chem. Toxicol. 1984;7(3):229–241. doi: 10.3109/01480548409035105. [DOI] [PubMed] [Google Scholar]
- 36.Gastaldelli A., Baldi S., Pettiti M., Toschi E., Camastra S., Natali A., Landau B.R., Ferrannini E. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes. 2000;49(8):1367–1373. doi: 10.2337/diabetes.49.8.1367. [DOI] [PubMed] [Google Scholar]
- 37.Blumenthal S.S., Ren L., Lewand D.L., Krezoski S.K., Petering D.H. Cadmium decreases SGLT1 messenger RNA in mouse kidney cells. Toxicol. Appl. Pharmacol. 1998;149(1):49–54. doi: 10.1006/taap.1997.8353. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson T., Rorsman F., Berggren P.O., Hellman B. Accumulation of cadmium in pancreatic beta cells is similar to that of calcium in being stimulated by both glucose and high potassium. Biochim. Biophys. Acta. 1986;888(3):270–277. doi: 10.1016/0167-4889(86)90225-9. [DOI] [PubMed] [Google Scholar]
- 39.Jin T., Nordberg G.F., Sehlin J., Leffler P., Wu J. The susceptibility of spontaneously diabetic mice to cadmium-metallothionein nephrotoxicity. Toxicology. 1994;89(2):81–90. doi: 10.1016/0300-483X(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 40.Jezek P., Dlaskova A., Plecita-Hlavata L. Redox homeostasis in pancreatic beta cells. 2015. [DOI] [PMC free article] [PubMed]
- 41.Takebayashi S., Jimi S., Segawa M., Takaki A. Mitochondrial DNA deletion of proximal tubules is the result of itai-itai disease. Clin. Exp. Nephrol. 2003;7(1):18–26. doi: 10.1007/s101570300002. [DOI] [PubMed] [Google Scholar]
- 42.Sato H., Siow R.C., Bartlett S., Taketani S., Ishii T., Bannai S., Mann G.E. Expression of stress proteins heme oxygenase-1 and -2 in acute pancreatitis and pancreatic islet betaTC3 and acinar AR42J cells. FEBS Lett. 1997;405(2):219–223. doi: 10.1016/S0014-5793(97)00191-9. [DOI] [PubMed] [Google Scholar]
- 43.Yang B., Fu J., Zheng H., Xue P., Yarborough K., Woods C.G., Hou Y., Zhang Q., Andersen M.E., Pi J. Deficiency in the nuclear factor E2-related factor 2 renders pancreatic β-cells vulnerable to arsenic-induced cell damage. Toxicol. Appl. Pharmacol. 2012;264(3):315–323. doi: 10.1016/j.taap.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavazzo P., Morelli E., Marchetti C. Susceptibility of insulinoma cells to cadmium and modulation by L-type calcium channels. Biometals. 2005;18(2):131–142. doi: 10.1007/s10534-004-5789-1. [DOI] [PubMed] [Google Scholar]
- 45.Rogers G.J., Hodgkin M.N., Squires P.E. E-cadherin and cell adhesion: a role in architecture and function in the pancreatic islet. Cell. Physiol. Biochem. 2007;20(6):987–994. doi: 10.1159/000110459. [DOI] [PubMed] [Google Scholar]
- 46.Jaques F., Jousset H., Tomas A., Prost A.L., Wollheim C.B., Irminger J.C., Demaurex N., Halban P.A. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology. 2008;149(5):2494–2505. doi: 10.1210/en.2007-0974. [DOI] [PubMed] [Google Scholar]
- 47.Bosco D., Rouiller D.G., Halban P.A. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J. Endocrinol. 2007;194(1):21–29. doi: 10.1677/JOE-06-0169. [DOI] [PubMed] [Google Scholar]
- 48.Wells J.M., Esni F., Boivin G.P., Aronow B.J., Stuart W., Combs C., Sklenka A., Leach S.D., Lowy A.M. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev. Biol. 2007;7:4–22. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prozialeck W.C., Lamar P.C., Lynch S.M. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003;189(3):180–195. doi: 10.1016/S0041-008X(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 50.Chakraborty P.K., Scharner B., Jurasovic J., Messner B., Bernhard D., Thévenod F. Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicol. Lett. 2010;198(1):69–76. doi: 10.1016/j.toxlet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Thévenod F., Wolff N.A., Bork U., Lee W.K., Abouhamed M. Cadmium induces nuclear translocation of beta-catenin and increases expression of c-myc and Abcb1a in kidney proximal tubule cells. Biometals. 2007;20(5):807–820. doi: 10.1007/s10534-006-9044-9. [DOI] [PubMed] [Google Scholar]
- 52.Pearson C.A., Lamar P.C., Prozialeck W.C. Effects of cadmium on E-cadherin and VE-cadherin in mouse lung. Life Sci. 2003;72(11):1303–1320. doi: 10.1016/S0024-3205(02)02379-2. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson W.J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman J. 20 years of leptin: leptin at 20: an overview. J. Endocrinol. 2014;223(1):T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 56.Kieffer T.J., Habener J.F. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2000;278(1):E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami T., Nishiyama K., Kadota Y., Sato M., Inoue M., Suzuki S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol. Appl. Pharmacol. 2013;272(3):625–636. doi: 10.1016/j.taap.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Prozialeck W.C., Edwards J.R., Vaidya V.S., Bonventre J.V. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol. Appl. Pharmacol. 2009;238(3):301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padilla M.A., Elobeid M., Ruden D.M., Allison D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public Health. 2010;7(9):3332–3347. doi: 10.3390/ijerph7093332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujita Y., Wideman R.D., Asadi A., Yang G.K., Baker R., Webber T., Zhang T., Wang R., Ao Z., Warnock G.L., Kwok Y.N., Kieffer T.J. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology. 2010;138(5):1966–1975. doi: 10.1053/j.gastro.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 61.Lonovics J., Guzman S., Devitt P., Hejtmancik K.E., Suddith R.L., Rayford P.L., Thompson J.C. Release of pancreatic polypeptide in humans by infusion of cholecystokinin. Gastroenterology. 1980;79(5 Pt 1):817–822. [PubMed] [Google Scholar]
- 62.Xu F., Yin X.M., Wang Y. The association between amount of cigarettes smoked and overweight, central obesity among Chinese adults in Nanjing, China. Asia Pac. J. Clin. Nutr. 2007;16(2):240–247. [PubMed] [Google Scholar]