Abstract
Plasmacytic differentiation may occur in almost all small B cell lymphomas (SBLs), although it varies from being uniformly present (as in lymphoplasmacytic lymphoma (LPL)) to very uncommon (as in mantle cell lymphomas (MCLs)). The discovery of MYD88 L265P mutations in the vast majority of LPLs has had a major impact on the study of these lymphomas. Review of the cases contributed to the 2014 European Association for Haematopathology/Society for Hematopathology slide workshop illustrated how mutational testing has helped refine the diagnostic criteria for LPL, emphasizing the importance of identifying a clonal monotonous lymphoplasmacytic population and highlighting how LPL can still be diagnosed with extensive nodal architectural effacement, very subtle plasmacytic differentiation, follicular colonization, or uncommon phenotypes such as CD5 or CD10 expression. MYD88 L265P mutations were found in 11/11 LPL cases versus only 2 of 28 other SBLs included in its differential diagnosis. Mutational testing also helped to exclude other cases that would have been considered LPL in the past. The workshop also highlighted how plasmacytic differentiation can occur in chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, SOX11 negative MCL, and particularly in marginal zone lymphomas, all of which can cause diagnostic confusion with LPL. The cases also highlighted the difficulty in distinguishing lymphomas with marked plasmacytic differentiation from plasma cell neoplasms. Some SBLs with plasmacytic differentiation can be associated with amyloid, other immunoglobulin deposition, or crystal-storing histiocytosis, which may obscure the underlying neoplasm. Finally, although generally indolent, LPL may transform, with the workshop cases suggesting a role for TP53 abnormalities.
Keywords: Lymphoplasmacytic lymphoma, MYD88, Marginal zone lymphoma, Mantle cell lymphoma, Chronic lymphocytic leukemia, Follicular lymphoma, Plasmacytic differentiation
Introduction
Plasma cells are the ultimate effector cells of the humoral immune system, developing from lymphoid cells. While in many cases malignant lymphomas represent a block in the normal maturational sequence, the block is incomplete in a variable proportion of the different small B cell lymphomas (SBLs), ranging from some in which plasma cell differentiation is expected (lymphoplasmacytic lymphoma (LPL)) to others in which it is very infrequent (mantle cell lymphoma (MCL)). Furthermore, the extent of plasmacytic differentiation can vary from minimal, only recognizable with the aid of immunohistochemical stains, to very extensive with little or perhaps no remaining lymphoid component. Thus, plasmacytic differentiation does not define any specific type of B cell lymphoma, and their categorization should be based on the more specific type of lymphoid cells present, as assessed from their morphologic, phenotypic, and molecular/cytogenetic features and aided by the clinical presentation. This manuscript summarizes the 2014 European Association for Haematopathology (EAHP)/Society for Hematopathology (SH) slide workshop sessions that dealt with SBL with plasmacytic differentiation. It highlights the contributions of next generation sequencing with the recent discovery of MYD88 L265P mutations in the vast majority of LPLs and relatively few other SBLs. It will concentrate on the issues addressed at the workshop with more comprehensive reviews of LPL published elsewhere [9, 27, 45, 53].
Lymphoplasmacytic lymphoma and the impact of MYD88 L265P mutations
According to the 2008 monograph on the WHO classification of hematolymphoid tumors, LPL “is a neoplasm of small B lymphocytes, plasmacytoid lymphocytes, and plasma cells, usually involving bone marrow (BM), sometimes lymph nodes and spleen, which does not fulfill the criteria for any of the other small B cell lymphoid neoplasms that may also have plasmacytic differentiation. Because the distinction between LPL and one of these other lymphomas, especially some marginal zone lymphomas (MZLs), is not always clear-cut, some cases may need to be diagnosed as a small B cell lymphoma with plasmacytic differentiation and a differential diagnosis provided” [49]. Thus, it is a diagnosis of exclusion, in part as “No specific chromosomal or oncogene abnormalities are recognized…” [49]. Deletion 6q, reported in >50 % BM-based LPL, and trisomy 4, reported in approximately 20 % of LPL, are not specific findings and were both found in at least one of the workshop cases (case 267, submitted by K. Gill) (Fig. 1) [7, 52].
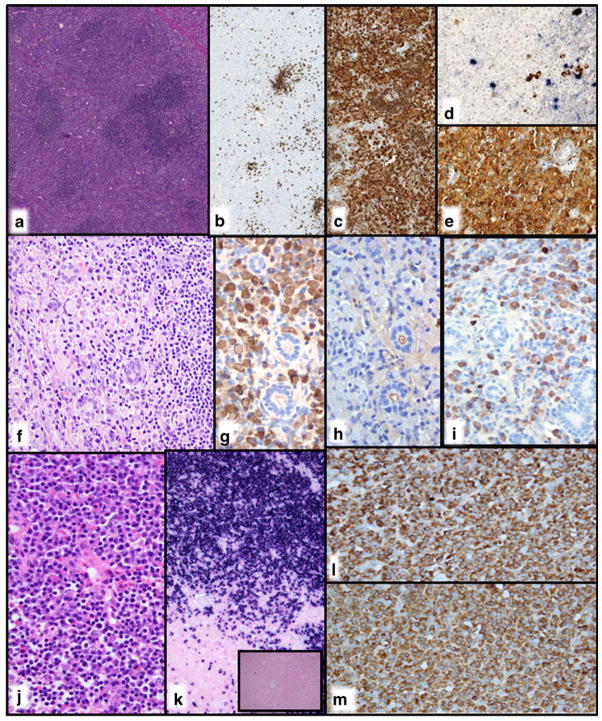
Fig. 1.
CD10+ lymphoplasmacytic lymphoma with MYD88 L265P mutation and typical cytogenetic findings. a Note the monotonous cell population between the widely patent sinuses, typical for LPL. b There are many small lymphoid cells admixed with obvious plasmacytoid and plasma cells. The infiltrate is c CD20+ and d CD10+. e Classical cytogenetics demonstrated 47,XX,+4,i(6)(p10) in 6 of 19 cells. The latter abnormality is equivalent to deletion 6q (case 267, courtesy of K. Gill)
The lack of a LPL-associated molecular marker is now a thing of the past with the discovery of MYD88 L265P mutations in the vast majority of LPLs/Waldenström macroglobulinemia (WM) [56]. Although some studies have reported more variable results, the literature supports the very strong association of this mutation with LPL and its presence in a subset of IgM but not IgG or IgA monoclonal gammopathy of undetermined significance and not in plasma cell myelomas (PCMs) even of IgM type (Fig. 2). MYD88 L265P mutations are also reported in a small proportion of other SBLs and a subset of large B cell lymphomas of non-germinal center/activated B cell type, particularly those of leg type and those at immune privileged sites (CNS and testis) [25, 36, 62]. The variable results for the reported frequency of MYD88 mutation in LPL and other lymphoid neoplasms relate in part to use of detection methods with different sensitivities, differences in the proportion of neoplastic cells in the samples studied, possible inclusion of misdiagnosed cases with a lack of tissue confirmation in some studies, and some results based on investigation of very few cases. Varying diagnostic criteria used in the studies of non-LPL SBLs will greatly affect the frequency of finding MYD88 mutations, as overdiagnosis of LPL will result in a smaller proportion of MYD88-mutated cases and underdiagnosis will result in more non-LPLs demonstrating the mutation. Among the workshop cases tested, MYD88 L265P mutation was present in 11/11 LPLs (including three with transformation), 1/13 nodal marginal zone lymphomas (NMZL, including none of five with plasmacytic differentiation), 0/5 other SBLs (two extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (MALT), two splenic marginal zone lymphomas (SMZLs), and one unclassified), 0/4 chronic lymphocytic leukemia/small lymphocytic lymphomas (CLL/SLL), 0/3 follicular lymphoma (FL), and 1/3 MCLs with plasmacytic differentiation.
Fig. 2.
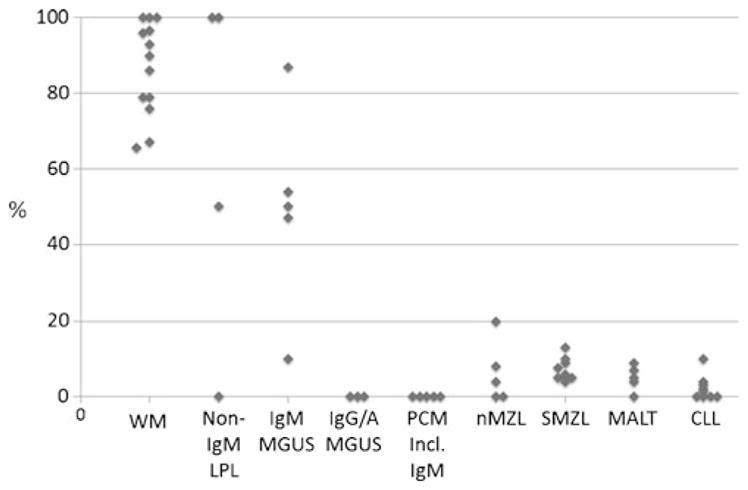
Frequency of MYD88 L265P mutations in B cell neoplasms. Compilation of numerous studies reported in the literature demonstrates that many but not all LPL have MYD88 L265P mutations even if they are not IgM+. Some cases of IgM MGUS are mutated but not IGG/A MGUS nor myeloma even if IgM+. A small proportion of other small B cell lymphomas are mutated (see text). In addition, up to about one third of non-GC/ABC-type DLBCL have MYD88 L265P mutations, with a higher proportion of leg-type DLBCL, CNS, and testicular DLBCL (not illustrated in figure). References available upon request
The workshop cases illustrated many of the classic histologic findings in tissue-based LPL: widely patent sinuses, sparse follicles, prominent plasma cells (sometimes with Dutcher bodies), hemosiderin, and increased mast cells (Figs. 1, 3, and 4a–d). They also illustrated the impact that MYD88 mutational status has had on our diagnostic criteria for LPL. Consistent with the conclusions of a recently published study, the workshop cases highlighted the importance of a relatively monotonous lymphoplasmacytic proliferation in the diagnosis of LPL, the subtle nature of the plasmacytic component in some cases, extensive architectural effacement in some cases, and significant follicular colonization in a minority of cases (Figs. 1, 3, and 4a–d) [19]. Caution is advised since, in addition to a paucity of frank plasma cells, polytypic plasma cells are present in some cases, a finding that would usually (but inappropriately) be taken to exclude LPL. While the majority of the LPLs were present in lymph nodes (with bone marrow involvement in five of six cases with available data), there were two ocular LPLs (cases 80 and 81, submitted by L. Venkatraman and M. Catherwood) and one in a myelolipoma (case 292, A. Staiger). One of the ocular cases is similar to one from the 2009 SH/EAHP workshop representing a recurrence in a patient known to have LPL/WM [27]. Both had prominent follicular colonization, and one had polykaryocytes as are typically found in ocular MALT lymphomas [44]. The diagnosis of LPL rested largely on the monotonous lymphoplasmacytic infiltrate, the MYD88 L265P mutation, and in one case, the previously documented WM.
Fig. 3.
CD5+ lymphoplasmacytic lymphoma with MYD88 L265P mutation and limited morphologic plasmacytic features. a Note the monotonous cell population between the widely patent sinuses, typical for LPL. b There are numerous small lymphoid cells and focal hemosiderin. c Tryptase stain highlights prominent mast cells. The B cell population is d CD3−, e CD5+, and f LEF1− (positive T cells are present). g An IgM stain highlights the mostly perisinus plasmacytic component (case 190, courtesy of X. Zhao et al.)
Fig. 4.
a–d Lymphoplasmacytic lymphoma with MYD88 L265P mutation, marked follicular colonization, and extensive architectural effacement. a There is architectural effacement with some hyalinized small vessels and other sclerosis. b The CD21 immunohistochemical stain highlights the striking follicular colonization. c Small lymphocytes with admixed plasmacytoid and plasmacytic cells as well as hemosiderin are present. d The kappa stain showed varying degrees of cytoplasmic positivity and highlighted Dutcher bodies (arrows). A lambda stain showed only non-specific staining (not shown) (case 80, courtesy of L. Venkatraman et al.). e Favor mantle cell lymphoma (cyclin D1+ but without demonstrable CCND1 rearrangement) with plasmacytic differentiation. Note how easily this lymphoma could be confused with a lymphoplasmacytic lymphoma, with its lymphoplasmacytic proliferation and a Dutcher body (arrow). The cyclin D1 staining was considered incompatible with the diagnosis of lymphoplasmacytic lymphoma or marginal zone lymphoma and the wild-type MYD88 also very consistent with the panel’s diagnosis (case 46, courtesy of M. R. Ashton-Key)
MYD88 mutational studies also suggest that some cases formerly considered LPL should be excluded from that category. Specifically, other than in cases undergoing transformation (see discussion below), the lack of MYD88 mutations in “polymorphic LPL” suggests that these cases are better classified as something else [19]. Similarly, gamma heavy chain disease, which can resemble LPL, also lacks the mutation and thus does not represent a form of LPL [18]. It has been suggested that the lymphoplasmacytic proliferations associated with primary cold agglutinin disease are also not MYD88 mutated [38, 47], but 27 % of MYD88-mutated WM in one study were reported to have associated cold agglutinin disease [37]. Finally, it has been reported that patients with WM lacking the MYD88 L265P mutation have an adverse prognosis and a lower response to ibrutinib [54, 55]. Cases of LPL with wild-type MYD88 are, however, more likely to be diagnosed in a less uniform fashion. Furthermore, as also illustrated at the workshop, some SBLs with plasmacytic differentiation and wild-type MYD88 must remain unclassifiable.
Although not a focus of the workshop, it is also of interest that WHIM-like CXCR4 mutations of either nonsense (most frequently CXCR4S338X) or frameshift type are present in approximately 27–36 % of WM/LPL, 20 % of IgM monoclonal gammopathy of undetermined significance (MGUS), and in only a very small proportion of some other B cell lymphomas [20, 41, 47, 54, 55]. These cases have some clinicopathologic associations and therapeutic implications, although survival differences have not been documented.
LPL is phenotypically diverse
The 2009 SH/EAHP workshop highlighted how some LPLs are CD5 (17 %), CD10 (16 %), and/or CD23 (58 %) positive (complete discussion in reference) [27]. The LPLs presented in Istanbul, which all had MYD88 L265P mutations, once again highlighted this point with 4/8 (50 %) CD10+ (Fig. 1d) in the absence of BCL6 and in one case, also LMO2. Although often diffuse and sometimes with plasma cell positivity, the CD10 positivity in one case was restricted to BCL2+ and BCL6 essentially negative follicles (case 222, submitted by M. Parrens). In the absence of evidence for a coexisting FL, this might represent follicular colonization, with CD10 upregulation in the follicle environment. These cases emphasize that not all CD10+ B cell lymphomas are of germinal center cell type, a potential diagnostic trap in making major diagnostic decisions based on flow cytometric studies in fine needle aspirations. Only 1/8 (13 %) LPLs in this workshop was CD5+ (Fig. 3e) (case 190, submitted by X. Zhao), and a different single case was focally CD23+ (case 200, submitted by L. Hock). Some also report CD25 expression to be common in LPL, potentially causing confusion with hairy cell leukemia (HCL), except that other HCL markers are negative [35]. Phenotypic studies are also useful in distinguishing the plasma cells in LPL from those in a true plasma cell neoplasms with the usual finding of CD45+ and CD19+ plasma cells in LPL that are more likely to be PAX5+ and IRF4/MUM1− than the plasma cells in a plasma cell neoplasm (or MZL) [27, 40, 42].
When should testing for MYD88 mutations be performed?
Testing for MYD88 mutations is of greatest benefit in SBLs with plasmacytic differentiation in which LPL is a strong possibility but where the findings are not definitive. A positive result should lead to a diagnosis of LPL, whereas a negative result would preclude definitive classification. If a diagnosis of LPL is not in the differential diagnosis, evaluation of MYD88 is not recommended at this time because a small proportion of non-LPLs have MYD88 L265P mutations, and therefore, a positive result would not alter the diagnosis [19, 30]. Mutational testing might be of value, even in definite LPL, if its presence was required for a more specific targeted therapy or if, as noted above, its absence implies a more aggressive disease [54, 55]. Mutational testing can also be helpful if PCM is in the differential diagnosis since, even if IgM+, it lacks MYD88 L265P mutations.
Plasmacytic differentiation in small B cell lymphomas, other than LPL and marginal zone lymphomas
Chronic lymphocytic leukemia/small lymphocytic lymphoma
Some degree of plasmacytic differentiation is well-recognized in CLL/SLL even though the vast majority of cases lack differentiation to frank plasma cells. In the past, some CLLs/SLLs were classified as “lymphoplasmacytoid” lymphoma in the Kiel classification because of this (as opposed to LPL which had frank plasma cells). Cytoplasmic immunoglobulin can be documented in paraffin-embedded sections in the majority of CLLs/SLLs, and proliferation centers characteristically are IRF4/MUM1+ [51]. PRDM1/BLIMP-1 is present in plasma cell neoplasms, LPL, and a significant number of diffuse large B cell lymphomas (DLBCLs), with weak reactivity reported in 15 % of CLLs/SLLs [11]. In vitro differentiation of CLL cells into immunoglobulin-secreting cells is also associated with increased PRDM1/BLIMP-1 expression and decreased LEF1 [10, 17]. CLL lacking recognizable proliferation centers, as in one of the two workshop cases, may be difficult to distinguish from LPL since CD5 and CD23 positivities do not exclude LPL. The plasmacytic differentiation may be largely expressed phenotypically without morphologically obvious plasma cells (case 78, submitted by D. Martinez) or with easily recognized plasma cells (case 182, submitted by J. R. Goodlad). Proliferation centers were not identified in the latter case; however, the small-cell component had a characteristic CLL phenotype. Plasmacytic differentiation in the former case (“accelerated” CLL [14]) was documented by IRF4/MUM1 that was mostly in the proliferation centers, cytoplasmic lambda light chain expression, and diffuse PRDM1/BLIMP1 expression (Fig. 5). At least most of the plasmacytic cells in both cases were CD138 negative. Distinction from LPL was aided in both cases by finding wild-type MYD88 and LEF1 immunoreactivity. Among the SBL, LEF1 is a very sensitive and specific marker for CLL/SLL [50]. The possibility that the case without proliferation centers might represent a type of tissue-based monoclonal B cell lymphocytosis was raised as it presented with splenic but not nodal or peripheral blood involvement [13]. The patient has remained well for 8 years following splenectomy.
Fig. 5.
Accelerated CLL with plasmacytic differentiation. a Note the prominent proliferation center. Immunohistochemical stains demonstrated b minimal staining for kappa but c numerous cells with cytoplasmic lambda staining and d extensive positivity for BLIMP1 (case 89, courtesy of D. Martinez et al.)
Follicular lymphoma
Plasmacytic differentiation in FL is rare with one form having predominantly interfollicular plasmacytic cells and usually a BCL2 rearrangement and the other having predominantly intrafollicular/perifollicular plasma cells, usually without a BCL2 rearrangement in either component [15]. Among the workshop cases, two FLs had predominantly interfollicular plasmacytic differentiation, one of which had a BCL2 rearrangement, and two FLs had prominent intrafollicular plasmacytic differentiation, one without a BCL2 rearrangement and one with both BCL2 and BCL6 rearrangements (case 150, submitted by M. Kinney) (Fig. 6). Two previously reported BCL2 and BCL6 rearranged cases have also had intrafollicular plasmacytic differentiation [15, 61]. As highlighted in the workshop, the degree of plasmacytic differentiation varied, ranging from prominent plasma cells seen in the routine sections to cases only recognized with the aid of immunohistochemical stains and not necessarily with CD138 expression. Dutcher bodies (and Mott cells) were seen in one case (Fig. 6b, e, f) [15, 61]. CD10 was negative in two of the workshop cases (one CD5+), and BCL6 was not clearly positive in either with one of these cases raising the difficult differential of a MZL with prominent follicular colonization (case 326, submitted by J. Song) and the other HGAL+ (case 107, submitted by D. Ruijs), further supporting but not proving a follicular origin (see Xerri et al.’s workshop report). Although unusual, FL with plasmacytic differentiation may lack CD10 and, probably less often, BCL6 expression [15]. BCL2 protein is usually but not always positive with 2/14 previously reported and 1/3 workshop cases being negative (in spite of BCL2 rearrangement). Cases of FL with plasmacytic differentiation must be distinguished from cases of follicular hyperplasia with monotypic germinal centers [31] as illustrated in case 316 submitted by T. Quinto, in which a subset of otherwise hyperplastic-appearing CD10+ follicles showed very strong cytoplasmic IgM kappa. There was no BCL2 translocation by fluorescence in situ hybridization (FISH) and no evidence of a clonal IgH rearrangement by PCR.
Fig. 6.
Follicular lymphoma with plasmacytic differentiation and BCL2 and BCL6 rearrangements. a Architectural effacement by a somewhat ill-defined follicular proliferation is present. b The neoplastic germinal center includes both Dutcher bodies (short arrow) and Mott cells (longer arrow). c A CD21 stain confirms the follicular architecture. d The CD138 stain highlights the prominent intrafollicular and interfollicular plasmacytic population. There is cytoplasmic e IgM- and f kappa-restricted positivity. Both IgM and kappa stains highlighted the presence of Dutcher bodies (arrows) (case 150, courtesy of M. Kinney)
Mantle cell lymphoma
MCL typically does not have plasmacytic differentiation; however, seven cases with focal clonally related plasmacytic differentiation have been reported [8, 32, 59]. These cases had a clinical presentation similar to classical MCL with nodal (n=4), extranodal (n=4), splenic (n=2), and bone marrow involvement (n=6). The growth pattern was nodular in three cases, diffuse in two cases, and mantle zone in two cases. All cases were CD5 positive as was the plasma cell component in two cases. The t(11;14) translocation and cyclin D1 expression were demonstrated in the seven cases, but cyclin D1 expression was only detected in the plasma cell component in three of them. The significance of this surprising finding is uncertain. In the five patients with clinical follow-up, one died of the disease at 26 months and the others were alive with disease from 24 to 42 months.
Four such workshop cases helped to further characterize MCL with plasmacytic differentiation. One case, whose diagnosis was debatable, was composed almost exclusively of cyclin D1+ plasma cells and is discussed below (case 49, submitted by F. Sen). In the three other cases, the plasma or lymphoplasmacytic cells were intermingled with a small-cell lymphoid component that could be diagnosed as MCL (case 40, submitted by D. de Jong, case 46 by M.R. Ashton-Key, and case 89 by I. Ribera-Cortada) (Figs. 4e and 7).
Fig. 7.
Mantle cell lymphoma with plasmacytic differentiation and MYD88 mutation. a Mantle cell lymphoma with a residual naked germinal center. b The cells were small with a round nucleus, and some had Dutcher bodies (arrow). c The neoplastic cells were SOX11-negative but d strongly positive for cyclin D1. e Cells with Dutcher bodies were also cyclin D1-positive (arrow) (case 89, courtesy of I. Ribera-Cortada et al.)
Two of these three cases (cases 46 and 89) showed a sub-population of atypical small cells with lymphoplasmacytic differentiation, some with Dutcher bodies, whereas the third (case 40) had a component of monotypic plasma cells and amyloid deposition. They were strongly cyclin D1 positive in the small cells, cells with plasmacytic differentiation and plasma cells (Fig. 7). The small cells were strongly positive for CD20 and BCL2 but were negative for CD5. Strong cytoplasmic kappa light chain restriction was present, with IgM in two and no heavy chain in one (case 40). The t(11;14) could be demonstrated in cases 40 and 89 but not in case 46, even with break apart FISH probes. This last patient had significant radiation exposure from nuclear testing more than 50 years before and had a past history of several malignancies. One of the three cases had a MYD88 L256P mutation (case 89).
Two of these three cases presented with nodal involvement (cases 40 and 89), whereas the third developed lymphadenopathy after a previous 7-year history of lymphocytosis initially diagnosed as atypical CLL but later re-interpreted as MCL due to the strong cyclin D1 expression that was also present in the initial bone marrow biopsy. The bone marrow was involved in the three cases, and splenic (case 46) and gastric infiltration (case 89) were seen in the course of the disease in two of them. One patient (case 40) was treated with chemotherapy at diagnosis, received several treatments at different relapses including allogenic bone marrow transplant, and was alive with disease 12 years after diagnosis. Treatment was deferred in the other two patients for 2 and 7 years. The first patient was alive with disease at 4.7 years, and the second died of disease at 10 years.
The mechanisms underlying plasmacytic differentiation in MCL are not well known. A recent study on the oncogenic mechanisms of SOX11, a transcription factor overexpressed in most MCL, showed that it directly regulates PAX5 and may promote tumor growth [57]. PAX5 maintains the B cell identity and is downregulated when the cell starts the plasma cell differentiation program. Experimental silencing of SOX11 in MCL cell lines led to downregulation of PAX5 and shift from a mature B cell phenotype towards early plasmacytic differentiation with upregulation of the plasma cell antigens BLIMP1 and XBP1. These observations suggested that SOX11 could influence MCL pathogenesis by blocking terminal B cell differentiation [57]. We therefore hypothesized that MCL with plasmacytic differentiation most likely would be SOX11 negative. The panel therefore performed SOX11 staining in the four cases, and all were negative (Fig. 7c). These findings are concordant with a recent larger study (which included case 89), showing that plasma cell and terminal B cell differentiation in MCL mainly occur in SOX11-negative tumors [39].
The three cases presented in the workshop, together with those previously published, illustrate how easily these MCLs can be confused with other SBLs with plasmacytic differentiation, particularly those that are CD5 negative and have a paraprotein [8, 39, 59]. However, the clinical presentation with generalized lymphadenopathy, and extranodal involvement, splenomegaly, and leukemic expression in some of the cases, may suggest the diagnosis of MCL that can be confirmed by demonstrating cyclin D1 expression. The difficulty in the differential diagnosis was well demonstrated by case 89 that had an IgM paraprotein. MYD88 mutation suggested a LPL with secondary acquisition of t(11;14) leading to cyclin D1 overexpression, or a MCL with plasmacytic differentiation, possibly related to secondary acquisition of the MYD88 mutation. It was not possible to determine which genetic event occurred first. The workshop also did include two cases of CLL in which the t(11;14) was acquired (case 20, submitted by I. Oschlies and case 98, submitted by I. Schliemann), indicating that although rare, t(11;14) might be a secondary event in the evolution of SBL.
Whether case 89 should be considered a MCL with plasmacytic differentiation or LPL with cyclin D1 overexpression is debatable. However, plasmacytic differentiation is a common phenomenon observed in many subtypes of SBL that must be diagnosed according to the characteristics of the lymphoid component. In addition, although the t(11;14) is found in both MCL and a minority of myelomas, it is more critical in defining entities than MYD88 mutations which are present in a much broader spectrum of B cell lymphoid neoplasms. The other two MCLs with plasmacytic differentiation in the workshop, as well as six additional recently published cases, lacked MYD88 mutation, in addition to more than 100 other MCLs investigated for this mutation [39]. Another differential diagnosis when there is marked plasmacytic differentiation is PCM with t(11,14). However, the clinical presentation with generalized lymphadenopathy, extranodal involvement, and lack of lytic bone lesions or hypercalcemia should favor the diagnosis of MCL.
Although the number of cases studied is limited, the clinical evolution of MCL with plasmacytic differentiation seems similar to the frequently indolent behavior seen in other SOX11-negative MCLs without TP53 mutations [39]. Two cases in the workshop and six additional published cases were not treated with chemotherapy for two or more years, and some of them had a long survival even with persistence of the disease.
Marginal zone lymphomas (with an emphasis on plasmacytic differentiation)
The workshop, which included 13 NMZLs, 9 MALT lymphomas, and 4 SMZLs, highlighted some of the difficulties in making these diagnoses, particularly in the face of plasmacytic differentiation and non-specific phenotypic and molecular findings. It also highlighted how newer mutational studies may be very helpful, even if many of the mutations only occur in a minority of cases [43].
NMZL is a SBL that “morphologically resembles lymph nodes involved by MZL of extranodal or splenic types but without evidence of extranodal or splenic disease” [49]. Because its growth pattern and cytologic appearance is variable and without a unique phenotype, it is sometimes used as a wastebasket for SBLs that are otherwise unclassifiable [2, 4, 46]. Although IRTA-1 expression is reported to help identify NMZL, the antibody is not commercially available [2]. The differential diagnosis for NMZL includes all the SBL. Distinction of NMZL with IgM+ plasmacytic differentiation from LPL, as seen in four workshop cases (cases 69, 186, 223, and 302), has been particularly difficult; however, the lack of MYD88 mutation in these four cases and in the vast majority of NMZLs (as reported in the literature) helped make the correct diagnosis (Fig. 8a–e). Caution is advised as the workshop included one NZML with MYD88 L265P mutation (case 288, submitted by M. Klimkowska) (Fig. 8f–i) and not all LPLs have a demonstrable mutation. Another confounding feature in NMZL is the CD5 and/or CD23 expression seen in a small subset raising the possibility of CLL/SLL or MCL. CD5 expression in NZML has been associated with more frequent dissemination [22]. Reactive hyperplasias may also mimic a NMZL when there are very prominent monocytoid B cell areas as is typically seen with CMV infection (case 187, submitted by R. Batra).
Fig. 8.
Nodal marginal zone lymphomas with plasmacytic differentiation. a Note the typical marginal zone growth pattern in this NMZL with wild-type MYD88 b with numerous CD20 positive B cells c that outside of the germinal center express CD5. d The CD3 highlights the benign T cells. e The IgM immunostain marks both the neoplastic B cells and a small number of neoplastic plasma cells (a–e, case 302, courtesy of S. Isiksoy). (f) This NMZL with a MYD88 L265P mutation also had a typical marginal zone growth pattern. g Note the CD20 positive B cells in the follicle and at the periphery of the image (red), the surrounding CD3 positive T cells (brown), and the presence of cells marking with neither. h A CD138 stain highlights the prominent plasma cells outside the nodules. i There was also focally marked p53 expression (f–i, case 288, courtesy of M. Klimkowska)
EMZL also includes a significant minority of cases with plasmacytic differentiation leading to diagnostic difficulties. In one workshop case of MALT lymphoma associated with Hashimoto thyroiditis, the plasma cells only expressed gamma heavy chain as seen in gamma heavy chain disease but there were no serum Ig abnormalities (case 270, submitted by E. M. Maggio) (Fig. 9a–e). The reported absence of MYD88 L265P mutations in gamma heavy chain disease helps in the distinction from LPL [18]. The workshop also highlighted how MALT lymphomas can mimic IgG4-related disease or arise in the setting of IgG4-related disease, emphasizing the importance of establishing whether or not the IgG4+ plasma cells are light chain class restricted. The skin has the highest proportion of IgG4+ MZLs [3]. IgG4+ lymphomas as well as IgG4-related disease are also well-described in the ocular adnexae [5, 33], in association with chronic sclerosing sialadenitis [12] (case 264, submitted by J. M. Jaso) (Fig. 9f–i) and in the dura (case 255, courtesy of T. Gindin) (Fig. 9j–m), where one third of the MALT lymphomas are reported to be IgG4+ [58, 60].
Fig. 9.
MALT lymphomas with plasmacytic differentiation. a This MALT lymphoma of the thyroid from a 52-year-old man with a history of Hashimoto’s thyroiditis had b very limited CD20 but c marked CD79a expression. d The plasmacytic cells marked with neither kappa or lambda in this double in situ hybridization stain but e showed extensive IgG positivity (a–e, case 270, courtesy of E. M. Maggio). f This MALT lymphoma that was associated with chronic sclerosing sialadenitis had g, h kappa monotypic plasma cells with i about 36 % positive for IgG4 (f–i, case 264, courtesy of J. M. Jaso). j This dural MALT lymphoma included numerous plasma cells that k were kappa monotypic (inset lambda) based on in situ hybridization stains and were l IgG and m IgG4 positive (case 255, courtesy of T. Gindin)
The diagnosis of SMZL presents diagnostic challenges, particularly when it is rendered without pathologic examination of the spleen. This may complicate some of the published MYD88 data. A major diagnostic pitfall is the SMZL that have plasmacytic differentiation with IgM paraproteinemia (seen in 15 % of cases at presentation), raising the possibility of LPL/WM [29]. The presence of a typical biphasic cytologic appearance, as seen in case 178, submitted by M. A. Piris, can be helpful in arriving at the correct diagnosis (Fig. 10). The absence of a MYD88 mutation can also be helpful, but up to about 15 % of SMZLs have been reported to have MYD88 mutations and with MYD88-mutated SMZL more likely to have an IgM paraprotein and plasmacytic differentiation [29]. The presence of KLF2 mutation (found in 42 % of SMZL) as well as the presence of 7q deletions also can help to establish the diagnosis of SMZL even though these genetic changes can uncommonly be found in other SBLs [6]. NOTCH2 mutations have also been associated with SMZL but are present in only about 25 % of cases and not a specific finding [24]. Although we believe that the diagnosis of SMZL can be made with the greatest confidence from splenectomy specimens, most cases are being diagnosed from bone marrow examinations, together with the clinicopathologic findings and ancillary studies. It is recognized, however, that many of the SMZL-associated molecular/cytogenetic abnormalities are present in a minority of cases and none are completely specific.
Fig. 10.
Splenic marginal zone lymphoma with plasmacytic differentiation and wild-type MYD88. a Note the typical biphasic pattern but with b focally numerous plasma cells that were c IgM positive (case 178, courtesy of M. A. Piris)
Can you have a lymphoma with complete plasmacytic differentiation and how is that different from a plasma cell neoplasm?
Given the variable extent of plasmacytic differentiation in many types of SBL, one must consider the possibility that it could be extreme and total. For example, one of the previously reported FLs with plasmacytic differentiation was indistinguishable from myeloma in the bone marrow [15] and elimination of the lymphoid but not the plasmacytic component in LPL following treatment has been reported [27]. In the case referred to above, a pure plasma cell neoplasm associated with amyloid presented as nodal disease without bone marrow involvement and without a paraprotein (case 49, submitted by F. Sen). There were scattered non-neoplastic follicles but no neoplastic lymphoid component. The cyclin D1 positivity and t(11;14) present were findings that in a plasmacytic neoplasm suggest PCM, but other features of myeloma were lacking. The panel recognized this as a plasma cell neoplasm but could not determine if it was more closely related to a MCL (with extreme plasmacytic differentiation) or PCM. Another unusual case of a pure CD10+ neoplastic plasma cell proliferation in the epiglottis and lymph nodes associated with an IgGλ paraprotein raised a similar issue (case 311, submitted by G. Kaygusuz). The patient did not have bone lesions, bone marrow involvement, or other findings to suggest PCM. As with the other cases, the plasma cells surrounded a moderate number of reactive lymphoid follicles. While the panel favored the diagnosis of an extramedullary plasmacytoma, one must also consider the possibility of a MZL with extreme plasmacytic differentiation [21]. The plasma cells were CD45+, a feature more commonly found in a plasmacytic component of a B cell lymphoma. Both of these cases highlight how hematopathologic diagnoses require a multiparameter approach including correlation with the clinical findings and also how definitive diagnoses are sometimes beyond our reach.
Amyloid and immunoglobulin-related deposits in lymphomas with plasmacytic differentiation
Abnormal intracellular and extracellular immunoglobulin (Ig) deposition is a rare but well-recognized phenomenon in B cell lymphomas with plasmacytic differentiation. The deposits can take a number of physical forms including extracellular deposition of whole Ig molecules, amyloid light-chain (AL) amyloidosis, and accumulation of crystalized Ig in reactive histiocytes (crystal-storing histiocytosis (CSH)). In the context of a B cell lymphoma, the deposition tends to remain localized to the tumor site only rarely causing systemic complications [16]. Eight workshop cases showed abnormal Ig deposition including four cases of localized AL amyloidosis (one probable LPL, one MCL with marked plasmacytic differentiation, one MCL with marked plasmacytic differentiation versus PCM, and one MALT lymphoma with plasmacytic differentiation), two cases of CSH (one MALT lymphoma and one NMZL with plasmacytic differentiation), and two cases of extracellular immunoglobulin deposition (one difficult-to-classify SBL with plasmacytic differentiation and one NMZL with plasmacytic differentiation).
Case 312 submitted by K. Grogg was a patient with a history of Sjögren’s syndrome and cutaneous and pulmonary MALT lymphoma with extensive localized amyloidosis. The lymphoma component in both biopsies was subtle, with only a few small B cell aggregates and a few clusters of kappa light chain-restricted plasma cells. Proteomic analysis of the amyloid deposits showed, in addition to pathogenic clonal kappa light chain, a significant component of IgG1 heavy chain. This is a distinguishing feature of localized AL amyloidosis caused by MALT lymphoma where the Ig heavy chain is frequently incorporated into the amyloid plaque. In contrast, in systemic AL amyloidosis, Ig light chains without a heavy-chain component are deposited at distant sites [16].
Case 175 submitted by A. Chiu was a pulmonary MALT lymphoma with plasmacytic differentiation and extensive CSH. Proteomic analysis showed that the crystals were composed of Ig kappa chain variable region. This is consistent with other published studies suggesting that a truncated product of Ig heavy or light chain forms the crystals [23, 34]. The truncation is most likely a posttranslational event as intact Ig heavy and light chains can be shown in the neoplastic plasma cells.
Progression of lymphoplasmacytic lymphomas
Although LPL is indolent, transformation may occur, with a frequency of 5–13 %, portending a more aggressive clinical course [26, 48]. Three cases submitted to the workshop, all confirmed to have MYD88 mutation, highlighted the transformational process and the importance of TP53 abnormalities in this setting.
As demonstrated by the workshop cases, progression and overt transformation may occur soon or many years after the diagnosis of LPL and has even been reported to occur at the time of diagnosis [26]. Transformation may develop suddenly or as part of a slower-paced progression. Histologically, when clusters and sheets of large transformed cells are present, a diagnosis of “transformation to diffuse large B cell lymphoma” is straightforward. Other clues to the presence of transformation are readily found mitotic figures or a high Ki-67 index. Case 144 (submitted by C. Soderquist) was incidentally discovered to have LPL and an IgM paraproteinemia, which was managed by observation. However, 2 months later, the patient developed severe epistaxis, worsening anemia and thrombocytopenia, and axillary lymphadenopathy. Lymph node biopsy showed areas of LPL and other areas with very prominent large lymphoid cells easily recognized as transformation to DLBCL (Fig. 11).
Fig. 11.
Histologic, immunophenotypic, and molecular features of transformed LPL. a Axillary lymph node showing architectural distortion by small lymphocytes, plasmacytoid lymphocytes, and plasma cells. b Focally, there are clusters of large atypical cells with open chromatin and conspicuous nucleoli. Mitotic figures are prominent. c There are more abundant p53-positive cells in the large-cell component (right half). d Targeted massively parallel sequencing reveals the characteristic point mutation in MYD88 (L265P) with an allele frequency of 28 % and e a deletion of three nucleotides in TP53 (V218del) with an allele frequency of 49 % (case 144, courtesy of C. Soderquist)
Greater diagnostic challenges are encountered when there is just an increased proportion of large transformed cells admixed with non-transformed LPL cells since up to 10 % large transformed cells can be present in typical LPL [1]. With no established criteria for transformation in the absence of numerous transformed cells, we recommend labeling cases with intermediate numbers of transformed cells as “progressed LPL.” These cases may be more aggressive, and many likely correspond to “polymorphic immunocytoma” of the Kiel classification [1, 26, 28].
Case 113 (submitted by L. Quintanilla-Martinez) originally presented with IgM monoclonal gammopathy of undetermined significance, followed 5 years later by typical LPL/WM based on a bone marrow biopsy. Four years later, the patient developed a rapidly enlarging breast mass. The disease was refractory to chemotherapy, and the patient died after a few months. The breast biopsy showed small lymphocytes, lymphoplasmacytoid cells, and plasma cells admixed with many large lymphoid cells in some areas. Ki67 index was almost 100 % in areas rich in large cells. Molecular analysis demonstrated identical IgH rearrangement in the bone marrow with LPL and in the transformed tumor in the breast. Case 185 (submitted by R. E. Felgar) presented originally as WM, with LPL documented in the marrow. Treatment resulted in a reduced disease burden. Seven years later, the patient presented with enlarging lymph nodes. She did not respond to Velcade. Repeat lymph node biopsy later that same year showed predominantly a small lymphoid cell infiltrate without significant plasmacytic differentiation but with interspersed tingible body macrophages and admixed large to medium-sized transformed cells.
Information on the molecular basis for transformation is limited in the literature, but the workshop cases suggest that TP53 deletion and/or mutation play an important role, although the abnormalities may precede histologic and clinical transformation. Case 144 had a TP53 mutation in the DLBCL, and case 185 with progressed LPL had a TP53 deletion. Case 113 was interesting in that the bone marrow biopsy with LPL had only a subset of cells expressing p53, whereas both the smaller- and larger-cell areas in the transformed biopsy showed >80 % strongly p53+ cells. Both biopsies, however, had a deletion in TP53.
Conclusions
The workshop highlighted that many types of SBL can have plasmacytic differentiation with the diagnosis relying heavily on the characterization of the lymphoid component, including phenotypic, cytogenetic/molecular, and sometimes clinical findings. MYD88 mutational analysis is very helpful in this setting, even if not pathognomonic for LPL, since MYD88 L265P mutations are also present in a small proportion of other SBLs. This mutation has helped to refine the diagnostic criteria for LPL, leading to the exclusion of some cases formerly under this umbrella and inclusion of others that may have been previously diagnosed as MZL. Nevertheless, even in 2015, some SBLs with plasmacytic differentiation still defy classification.
The workshop cases also highlighted additional clinicopathologic and biologic features of SBLs with plasmacytic differentiation. Lymphoid neoplasms with CCND1 rearrangements and plasmacytic differentiation may be related to SOX11 negative MCL. Whether rare nodal neoplasms with CCND1 rearrangements and total plasmacytic differentiation represent examples of a SOX11 negative MCL remains uncertain. Some SBLs with plasmacytic differentiation also may be challenging to diagnose because they have extensive amyloid or other immunoglobulin deposition or numerous crystal-storing histiocytes.
Progression in LPL is not common but does occur with a gradual or more precipitous increase in the proportion of large transformed cells. Based on the workshop cases, progression is often associated with TP53 abnormalities.
Acknowledgments
The authors are very grateful to the participants who contributed a large number of very interesting and informative cases to this workshop and for the use of their cases and images in this review.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Andriko JA, Swerdlow SH, Aguilera NI, Abbondanzo SL. Is lymphoplasmacytic lymphoma/immunocytoma a distinct entity? A clinicopathologic study of 20 cases. Am J Surg Pathol. 2001;25:742–751. doi: 10.1097/00000478-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bob R, Falini B, Marafioti T, Paterson JC, Pileri S, Stein H. Nodal reactive and neoplastic proliferation of monocytoid and marginal zone B cells: an immunoarchitectural and molecular study highlighting the relevance of IRTA1 and T-bet as positive markers. Histopathology. 2013;63:482–498. doi: 10.1111/his.12160. [DOI] [PubMed] [Google Scholar]
- 3.Brenner I, Roth S, Puppe B, Wobser M, Rosenwald A, Geissinger E. Primary cutaneous marginal zone lymphomas with plasmacytic differentiation show frequent IgG4 expression. Mod Pathol. 2013;26:1568–1576. doi: 10.1038/modpathol.2013.106. [DOI] [PubMed] [Google Scholar]
- 4.Campo E, Miquel R, Krenacs L, Sorbara L, Raffeld M, Jaffe ES. Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol. 1999;23:59–68. doi: 10.1097/00000478-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, Lo YF, Chan WK, Chan JK. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:1159–1167. doi: 10.1097/PAS.0b013e31816148ad. [DOI] [PubMed] [Google Scholar]
- 6.Clipson A, Wang M, de Leval L, Ashton-Key M, Wotherspoon A, Vassiliou G, Bolli N, Grove C, Moody S, Escudero-Ibarz L, Gundem G, Brugger K, Xue X. KLF2 mutation is the most frequent somatic change in splenic marginal zone lymphoma and identifies a subset with distinct genotype. Blood. 2015;29:1177–1185. doi: 10.1038/leu.2014.330. [DOI] [PubMed] [Google Scholar]
- 7.Cook JR, Aguilera NI, Reshmi S, Huang X, Yu Z, Gollin SM, Abbondanzo SL, Swerdlow SH. Deletion 6q is not a characteristic marker of nodal lymphoplasmacytic lymphoma. Cancer Genet Cytogenet. 2005;162:85–88. doi: 10.1016/j.cancergencyto.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Cooper CL, Joshua DE, Lee CS, Eyers AA, Cooper WA. Extranodal plasmablastic lymphoma arising in mantle cell lymphoma. Histopathology. 2007;51:856–859. doi: 10.1111/j.1365-2559.2007.02859.x. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Kastritis E, Owen RG, Kyle RA, Landgren O, Morra E, Leleu X, Garcia-Sanz R, Munshi N, Anderson KC, Terpos E, Ghobrial IM, Morel P, Maloney D, Rummel M, Leblond V, Advani RH, Gertz MA, Kyriakou C, Thomas SK, Barlogie B, Gregory SA, Kimby E, Merlini G, Treon SP. Treatment recommendations for patients with Waldenstrom macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood. 2014;124:1404–1411. doi: 10.1182/blood-2014-03-565135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth A, Glenn M, Slupsky JR, Packham G, Kalakonda N. Variable induction of PRDM1 and differentiation in chronic lymphocytic leukemia is associated with anergy. Blood. 2014;123:3277–3285. doi: 10.1182/blood-2013-11-539049. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JF, Roncador G, Garcia JF, Sanz AI, Maestre L, Lucas E, Montes-Moreno S, Fernandez Victoria R, Martinez-Torrecuadrara JL, Marafioti T, Mason DY, Piris MA. PRDM1/BLIMP-1 expression in multiple B and T-cell lymphoma. Haematologica. 2006;91:467–474. [PubMed] [Google Scholar]
- 12.Geyer JT, Ferry JA, Harris NL, Stone JH, Zukerberg LR, Lauwers GY, Pilch BZ, Deshpande V. Chronic sclerosing sialadenitis (Kuttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 13.Gibson SE, Swerdlow SH, Ferry JA, Surti U, Dal Cin P, Harris NL, Hasserjian RP. Reassessment of small lymphocytic lymphoma in the era of monoclonal B-cell lymphocytosis. Haematologica. 2011;96:1144–1152. doi: 10.3324/haematol.2011.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giné E, Martinez A, Villamor N, López-Guillermo A, Camos M, Martinez D, Esteve J, Calvo X, Muntañola A, Abrisqueta P, Rozman M, Rozman C, Bosch F, Campo E, Montserrat E. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95:1526–1533. doi: 10.3324/haematol.2010.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradowski JF, Jaffe ES, Warnke RA, Pittaluga S, Surti U, Gole LA, Swerdlow SH. Follicular lymphomas with plasmacytic differentiation include two subtypes. Mod Pathol. 2010;23:71–79. doi: 10.1038/modpathol.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grogg KL, Aubry MC, Vrana JA, Theis JD, Dogan A. Nodular pulmonary amyloidosis is characterized by localized immunoglobulin deposition and is frequently associated with an indolent B-cell lymphoproliferative disorder. Am J Surg Pathol. 2013;37:406–412. doi: 10.1097/PAS.0b013e318272fe19. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez A, Jr, Arendt BK, Tschumper RC, Kay NE, Zent CS, Jelinek DF. Differentiation of chronic lymphocytic leukemia B cells into immunoglobulin secreting cells decreases LEF-1 expression. PLoS One. 2011;6:e26056. doi: 10.1371/journal.pone.0026056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamadeh F, MacNamara S, Bacon CM, Sohani AR, Swerdlow SH, Cook JR. Gamma heavy chain disease lacks the MYD88 L265p mutation associated with lymphoplasmacytic lymphoma. Haematologica. 2014;99:e154–e155. doi: 10.3324/haematol.2014.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamadeh F, MacNamara SP, Aguilera NS, Swerdlow SH, Cook JR. MYD88 L265P mutation analysis helps define nodal lymphoplasmacytic lymphoma. Mod Pathol. 2015;28:564–574. doi: 10.1038/modpathol.2014.120. [DOI] [PubMed] [Google Scholar]
- 20.Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C, Patterson CJ, Sheehy P, Treon SP. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123:1637–1646. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 21.Hussong JW, Perkins SL, Schnitzer B, Hargreaves H, Frizzera G. Extramedullary plasmacytoma. A form of marginal zone cell lymphoma? Am J Clin Pathol. 1999;111:111–116. doi: 10.1093/ajcp/111.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Jaso JM, Yin CC, Wang SA, Miranda RN, Jabcuga CE, Chen L, Medeiros LJ. Clinicopathologic features of CD5-positive nodal marginal zone lymphoma. Am J Clin Pathol. 2013;140:693–700. doi: 10.1309/ajcpemvxes72duif. [DOI] [PubMed] [Google Scholar]
- 23.Kanagal-Shamanna R, Xu-Monette ZY, Miranda RN, Dogan A, Zou D, Luthra R, Weber D, O’Malley DP, Jorgensen JL, Khoury JD, Bueso-Ramos CE, Orlowski RZ, Medeiros JL, Young KH. Crystal-storing histiocytosis: a clinicopathologic study of 13 cases. Histopathology. 2015 doi: 10.1111/his.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiel MJ, Velusamy T, Betz BL, Zhao L, Weigelin HG, Chiang MY, Huebner-Chan DR, Bailey NG, Yang DT, Bhagat G, Miranda RN, Bahler DW, Medeiros LJ, Lim MS, Elenitoba-Johnson KS. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med. 2012 doi: 10.1084/jem.20120910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraan W, Horlings HM, van Keimpema M, Schilder-Tol EJ, Oud ME, Scheepstra C, Kluin PM, Kersten MJ, Spaargaren M, Pals ST. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. doi: 10.1038/bcj.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin P, Mansoor A, Bueso-Ramos C, Hao S, Lai R, Medeiros LJ. Diffuse large B-cell lymphoma occurring in patients with lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Clinicopathologic features of 12 cases. Am J Clin Pathol. 2003;120:246–253. doi: 10.1309/R01V-XG46-MFCD-VNHL. [DOI] [PubMed] [Google Scholar]
- 27.Lin P, Molina TJ, Cook JR, Swerdlow SH. Lymphoplasmacytic lymphoma and other non-marginal zone lymphomas with plasmacytic differentiation. Am J Clin Pathol. 2011;136:195–210. doi: 10.1309/AJCP8FOIVTB6LBER. [DOI] [PubMed] [Google Scholar]
- 28.Mansoor A, Medeiros LJ, Weber DM, Alexanian R, Hayes K, Jones D, Lai R, Glassman A, Bueso-Ramos CE. Cytogenetic findings in lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Chromosomal abnormalities are associated with the polymorphous subtype and an aggressive clinical course. Am J Clin Pathol. 2001;116:543–549. doi: 10.1309/6U88-357U-UKJ5-YPT3. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Lopez A, Curiel-Olmo S, Mollejo M, Cereceda L, Martinez N, Montes-Moreno S, Almaraz C, Revert JB, Piris MA. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol. 2015;39:644–651. doi: 10.1097/pas.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Trillos A, Pinyol M, Navarro A, Aymerich M, Jares P, Juan M, Rozman M, Colomer D, Delgado J, Gine E, Gonzalez-Diaz M, Hernandez-Rivas JM, Colado E, Rayon C, Payer AR, Terol MJ, Navarro B, Quesada V, Puente XS, Rozman C, Lopez-Otin C, Campo E, Lopez-Guillermo A, Villamor N. Mutations in TLR/MYD88 pathway identify a subset of young chronic lymphocytic leukemia patients with favorable outcome. Blood. 2014;123:3790–3796. doi: 10.1182/blood-2013-12-543306. [DOI] [PubMed] [Google Scholar]
- 31.Nam-Cha SH, San-Millan B, Mollejo M, Garcia-Cosio M, Garijo G, Gomez M, Warnke RA, Jaffe ES, Piris MA. Light-chain-restricted germinal centres in reactive lymphadenitis: report of eight cases. Histopathology. 2008;52:436–444. doi: 10.1111/j.1365-2559.2008.02965.x. [DOI] [PubMed] [Google Scholar]
- 32.Naushad H, Choi WW, Page CJ, Sanger WG, Weisenburger DD, Aoun P. Mantle cell lymphoma with flow cytometric evidence of clonal plasmacytic differentiation: a case report. Cytometry B Clin Cytom. 2009;76:218–224. doi: 10.1002/cyto.b.20463. [DOI] [PubMed] [Google Scholar]
- 33.Ohno K, Sato Y, Ohshima K-i, Takata K, Miyata-Takata T, Takeuchi M, Gion Y, Tachibana T, Orita Y, Ito T, Swerdlow SH, Yoshino T. A subset of ocular adnexal marginal zone lymphomas may arise in association with IgG4-related disease. Sci Rep. 2015;5:13539. doi: 10.1038/srep13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr BA, Gallia GL, Dogan A, Rodriguez FJ. IgA/kappa-restricted crystal storing histiocytosis involving the central nervous system characterized by proteomic analysis. Clin Neuropathol. 2014;33:23–28. doi: 10.5414/np300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paiva B, Montes MC, Garcia-Sanz R, Ocio EM, Alonso J, de Las HN, Escalante F, Cuello R, de Coca AG, Galende J, Hernandez J, Sierra M, Martin A, Pardal E, Barez A, Alonso J, Suarez L, Gonzalez-Lopez TJ, Perez JJ, Orfao A, Vidriales MB, San Miguel JF. Multiparameter flow cytometry for the identification of the Waldenstrom’s clone in IgM-MGUS and Waldenstrom’s macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia. 2014;28:166–173. doi: 10.1038/leu.2013.124. [DOI] [PubMed] [Google Scholar]
- 36.Pham-Ledard A, Beylot-Barry M, Barbe C, Leduc M, Petrella T, Vergier B, Martinez F, Cappellen D, Merlio JP, Grange F. High frequency and clinical prognostic value of MYD88 L265P mutation in primary cutaneous diffuse large B-cell lymphoma, leg-type. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2014.821. [DOI] [PubMed] [Google Scholar]
- 37.Poulain S, Roumier C, Decambron A, Renneville A, Herbaux C, Bertrand E, Tricot S, Daudignon A, Galiegue-Zouitina S, Soenen V, Theisen O, Grardel N, Nibourel O, Roche-Lestienne C, Quesnel B, Duthilleul P, Preudhomme C, Leleu X. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood. 2013;121:4504–4511. doi: 10.1182/blood-2012-06-436329. [DOI] [PubMed] [Google Scholar]
- 38.Randen U, Troen G, Tierens A, Steen C, Warsame A, Beiske K, Tjonnfjord GE, Berentsen S, Delabie J. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99:497–504. doi: 10.3324/haematol.2013.091702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribera-Cortada I, Martinez D, Amador V, Royo C, Navarro A, Beà S, Gine E, Leval L, Serrano S, Wotherspoon A, Colomer D, Martinez A, Campo E. Plasma cell and terminal B-cell differentiation in mantle cell lymphoma mainly occur in the SOX11-negative subtype. Mod Pathol. 2015 doi: 10.1038/modpathol.2015.99. [DOI] [PubMed] [Google Scholar]
- 40.Roberts MJ, Chadburn A, Ma S, Hyjek E, Peterson LC. Nuclear protein dysregulation in lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Am J Clin Pathol. 2013;139:210–219. doi: 10.1309/ajcp0ygm8blfyhjy. [DOI] [PubMed] [Google Scholar]
- 41.Roccaro AM, Sacco A, Jimenez C, Maiso P, Moschetta M, Mishima Y, Aljawai Y, Sahin I, Kuhne M, Cardarelli P, Cohen L, San Miguel JF, Garcia-Sanz R, Ghobrial IM. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood. 2014;123:4120–4131. doi: 10.1182/blood-2014-03-564583. [DOI] [PubMed] [Google Scholar]
- 42.Rosado FG, Morice WG, He R, Howard MT, Timm M, McPhail ED. Immunophenotypic features by multiparameter flow cytometry can help distinguish low grade B-cell lymphomas with plasmacytic differentiation from plasma cell proliferative disorders with an unrelated clonal B-cell process. Br J Haematol. 2015;169:368–376. doi: 10.1111/bjh.13303. [DOI] [PubMed] [Google Scholar]
- 43.Rossi D, Ciardullo C, Gaidano G. Genetic aberrations of signaling pathways in lymphomagenesis: revelations from next generation sequencing studies. Semin Cancer Biol. 2013;23:422–430. doi: 10.1016/j.semcancer.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz A, Reischl U, Swerdlow SH, Hartke M, Streubel B, Procop G, Tubbs RR, Cook JR. Extranodal marginal zone B-cell lymphomas of the ocular adnexa: multiparameter analysis of 34 cases including interphase molecular cytogenetics and PCR for Chlamydia psittaci. Am J Surg Pathol. 2007;31:792–802. doi: 10.1097/01.pas.0000249445.28713.88. [DOI] [PubMed] [Google Scholar]
- 45.Sahin I, Leblebjian H, Treon SP, Ghobrial IM. Waldenstrom macroglobulinemia: from biology to treatment. Expert Rev Hematol. 2014;7:157–168. doi: 10.1586/17474086.2014.871494. [DOI] [PubMed] [Google Scholar]
- 46.Salama ME, Lossos IS, Warnke RA, Natkunam Y. Immunoarchitectural patterns in nodal marginal zone B-cell lymphoma. Am J Clin Pathol. 2009;132:39–49. doi: 10.1309/AJCPZQ1GXBBNG8OG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt J, Federmann B, Schindler N, Steinhilber J, Bonzheim I, Fend F, Quintanilla-Martinez L. MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol. 2015 doi: 10.1111/bjh.13361. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow SH, Berger F, Pileri SA, Harris NL, Jaffe ES, Stein H. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC; Lyon: 2008. pp. 194–195. [Google Scholar]
- 49.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC; Lyon: 2008. [Google Scholar]
- 50.Tandon B, Peterson L, Gao J, Nelson B, Ma S, Rosen S, Chen YH. Nuclear overexpression of lymphoid-enhancer-binding factor 1 identifies chronic lymphocytic leukemia/small lymphocytic lymphoma in small B-cell lymphomas. Mod Pathol. 2011;24:1433–1443. doi: 10.1038/modpathol.2011.103. [DOI] [PubMed] [Google Scholar]
- 51.Tandon B, Swerdlow SH, Hasserjian RP, Surti U, Gibson SE. Chronic lymphocytic leukemia/small lymphocytic lymphoma: another neoplasm related to the B-cell follicle? Leuk Lymphoma. 2015:1–9. doi: 10.3109/10428194.2015.1037759. [DOI] [PubMed] [Google Scholar]
- 52.Terre C, Nguyen-Khac F, Barin C, Mozziconacci MJ, Eclache V, Leonard C, Chapiro E, Farhat H, Bouyon A, Rousselot P, Choquet S, Spentchian M, Dubreuil P, Leblond V, Castaigne S. Trisomy 4, a new chromosomal abnormality in Waldenstrom’s macroglobulinemia: a study of 39 cases. Leukemia. 2006;20:1634–1636. doi: 10.1038/sj.leu.2404314. [DOI] [PubMed] [Google Scholar]
- 53.Treon SP. How I treat Waldenstrom macroglobulinemia. Blood. 2015;126:721–732. doi: 10.1182/blood-2015-01-553974. [DOI] [PubMed] [Google Scholar]
- 54.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–2796. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 55.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC, Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR, Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML, Advani RH. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 56.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL, Laramie JM, Skifter DA, Lincoln SE, Hunter ZR. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 57.Vegliante MC, Palomero J, Perez-Galan P, Roue G, Castellano G, Navarro A, Clot G, Moros A, Suarez-Cisneros H, Bea S, Hernandez L, Enjuanes A, Jares P, Villamor N, Colomer D, Martin-Subero JI, Campo E, Amador V. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121:2175–2185. doi: 10.1182/blood-2012-06-438937. [DOI] [PubMed] [Google Scholar]
- 58.Venkataraman G, Rizzo KA, Chavez JJ, Streubel B, Raffeld M, Jaffe ES, Pittaluga S. Marginal zone lymphomas involving meningeal dura: possible link to IgG4-related diseases. Mod Pathol. 2011;24:355–366. doi: 10.1038/modpathol.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Visco C, Hoeller S, Malik JT, Xu-Monette ZY, Wiggins ML, Liu J, Sanger WG, Liu Z, Chang J, Ranheim EA, Gradowski JF, Serrano S, Wang HY, Liu Q, Dave S, Olsen B, Gascoyne RD, Campo E, Swerdlow SH, Chan WC, Tzankov A, Young KH. Molecular characteristics of mantle cell lymphoma presenting with clonal plasma cell component. Am J Surg Pathol. 2011;35:177–189. doi: 10.1097/PAS.0b013e3182049a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, Deshpande V, Stone JH. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore) 2013;92:206–216. doi: 10.1097/MD.0b013e31829cce35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang E, Stoecker M, Burchette J, Rehder C. Follicular lymphoma with prominent Dutcher body formation: a pathologic study of 3 cases in comparison with nodal or splenic lymphoplasmacytic lymphoma and marginal zone lymphoma. Hum Pathol. 2012;43:2001–2011. doi: 10.1016/j.humpath.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Yamada S, Ishida Y, Matsuno A, Yamazaki K. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma. 2015:1–5. doi: 10.3109/10428194.2014.979413. [DOI] [PubMed] [Google Scholar]