Abstract
Moderate-to-severe traumatic brain injury (TBI) remains a major global challenge, with rising incidence, unchanging mortality and lifelong impairments. State-of-the-science reviews are important for research planning and clinical decision support. This review aimed to identify randomized controlled trials (RCTs) evaluating interventions for acute management of moderate/severe TBI, synthesize key RCT characteristics and findings, and determine their implications on clinical practice and future research. RCTs were identified through comprehensive database and other searches. Key characteristics, outcomes, risk of bias, and analysis approach were extracted. Data were narratively synthesized, with a focus on robust (multi-center, low risk of bias, n > 100) RCTs, and three-dimensional graphical figures also were used to explore relationships between RCT characteristics and findings. A total of 207 RCTs were identified. The 191 completed RCTs enrolled 35,340 participants (median, 66). Most (72%) were single center and enrolled less than 100 participants (69%). There were 26 robust RCTs across 18 different interventions. For 74% of 392 comparisons across all included RCTs, there was no significant difference between groups. Positive findings were broadly distributed with respect to RCT characteristics. Less than one-third of RCTs demonstrated low risk of bias for random sequence generation or allocation concealment, less than one-quarter used covariate adjustment, and only 7% employed an ordinal analysis approach. Considerable investment of resources in producing 191 completed RCTs for acute TBI management has resulted in very little translatable evidence. This may result from broad distribution of research effort, small samples, preponderance of single-center RCTs, and methodological shortcomings. More sophisticated RCT design, large multi-center RCTs in priority areas, increased focus on pre-clinical research, and alternatives to RCTs, such as comparative effectiveness research and precision medicine, are needed to fully realize the potential of acute TBI research to benefit patients.
Key words: : clinical trial, review, traumatic brain injury
Introduction
Moderate to severe traumatic brain injury (TBI) remains a significant global heath challenge. Estimates of TBI incidence vary from under 100 to over 700 per 100,000 head of population due to variability in TBI definition and incomplete collection of incidence and outcome data.1–3 Despite these limitations, there are sufficient data to demonstrate a rise in TBI incidence, driven by increased use of motor vehicles in developing countries and falls in aging global population.2,4–6
Outcomes following TBI have not changed substantially over the last 25 years, as reflected by meta-analyses demonstrating weighted mortality rates of 36–42% and unfavorable outcome rates of 52–60% for severe TBI (Glasgow Coma Scale (GCS) score ≤8).7 Various reasons for this lack of progress have been postulated. A rise in the elderly population, who are at greater risk of mortality due to comorbidities, may be offsetting improved outcomes expected from implementation of evidence-based TBI guidelines.2 The complexity and heterogeneity of the disease and limitations of conventional statistical analysis may also explain why TBI trials have not shown beneficial effect of treatments.8
Survivors of moderate-to-severe TBI (GCS ≤12) face reduced life expectancy9,10 and long-term deficits in physical, cognitive, behavioral, and social function,5,11–14 which carry substantial costs to quality of life5 and society.6 Given these substantial personal, financial, and societal impacts, a comprehensive “state-of-the-science” overview of TBI research is warranted. Unlike systematic reviews focusing on one intervention, overviews can facilitate identification of the nature and findings of research across an entire field of enquiry.15,16 Such overviews also are critical for research synthesis, planning, translation, and clinical decision support.17 Existing TBI research overviews are limited in use of systematic search18,19 and/or scope.18–23 Therefore, this overview aimed to:
• Systematically identify RCTs evaluating interventions for acute management of moderate to severe TBI;
• Describe their key characteristics, findings, and risk of bias; and
• Discuss the implications of overview findings for clinical practice and research.
This overview updates and expands upon previous large-scale overviews published by the author team.7,24,25
Methods
A full description of study methods is provided in Appendix 1. The comprehensive systematic search for primary studies from our previously published review7 was updated to November 2013, and our search of the World Health Organization International Clinical RCTs Registry Platform26 updated to March 2015. We examined reference lists of all systematic reviews within a comprehensive TBI systematic review database, updated to March 2015, for further RCTs.27 Included full text publications from the previous review (n = 143),7 as well as titles, abstracts, and full-text publications from the update search outlined above, were screened to identify RCTs of acute interventions for adults or children with moderate to severe TBI.
RCTs were categorized in Microsoft® Excel® for Mac 201128 under 11 intervention categories:
1. Airway, ventilation, and oxygenation strategies
2. Fluid management
3. Hypothermia/normothermia
4. Intracranial pressure (ICP), cerebral perfusion pressure (CPP), and blood pressure (BP) management (including hyperosmolar therapies)
5. Nutrition and glucose management
6. Pharmacological therapies (not elsewhere defined)
7. Pre-hospital care
8. Sedation, pain management, anesthesia, and arousal
9. Seizure prophylaxis
10. Steroids
11. Surgery
For all eligible RCTs, key characteristics (year of publication, country, number of participants/centers, GCS score of participants, intervention, and comparison) were extracted. For completed RCTs:
- • Study findings were extracted for:
- ○ Clinical outcomes of interest: mortality, Glasgow Outcome Score (GOS), Glasgow Outcome Score extended (GOSE)
- ○ Early surrogate end-points: intracranial pressure (ICP), hospital and/or intensive care unit (ICU) length of stay (LOS)
• For two clinical outcomes of interest (early mortality, 6-month GOS), three-dimensional graphical analysis was undertaken to explore associations between RCT characteristics and outcomes;
• Risk of bias was evaluated based upon two key domains of the Cochrane Risk of Bias Tool: random sequence generation and allocation concealment29; and
• The use of two advanced statistical approaches for dealing with the heterogeneity inherent to TBI populations were recorded: covariate adjustment and (for studies measuring GOS/GOSE) ordinal analysis.
Findings of completed adult RCTs were narratively summarized, with an emphasis on robust RCTs. For the purpose of this overview, “robust” was defined as:
• Multi-center, due to the importance of multi-center design on generalizability of RCT findings.30,31 Further, it has been demonstrated in critical care literature that interventions found to be effective in single-center trials have subsequently been found in multi-center studies to be ineffective or harmful.31 This phenomenon is also evidenced by a meta-epidemiological analysis showing that clinical trials with continuous outcomes showed slightly larger intervention effects than did multi-center trials32;
• Low risk of bias in random sequence generation and / or allocation concealment, as there is empirical evidence of the influence of these types of bias on RCT outcomes33; and
• Over 100 participants, as it has been demonstrated that TBI RCTs above this size have a significantly different effect size to TBI RCTs below this size.34
Role of the funding source
The sponsors of this study had no role in study design; collection, analysis, and interpretation of data; writing of this manuscript, or the decision to submit this manuscript for publication. The corresponding author (PB) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
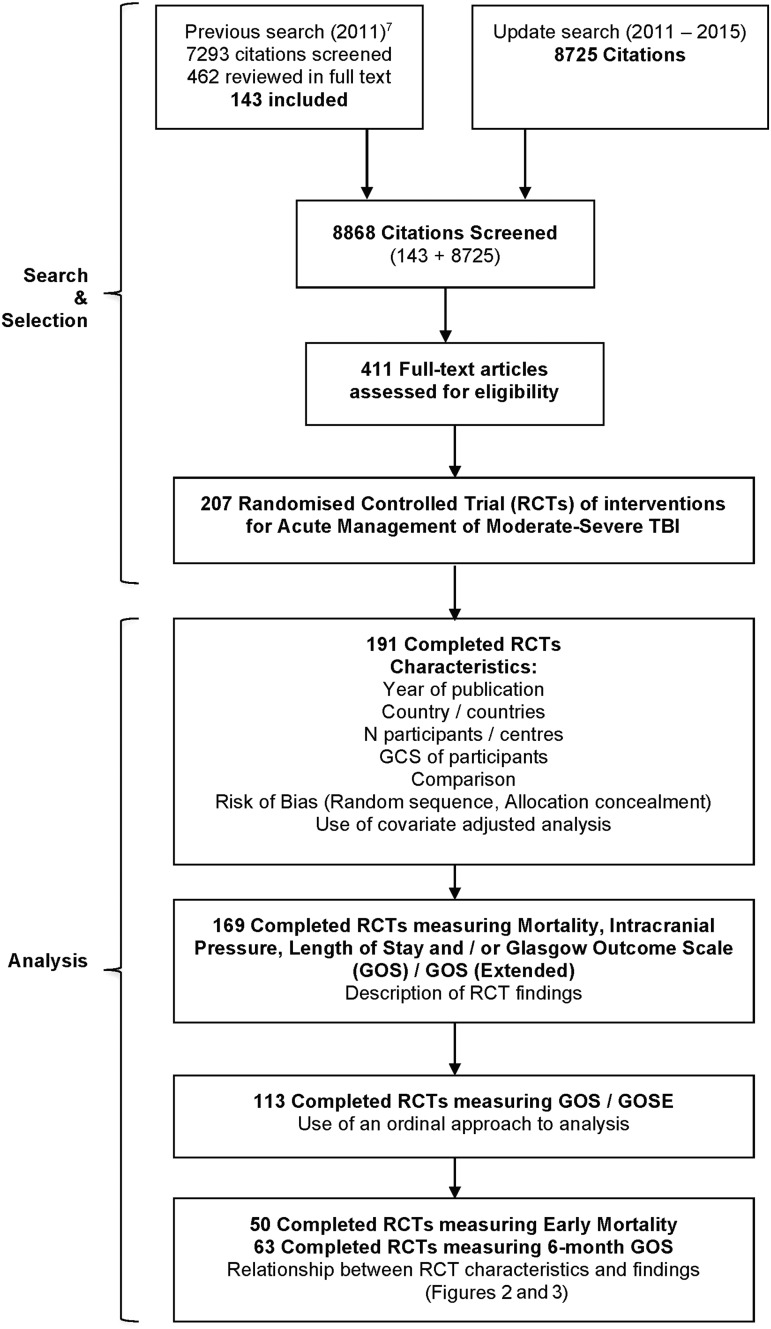
Search and study selection yielded 207 RCTs, of which 191 were complete (180 adult, 11 pediatric) and 169 made statistical comparisons between groups for at least one outcome of interest (Fig. 1). Supplementary Table 1 (see online supplementary material at www.liebertpub.com) contains all data extracted from all 207 RCTs. Of all 173 single-country RCTs, 71 (41%) were from the United States, 23 (13%) from China, nine (5%) from the United Kingdom, and eight (5%) each from France and Japan. Thirty-five RCTs (17%) were the sole study of an intervention, including 14 of pharmacological agents and seven of nutritional agents.
FIG. 1.
Search, selection, and analysis process.
Characteristics of completed RCTs (Table 1)
Table 1.
Characteristics and Risk of Bias by Intervention Category for Completed RCTs
| TOTAL | Airway/Vent/Oxy | Fluid Mx | Hypothermia | ICP/CPP/BP Mx including hyperosmolar | Nutrition/glucose Mx | Pharmacological therapies | Pre-hospital care | Sedation/pain Mx/anesthesia | Seizure prophylaxis | Steroids | Surgery | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Trials | 191 | 10 | 3 | 35 | 17 | 26 | 39 | 7 | 22 | 4 | 19 | 9 |
| N Participants | 35,340 | 646 | 580 | 3,351 | 1,266 | 1,594 | 9,826 | 2,155 | 904 | 1,191 | 12,455 | 1,372 |
| Population | ||||||||||||
| Adult | 180 | 9 | 3 | 31 | 15 | 25 | 39 | 7 | 21 | 4 | 18 | 8 |
| Pediatric | 11 | 1 | 0 | 4 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Number of participants | ||||||||||||
| Small (≤ 100) | 132 | 8 | 2 | 29 | 13 | 23 | 20 | 3 | 20 | 0 | 10 | 4 |
| Medium (101-500) | 51 | 2 | 1 | 6 | 4 | 3 | 13 | 3 | 2 | 4 | 8 | 5 |
| Large (> 500) | 8 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 1 | 0 |
| Year published | ||||||||||||
| Pre-1980 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 |
| 1980–1989 | 24 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 6 | 2 | 11 | 0 |
| 1990–1999 | 47 | 2 | 0 | 8 | 5 | 9 | 11 | 1 | 8 | 1 | 2 | 0 |
| 2000–2009 | 70 | 3 | 3 | 17 | 8 | 9 | 17 | 2 | 6 | 1 | 1 | 3 |
| 2010 onwards | 44 | 4 | 0 | 10 | 3 | 6 | 9 | 4 | 1 | 0 | 1 | 6 |
| Risk of bias: Random sequence | ||||||||||||
| High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unclear | 132 | 8 | 0 | 29 | 11 | 16 | 23 | 3 | 17 | 3 | 18 | 4 |
| Low | 59 | 2 | 3 | 6 | 6 | 10 | 16 | 4 | 5 | 1 | 1 | 5 |
| Risk of bias: Allocation concealment | ||||||||||||
| High | 5 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| Unclear | 124 | 9 | 1 | 30 | 11 | 18 | 18 | 1 | 16 | 1 | 14 | 4 |
| Low | 62 | 1 | 2 | 5 | 5 | 8 | 20 | 6 | 5 | 3 | 4 | 3 |
| Covariate adjustment (n = 185; 6 not reported) | ||||||||||||
| Yes | 44 | 4 | 2 | 5 | 2 | 4 | 16 | 0 | 3 | 2 | 3 | 3 |
| No | 141 | 6 | 1 | 28 | 15 | 22 | 22 | 7 | 19 | 2 | 14 | 5 |
| Ordinal approach (n = 111; 77 not applicable, 3 not reported) | ||||||||||||
| Yes | 8 | 1 | 0 | 1 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 1 |
| No | 103 | 3 | 2 | 25 | 10 | 8 | 22 | 6 | 9 | 0 | 11 | 7 |
RCT, randomized controlled trial; Vent, ventilation; Oxy, oxygenation: Mx, management; ICP, intracranial pressure; CPP, cerebral perfusion pressure; BP, blood pressure.
Most completed RCTs (67%) were published from 2000 onwards. The most frequently studied intervention categories were “pharmacological therapies” (39 RCTs), “hypothermia” (35 RCTs), and “nutrition/glucose management” (26 RCTs).
The 191 completed RCTs collectively enrolled 35,340 participants, with a median of 66 participants (range, 4–10,008). Most RCTs recruited participants with severe TBI (range or mean/median GCS score ≤8). Over two-thirds (132; 69%) of RCTs had fewer than 100 participants and almost three-quarters (138; 72%) were single center. The rate of RCTs published almost tripled since 1980, from 2.4 per year in the 1980s to more than seven per year from 2000 onwards. This does not include the 16 ongoing trials, which contribute to a total RCT rate (published + ongoing) of 12 per year from 2010–2015. Risk of bias for random sequence generation was “unclear” for 132 RCTs (69%) and “low” for 59 (31%). Risk of bias for allocation concealment was “high” for five RCTs (3%), “unclear” for 124 (65%), and “low”‘ for 62 (32%). Covariate adjustment was performed in 44 (24%) of 185 RCTs when this data were available; an ordinal approach to analysis was employed in eight (7%) of 111 applicable RCTs.
RCT findings overall (Table 2)
Table 2.
Outcomes for Completed RCTs (n = 169)
| RCT findings (% of RCTs measuring outcome)** | ||||
|---|---|---|---|---|
| Outcome | N RCTs reporting outcome (% of RCTs)* | Superior | No difference | Inferior |
| Mortality: early (≤ 1 month) | 49 (29%) | 5 (10%) | 44 (90%) | 0 (0) |
| Mortality: late (> 1 month) | 74 (44%) | 17 (23%)^ | 56 (76%)^ | 3 (4%) |
| Mortality: time not stated | 19 (11%) | 2 (11%) | 18 (95%)^ | 0 (0) |
| ICP | 76 (45%) | 30 (39%)^ | 45 (59%)^ | 3 (4%) |
| Length of stay: hospital | 20 (12%) | 0 (0) | 20 (100%) | 0 (0) |
| Length of stay: ICU | 24 (14%) | 7 (29%) | 16 (67%) | 1 (4%) |
| GOS: 6-month | 63 (37%) | 13 (21%) | 51 (81%)^ | 2 (3%)^ |
| GOS: all other time-points | 42 (25%) | 13 (31%) | 29 (69%) | 0 (0) |
| GOSE: 6-month | 11 (7%) | 2 (18%) | 9 (82%)^ | 1 (9%) |
| GOSE: all other time-points | 5 (3%) | 2 (40%) | 3 (60%) | 0 (0) |
| TOTAL (% of all 392 outcomes measured) | 91 (23%) | 291 (74%) | 10 (3%) | |
| TOTAL: CLINICAL OUTCOMES (Mortality, GOS, GOSE: 270 outcomes measured) | 54 (20%) | 210 (78%) | 6 (2%) | |
| TOTAL: SURROGATE ENDPOINTS (ICP, Length of Stay: 122 outcomes measured) | 37 (30%) | 81 (66%) | 4 (3%) | |
Will not total 100, as many RCTs measured ≥1 outcome of interest.
May not total 100 due to rounding.
Multiple measures of outcome with split findings; therefore, total n > total RCTs measuring outcome and total % > 100.
RCT, randomized controlled trial; ICU, intensive care unit; GOS, Glasgow Outcome Score; GOSE, Glasgow Outcome Score-Extended; ICP, intracranial pressure.
The most frequently measured outcomes were ICP (76 RCTs; 45% of total completed RCTs), late mortality (74; 44%), 6-month GOS (63; 37%), and early mortality (49; 29%). For the 392 statistical comparisons between groups on all outcomes of interest across all included RCTs, 74% (291 comparisons) found no significant difference between groups; 23% (91) found the intervention to be superior to control and 3% (10) found the intervention to be inferior to control. The outcomes with the highest proportion of superior findings were GOSE at time-points other than 6 months (two RCTs; 40% of all RCTs measuring this outcome), ICP (30; 39%), GOS at time-points other than 6 months (13; 31%), and ICU LOS (7; 29%). Conversely, all 20 of the RCTs measuring hospital LOS detected no difference between groups. Other outcomes with a high percentage of equivocal findings were the three mortality outcomes (76%-95%) and 6-month GOS/GOSE (81%, 82%). The rate of superior findings was lower for clinical outcomes (mortality, GOS/GOSE; 20%) than for surrogate end-points (ICP, LOS; 30%); conversely, the rate of equivocal findings was higher for clinical outcomes, compared with surrogate end-points (78% vs. 66%).
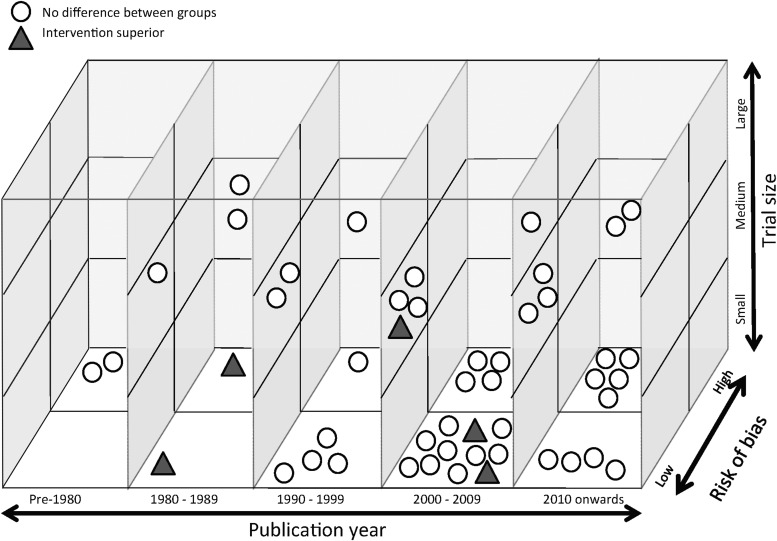
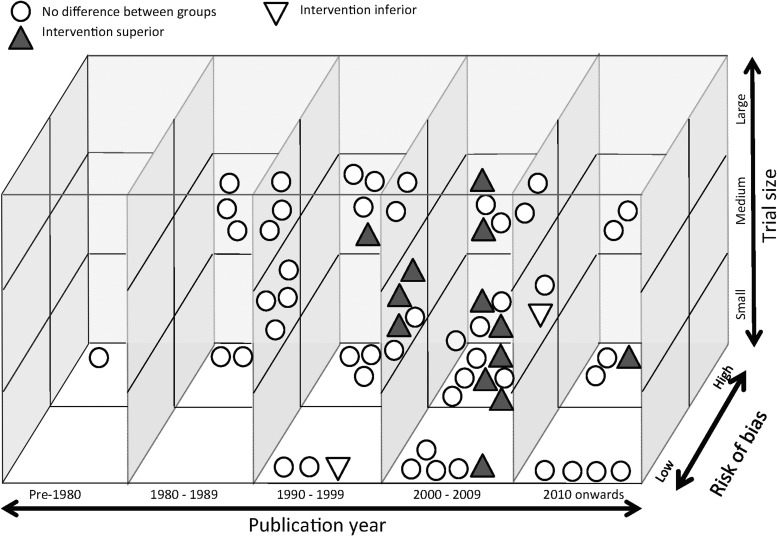
Three-dimensional graphical analysis of findings against RCT characteristics for early mortality and 6-month GOS (Fig. 2 and Fig. 3, respectively)
FIG. 2.
Three-dimensional graphical analysis of early mortality findings against randomized controlled trial (RCT) characteristics (49 RCTs, 49 outcome measurements).
○No difference between groups
▲Intervention superior
FIG. 3.
Three-dimensional graphical analysis of Glasgow Outcome Score findings against randomized controlled trial (RCT) characteristics (63 RCTs, 66 outcome measurements; only 64 are represented, as there were two RCTs in which these were two separate comparisons resulting in no difference between groups).
○No difference between groups
▲Intervention superior
▽Intervention inferior
The five positive early mortality RCTs were broadly distributed with respect to publication year, risk of bias and RCT size. The 13 positive 6-month GOS RCTs showed some clustering for year of publication (11 were published between 2000 and 2009) and intervention (four investigated hypothermia), but were otherwise also broadly distributed with respect to RCT characteristics.
RCT findings and clinical commentary by intervention category (Table 3)
Table 3.
Summary of Robust (Multi-Center, Low Risk of Bias, n > 100) Acute TBI RCTs (Multiple RCTs for an Intervention Highlighted)
| Study Characteristics | Study findings | Risk of bias | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country (N countries) | Number (Number of centers >1) | Comparison | GCS (mean): Md = Median, R = Range) | Mortality: early (≤1 month) | Mortality: late (> 1 month) | Mortality: NR | ICP* | Length of stay: ICU | Length of stay: hosp. | GOS (E = GOSE)** 6 month unless stated | Random seq. | Allocation concealment | Covariate adjustment | Ordinal approach |
| Fluid management [1] | |||||||||||||||
| Myburgh 2007 | 2 | 460 (16) | Saline vs. albumin | 7 (Md) | S | S | S (E, 24) | L | L | Y | N | ||||
| Hypothermia [1] | |||||||||||||||
| Andrews 2015 | 18 | 387 (47) | Hypothermia vs. normothermia | 3 – 15 (R) | I | ND | ND | I (E) | L | L | Y | Y | |||
| Management guided by ICP [1] | |||||||||||||||
| Chesnut 2012 | 2 | 324 (6) | ICP-guided Mx vs. clinical-imaging guided Mx | 3 – 8 (R) | ND | ND | ND (E) | L | U | Y | Y | ||||
| Pharmacological therapies not elsewhere defined [13] | |||||||||||||||
| Skolnick 2014 | 21 | 1195 (140) | Progesterone vs. placebo | 3 – 8 (R) | ND | ND, ND (E) | L | L | Y | Y | |||||
| Wright 2014 | USA | 882 (49) | Progesterone vs. placebo | 4 – 12 (R) | ND | ND (E) | L | L | Y | Y | |||||
| Shakur 2009 | 8 | 228 (15) | Low-, medium-, or high-dose Anatibant vs. placebo | 7·5 / 7·6 | ND | L | L | Y | |||||||
| Marmarou 1999 | USA | 139 (31) | Bradycor vs. placebo | 6·0 / 6·1 | ND | ND | ND | L | L | Y | N | ||||
| Euro. Study Group 1994 | 13 | 852 (21) | Nimodipine vs. placebo | NR | ND | ND | U | L | Y | N | |||||
| Teasdale 1992 | 3 | 352 (7) | Nimodipine vs. placebo | NR | ND | ND | ND | U | L | N | N | ||||
| Perel 2012 | 2 | 170 (10) | Tranexamic acid vs. placebo | 10·5 / 10·5 | ND | U | L | Y | |||||||
| Robertson 2014 | USA | 200 (2) | Erythropoetin vs. placebo | NR | ND | ND | U | L | L | ||||||
| Maas 2006 | 15 | 861 (15) | Dexanabinol vs. placebo | NR | ND | ND | ND | L | L | L | |||||
| Yurkewicz 2005 | USA | 404 (40) | Traxopodil vs. placebo | 4 – 8 (R) | ND | ND | L | U | L | ||||||
| Morris 1999 | 6 | 693 (99) | Selfotel vs. control [ceased; mortality / adverse events] | 6·1 / 6·1 | ND | ND | U | L | L | ||||||
| Marshall 1998 | 15 | 1120 (15) | Tirilazad vs. placebo | 4 – 12 (R) | ND | ND | L | U | L | ||||||
| Young 1996 | USA | 463 (29) | High-dose pegorgotein vs. placebo | 5·6 / 5·3 | ND | ND | U | L | L | ||||||
| Low-dose pegorgotein vs. placebo | 5·5 / 5·3 | ND | ND | ||||||||||||
| Pre-hospital cazre [3] | |||||||||||||||
| Bernard 2010 | Australia | 312 (4) | RSI vs non-invasive ventilation | 5·0 | ND | ND | ND | ND | S (E) | L | L | N | N | ||
| Morrison 2011 | Canada | 113 (2) | HTS + dextran vs. normal saline | ≤ 8 | ND | ND | ND (E, 4) | U | L | N | N | ||||
| Bulger 2010 | 2 | 1331 (11) | HTS + dextran vs. HTS vs. normal saline | 4·9 / 5·0 / 5·0 | ND | ND | ND | ND | ND | L | L | N | N | ||
| Seizure Prophylaxis [1] | |||||||||||||||
| McQueen 1983 | UK | 164 (2) | Phenytoin vs. placebo | NR | ND | U | L | N | |||||||
| Steroids [3] | |||||||||||||||
| Asehnoune 2014 | France | 336 (19) | Hydrocortisone + fludrocortisone vs. placebo | ≤ 8 | ND | ND | ND | L | L | Y | |||||
| Edwards 2005 | 49 | 10,008 (239) | Methylprednisolone vs. placebo | 3 – 14 (R) | I | ND | U | L | N | N | |||||
| Grumme 1995 | 2 | 396 (9) | Triamcinolone vs. placebo | Most <8 | ND | ND | ND (12) | U | L | N | N | ||||
| Surgery [3] | |||||||||||||||
| Jiang 2005 | China | 486 (5) | Standard large craniectomy vs. limited craniectomy | 5·2 / 5·3 | S | S | S | L | L | N | N | ||||
| Mendelow 2015 | 13 | 170 (31) | Early surgery (hematoma) vs. standard care | <8 – 15 (R) | S | ND, ND (E) | L | H | Y | N | |||||
| Cooper 2011 | 3 | 155 (15) | DC vs. standard care | 5 / 6 (Md) | ND | S | S | ND | I | L | L | Y | Y | ||
There is variability in how RCTs measure and report intracranial pressure (ICP). For this table, statistical significance was judged according to the overall ICP results as reported
GOS (Glasgow Outcome Scale)/GOSE (Glasgow Outcome Scale-Extended) = proportion of favorable outcome (e.g., GOS 4 or 5) between groups unless otherwise stated.
DC, decompressive craniectomy; Random Seq., random sequence generation; H, high risk of bias; Hosp., hospital; HTS, hypertonic saline; ICU, intensive care unit; I, intervention inferior (statistically significant difference favoring control, non-bold in comparison column); L, low; Mx, management; N, no; ND, no statistically significant difference between groups; NR, not reported; RSI, rapid sequence intubation; S, intervention superior (statistically significant difference favoring intervention, bold in comparison column); SA, South Africa; U, unclear; UK, United Kingdom; USA, United States of America; Y, yes.
There were 26 RCTs classified as robust (multi-center, low risk of bias; n > 100) across 18 different interventions and eight intervention areas.35–59,71 Table 3 presents a summary of trial characteristics, findings, risk of bias, and analysis approaches for these RCTs. The following commentary by intervention category draws upon all completed adult RCTs with a focus on those classified as robust. The intention of this commentary is primarily to reflect upon the findings of the identified trials, not to provide detailed clinical guidance, for which readers should refer to clinical practice guidelines, such as those of the Brain Trauma Foundation (www.braintrauma.org).
Airway, ventilation, and oxygenation strategies (12 RCTs, including one pediatric, two ongoing)
Although the use of hyperbaric oxygen has been investigated in seven RCTs, these were all single-center and most (five) had a sample size under 100. Hence, none were classified as robust. Demonstrated associations between the occurrence of hypoxia and poorer outcome, combined with pathophysiologic insight provide a strong basis for avoidance of hypoxia, and there is a consensus (admittedly based on less rigorous evidence) that extreme hypocarbia and hypercarbia are undesirable in early TBI, although thresholds for harm are debated.60 However, supranormal oxygen levels (induced by normobaric hyperoxia or hyperbaric oxygen administration) have some potential for harm and a careful balance should be sought between benefit and risk.61 It is unknown if hyperbaric hyperoxia has any advantage over normobaric hyperoxia and further, hyperbaric chambers that can accommodate critically ill patients are unavailable in most settings.7 An area that may emerge as a key research focus is the use of lung protective ventilation strategies in critically ill TBI patients. A further two completed adult RCTs investigating airway, ventilation, and oxygenation strategies were identified, of which one62 found hyperventilation to be inferior to hyperventilation + tromethamine and normoventilation on 6-month GOS.
Fluid management (three RCTs)
One fluid management RCT was classified as robust.35 The use of albumin sharply declined following publication of this post hoc analysis of patients with TBI included in the saline versus albumin fluid evaluation (SAFE) study, which demonstrated that fluid resuscitation with albumin was associated with higher mortality rates than resuscitation with saline.35 However, it is less clear whether albumin should/can be used when TBI co-exists with an extracranial diagnosis (such as sepsis) where albumin may be indicated. A further two completed adult RCTs of fluid management were identified, of which one63 reported lower mortality in patients receiving fresh frozen plasma, compared with placebo.
Hypothermia/normothermia (37 RCTs, including four pediatric, two ongoing)
Despite the large number of RCTs for hypothermia, only one was classified as robust—the recently published Eurotherm3235 study.59 This RCT showed worse outcomes in patients treated with hypothermia titrated to ICP control (both in terms of intensity and duration). A very large proportion of hypothermia RCTs (24/29; 83%) were either small (n < 100), or had uncertain risk of bias in both domains; 23 RCTs were single-center. There is currently some interest in the use of local brain cooling, but evidence to support this is limited to a single-center study from China.64 One safety and feasibility RCT investigating advanced fever control also was identified65 but this did not report significant results. Interpretation of the Eurotherm3235 findings in the context of related hypothermia trials requires a nuanced understanding of approaches to hypothermia and their rationale. In Eurotherm3235, hypothermia was used as an early intervention, rather than a rescue therapy for refractory intracranial hypertension (which is how it has been used by many centers). As a consequence, while results of ongoing studies are awaited, therapeutic hypothermia may still be used ahead of barbiturates by some centers in patients where ICP control is difficult. Guidance regarding hypothermia use in this clinical context is still not addressed by the available evidence base.
Other ongoing trials also address similar important issues in understanding and application of hypothermia, namely optimized prophylactic hypothermia using pre-hospital initiation; extended usage beyond 72 h and slow rewarming adjusted to clinical tolerance. The results of these trials may provide additional guidance on hypothermia use in TBI. In the meantime, the use of mild hypothermia remains a treatment option for refractory intracranial hypertension. However, maintaining hypothermia in TBI patients is complex, and when considering use of hypothermia, a careful assessment of risks and benefits is necessary.
Intracranial pressure (ICP), cerebral perfusion pressure (CPP), and blood pressure (BP) management including hyperosmolar therapies (18 RCTs, including two pediatric, one ongoing)
The only RCT classified as robust was the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST-TRIP) Trial,36 which showed no difference in outcome within patients with TBI treated according to ICP monitoring versus those in whom “ICP targeted” therapy was based on clinical observation. This finding, combined with increased availability and use of CT scanning, has caused some doubts about the benefits of ICP monitoring. However, concerns about generalizability of this RCT have been acknowledged by its authors.66,67 Many clinicians also have emphasized the contribution of ICP monitoring to a better characterization of the pathophysiology, thus facilitating precision medicine approaches. The five hypertonic saline RCTs were all small. The three mannitol RCTs by Cruz and colleagues are all single-center and further, the validity of the data reported has been questioned.68,69 Due to these characteristics, combined with the absence of clear outcome benefit from hyperosmotic agent or dose, choice of hypertonic saline or mannitol continues to be guided by local clinical preference. Two small completed adult RCTs in ICP/CPP/BP management also were identified, one of which reported 3ml of CSF drainage to be superior to 1 mL or 2 mL of cerebrospinal fluid (CSF) drainage on intracranial pressure (ICP).70
Nutrition and glucose management (26 RCTs, including one pediatric)
None of the nutrition RCTs was classified as robust; all were single-center and only two had a sample size of over 100. Therefore, the benefits of any particular nutritional approach in TBI are unproven, and most centers base management decisions on evidence from general critical care.
Pharmacological therapies not elsewhere defined (47 RCTs, including eight ongoing)
Thirteen RCTs investigating pharmacological therapies were classified as robust. These were split across nine different pharmacological agents, and none reported positive results for any of the outcomes of interest. Positive findings were sporadic across the remaining RCTs. Hence, despite a large number of RCTs, no pharmacologic treatment has been shown to be consistently effective in RCTs. While some smaller phase II trials showed efficacy of various agents, this efficacy was not confirmed in large scale phase III studies. This observation raises the question of whether targeting a single mechanism is appropriate given the heterogeneity of TBI populations.
Pre-hospital care (seven RCTs)
One robust RCT was identified, reporting that pre-hospital intubation was superior to non-invasive ventilation on 6-month GOS. Pre-hospital intubation is therefore recommended, provided that paramedics are well trained, maintain skills, and end-tidal CO2 is monitored. The six pre-hospital RCTs on hypertonic solutions have not shown benefit. Future RCTs of hypertonic solutions should consider if late outcomes such as GOS are appropriate given this is a very early intervention.
Sedation, pain management, anesthesia, and arousal (22 RCTs, including one pediatric)
No robust RCTs were identified in this category and the majority (18) of the 21 RCTs on sedation, pain management, anesthesia, and arousal have compared effectiveness between agents and found no differences. This implies that the choice of agent cannot be determined based on evidence but should be targeted toward patient needs and determined by physician preference.
Seizure prophylaxis (four RCTs)
One of the three RCTs was identified as robust, but none of the three RCTs reported differences between groups on any of the outcomes of interest. There are increasing trends to move away from phenytoin to newer agents, such as levetiracetam, for seizure prophylaxis in TBI, based on tolerability, drug interactions, and impact on long-term outcome with the latter. There is an urgent need for robust evidence to support or refute the benefit of such change in practice.
Steroids (19 RCTs, including one pediatric)
Of the three robust RCTs in this category, one—the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH) trial55—reported methylprednisolone to be inferior to placebo on late mortality. The very large sample size (10,008) of this trial provides strong evidence that using steroids in all-comers with TBI is harmful. However, steroids may be indicated where anterior pituitary insufficiency is suspected or known in the setting of TBI.7
Surgery (eight RCTs)
Three robust RCTs were identified. Conflicting findings between these trials have contributed to continuing debate on the indications and benefits of early contusion surgery or decompressive craniectomy (DC). The Decompressive Craniectomy in Patients With Severe Traumatic Brain Injury (DECRA) trial71 found positive effects for ICP and ICU LOS, but worse 6-month GOS in the DC group. This might imply that more aggressive medical ICP control with barbiturates may now the preferred third tier therapy for refractory intracranial hypertension, since complications from craniectomy appear to outweigh potential benefits and also in light of the recently published Eurotherm3235 findings.59 However, while DECRA provides valuable evidence on the use of decompressive craniectomy as an early intervention, the trial provides no guidance on its use as rescue therapy for refractory intracranial hypertension. As a consequence, while results of ongoing studies are awaited, decompressive craniectomy may still be used by some centers in this latter context.
Further, the DECRA trial involved bifrontotemporoparietal craniectomy for patients with diffuse cerebral swelling. The results are not generalizable to patients with acute subdural hematoma or other pathology for which unilateral decompressive craniectomy is frequently performed. The recent Surgical Trial in Traumatic Intracerebral Haemorrhage (STITCH(TRAUMA))58 was prematurely halted by the funding agency because of slow recruitment. On analysis, a strong trend to benefit of early surgery of contusions in patients with a GCS of 9-12 was noted. Given the variation in findings in surgical trials and the dearth of robust trials, the outcomes of the three ongoing RCTs are keenly anticipated, and further large trials are needed. A challenge towards the future will be to confidently identify patients with subtypes of TBI that may benefit from surgical interventions. In the meantime, decompressive craniectomy remains a management option:
• As an early means of ICP control, especially when performed in conjunction with the removal of an intracranial hematoma;
• As a late salvage procedure in situations of intractable ICP.
The challenge of expanding the surgical research evidence base is exacerbated by the relative expense and less lucrative financial returns of surgical, compared with pharmacological trials. This may explain the differences in volume of evidence between these two intervention categories.
Pediatric RCTs
There were 11 completed pediatric RCTs among the 207 RCTs eligible for this review, comprising:
• One small (n = 9) RCT investigating temperature-corrected versus uncorrected ventilatory management, reporting no difference on ICP72;
• Four hypothermia vs. normothermia RCTs,73–76 including the recent “Cool Kids” RCT74 that was ceased for futility, reporting no difference between groups on mortality, GOS, ICP (three RCTs) and LOS (one RCT);
• One RCT of immune-enhancing formula, reporting no difference between groups on early mortality or hospital LOS77;
• Two small (n < 33), single center RCTs of hypertonic saline vs. normal saline78/lactated Ringer's solution,79 which found mixed results on ICP (one positive, one with no statistically significant difference between groups), positive results on ICU LOS (one RCT) and no difference between groups on mortality and hospital LOS (one RCT);
• One RCT of phenobarbitone, which reported no difference on mortality or ICP80;
• One RCT of dexamethasone, which reported no difference on ICP or 6-month GOS81;
• One RCT of decompressive craniectomy (DC) versus standard care, which did not report any difference between groups on ICP, ICU, or hospital LOS or GOS.82
Ongoing RCTs
There were 16 ongoing RCTs among the 207 RCTs eligible for this review, comprising:
• Two multi-center RCTs investigating 80% versus 50% FiO2 (planned n = 68, n centers not reported)83 and management guided by partial pressure of oxygen in brain tissue (PbrO2; planned n = 182, four centers)84;
• Two medium-to-large RCTs of hypothermia versus normothermia,85,86 including the Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury (POLAR) RCT85,87;
• One multi-center RCT of vasopressin versus catecholamines for ICP control (planned n = 200, number of centers not reported)88;
- • Eight pharmacological RCTs:
• Three RCTs of surgery: decompressive craniectomy (DC) versus standard care (Randomised Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure [RESCUEicp]; planned n = 400, 40 centers),99–101 DC versus craniotomy (Randomised Evaluation of Surgery with Craniectomy for patients Undergoing Evacuation of Acute Subdural Haematoma [RESCUE-ASDH]; planned n = 990, 12 centers),102,103 and bypass to specialist neurosurgical center versus control (Head Injury Transportation Straight to Neurosurgery [HITS-NS];planned n = 700, three centers).104,105
Discussion
This is the most comprehensive known overview of RCTs investigating acute interventions for moderate-to-severe TBI. Strengths of this review include use of comprehensive database and other platform searching to identify, evaluate, and synthesize 207 RCTs across 11 intervention areas; inclusion of pediatric and ongoing RCTs; and evaluation of risk of bias, use of covariate adjustment, and use of an ordinal approach to analysis. The outputs of this review can be of considerable value to the acute TBI research and clinical community, for example, to inform future systematic reviews and clinical practice guidelines or aid in strategic research planning to optimize RCT focus, quality and impact, especially if the searches are re-run to create a “living” acute TBI RCT repository.
State-of-the-science overviews across multiple intervention areas are necessarily less in-depth than single-intervention systematic reviews, and a number of factors should therefore be borne in mind when interpreting overview findings. Although risk of bias was evaluated, only two of seven Cochrane domains were assessed. A more rigorous quality analysis may have categorized more RCTs as being high risk of bias. However, this was beyond the resources available due to the volume of literature included in this overview. Scoping studies such as this overview are in part defined by an emphasis on covering large volumes of literature without undertaking any quality appraisal.15,17 Quality appraisal is usually confined to systematic reviews, which have much smaller yields focusing on a narrow topic area. This is reflected in previously published multi-topic TBI overviews, none of which have undertaken any risk of bias or quality evaluation.7,18–25 In this context, the decision to review two of the seven Cochrane risk of bias domains represents a substantial advance on comparable overviews.
Further, the domains evaluated (random sequence generation and allocation concealment) have been empirically shown to influence RCT outcomes.33 While the RCTs were broadly grouped into intervention areas, there are many variations in intervention protocols (for example, cooling and rewarming rates in hypothermia), type and severity of injury (diffuse vs. focal) and control condition within these groupings. Broad intervention categorization was necessary to enable meaningful interpretation of such a large body of literature. The intervention categories were arbitrary; however, category development was iterative and drew upon an international research team with considerable clinical and research experience and expertise. Formal evaluation of heterogeneity should be undertaken prior to any meta-analysis and both of these were beyond the scope of this review. Meta-analysis can effectively increase the statistical power of several small trials, and this pooling can detect otherwise unseen statistically significant treatment effects.106
The approach taken to identify all RCTs measuring an outcome of interest, whether or not this was the primary outcome, is consistent with Cochrane systematic review methodology.107 However, combined with the small sample sizes observed, this means that not all of the identified RCTs were adequately powered to measure the outcomes of interest to this overview. Due to the volume of literature screened for this overview, it was not feasible to employ two researchers to independently screen citations and full text publications. However, this limitation was offset by use of explicit inclusion criteria developed by two authors (PB, AS) and independent screening by a second reviewer in all cases where there was uncertainty regarding inclusion. Finally, because it was not feasible to catalogue all primary outcomes across all included RCTs, some conclusions are limited—for example, there are no positive findings in the four RCTs of seizure prevention for the outcomes of interest; however, the outcome of seizure control is more relevant for guiding use of seizure prevention agents.
This overview found that no statistically significant difference between groups was found for 74% of comparisons across all outcomes of interest in the included RCTs. It should be emphasized that a finding of no difference between groups does not equate to statistical proof of absence of effect, and may be a function of small sample size or methodological shortcomings. Notwithstanding this, lack of successful translation from laboratory-based research to effective TBI treatments also was noted in our previous reviews.7,24,25 It appears from our graphical analysis that lack of positive RCT findings is not a function of trial characteristics including sample size and risk of bias. This finding is supported by the recent publication of two large RCTs showing no difference between progesterone and placebo on 6-month GOS or late mortality.37,38 As noted by Schwamm,108 both were high-quality RCTs underpinned by phase II trials. However, Schwamm contends that the progesterone findings reflect a raft of overarching TBI trial design issues that collectively mitigate against positive findings in phase III trials—slow enrolment, optimistic effect sizes, good outcomes in placebo groups, and insufficient phase II data to support robust sample-size estimates.108
Despite the considerable investment of resources and effort in producing 191 completed RCTs for early management of TBI, very little translatable evidence has been generated. This is highlighted by the high proportion of equivocal findings, the existence of only 26 robust (multi-center, low risk of bias, n > 100) RCTs spread across 18 different interventions, and the finding that 35 RCTs were the sole study of an intervention. For most intervention areas, there is insufficient consistency in study characteristics and/or findings to draw robust clinical implications from the RCT findings. Even in hypothermia, with more than 30 RCTs investigating systemic hypothermia versus normothermia, there is only one completed multi-center, low risk of bias RCT. Therefore, systematic hypothermia reviews recommended further, higher-quality RCTs.109
Covariate adjustment was performed in less than one-quarter of RCTs, and only eight of 111 eligible RCTs employed an ordinal approach to analysis. This reflects the common use of insensitive methodology in the majority of trials in TBI. The importance of dealing with the clinical heterogeneity of TBI populations was recognized by the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) study group.110 Extensive methodological analysis performed by this study group showed that applying covariate adjustment together with ordinal analysis could reduce the sample size requirement by as much as 50%. These findings, based upon simulation studies, were validated analysis of the CRASH trial data.111 The IMPACT recommendations are primarily targeted toward efficacy analysis of phase III studies, but the importance of covariate adjustment in phase II trials should be emphasized, as the risk of imbalances between treatment and placebo populations concerning baseline characteristics is higher in these smaller studies.
Although it is clear that more high-quality evidence strategically targeted at high-priority intervention areas must be generated, there is a clinical imperative to draw upon evidence of at least reasonable quality that might rationally guide practice in the interim. However, an analysis by Ioannidis112 of 49 highly cited clinical research studies (including 43 RCTs) found that following publication of these top-citing RCTs of medical interventions, 16% of subsequent trials of the same interventions contradicted the initial RCT findings, and a further 16% reported weaker effects. Further, RCTs that were not successfully replicated had significantly smaller sample sizes than RCTs in which findings were replicated (p = 0 · 009). He concluded that “evidence from recent trials, no matter how impressive, should be interpreted with caution, when only one trial is available.”112 This view is supported by leaders in the field of knowledge translation—the science of implementing research into practice—who note that, with the exception of very large RCTs, single RCTs do not warrant changes in healthcare practice or policy.113
This is further reinforced by the current review findings regarding sample size and number of centers. The 191 completed RCTs collectively enrolled more than 35,000 participants; however, the majority of RCTs in this review were single center trials with small (< 100) sample sizes; only 14 RCTs enrolled more than 500 participants, including the CRASH trial (n = 10,008). This is consistent with a previous review by Li and colleagues that found that the majority of interventional studies in both TBI and stroke were single-center, but that stroke had a much higher percentage of international studies.25 A further multi-intervention TBI overview24 reported that six of nine single center studies found positive treatment effects, compared with four of 24 multi-center studies. The authors stated that greater treatment standardization and increased recruitment may help counter the center effects in multi-center trials. Although analysis of statistical power of RCTs was beyond the scope of this review, the finding that most trials recruited fewer than 100 participants indicates a high likelihood of underpowered RCTs and risk of finding spurious treatment effects. This is consistent with a previous TBI trials overview, which argued that this reduces clinical translatability of research findings and negatively influences further trials due to overestimation of effect size.25 Halpern and colleagues114 argue that underpowered trials are only ethically justifiable in two situations—small trials in rare diseases, where there is an explicit plan to meta-analyze results alongside similar trials; and early-phase trials designed to guide an adequately-powered phase III trial. Button and colleagues115 conducted an analysis of 48 diverse neuroscience meta-analyses, which showed that the average statistical power of neuroscience studies was between approximately 8% and 31%. The authors mirrored the ethical concerns of Halpern and colleagues.114
An alternative view to producing more large RCTs is to alter the overall research approach to reflect the complexity of the TBI population. While high-quality RCTs remain the bedrock of evidence-based medicine, they are best suited to providing guidance regarding the use of unchanging interventions in patients with homogenous diagnostic categories and well-understood pathophysiological mechanisms. However, they provide imperfect guidance in conditions that are mechanistically heterogeneous and where therapies may require complex titration, such as in the ICU management of severe TBI. The recognition that current approaches to classifying TBI are suboptimal116 has led to current studies117 that seek to apply precision medicine approaches to classifying TBI in an effort to identify subgroups of patients who have more homogenous pathophysiology and, potentially, responses to therapy.118 The challenge will be to provide robust evidence that can be used to guide management in these smaller patient subgroups, where currently specified sample sizes for RCTs will be even more difficult to achieve. However, it is hoped that the homogeneity of study populations in this setting may allow smaller sample sizes. A recent overview of acute brain injury neuroprotection research also has highlighted methodological issues in the translation of animal studies to clinical trials, including the flawed assumption that disease mechanisms identified in animal models translate to humans. Echoing the challenge of accurate TBI classification, the authors recommend use of comprehensive cerebral and biochemical physiology monitoring to challenge such assumptions prior to definitive human trials, as well as greater collaboration between preclinical and clinical researchers.119
Conclusion
This state-of-the-science overview of 207 RCTs of acute TBI interventions found that RCTs were spread across a broad range of interventions and were predominantly small and single-center. For almost three-quarters of all outcomes of interest measured, no difference was found between intervention and control groups, and this appears not to be a function of trial size, risk of bias or intervention category. Less than one -third of RCTs demonstrated low risk of bias for two important domains (random sequence generation and allocation concealment); less than one-quarter used covariate adjustment; and only 7% used an ordinal approach to analysis.
These findings hamper translation of acute TBI research findings into clinical practice and policy. Authors in the field have recommended a number of strategies to address these issues, including more sophisticated approaches to trial design24,25,120; multi-center and multi-ethnicity recruitment to enhance generalizability25; greater research collaboration, co-ordination and standardization of pre-clinical research24,25,108; and alternatives to traditional RCTs, such as comparative effectiveness research and precision medicine approaches.25,118,121 Adaptive trials, which enable planned modifications to trials based upon emerging data, are another alternative approach, that could increase efficiency of TBI trials. However, care should be taken with this approach as such adaptations can cause bias if not properly implemented or underpinned by appropriate statistical assumptions.122
Li and colleagues emphasize the lessons that can be learned from other fields including stroke and oncology, where major clinical advances have been underpinned by large, multi-center trials based upon robust pre-clinical evidence and hypotheses.25 Enacting such recommendations is a complex task involving substantial challenges, for example in obtaining funding for large trials,25 and prioritizing research topics. Success in navigating these challenges—or completely changing the approach to evidence generation in this field through Comparative Effectiveness Research, precision medicine, or other research designs—is critical to optimizing the lives of those affected by moderate-to-severe TBI.
Supplementary Material
Appendix 1: Search, Selection, and Data Extraction Methods
Search strategy
To identify randomized controlled trials (RCTs), the comprehensive systematic search from our previously published review7 was updated:
• The Medline (OVID), All Evidence Based Medicine Reviews (OVID) and EMBASE databases from 2011 to November 2013 using the core terms “brain injuries,” “craniocerebral trauma,” and “traumatic brain injury” and keywords for decompressive craniectomy and hematoma evacuation; steroids; hemostatic drugs, including tranexamic acid; therapeutic hypothermia; hyperoxia and stem cells/regenerative therapies. Reference lists of relevant primary studies and reviews were used to identify further potentially relevant citations.
• The World Health Organization International Clinical RCTs Registry Platform26 was searched covering the period January 1, 2012, to March 2015, using search terms for tranexamic acid/steroids; therapeutic hypothermia; hyperoxia; stem cells/regenerative therapies and surgery.
We expanded our search from that in the previous review by examining reference lists of all systematic reviews within a comprehensive, up to date online traumatic brain injury systematic review database.27 All searches were limited to English language studies in human beings.
Study selection
Included full text publications from the previous review (n = 143), as well as titles, abstracts and full text publications from the update search outlined above, were screened against the following inclusion criteria:
• Population: Moderate-to-severe traumatic brain injury, defined as first Glasgow Coma Scale (GCS) score ≤13, loss of consciousness (LOC) >30 mins and/or post-traumatic amnesia >24 h.5,124,125 Studies including subjects with mild traumatic brain injury (TBI; GCS >13) were only included if they comprised less than 50% of the total cohort. Adult and pediatric studies (defined as >50% participants under 18 years) were included. Studies containing non-TBI patients were excluded.
• Study type: Randomized controlled trials (RCTs), as defined in the Cochrane Handbook for Systematic Reviews of Interventions.108 RCTs where the method of assignment to groups was ambiguous or quasi-random were excluded; protocols for ongoing RCTs were included; preliminary RCT findings were included unless a subsequent publication of the entire RCT cohort was available; post hoc analysis of an entire TBI RCT cohort was included; post hoc analysis of a TBI RCT subset or examining a different intervention to the RCT was excluded
• Intervention: Any intervention provided in the acute phase of care (pre-hospital/acute hospital) as defined by Hirshon and colleagues in the Bulletin of the World Health Organization:126 “…the health system components, or care delivery platforms, used to treat sudden, often unexpected, urgent or emergent episodes of injury and illness that can lead to death or disability without rapid intervention…including emergency medicine, trauma care, pre-hospital emergency care, acute care surgery, critical care, urgent care and short-term inpatient stabilization” (p. 386).126 RCTs in a rehabilitation (i.e., post-acute) setting were excluded.
• Publication status: English language; peer-reviewed journal, conference abstract/presentation, book chapter. Websites referring to RCT registration or protocols were included.
All titles, abstracts and full text publications were screened by one investigator (PB) and the RCTs search and screening was undertaken by a second person (Nathan Kuk). Where there was uncertainty regarding the inclusion of a RCT following full text review, an independent investigator (AS) screened the full text publication, with differences resolved by consensus discussion.
Data extraction
The following data were extracted from eligible RCTs:
• Study characteristics: Country, number of centers, number randomized, comparison, GCS by group, age (adult, >50% of participants ≥18 years; pediatric, >50% of participants <18 years)
- • Study findings: Results of between-group analysis—superior (S; statistically significant result favoring intervention)/no difference (ND; no difference between groups detected); Inferior (I; statistically significant result favoring control/placebo). Where statistical significance was not reported, this was calculated based upon the raw data information using RevMan software127 for the following outcomes:
- ○ Mortality: Early (in-hospital or ≤ 1 month); late (> 1 month), not specified.
- ○ Mortality outcomes were generated from GOS score if not reported.
- ○ Intracranial pressure (ICP): ICP measures and time-points were recorded. Where there were multiple measures of ICP within the same RCT, statistical significance was judged according to the results as reported.
- ○ Length of stay: Intensive care unit, hospital
- ○ Glasgow Outcome Score (GOS)/Glasgow Outcome Score-Extended (GOSE). GOS was dichotomized into “favorable” (GOS 4 and 5) and “unfavorable” (GOS 1-3)
• Risk of Bias: Evaluated using two key domains of the Cochrane Risk of Bias Tool—Random Sequence Generation and Allocation Concealment.29 The adjudging of risk of bias for each domain (“low,” “unclear,” or “high”) was undertaken based upon the guidelines in the Cochrane Handbook.33 Data extraction and risk of bias appraisal were undertaken by two investigators (PB and AS). To ensure consistency across studies, a data extraction protocol was developed and piloted.
• Use of covariate adjustment (yes/no) and (if measuring GOS/GOSE) ordinal outcome measures (yes/no). Ordinal outcome measures are often collapsed into a binary scale. The IMPACT recommendations111,128 for improving the design and analysis of clinical trials in TBI state that clinical trials should incorporate pre-specified covariate adjustment to mitigate the effects of heterogeneity, and apply an ordinal approach by means of either sliding dichotomy or proportional odds methodology. These approaches have the potential to increase statistical power by 50%. One reviewer (FR) screened all included clinical trials for application of either covariate adjustment and/or ordinal approach in statistical analysis of outcome. Data extracted on covariate adjustment included type and number of covariates and statistical method. Distinction was made between covariate adjustment related to clinical outcome measures at follow up (GOS, mortality, DRS) and covariate adjustment applied for analysis of early end-points (ICP, PbtO2, clinical events such as pneumonia). Information on the ordinal approach was verified in trials in which the GOS(E) was applied and data extracted included the statistical method used (sliding dichotomy or proportional odds methodology). Any indistinctness was discussed with a second reviewer (AM) and disagreements were resolved by discussion within the author team.
Acknowledgments
Funding: This work was supported by the Victorian Transport Accident Commission (TAC)—funded Centre of Excellence in Traumatic Brain Injury Research (CETBIR), as part of a program titled “Harnessing Victoria's Neurotrauma expertise: Promoting excellence and realising value” (Project Code: DP 186).
Part of this work was performed in the context of CENTER-TBI (Comparative European Neurotrauma Effectiveness Research in Traumatic Brain Injury), supported by the Framework 7 program of the European Union (Grant number: 602150-2). David Menon is supported by a Senior Investigator Award from the National Institute for Health Research (UK).
Personnel: Florence C.M. Reith, MD, Department of Neurosurgery, Antwerp University Hospital, Edegem, Belgium—data extraction for covariate adjustment and ordinal approach; Nathan Timothy Kuk, Monash Medical Centre, Centre for Inflammatory Diseases, Clayton, Victoria, Australia—assistance with screening of titles/abstracts, undertook searching and selection for ongoing RCTs
Author contributions: Peter Bragge, Russell Gruen, and Anneliese Synnot drafted the review design, based upon that previously published in the Lancet by Rosenfeld and colleagues.7 All authors participated in refinement of the review design. Peter Bragge and Anneliese Synnot undertook literature search, study selection, and data extraction; Nathan Timothy Kuk (acknowledged with permission above) assisted with screening of titles/abstracts and undertook searching and selection for ongoing RCTs; Andrew Maas led data extraction and interpretation pertaining to evaluation of covariate adjustment and ordinal approach; Florence Reith (acknowledged with permission above) undertook data extraction for covariate adjustment and ordinal approach.
Peter Bragge drafted the manuscript, Figure 1 and all tables; Anneliese Synnot drafted Figures 2 and 3.
All authors participated in resolution of or decisions on inclusion/exclusion of trials as required; writing and review of subsequent drafts of the manuscript, figures, and tables; data analysis and interpretation; and approval of final version of manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abelson-Mitchell N. (2008). Epidemiology and prevention of head injuries: literature review. J. Clin. Nurs. 17, 46–57 [DOI] [PubMed] [Google Scholar]
- 2.Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 3.Feigin V.L., Theadom A., Barker-Collo S., Starkey N.J., McPherson K., Kahan M., Dowell A., Brown P., Parag V., Kydd R., Jones K., Jones A., and Ameratunga S; BIONIC Study Group. (2013). Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 12, 53–64 [DOI] [PubMed] [Google Scholar]
- 4.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 5.Khan F., Baguley I.J., and Cameron I.D. (2003). 4: Rehabilitation after traumatic brain injury. Med. J. Aust. 178, 290–295 [DOI] [PubMed] [Google Scholar]
- 6.Faul M., Wald M., Rutland-Brown W., Sullivent E., and Sattin R. (2007). Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J. Trauma 63, 1271–1278 [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld J., Maas A., Bragge P., Morganti-Kossman M., Manley G., and Gruen R. (2012). Early management of severe traumatic brain injury. Lancet 380, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 8.Maas A.I., Steyerberg E.W., Murray G.D., Bullock R., Baethmann A., Marshall L.F., and Teasdale G.M. (1999). Why have recent trials of neuroprotective agents in head injury failed to show convincing efficacy? A pragmatic analysis and theoretical considerations. Neurosurgery 44, 1286–1298 [PubMed] [Google Scholar]
- 9.Baguley I.J., Nott M.T., Howle A.A., Simpson G.K., Browne S., King A.C., Cotter R.E., and Hodgkinson A. (2012). Late mortality after severe traumatic brain injury in New South Wales: a multicentre study. Med. J. Aust. 196, 40–45 [DOI] [PubMed] [Google Scholar]
- 10.Spitz G., Downing M., McKenzie D., and Ponsford J. (2015). Mortality following traumatic brain injury inpatient rehabilitation. J. Neurotrauma. 32, 1272–1280 [DOI] [PubMed] [Google Scholar]
- 11.Kalechstein A.D., Newton T.F., and van Gorp W.G. (2003). Neurocognitive functioning is associated with employment status: a quantitative review. J. Clin. Exp. Neuropsychol. 25, 1186–1191 [DOI] [PubMed] [Google Scholar]
- 12.Ponsford J., Draper K., and Schonberger M. (2008). Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J. Int. Neuropsychol. Soc. 14, 233–242 [DOI] [PubMed] [Google Scholar]
- 13.Satz P., Zaucha K., Forney D.L., McCleary C., Asarnow R.F., Light R., Levin H., Kelly D., Bergsneider M., Hovda D., Martin N., Caron M.J., Namerow N., and Becker D. (1998). Neuropsychological, psychosocial and vocational correlates of the Glasgow Outcome Scale at 6 months post-injury: a study of moderate to severe traumatic brain injury patients. Brain Inj. 12, 555–567 [DOI] [PubMed] [Google Scholar]
- 14.Rimel R.W., Giordani B., Barth J.T., and Jane J.A. (1982). Moderate head injury: completing the clinical spectrum of brain trauma. Neurosurgery 11, 344–351 [DOI] [PubMed] [Google Scholar]
- 15.Arksey H. and O'Malley L. (2005). Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 8, 19–32 [Google Scholar]
- 16.Katz D.L., Williams A.L., Girard C., Goodman J., Comerford B., Behrman A., and Bracken M.B. (2003). The evidence base for complementary and alternative medicine: methods of Evidence Mapping with application to CAM. Alt. Ther. Health Med. 9, 22–30 [PubMed] [Google Scholar]
- 17.Bragge P., Clavisi O., Turner T., Tavender E., Collie A., and Gruen R.L. (2011). The Global Evidence Mapping Initiative: scoping research in broad topic areas. BMC Med. Res. Methodol. 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boer C., Franschman G., and Loer S.A. (2012). Prehospital management of severe traumatic brain injury: concepts and ongoing controversies. Curr. Opin. Anaesthesiol. 25, 556–562 [DOI] [PubMed] [Google Scholar]
- 19.Wijayatilake D.S., Shepherd S.J., and Sherren P.B. (2012). Updates in the management of intracranial pressure in traumatic brain injury. Curr. Opin. Anaesthesiol. 25, 540–547 [DOI] [PubMed] [Google Scholar]
- 20.Gupta N., Pandia M.P., and Dash H.H. (2013). Research studies that have influenced practice of neuroanesthesiology in recent years: a literature review. Indian J. Anaesth. 57, 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConeghy K.W., Hatton J., Hughes L., and Cook A.M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636 [DOI] [PubMed] [Google Scholar]
- 22.Lu J., Gary K.W., Neimeier J.P., Ward J., and Lapane K.L. (2012). Randomized controlled trials in adult traumatic brain injury. Brain Inj. 26, 1523–1548 [DOI] [PubMed] [Google Scholar]
- 23.Tsang K.K. and Whitfield P.C. (2012). Traumatic brain injury: review of current management strategies. Br. j. Oral Maxillofac. Surg. 50, 298–308 [DOI] [PubMed] [Google Scholar]
- 24.Maas A.I., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L.M., Menon D.K., and Janowitz T. (2014). Cross-sectional analysis of data from the U.S. clinical trials database reveals poor translational clinical trial effort for traumatic brain injury, compared with stroke. PloS One 9, e84336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (2015). International Clinical Trials Registry Platform (ICTRP). Available at: www.who.int/ictrp/en Accessed February15, 2016
- 27.(2014). Evidence Map: Research in Context. Available at: evidencemap.org Accessed February15, 2016. Check
- 28.Microsoft Corporation (2010). Microsoft® Excel® for Mac 2011 Microsoft Corporation
- 29.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., and Sterne J.A.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellomo R. and Bagshaw S.M. (2006). Evidence-based medicine: classifying the evidence from clinical trials–the need to consider other dimensions. Crit. Care 10, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellomo R., Warrillow S.J., and Reade M.C. (2009). Why we should be wary of single-center trials. Crit Care Med 37, 3114–3119 [DOI] [PubMed] [Google Scholar]
- 32.Bafeta A., Dechartres A., Trinquart L., Yavchitz A., Boutron I., and Ravaud P. (2012). Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ 344, e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J., Altman D., and Sterne J. (2011). Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Higgins J., and Green S. (eds). The Cochrane Collaboration: London [Google Scholar]
- 34.Wilkes M.M. and Navickis R.J. (2001). Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 135, 149–164 [DOI] [PubMed] [Google Scholar]
- 35.Myburgh J., Cooper D.J., Finfer S., Bellomo R., Norton R., Bishop N., Kai Lo S., and Vallance S. (2007). Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 357, 874–884 [DOI] [PubMed] [Google Scholar]
- 36.Chesnut R.M., Temkin N., Carney N., Dikmen S., Rondina C., Videtta W., Petroni G., Lujan S., Pridgeon J., Barber J., Machamer J., Chaddock K., Celix J.M., Cherner M., and Hendrix T. (2012). A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 367, 2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., and Stocchetti N.; SYNAPSE Trial Investigators. (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 38.Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., and Barsan W.G.; NETT Investigators. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakur H., Andrews P., Asser T., Balica L., Boeriu C., Quintero J.D., Dewan Y., Druwe P., Fletcher O., Frost C., Hartzenberg B., Mantilla J.M., Murillo-Cabezas F., Pachl J., Ravi R.R., Ratsep I., Sampaio C., Singh M., Svoboda P., and Roberts I. (2009). The BRAIN TRIAL: a randomised, placebo controlled trial of a Bradykinin B2 receptor antagonist (Anatibant) in patients with traumatic brain injury. Trials 10, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marmarou A., Nichols J., Burgess J., Newell D., Troha J., Burnham D., and Pitts L. (1999). Effects of the bradykinin antagonist Bradycor (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Injury Consortium Study Group. J. Neurotrauma 16, 431–444 [DOI] [PubMed] [Google Scholar]
- 41.Teasdale G., Bailey I., Bell A., Gray J., Gullan R., Heiskanan O., Marks P.V., Marsh H., Mendelow D.A., and Murray G., et al. (1992). A randomized trial of nimodipine in severe head injury: HIT I. British/Finnish Co-operative Head Injury Trial Group. J Neurotrauma 9 Suppl 2, S545–S550 [PubMed] [Google Scholar]
- 42.The European Study Group on Nimodipine in Severe Head Injury. (1994). A multicenter trial of the efficacy of nimodipine on outcome after severe head injury. J. Neurosurg. 80, 797–804 [DOI] [PubMed] [Google Scholar]
- 43.Perel P., Al-Shahi Salman R., Kawahara T., Morris Z., Prieto-Merino D., Roberts I., Sandercock P., Shakur H., and Wardlaw J. (2012). CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury—a nested randomised, placebo-controlled trial. Health Technol. Assess. 16, iii–xii, 1–54 [DOI] [PubMed] [Google Scholar]
- 44.Robertson C., Hannay H., Yamal J., Gopinath S., Goodman J., andTilley B.; Epo Severe TBI Trial Investigators, Baldwin A., Rivera Lara L., Saucedo-Crespo H., Ahmed O., Sadasivan S., Ponce L., Cruz-Navarro J., Shahin H., Aisiku I.P., Doshi P., Valadka A., Neipert L., Waguspack J.M., Rubin M.L., Benoit J.S., and Swank P. (2014). Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 312, 36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maas A.I., Murray G., Henney H., 3rd, Kassem N., Legrand V., Mangelus M., Muizelaar J.P., Stocchetti N., and Knoller N. (2006). Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 5, 38–45 [DOI] [PubMed] [Google Scholar]
- 46.Yurkewicz L., Weaver J., Bullock M.R., and Marshall L.F. (2005). The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma 22, 1428–1443 [DOI] [PubMed] [Google Scholar]
- 47.Morris G.F., Bullock R., Marshall S.B., Marmarou A., Maas A., and Marshall L.F. (1999). Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J. Neurosurg. 91, 737–743 [DOI] [PubMed] [Google Scholar]
- 48.Marshall L.F., Maas A.I., Marshall S.B., Bricolo A., Fearnside M., Iannotti F., Klauber M.R., Lagarrigue J., Lobato R., Persson L., Pickard J.D., Piek J., Servadei F., Wellis G.N., Morris G.F., Means E.D., and Musch B. (1998). A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J. Neurosurg. 89, 519–525 [DOI] [PubMed] [Google Scholar]
- 49.Young B., Runge J.W., Waxman K.S., Harrington T., Wilberger J., Muizelaar J.P., Boddy A., and Kupiec J.W. (1996). Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. JAMA 276, 538–543 [PubMed] [Google Scholar]
- 50.Bernard S.A., Nguyen V., Cameron P., Masci K., Fitzgerald M., Cooper D.J., Walker T., Std B.P., Myles P., Murray L., David Taylor, Smith K., Patrick I., Edington J., Bacon A., Rosenfeld J.V., and Judson R. (2010). Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann. Surg. 252, 959–965 [DOI] [PubMed] [Google Scholar]
- 51.Morrison L.J., Baker A.J., Rhind S.G., Kiss A., MacDonald R.D., Schwartz B., Perreira T., Simitciu M., Trompeo A., Black S.E., Stuss D.T., and Rizoli S.B. (2011). The Toronto prehospital hypertonic resuscitation–head injury and multiorgan dysfunction trial: feasibility study of a randomized controlled trial. J. Crit. Care 26, 363–372 [DOI] [PubMed] [Google Scholar]
- 52.Bulger E.M., May S., Brasel K.J., Schreiber M., Kerby J.D., Tisherman S.A., Newgard C., Slutsky A., Coimbra R., Emerson S., Minei J.P., Bardarson B., Kudenchuk P., Baker A., Christenson J., Idris A., Davis D., Fabian T.C., Aufderheide T.P., Callaway C., Williams C., Banek J., Vaillancourt C., van Heest R., Sopko G., Hata J.S., and Hoyt D.B. (2010). Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA 304, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQueen J.K., Blackwood D.H., Harris P., Kalbag R.M., and Johnson A.L. (1983). Low risk of late post-traumatic seizures following severe head injury: implications for clinical trials of prophylaxis. J. Neurol. Neurosurg. Psychiatry 46, 899–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asehnoune K., Seguin P., Allary J., Feuillet F., Lasocki S., Cook F., Floch H., Chabanne R., Geeraerts T., Roger C., Perrigault P.F., Hanouz J.L., Lukaszewicz A.C., Biais M., Boucheix P., Dahyot-Fizelier C., Capdevila X., Mahe P.J., Le Maguet P., Paugam-Burtz C., Gergaud S., Plaud B., Constantin J.M., Malledant Y., Flet L., Sebille V., Roquilly A., and Corti T.C.S.G. (2014). Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet. Respir. Med. 2, 706–716 [DOI] [PubMed] [Google Scholar]
- 55.Edwards P., Arango M., Balica L., Cottingham R., El-Sayed H., Farrell B., Fernandes J., Gogichaisvili T., Golden N., Hartzenberg B., Husain M., Ulloa M.I., Jerbi Z., Khamis H., Komolafe E., Laloe V., Lomas G., Ludwig S., Mazairac G., Munoz Sanchez Mde L., Nasi L., Olldashi F., Plunkett P., Roberts I., Sandercock P., Shakur H., Soler C., Stocker R., Svoboda P., Trenkler S., Venkataramana N.K., Wasserberg J., Yates D., and Yutthakasemsunt S. (2005). Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 365, 1957–1959 [DOI] [PubMed] [Google Scholar]
- 56.Grumme T., Baethmann A., Kolodziejczyk D., Krimmer J., Fischer M., von Eisenhart Rothe B., Pelka R., Bennefeld H., Pollauer E., and Kostron H., et al. (1995). Treatment of patients with severe head injury by triamcinolone: a prospective, controlled multicenter clinical trial of 396 cases. Res. Exp. Med (Berl). 195, 217–229 [DOI] [PubMed] [Google Scholar]
- 57.Jiang J.Y., Xu W., Li W.P., Xu W.H., Zhang J., Bao Y.H., Ying Y.H., and Luo Q.Z. (2005). Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J. Neurotrauma 22, 623–628 [DOI] [PubMed] [Google Scholar]
- 58.Mendelow A.D., Gregson B.A., Rowan E.N., Francis R., McColl E., McNamee P., Chambers I., Unterberg A.W., Boyers D., and Mitchell P. (2015). Early surgery versus initial conservative treatment in patients with traumatic intracerebral haemorrhage [STITCH(Trauma)]: the first randomised trial. J. Neurotrauma. 32, 1312–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews P.J., Sinclair H.L., Rodriguez A., Harris B.A., Battison C.G., Rhodes J.K., and Murray G.D.; Eurotherm3235 Trial Collaborators. (2015). Hypothermia for intracranial hypertension after traumatic brain injury. N. Engl. J. Med. 373, 2403–2412 [DOI] [PubMed] [Google Scholar]
- 60.Stocchetti N. and Maas A.I. (2014). Traumatic intracranial hypertension. N. Engl. J. Med. 370, 2121–2130 [DOI] [PubMed] [Google Scholar]
- 61.Helmerhorst H.J., Roos-Blom M.J., van Westerloo D.J., and de Jonge E. (2015). Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit. Care Med. 43, 1508–1519 [DOI] [PubMed] [Google Scholar]
- 62.Muizelaar J.P., Marmarou A., Ward J.D., Kontos H.A., Choi S.C., Becker D.P., Gruemer H., and Young H.F. (1991). Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J. Neurosurg. 75, 731–739 [DOI] [PubMed] [Google Scholar]
- 63.Etemadrezaie H., Baharvahdat H., Shariati Z., Lari S.M., Shakeri M.T., and Ganjeifar B. (2007). The effect of fresh frozen plasma in severe closed head injury. Clin. Neurol. Neurosurg. 109, 166–171 [DOI] [PubMed] [Google Scholar]
- 64.Liu W.G., Qiu W.S., Zhang Y., Wang W.M., Lu F., and Yang X.F. (2006). Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J. Int. Med. Res. 34, 58–64 [DOI] [PubMed] [Google Scholar]
- 65.Sadaka F., Krause K., Steska M., Wilcox M., and O'Brien J. (2013). Induced normothermia after severe traumatic brain injury: safety and feasibility study. Neurocrit. Care 19, S13 [Google Scholar]
- 66.Rubiano A.M. and Puyana J.C. (2013). Intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 368, 1748. [DOI] [PubMed] [Google Scholar]
- 67.Chesnut R.M., Petroni G., and Rondina C. (2013). Intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 368, 1751–1752 [DOI] [PubMed] [Google Scholar]
- 68.Roberts I., Smith R., and Evans S. (2007). Doubts over head injury studies. BMJ 334, 392–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young C. and Godlee F. (2007). Managing suspected research misconduct. BMJ 334, 378–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerr M.E., Weber B.B., Sereika S.M., Wilberger J., and Marion D.W. (2001). Dose response to cerebrospinal fluid drainage on cerebral perfusion in traumatic brain-injured adults. Neurosurg. Focus 11, E1. [DOI] [PubMed] [Google Scholar]
- 71.Cooper D.J., Rosenfeld J.V., Murray L., Arabi Y.M., Davies A.R., D'Urso P., Kossmann T., Ponsford J., Seppelt I., Reilly P., and Wolfe R.; DECRA Trial Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. (2011). Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 364, 1493–1502 [DOI] [PubMed] [Google Scholar]
- 72.Schibler A. and Humphreys S. (2012). Increased brain tissue oxygen tension in children with traumatic brain injury using temperature-corrected guided ventilation during prophylactic hypothermia. Crit. Care Resusc 14, 20–24 [PubMed] [Google Scholar]
- 73.Adelson P.D., Ragheb J., Kanev P., Brockmeyer D., Beers S.R., Brown S.D., Cassidy L.D., Chang Y., and Levin H. (2005). Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 56, 740–754 [DOI] [PubMed] [Google Scholar]
- 74.Adelson P.D., Wisniewski S.R., Beca J., Brown S.D., Bell M., Muizelaar J.P., Okada P., Beers S.R., Balasubramani G.K., and Hirtz D.; Paediatric Traumatic Brain Injury Consortium. (2013). Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 12, 546–553 [DOI] [PubMed] [Google Scholar]
- 75.Hutchison J.S., Frndova H., Lo T.Y.M., and Guerguerian A.M.; Hypothermia Pediatric Head Injury Trial Investigators; Canadian Critical Care Trials Group. (2011). Impact of hypotension and low cerebral perfusion pressure on outcomes in children treated with hypothermia therapy following severe traumatic brain injury: a post hoc analysis of the Hypothermia Pediatric Head Injury Trial. Dev. Neurosci. 32, 406–412 [DOI] [PubMed] [Google Scholar]
- 76.Biswas A.K., Bruce D.A., Sklar F.H., Bokovoy J.L., and Sommerauer J.F. (2002). Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit. Care Med. 30, 2742–2751 [DOI] [PubMed] [Google Scholar]
- 77.Briassoulis G., Filippou O., Kanariou M., Papassotiriou I., and Hatzis T. (2006). Temporal nutritional and inflammatory changes in children with severe head injury fed a regular or an immune-enhancing diet: a randomized, controlled trial. Pediatr. Crit. Care Med. 7, 56–62 [DOI] [PubMed] [Google Scholar]
- 78.Fisher B., Thomas D., and Peterson B. (1992). Hypertonic saline lowers raised intracranial pressure in children after head trauma. J. Neurosurg. Anesthesiol. 4, 4–10 [DOI] [PubMed] [Google Scholar]
- 79.Simma B., Burger R., Falk M., Sacher P., and Fanconi S. (1998). A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer's solution versus hypertonic saline. Crit. Care Med. 26, 1265–1270 [DOI] [PubMed] [Google Scholar]
- 80.Bohn D., Swan P., Sides C., and Hoffman H. (1989). High-dose barbiturate therapy in the management of severe paediatric head injury: a randomised controlled trial. 1989;S118:17. Crit. Care Med. 17, S118 [Google Scholar]
- 81.Fanconi S., Kloti J., Meuli M., Zaugg H., and Zachmann M. (1988). Dexamethasone therapy and endogenous cortisol production in severe pediatric head injury. Intensive Care Med. 14, 163–166 [DOI] [PubMed] [Google Scholar]
- 82.Taylor A., Butt W., Rosenfeld J., Shann F., Ditchfield M., Lewis E., Klug G., Wallace D., Henning R., and Tibballs J. (2001). A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child Nerv. Syst,. 17, 154–162 [DOI] [PubMed] [Google Scholar]
- 83.Taher A. (2013). Effect of hyperoxia with FIO2 80% in comparison with FIO2 50% on Glascow Outcome Scale, Barthel Index and Modified Ranking Scale in patients with severe traumatic brain injury: a double blinded randomized clinical trial. Available at www.irct.ir/searchresult.php?id=9014&number=17 Accessed March3, 2016
- 84.Diaz-Arrastia R. (2012). Brain Tissue Oxygen Monitoring in Traumatic Brain Injury (TBI) (BOOST 2). Available at: https://clinicaltrials.gov/ct2/show/NCT00974259 Accessed February15, 2016
- 85.Cooper D.J., Joyce C., Macisaac C., Webb S., Pilifloury S., McArthur C., and Frengley R. (2015). The prophylactic hypothermia trial to lessen traumatic brain injury (POLAR-RCT). Available at: https://clinicaltrials.gov/ct2/show/NCT00987688 Accessed February15, 2016
- 86.Jiang J. (2014). Randomized Controlled Trial of Long-term Mild Hypothermia for Severe Traumatic Brain Injury (LTH-I). Available at: https://clinicaltrials.gov/ct2/show/NCT01886222 Accessed February15,2016
- 87.Moore E.M., Nichol A.D., Bernard S.A., and Bellomo R. (2011a). Therapeutic hypothermia: benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury. Injury 42, 843–854 [DOI] [PubMed] [Google Scholar]
- 88.Proctor K. (2014). Vasopressin Versus Catecholamines for Cerebral Perfusion Pressure Control in Brain Injured Trauma Patients (AVP). Available at: https://clinicaltrials.gov/ct2/show/NCT00795366 Accessed February15,2016
- 89.Fakharian E. (2013). The effect of tranexamic acid on prevention of increased hemorrhage in traumatic brain injury. Available at www.irct.ir/searchresult.php?id=2854&number=6 Accessed March3, 2016
- 90.Shakur H. (2013). Clinical randomisation of an antifibrinolytic in significant head injury (CRASH-3). Available at: https://clinicaltrials.gov/ct2/show/NCT01402882 Accessed February15,2016
- 91.Gruen R. (2014). Pre-hospital Anti-fibrinolytics for Traumatic Coagulopathy and Haemorrhage (The PATCH Study). Available at: https://clinicaltrials.gov/ct2/show/NCT02187120 Accessed February15,2016
- 92.Mahdian M. (2013). Comparison of the effects of erythropoietin and placebo on lesion size and outcome (Glasgow Outcome Score) in the severe brain-injured patients. Available at www.irct.ir/searchresult.php?id=14138&number=1 Accessed March3,2016
- 93.Moore E.M., Bellomo R., and Nichol A.D. (2011). Erythropoietin as a novel brain and kidney protective agent. Anaesth. Intensive Care 39, 356–372 [DOI] [PubMed] [Google Scholar]
- 94.Bellomo R. (2013). Erythropoietin in Traumatic Brain Injury (EPO-TBI). Available at: https://clinicaltrials.gov/ct2/show/NCT00987454 Accessed February15,2016
- 95.Eisenberg H. (2014). Glyburide (RP-1127) for traumatic brain injury (TBI). Available at: https://clinicaltrials.gov/ct2/show/NCT01454154 Accessed February15,2016
- 96.Bullock R. (2014). Study of NNZ-2566 in patients with traumatic brain injury (INTREPID2566). Available at: https://clinicaltrials.gov/ct2/show/NCT00805818 Accessed February15,2016
- 97.Wigginton J. (2014). Resuscitative endocrinology: single-dose clinical uses for estrogen—traumatic brain injury (RESCUE - TBI). Available at: https://clinicaltrials.gov/ct2/show/NCT00973674 Accessed February15,2016
- 98.Patel M.B., McKenna J.W., Alvarez J.M., Sugiura A., Jenkins J.M., Guillamondegui O.D., and Pandharipande P.P. (2012). Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials 13, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hutchinson P. (2013). Randomised Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure (ICP) (www.RESCUEicp.com). Available at: www.controlled-trials.com/ISRCTN66202560 Accessed February15, 2016
- 100.Hutchinson P.J., Kolias A.G., Timofeev I., Corteen E., Czosnyka M., Menon D.K., Pickard J.D. and Kirkpatrick P.J. (2011). Update on the RESCUEicp decompressive craniectomy trial. Crit. Care 15, S111–S112 [Google Scholar]
- 101.Hutchinson P.J., Kolias A.G., Timofeev I., Corteen E.A., Li L.M., Ingham S.C., Czosnyka M., Pickard J.D., Menon D.K., and Kirkpatrick P.J. (2012). RESCUEicp study—A randomised controlled trial of decompressive craniectomy for refractory post-traumatic intracranial hypertension: A progress report. Br. J. Neurosurg. 26, 608–609 [Google Scholar]
- 102.Kolias A.G., Scotton W.J., Belli A., King A.T., Brennan P.M., Bulters D.O., Eljamel M.S., Wilson M.H., Papadopoulos M.C., Mendelow A.D., Menon D.K., and Hutchinson P.J.; UK Neurosurgical Research Network; RESCUE-ASDH collaborative group. (2013). Surgical management of acute subdural haematomas: current practice patterns in the United Kingdom and the Republic of Ireland. Br. J. Neurosurg. 27, 330–333 [DOI] [PubMed] [Google Scholar]
- 103.Kolias A.G., Timofeev I., Corteen E.A., Czosnyka M., Pickard J.D., Menon D.K., Kirkpatrick P.J., and Hutchinson P.J. (2013). Developing the evidence base in neurosurgery: the case of decompressive craniectomy following traumatic brain injury. Br. J. Neurosurg. 27, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lecky F. (2013). Head Injury Transportation Straight to Neurosurgery (HITS-NS): a cluster randomised feasibility study. Available at: www.controlled-trials.com/ISRCTN68087745 Accessed February15, 2016 [DOI] [PMC free article] [PubMed]
- 105.McClelland G., Pennington E., Byers S., Russell W., and Lecky F. (2015). The challenges of conducting prehospital research: successes and lessons learnt from the Head Injury Transportation Straight to Neurosurgery (HITS-NS) trial. Emerg. Med. J. 32, 663–664 [DOI] [PubMed] [Google Scholar]
- 106.Deeks J., Higgins J., and Altman D.; Cochrane Statistical Methods Group (2011). Chapter 9: Analyzing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Higgins J. and Green S. (eds). The Cochrane Collaboration: London [Google Scholar]
- 107.O'Connor D., Green S., and Higgins J. (2011). Chapter 5: Defining the review question and developing criteria for including studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Higgins J. and Green S. (eds). The Cochrane Collaboration: London [Google Scholar]
- 108.Schwamm L.H. (2014). Progesterone for traumatic brain injury–resisting the sirens' song. N. Engl. J. Med. 371, 2522–2523 [DOI] [PubMed] [Google Scholar]
- 109.Crossley S., Reid J., McLatchie R., Hayton J., Clark C., MacDougall M., and Andrews P.J. (2014). A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit. Care 18, R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maas A.I., Marmarou A., Murray G.D., Teasdale S.G., and Steyerberg E.W. (2007). Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma 24, 232–238 [DOI] [PubMed] [Google Scholar]
- 111.Maas A.I., Murray G.D., Roozenbeek B., Lingsma H.F., Butcher I., McHugh G.S., Weir J., Lu J., and Steyerberg E.W.; International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group. (2013). Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurol. 12, 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ioannidis J.P. (2005). Contradicted and initially stronger effects in highly cited clinical research. JAMA 294, 218–228 [DOI] [PubMed] [Google Scholar]
- 113.Grimshaw J.M., Eccles M.P., Lavis J.N., Hill S.J., and Squires J.E. (2012). Knowledge translation of research findings. Implement. Sci. 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halpern S.D., Karlawish J.H., and Berlin J.A. (2002). The continuing unethical conduct of underpowered clinical trials. JAMA 288, 358–362 [DOI] [PubMed] [Google Scholar]
- 115.Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., and Munafo M.R. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature reviews. Neuroscience 14, 365–376 [DOI] [PubMed] [Google Scholar]
- 116.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T.; Workshop Scientific Team and Advisory Panel Members. (2008). Classification of traumatic brain injury for targeted therapies. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maas A.I., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., Hill S., Legrand V., andSorgner A.; CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 118.Menon D.K. and Maas A.I. (2015). Traumatic brain injury in 2014. Progress, failures and new approaches for TBI research. Nat. Rev. Neurol. 11, 71–72 [DOI] [PubMed] [Google Scholar]
- 119.Stocchetti N., Taccone F.S., Citerio G., Pepe P.E., Le Roux P.D., Oddo M., Polderman K.H., Stevens R.D., Barsan W., Maas A.I., Meyfroidt G., Bell M.J., Silbergleit R., Vespa P.M., Faden A.I., Helbok R., Tisherman S., Zanier E.R., Valenzuela T., Wendon J., Menon D.K., and Vincent J.L. (2015). Neuroprotection in acute brain injury: an up-to-date review. Crit. Care 19, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roozenbeek B., Lingsma H.F., and Maas A.I. (2012). New considerations in the design of clinical trials for traumatic brain injury. Clin. Invest. 2, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maas A.I., Menon D.K., Lingsma H.F., Pineda J.A., Sandel M.E., and Manley G.T. (2012). Re-orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J. Neurotrauma 29, 32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kairalla J.A., Coffey C.S., Thomann M.A., and Muller K.E. (2012). Adaptive trial designs: a review of barriers and opportunities. Trials 13, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kay T., Harrington D., and Adams R. (1993). Definition of mild traumatic brain injury. J Head Trauma Rehabil 8, 86 - 87 [Google Scholar]
- 124.Rosenthal M., Griffith E., and Bond M. (1990). Rehabilitation of the Adult and Child With Traumatic Brain Injury. 2nd ed. FA Davis: Philadelphia [Google Scholar]
- 125.Lefebvre C., Manheimer E., and Glanville J. (2011). Chapter 6: Searching for studies: Box 6.3.a: Cochrane definitions and criteria for randomized controlled trials (RCTs) and controlled clinical trials (CCTs). In: Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0 [updated March 2011]. Higgins J. and Green S. (eds). The Cochrane Collaboration: London [Google Scholar]
- 126.Hirshon J.M., Risko N., Calvello E.J., Stewart de Ramirez S., Narayan M., Theodosis C., andO'Neill J.; Acute Care Research Collaborative at the University of Maryland Global Health Initiative. (2013). Health systems and services: the role of acute care. Bull World Health Organ. 91, 386–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.The Nordic Cochrane Centre (2014). Review Manager (RevMan). The Cochrane Collaboration: Copenhagen [Google Scholar]
- 128.Maas A.I., Steyerberg E.W., Marmarou A., McHugh G.S., Lingsma H.F., Butcher I., Lu J., Weir J., Roozenbeek B., and Murray G.D. (2010). IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 7, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.