Abstract
Transplant outcomes of autologous or allogeneic stem cell transplantation (SCT) have not been elucidated as a single cohort in non-Hodgkin lymphoma (NHL). We analyzed the outcomes of 270 adult recipients receiving auto (n=198) or allo-SCT (n=72) for NHL between year 2000 and 2010. Five-year overall survival for B-cell and T-cell NHL were 58% and 50%, respectively (allo-SCT 51% vs. 54% for B and T-cell NHL, and auto-SCT 60% vs. 47% for B and T-cell lymphoma, respectively) (p=NS). In multivariate analysis, number of chemotherapy regimens and disease status pre-SCT were independently associated with long-term outcome after SCT (for both auto and allo-SCT). We conclude that based on patient selection and disease related factors, the type of transplantation offered to patients can achieve long term survival highlighting the importance of further improvement in disease control and reducing procedure related mortality. The role of transplantation needs to be reevaluated in the era of targeted therapy.
Keywords: autologous stem cell transplant, allogeneic stem cell transplant, lymphoma
Introduction
Stem cell transplantation is frequently considered for eligible patients with NHL.1–6 Autologous-SCT (auto) is recommended for patients with either relapsed NHL or in first remission as consolidative therapy. Given the high rate of relapse seen even after chemotherapy and auto-SCT, and the potential benefit of a graft-versus-lymphoma (GVL) effect after allogeneic-SCT (allo) patients with NHL are frequently considered for allo-SCT. The outcomes of these patients in large prospective studies are lacking and current recommendations and timing of selection of auto or allo-SCT are influenced by variety of factors including patient or disease related factors, physician preference and intuitional practices.7–10
Registry data from the EBMT and the CIBMTR shows no plateau in the relapse rates post-autografting.11–13 Furthermore, the risk of second malignancies post auto-HCT is not insignificant, ranging from 5–15% in several studies. On the other hand, clinical evidence of a GVL effect after allo-SCT is suggested by a plateau in relapse risk that is reached 2–5 years following allo-SCT, indicating that a substantial proportion of lymphoma patients derive long-term disease control from transplantation.10;12;14
The only prospective comparison being conducted of auto and allo-SCT for relapsed NHL (low grade histology only) closed early as a result of poor accrual.15 Moreover, it would be impossible to perform comparative studies due to varied disease course among vast and heterogeneous NHL histologies. The comparisons based on retrospective analyses of registry data have shown a lower relapse rate and a longer progression-free survival after allo-SCT than after auto-SCT.14–17 The high NRM rate associated with myeloablative (MST) allo-SCT, however, offsets any potential survival benefits.12;14;18;19 Reduced-intensity conditioning (RIC) and non-myeloablative conditioning (NST) regimens are increasingly used in patients with NHL.5;6;20 These lower-intensity conditioning regimens (RIC or NST) reportedly have lower non-relapse mortality (NRM) and can be used in older patients with comorbidities. Lower-intensity regimens for allo-SCT use lower doses of conditioning chemotherapy and radiation and rely on an immune-mediated GVL effect for disease control.21;22 In the era of emerging novel therapies, the actual timing, optimal conditioning regimens and long term impact of the type of stem cell transplantation is unclear.
The primary objective of the present analysis was to define outcomes after SCT in patients with NHL and to correlate disease and treatment-related variables with outcomes in the rituximab era. We clarify that, this analysis does not attempt to compare directly the outcomes of subjects with NHL who received auto or allo-SCT.
Patients and methods
Two-hundred-seventy consecutive patients older than age 18 years with NHL receiving SCT between January 2000 and December 2010 at Vanderbilt University Medical Center adult transplant program, were included in this study (Table 1). All B-cell NHL patients received planned rituximab based chemotherapy pre-SCT. Patients were required to have chemotherapy-sensitive disease (or non-bulky stable disease [SD]) documented pre-SCT after induction or salvage chemotherapy. This study was approved by the Institutional Review Board of Vanderbilt University Medical Center. All patients provided informed consent in accordance to the Declaration of Helsinki.
Table 1.
Patient Characteristics
| Variable | Auto-SCT (n=198) |
Allo-SCT (n=72) |
|---|---|---|
| Male | 127 (64%) | 43 (60%) |
| Female | 71 (36%) | 29 (40%) |
| Median age | 52 (22–71) | 47 (22–65) |
| B-cell | 176 (89%) | 62 (86%) |
| T-cell | 22 (11%) | 10 (14%) |
| B-cell Indolent | 19 (25%) | 30 (48.2%) |
| B cell aggressive | 157 (75%) | 32 (51.8%) |
| Stage | ||
| I/II | 53 (27%) | 11 (15%) |
| III/IV | 145 (73%) | 61 (85%) |
| Number of prior regimens | ||
| ≤2 | 128 (65%) | 30 (42%) |
| >2 | 70 (35%) | 42 (58%) |
| Disease status prior to transplant | ||
| CR | 142 (70%) | 40 (56%) |
| PR/ SD | 56 (29%) | 32 (44%) |
| Prior radiation Yes | 19 (10%) | 15 (20%) |
| MRD | - | 45 |
| Unrelated stem cell source | - | 27 |
| RIC | - | 49 (68%) |
| MST | - | 23 (32%) |
Clinical information was reviewed, and baseline characteristics including common pre-transplant and transplant variable information was recorded.
NHL: histological subtypes
In B cell histological subtypes, diffuse large, mantle cell, transformed lymphoma were considered aggressive lymphomas while follicular lymphoma was indolent. All peripheral T cell lymphomas were considered aggressive in nature except ALK positive anaplastic large cell lymphoma. All pathology was reviewed and diagnosis confirmed at our institution.
Definitions and response criteria
Overall survival (OS) was defined as the time from date of transplantation to date of death or last follow up. Progression free survival (PFS), was calculated as the time interval between the date of transplantation and date at relapse, progression or death after transplantation. Patients alive without evidence of disease relapse or progression were censored at last follow-up, and PFS was summarized by a survival curve. Non-relapse mortality (NRM) was defined as death from any cause without evidence of lymphoma progression/relapse.
Response criteria were based on guidelines from the International workshop on non-Hodgkin lymphoma (NHL).23 Complete remission (CR) was defined as complete radiological regression of all previous measurable disease or bone marrow involvement. Partial response (PR) was defined as a reduction of 50% or greater reduction in the sum of the products of the longest and perpendicular diameter of measurable lesions. Progression was defined as an increase of ≥25% in the sites of lymphoma or development of new sites of lymphoma at any time after transplantation. Relapse was defined as recurrence of lymphoma after a complete response. Based on these criteria, all data were individually verified regarding the best response status prior to SCT.
Other outcomes analyzed include acute and chronic graft vs. host disease (GVHD) and cause of death. Acute GVHD was defined and graded based on the pattern and severity of organ involvement using established criteria.24 Chronic GVHD was defined as the development of any cGVHD based on clinical criteria.25;26 Both these events were summarized by the corresponding cumulative incidence estimate, with death without development of GVHD as the competing risk. The WHO criteria were used to define histological classification of NHL after 2001.
Transplantation procedures
Auto-SCT
Stem cells were mobilized using high dose chemotherapy (cyclophosphamide) and G-CSF or G-CSF (with or without plerixafor). CBV (cyclophosphamide 7200mg/m2, etoposide 2000 mg/m2, and BCNU 400 mg/ m2) was the most commonly used (87%) conditioning regimen in patients receiving auto-SCT. Patients with a histological diagnosis of mantle cell and peripheral T-cell lymphoma received auto-SCT in CR1. Patients with relapsed DLBCL underwent auto-SCT in CR2.
Allo-SCT
Forty nine patients received reduced intensity conditioning (RIC; fludarabine and busulfan [Flu Bu]= 39; fludarabine, cyclophosphamide, rituximab [FCR]= 10) and 23 myeloablative (MST) regimen followed by either matched related (n=45) or unrelated donor (n=27) stem cell transplantation. All patients received GVHD prophylaxis with calcineurin inhibitor and either methotrexate (myeloablative and FCR RIC regimen) or mycophenolate mofetil (Flu Bu RIC regimen).
Supportive care
All patients received standard supportive care per institutional guidelines. Standard antimicrobial prophylaxis, surveillance cultures, and treatment were administered per protocol.
Statistical Analysis
Patient, disease and transplant-related variables (Table 1) were described with median and range for continuous variables, and percent of total for categorical variables. Occurrence of aGVHD and cGVHD, NRM, and disease recurrence/progression were calculated using cumulative incidence estimates, taking into account the competing risk. Probabilities of PFS and OS were estimated from the time of transplantation using Kaplan-Meier analysis. Univariate analysis was performed with disease and transplantation related variables to see the impact on long term outcome on patients. Chi-Square test was used to determine the relationship between all categorical variables. For association between continuous variables and categories Mann–Whitney U rank sum tests were used. Multivariable analyses were performed using logistic regression or Cox proportional hazard regression models. The proportional hazards assumptions for all the variables were examined by testing time-dependent covariates. All reported p values were two-sided with statistical significance declared at p<0.05. Statistical analyses were performed using SPSS software (v.20) (IBM-SPSS, Chicago, IL, USA).
Results
Patient, disease, and transplantation related characteristics are presented in Table 1.
A total of 270 eligible patients underwent SCT for NHL from 1/2000 to 12/2010 included in analysis (auto-SCT=198, allo-SCT=72). The median age at transplant was 52 years for the entire group. The median age of patients receiving auto-SCT was 52 years (range, 22–71) and 47 years (range, 22–65) for allo-SCT recipients.
Majority of patients (76%) had advanced stage disease (Table 1). Fifty four (20%) received radiation therapy either before or after transplantation. The median number of prior regimens for allo-SCT were 3 (range 1–5) and 2 for auto-SCT (range 1 to 4). At disease relapse, 63% of patients received Ifosfamide, carboplatin and etoposide (ICE); 27% received etoposide, solumedrol, cytarabine, cisplatin (ESHAP) and 10% of patients received other therapies as salvage treatment.
One hundred eighty two (67%) patients were in CR at the time of SCT (auto=142 [70%]; allo=40 [56%]) and 88 (33%) patients had PR or SD disease pre-SCT (auto=56 [29%; allo=32 [44%]).
In total 238 patients underwent SCT for B-cell lymphoma (176 auto, 62 allo-SCT), and 32 for T-cell lymphoma (22 auto and 10 allo-SCT). The median interval from diagnosis to allo-SCT or auto-SCT was 1.4 year (range, 0.32–13.1) and 1.69 (range 0.38–13.7), respectively. The median follow up time for the entire cohort was 6.2 year. Eighty-one patients (41%) underwent auto-SCT within one year of diagnosis. Fifteen patients (6%) received prior auto-SCT. The median interval between auto-SCT and allo-SCT was 2.1 year (range, 0.5–8). The median number of prior regimens for allo-SCT were 3 (range 1–5) and 2 for auto-SCT (range 1 to 4).
Within the allo-SCT group (n=72), 45 received matched-related donor transplants, and 26 unrelated donor transplants; majority of patients (n=49) received reduced intensity conditioning regimen. The auto-SCT group predominantly received CBV as their conditioning regimen. The median follow-up of entire cohort was three year (range 0.6–11.6).
Outcomes
Patient outcomes and univariate analysis are summarized in Table 2
Table 2.
Factors associated with outcome after SCT for NHL: Univariate analysis
| Variable list | Overall survival N (%) |
PFS N (%) |
|
|---|---|---|---|
| Age | <median vs. | 81 (59.5%) | 66 (49%) |
| ≥median | 73 (54.4%) | 61 (45.5%) | |
| Male | 95 (55.8%) | 77 (45%) | |
| Female | 59 (59%) | 50 (50%) | |
| B-cell | 138 (57.9%) | 114 (47.8%) | |
| T-cell | 16 (50%) | 13 (41%) | |
| Indolent | 28 (58.3%) | 25 (52%) | |
| Aggressive | 104 (57.4%) | 85 (47%) | |
| Stage | I/II | 42 (65.6%) | 32 (50%) |
| III/IV | 112 (54.3%) | 95 (46.1%) | |
| Number of regimens | |||
| ≤2 | 100 (63.2%) p=0.018 | 83 (52.5%) p=0.036 | |
| >2 | 54 (48.2%) | 44 (39%) | |
| Disease status | |||
| CR | 112 (61.5%) p=0.018 | 94 (51.5%) p=0.037 | |
| PR/ SD | 42 (47.7%) | 33 (37%) | |
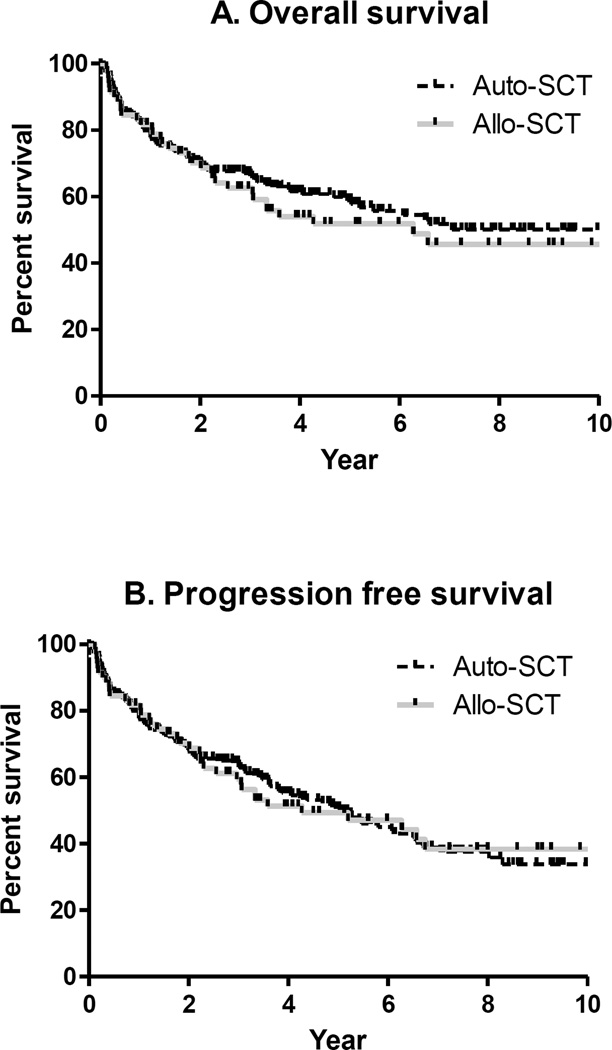
One hundred fifty four of the 270 patients were alive at the time of analysis. The 5 year OS of the entire cohort was 52% (95% CI 44–59%), (auto-SCT 59%, 95% CI 51–66%; allo-SCT 52%, 95% CI 40–63%; p=0.5; Figure 1A). Five year PFS for the entire cohort was 50% (95% CI 42–55%), (auto-SCT 51%, 95% CI 42–58%; allo-SCT 49%, 95% CI 37–60%; p=NS; Figure 1B).
Figure 1.
NRM rates were 10.5% (95% CI, 3.2%–19.2%) at 5 year after allo-SCT. Two year NRM for patients who received prior-auto was 0%.
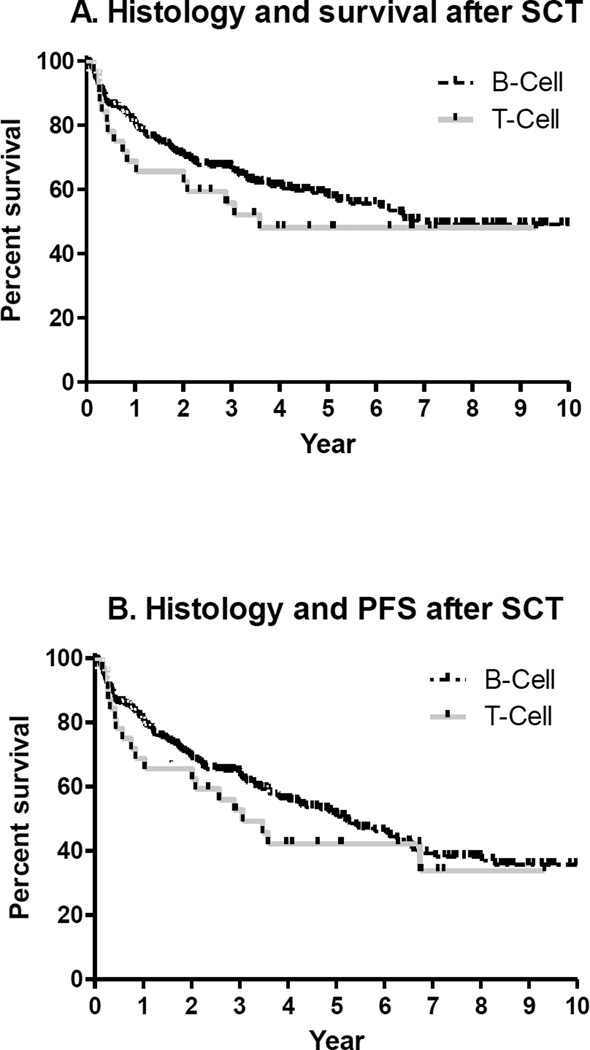
Five year OS rates for the B- cell and T-cell NHL were 58% (95% CI 51–65%) and 48% (95% CI 30–64%), respectively (Figure 2A). Five year PFS for the B-cell and T-cell NHL were 51% (95% CI 44–58%) and 42% (95% CI 25–59%), respectively (Figure 2B).
Figure 2.
Factors associated with transplant outcome
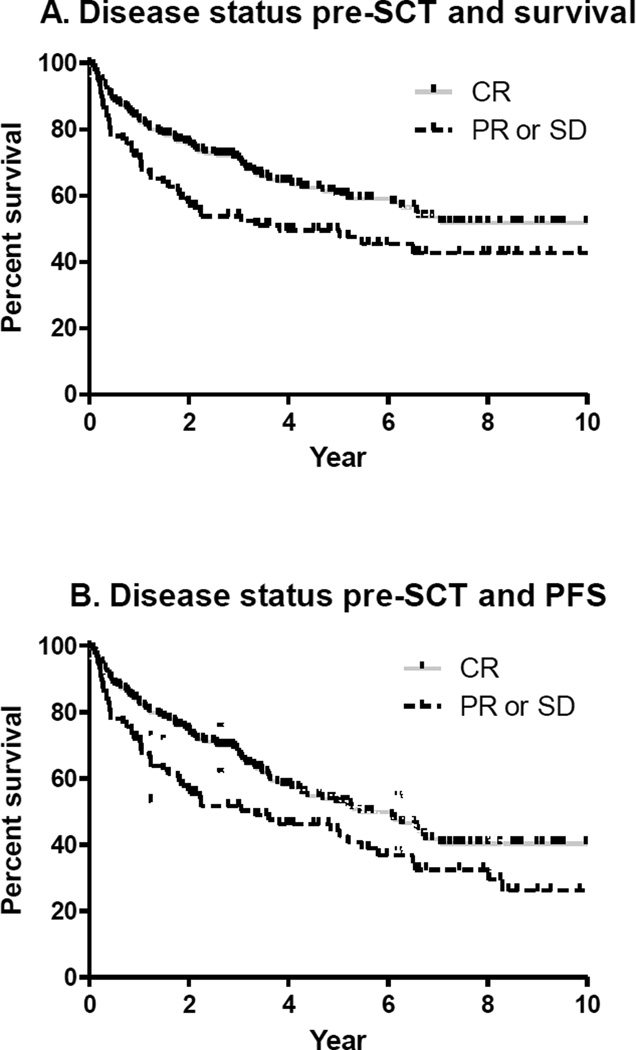
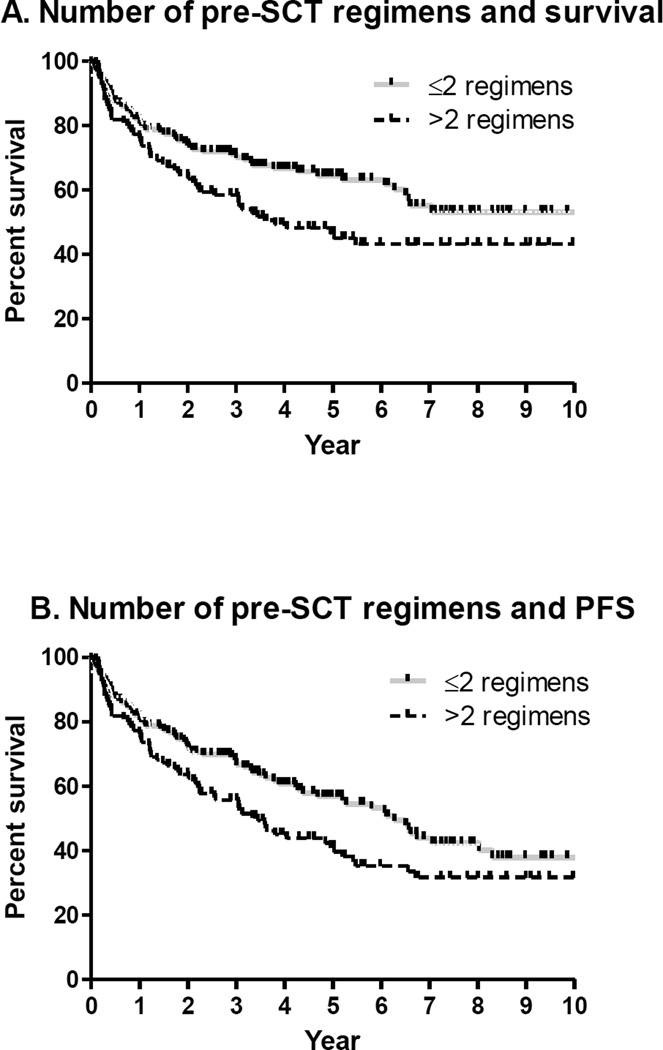
OS and PFS were significantly associated with disease status pre-SCT (Figure 3) and number of chemotherapy regimens pre-SCT (Figure 4). Differences remain significant when auto-SCT and allo-SCT groups were analyzed separately and for patients with B-cell NHL. Further analysis was restricted among T-cell NHL due to limited number of patients receiving allo-SCT.
Figure 3.
Figure 4.
Five year OS and PFS rates for patients in CR pre-SCT were 60% (95% CI 52–67%) and 52% (95% CI 44–60%) and for patients in PR or SD at transplant were 47% (95% CI 37–58%) and 43% (95% CI 32–54%), respectively (Figure 3).
Similarly, 5 year OS and PFS rates for patients ≤2 regimens pre-SCT were 65% (95% CI 54–72%) and 57% (95% CI 46–65%) and for patients receiving >2 regimens pre-SCT were 47% (95% CI 37–57%) and 41% (95% CI 31–51%), respectively (Figure 4).
There was no impact of acute or chronic GVHD on transplant outcome.
Multivariate Analyses
Table 3 presents the results of multivariate analysis of OS and PFS.
Table 3.
Multivariable analysis
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Number of regimens (≤2 vs. >2) | 1.55 | 1.07–2.23 | 0.01 | 1.43 | 1.03–1.99 | 0.03 |
| Disease status (CR vs. PR/ SD) | 1.56 | 1.08–2.27 | 0.01 | 1.50 | 1.07–2.11 | 0.01 |
OS and PFS were significantly correlated with the number of chemotherapy regimens pre-SCT (OS- HR 1.55, 95% CI 1.07– 2.23, p=0.01; PFS- HR 1.43, 95% CI 1.03– 1.99, p=0.03). Also, OS and PFS were significantly independently associated with disease status pre-SCT (OS- HR 1.56, 95% CI 1.08– 2.27, p=0.01; PFS- HR 1.50, 95% CI 1.07– 2.11, p=0.01).
Discussion
In this cohort of patients with a majority considered as high-risk for disease relapse, 49.5% of subjects were alive without disease progression at 5 year post SCT. Disease recurrence is still the most common cause of transplant failure. Risk factors that correlated with poor outcome were disease status and number of chemotherapy regimens pre-SCT. These results indicate that in the era of immuno-chemotherapy, the earlier transplantation is performed, the better the outcome. It remains to be seen if this observation continues to be applicable in the era of targeted therapy.
Interestingly, NRM in our series after allo-SCT was more favorable than previously reported.12;14 Historically, the limitation of allo-SCT has been NRM. In order to offer the curative allo-SCT treatment option for most patients, safer regimens with acceptable GVHD-associated morbidity and NRM are preferred. Among several RIC regimens, FCR regimen followed by either related or unrelated donor allo-SCT is safe and effective in B cell lymphoid malignancy.5;21;22 A recently published MDACC study showed excellent PS and OS (85% and 83%, respectively, after a median follow-up of 60 months) for relapsed FL after FCR RIC allo-SCT.22 The incidence of grade II-IV acute GVHD (aGVHD) was only 11%. We have also recently reported our results using FCR RIC allo-SCT in patients with CD20+ B-cell lymphoid malignancies with improved outcome and low GVHD and NRM.5 Lower intensity conditioning regimens have therefore been extended to older patients. As expected, subjects with more advanced disease and more aggressive histologies had greater risk of relapse.
Previous studies reporting on RIC or NST allo-SCT in patients with NHL who relapse after auto-SCT have included limited number of patients, with variable histologies and variable follow-up, limiting comparisons. Recently published, registry study showed 3 year probabilities of PFS and OS were 21% and 32%, respectively after RIC allo-SCT who had failed prior auto-SCT.27 Despite the lower intensity of the conditioning regimens, 3 year NRM was high at 44%. This is in contrast to our series, albeit small numbers, we observed 0% 2 year NRM in patients receiving allo-SCT after failed auto-SCT. Furthermore, our study population did not include all patients who relapsed after auto-HSCT and were eligible for RIC or NST allo-SCT. In fact, only a minority of patients who relapse after auto-SCT undergo allo-SCT. The reasons for this might be related to the lack of effective salvage therapies for NHL relapse, early mortality after relapse, ineligibility for allo-SCT, or patient-physician choice.
Most previous studies had limited statistical power to detect differences in outcomes among lymphoma subtypes.12;14;28 Similarly, survival was similar in patients with DLBCL, follicular cell lymphoma, and mantle cell lymphoma and T-cell lymphomas in the present study. More aggressive NHL histologies had reported outcomes approximating 20% PFS. In contrast, to selecting RIC or NST regimens to all NHL patients, we prefer MST regimens for eligible patients with aggressive histology. The outcome of aggressive NHL in our series is highly favorable likely due to selection of upfront MST allo-SCT for eligible patients with aggressive NHL.
Our interpretation of these results is limited due to the fact that our study population was subject to selection bias. We report the outcomes of patients who were able to undergo the planned procedure. We do not report outcomes on patients who were deemed ineligible either due to disease or patient characteristics. Despite individual physician bias in patient selection, it is important to recognize that we uniformly follow standard institutional protocol prior to recognizing an eligible patient.
The transplant outcomes are certainly less than optimal; more than 45% of patients relapsed after auto or allo-SCT for NHL. The recommendation for the type of SCT approach might be very challenging considering the availability of novel agents.29 It is certain that as agents that target pathways such as PI3-kinase, bruton tyrosine kinase, histone deacetylase or immunomodulators gain FDA approval for various NHL histologies, the role and timing of SCT becomes even more complex. Incorporating these agents as a maintenance strategy following RIC- allo or auto transplants is certainly an attractive strategy. We believe that future clinical trials should aim to consider these novel agents in the peri and post- transplant period.
Acknowledgments
Funding: This work was supported by National Center for Research Resources, National Institute of Health (grant # 5K-12 CA090625-09, N.R.)
Footnotes
Declaration of commercial interest: None
Reference List
- 1.Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy PL, Jr, Hahn T, Hassebroek A, et al. Trends in utilization and survival after autologous hematopoietic cell transplantation in North America from 1995 to 2005: Significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy N, Greer JP, Goodman S, et al. Consolidative therapy with stem cell transplantation improves survival of patients with mantle cell lymphoma after any induction regimen. Exp. Hematol. 2012;40:359–366. doi: 10.1016/j.exphem.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy N, Savani BN. Treatment options for transformed lymphoma: incorporating allogeneic stem cell transplantation in a multimodality approach. Biol. Blood Marrow Transplant. 2011;17:1265–1272. doi: 10.1016/j.bbmt.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinratanalab W, Reddy N, Greer JP, et al. Immunomodulatory nonablative conditioning regimen for B-cell lymphoid malignancies. Exp. Hematol. 2012;40:431–435. doi: 10.1016/j.exphem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khouri IF. Allogeneic stem cell transplantation in follicular lymphoma. Best. Pract. Res. Clin. Haematol. 2011;24:271–277. doi: 10.1016/j.beha.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary L, Kharfan-Dabaja MA, Hari P, Hamadani M. Is hematopoietic cell transplantation still a valid option for mantle cell lymphoma in first remission in the chemoimmunotherapy-era? Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.56. [DOI] [PubMed] [Google Scholar]
- 8.Oliansky DM, Czuczman M, Fisher RI, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol. Blood Marrow Transplant. 2011;17:20–47. doi: 10.1016/j.bbmt.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Oliansky DM, Gordon LI, King J, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: an evidence-based review. Biol. Blood Marrow Transplant. 2010;16:443–468. doi: 10.1016/j.bbmt.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Papageorgiou SG, Cwynarski K, Kottaridis PD. The role of allogeneic haematopoietic progenitor cell transplantation in patients with diffuse large B-cell non-Hodgkin lymphomas (DLBCL) Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2012.266. [DOI] [PubMed] [Google Scholar]
- 11.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 at: http://www.cibmtr.org. http://www.cibmtr.org. 2012. Ref Type: Electronic Citation. [Google Scholar]
- 12.Jantunen E, Sureda A. The evolving role of stem cell transplants in lymphomas. Biol. Blood Marrow Transplant. 2012;18:660–673. doi: 10.1016/j.bbmt.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 14.Ayala E, Tomblyn M. Hematopoietic cell transplantation for lymphomas. Cancer Control. 2011;18:246–257. doi: 10.1177/107327481101800405. [DOI] [PubMed] [Google Scholar]
- 15.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mounier N, Canals C, Gisselbrecht C, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant. 2012;18:788–793. doi: 10.1016/j.bbmt.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol. Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol. Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012 doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaise D, Farnault L, Faucher C, et al. Reduced-intensity conditioning with Fludarabin, oral Busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol. 2010 doi: 10.1016/j.exphem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Khouri IF. Reduced-intensity regimens in allogeneic stem-cell transplantation for non-Hodgkin lymphoma and chronic lymphocytic leukemia. Hematology. Am. Soc. Hematol. Educ. Program. 2006:390–397. doi: 10.1182/asheducation-2006.1.390. [DOI] [PubMed] [Google Scholar]
- 22.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 25.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am. J. Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 26.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freytes CO, Zhang MJ, Carreras J, et al. Outcome of lower-intensity allogeneic transplantation in non-Hodgkin lymphoma after autologous transplantation failure. Biol Blood Marrow Transplant. 2012;18:1255–1264. doi: 10.1016/j.bbmt.2011.12.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz N, Dreger P, Glass B, Sureda A. Allogeneic transplantation in lymphoma: current status. Haematologica. 2007;92:1533–1548. doi: 10.3324/haematol.11185. [DOI] [PubMed] [Google Scholar]
- 29.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–1503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]