Abstract
Anticorrelation between the default and dorsal attention networks is a central feature of human functional brain organization. Hallmarks of aging include impaired default network modulation and declining medial temporal lobe (MTL) function. However, it remains unclear if this anticorrelation is preserved into older adulthood during task performance, or how this is related to the intrinsic architecture of the brain. We hypothesized that older adults would show reduced within- and increased between-network functional connectivity (FC) across the default and dorsal attention networks. To test this hypothesis, we examined the effects of aging on task-related and intrinsic FC using fMRI during an autobiographical planning task known to engage the default network and during rest, respectively, with young (n=72) and older (n=79) participants. The task-related FC analysis revealed reduced anticorrelation with aging. At rest, there was a robust double dissociation, with older adults showing a pattern of reduced within-network FC, but increased between-network FC, across both networks, relative to young adults. Moreover, older adults showed reduced intrinsic resting-state FC of the MTL with both networks suggesting a fractionation of the MTL memory system in healthy aging. These findings demonstrate age-related dedifferentiation among these competitive large-scale networks during both task and rest, consistent with the idea that age-related changes are associated with a breakdown in the intrinsic functional architecture within and among large-scale brain networks.
Keywords: Default network, dorsal attention network, anticorrelation, aging, fMRI, resting-state functional connectivity, medial temporal lobe
Introduction
Anticorrelation between large-scale brain networks is a central feature of human functional brain organization (e.g. Fox et al., 2005; Fransson, 2005; Golland, Golland, Bentin, & Malach, 2008). Anticorrelation is observed as positive within-network functional connectivity (FC) concomitant with negative between-network FC of the dorsal attention and default networks (Fox et al., 2005). The dorsal attention network is composed of the frontal eye fields (FEF), the ventral frontal region PrCv (precentral ventral), middle temporal motion complex (MT+), inferior parietal sulcus (IPS), superior parietal lobule (SPL) and dorsolateral prefrontal cortex (DLPFC). Regions of the default network include medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), superior and inferior frontal gyrus, lateral temporal lobes, inferior parietal lobule (IPL), and the medial temporal lobes (MTL) (Fox et al., 2005), though there is evidence that the MTL may have a unique functional relationship with the default network (Eldaief, Halko, Buckner, & Pascual-Leone, 2011; Ward et al., 2014) and comprises sub-regions that have dissociable patterns of FC (Kahn, Andrews-Hanna, Vincent, Snyder, & Buckner, 2008). The dorsal attention and default networks are inversely engaged during externally and internally directed cognition, respectively (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010), and this reciprocal pattern of activity may serve as a critical neural substrate for flexibly allocating attentional resources and is important for healthy cognitive function (Whitfield-Gabrieli & Ford, 2012). Magnitude of anticorrelation is associated with externally-directed task performance in young adults (e.g. Hampson, Driesen, Roth, Gore, & Constable, 2010)
Numerous age-related changes are apparent in brain activation during externally oriented attention (Spreng, Wojtowicz, & Grady, 2010), which reliably engages the dorsal attention network in the young (Gusnard, Raichle, & Raichle, 2001; Spreng, Stevens, et al., 2010). Additionally, reductions in task-related default network suppression and altered FC have been observed with advancing age (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Grady et al., 2010; Hafkemeijer, van der Grond, & Rombouts, 2012; Sala-Llonch et al., 2012; Sambataro et al., 2010; Stevens, Hasher, Chiew, & Grady, 2008; Turner & Spreng, 2015). These findings complement whole brain resting-state FC (RSFC) observations of a dedifferentiation of network connectivity with age, with increases in RSFC between large-scale brain systems in older adults (Betzel et al., 2014; Chan, Park, Savalia, Petersen, & Wig, 2014; Geerligs, Renken, Saliasi, Maurits, & Lorist, 2015; Grady, Sarraf, Saverino, & Campbell, 2016; Meunier, Achard, Morcom, & Bullmore, 2009; Onoda & Yamaguchi, 2013). It is unclear, however, if the robust pattern of anticorrelated activity between the default and dorsal attention networks is preserved into older adulthood.
Chan et al. (2014) observed increased correlations between default and dorsal attention networks as one feature of a larger pattern of reduced network segregation in older adulthood (see also, Grady et al., 2016). However age-related changes in anticorrelation between networks were not investigated in this study. Reduced anticorrelations between default and dorsal attention networks have been reported in older adults (Betzel et al., 2014; Wu et al., 2011). However, the use of mean global signal regression (GSR) in these studies may have altered the interregional correlation differences between groups (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009; Saad et al., 2012), complicating the interpretation of negative correlation values (Gotts et al., 2013). More recently, preprocessing procedures that do not rely on GSR have revealed that the antagonism between medial and lateral prefrontal cortex is attenuated in older adults (Keller et al., 2015), but age-related changes in anticorrelation between the dorsal attention and default networks more broadly have not been reported.
Consistent with the critical role of the MTL in episodic memory (Squire et al., 2004) and the marked deficits in episodic memory in age-related dementia, several studies have demonstrated reduced RSFC of the MTL in particular with other default network regions in individuals with mild cognitive impairment (Das et al., 2013; Das et al., 2015; Jin, Pelak, & Cordes, 2012), and Alzheimer’s disease (for review see Hafkemeijer et al., 2012; Mevel et al., 2011), including patients in prodromal stages (Sperling et al., 2010; Wang et al., 2006). Episodic memory also shows declines in typical healthy aging (Grady, 2012) and there is evidence for MTL-cortical reductions in FC at rest with advancing age (Salami, Pudas & Nyberg, 2014). An earlier study, however, found that while some sub-systems of the default network showed differential patterns of RSFC between young and older adults, the MTL subsystem in particular, did not show any age-related differences (Campbell, Grigg, Saverino, Churchill, & Grady, 2013). Therefore, the extent to which FC of the MTL is altered in aging and potentially related to declines in memory remains unclear.
Spreng and Schacter (2012) assessed patterns of large-scale network activity in young and older adults during performance of an autobiographical planning task that engages the default network and a visuospatial planning task (the Tower of London) that engages the dorsal attention network (Spreng, Stevens, et al., 2010), consistent with the anti-correlated domains of internalized and externalized cognition. Older adults robustly engaged the default network during the autobiographical planning task, not different in magnitude than their younger counterparts (Spreng & Schacter, 2012, see also Figure 1). Unlike young adults, older adults had reduced suppression of the default network during visuospatial planning (Spreng & Schacter, 2012; Turner & Spreng, 2015). Thus, autobiographical planning provides a unique opportunity to examine FC patterns in older adults, and potential age-related changes in anticorrelation during a task known to engage the default network, without the confound of age-related differences in task-based activation patterns. In the current study, we examine a) the impact of age on task-related MPFC connectivity during autobiographical planning using a multivariate FC analysis; and b) patterns of RSFC in young and older adults, leveraging a preprocessing strategy that does not involve GSR. Together these analyses provide the first evidence that aging is associated with reduced anticorrelation between the default and dorsal attention networks during task and rest, and decreased intrinsic FC of the MTL across both networks.
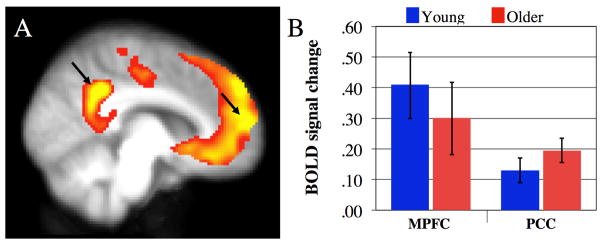
Figure 1.
Autobiographical planning task activation. A) Young and older adults robustly engaged the default network during autobiographical planning, relative to visuospatial planning (see Spreng & Schacter, 2012). B) MPFC and PCC activity was significantly elevated relative to fixation baseline in both young (tMPFC (17) = 3.89, p < . 001; tPCC (17) = 3.26, p < . 005) and older adults (tMPFC (16) = 2.56, p < .05; tPCC (17) = 3.80, p < . 001). No differences were observed in the magnitude of MPFC or PCC activation between groups (tMPFC (33) = 0.69, ns; tPCC (34) = 1.00, ns). Differences between groups in the connectivity profile thus cannot be attributed to differences in task-related brain activity.
Methods
Participants
All participants were healthy, with normal or corrected-to-normal visual acuity, and no history of psychiatric, neurological, or other medical illness that could compromise cognitive functions. All participants gave written informed consent in accordance with the Harvard Institutional Review Board or the Human Subjects Research Committee at Massachusetts General Hospital.
Experiment 1
Task fMRI data were collected from 18 young adults (mean age = 22.8 ± 2.4 years; range = 19–27; 9 women) and 18 older adults (mean age = 71.4 ± 4.0 years; range = 63–78; 9 women) previously reported by Spreng & Schacter (2012). Years of education were equivalent between groups (young = 15.9 ± 1.9 years; older = 15.9 ± 1.1 years). Older adults were high functioning, with a healthy mental status (Mini Mental Status Examination ≥ 27; mean = 28.4 ± 1.5), and not depressed (Geriatric Depression Scale ≤ 3.0; mean = 0.8 ± 0.9). Most participants were right-handed; one male in each group was left-handed. One older adult was excluded due to outlier MPFC BOLD signal activation > 5 SDs from the group mean.
Experiment 2
Resting-state fMRI data were collected from an independent sample of 54 young adults (mean age=24±3.8years;range=18-35;27women) and 61 older adults (mean age=74.6±3.8years;range=65-86;31women).Years of education were equivalent between groups(young = 16 ± 2.1 years; older = 16 ± 2.8 years). These older adults were high functioning, with a healthy mental status (Mini-Mental Status Examination ≥ 25; mean = 28.86 ± 1.2), and not depressed (Geriatric Depression Scale ≤ 4; mean = 0.7 ± 0.9).
Procedure
Experiment 1 – Task
The autobiographical planning task required participants to devise plans in order to meet specific goals in their personal futures. For example, “Exercise” constituted one of the goals in the autobiographical planning task. Participants viewed the goal and then saw two steps they could take toward achieving that goal (“make routine” and “walk more”) as well as an obstacle they needed to overcome in order to achieve the goal (“avoid injury”). They were instructed to integrate the steps and obstacles into a cohesive personal plan that would allow them to achieve the goal. Participants completed 24 autobiographical planning trials. The pacing of the task was such that the goal was presented for 5 seconds, followed by 20 seconds of plan formation where the goal, steps and obstacle were visible.
Experiment 2 – Rest
Participants were instructed to fixate on a centrally presented crosshair while blinking and breathing normally, to remain motionless, and to not fall asleep. Compliance was confirmed by verbal confirmation at the end of each run.
MRI data collection and preprocessing
Experiment 1
Brain imaging data were acquired at the Harvard Center for Brain Sciences with a 3.0 T Siemens TimTrio MRI scanner with a 32-channel head coil. Anatomical scans were acquired using a T1-weighted multi-echo volumetric MRI (TR = 2530 ms; TE’s = 1.64, 7.22ms; 7° flip angle; 1.0 mm isotropic voxels). Six 7-min task BOLD functional scans were acquired with a T2*-weighted EPI pulse sequence (TR = 2500ms; TE = 30ms; 85° flip angle; 39 axial slices parallel to the plane of the AC-PC; 3.0 × 3.0 × 2.5mm voxels with a 0.5mm gap).
Task fMRI data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). The first 4 volumes in each run were excluded from analyses to allow for T1-equilibration effects. Data were corrected for slice-dependent time shifts and for head motion within and across runs using a rigid body correction. During the autobiographical planning blocks, no differences in head motion were observed between younger and older adults in the six motion parameters (F (5,30) = 0.94, p = .472). The data were then normalized to a combined young-older brain template that approximated MNI atlas space in order to compare brain data across age groups (Buckner et al., 2004). The volumetric time series was then resampled at 2mm cubic voxels and spatially smoothed with an 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel.
Experiment 2
All brain imaging data were acquired at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital (Charlestown, MA) on a 3.0 T Siemens Magnetom TimTrio MRI scanner with a 12-channel head coil (Siemens Medical Solutions, Erlangen, Germany). Anatomical images were acquired using a high-resolution 3D magnetization-prepared rapid gradient echo sequence (MPRAGE: 128 sagittal slices; repetition time [TR] = 2530 ms; echo time [TE] = 3.45 ms; flip angle = 7°; voxel size = 1 × 1 × 1.33 mm). For one sample of young (n = 37) and older (n = 41) adults, functional images for 4 rest runs were collected using T2* gradient echo, echo planar imaging sensitive to BOLD contrast (TR = 2500 ms; TE = 30 ms; flip angle = 90°; voxel size = 4 mm isotropic) in 4 sets of 112 volumes per run acquired axially in 36 slices, yielding whole-brain coverage, for a total of 448 volumes (duration = 18 min 40 s). For a second sample of young (n = 18) and older (n = 32) adults, functional images for 2 rest runs were collected using T2* gradient echo, echo planar imaging sensitive to BOLD contrast (TR = 2500 ms; TE = 30 ms; flip angle = 90°; voxel size = 3 mm isotropic) in 2 sets of 124 volumes per run acquired axially in 36 slices with a 0.5 mm gap between slices, yielding whole-brain coverage, for a total of 248 volumes (duration = 10 min 20 s). To maximize statistical power, all young adults were combined and all older adults were combined to form two groups of large sample sizes (young: n = 55; older: n = 73) for all RSFC analyses.
Echo-planar images were preprocessed using the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996). For each run, the first four image-volumes were removed to allow for T1-equilibration effects. Large transients in the remaining volumes were removed through interpolation (3dDespike). Volumes were then slice-time corrected (3dTshift) and co-registered to the volume nearest the anatomical scan (3dVolreg). In RSFC analyses, artifacts in the data resulting from participant head motion are particularly problematic, especially when comparing samples across different populations (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Satterthwaite et al., 2012; Van Dijk, Sabuncu, & Buckner, 2012). Numerous data analysis techniques have been developed to address this potential problem; one approach involves a combination of GSR and censoring of volumes coinciding with periods of abrupt and/or excessive motion, known as “scrubbing” (Power et al., 2014). However, a number of studies have demonstrated that GSR can distort the results of RSFC analyses in several ways (Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012) and introduce spurious anticorrelations (Murphy et al., 2009). Scrubbing can involve discarding a substantial portion of data, often unevenly across groups, thus resulting in a loss of statistical power and the potential introduction of group confounds (Satterthwaite et al., 2013). A particularly effective approach to the removal of nuisance physiological, non-physiological, and motion-related artifacts is the ANATICOR procedure (Jo, Saad, Simmons, Milbury, & Cox, 2010). This approach has been shown to drastically reduce or virtually eliminate motion-related artifacts in resting-state time-series analyses (Jo et al., 2013). Further, the method eschews temporal filtering (e.g., bandpass filtering), which is ineffective at removing signals with frequencies above the Nyquist frequency (i.e., 0.5 × 1/TR = 0.2 Hz in the current study), such as cardiac and respiratory signals (Gotts et al., 2013; Van Dijk et al., 2010), and GSR, which can distort RSFC results in a number of detrimental ways (Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012). A modified version of the ANATICOR procedure was used here, as described previously (Fischl et al., 2002; Stevens, Tessler, Peng, & Martin, 2015). For each subject, the anatomical scan was segmented into tissue compartments using Freesurfer (Fischl et al., 2002). Then, ventricle, white-matter, and draining-vessel masks were created and eroded to prevent partial volume effects with gray matter. These masks were then applied to the volume-registered echo-planar image yielding pure nuisance time-series for the ventricles and draining-vessels, as well as local estimates of the white-matter BOLD signal averaged within a 15-mm radius sphere. To summarize, nuisance variables for each voxel’s time-series included: an average ventricle time-series, an average draining-vessel time-series, a local average white-matter time-series, six head motion parameter estimates, and the temporal derivative of each of the latter. All of the above nuisance time-series were detrended with fourth-order polynomials. Least-squares model fitted time-series of these nuisance variables were then subtracted from the voxel time-series, yielding a residual time-series that was used in all subsequent statistical analyses. To compare the differential effects of preprocessing techniques, we also analyzed the resting-state data using GSR combined with the scrubbing technique (GRS+Scrubbing) described by Power et al., (2012). Briefly, framewise displacement (FD: sum of absolute values of the differentials of the 3 translational and 3 rotational motion parameters) and signal change (DVARS) were calculated, and any volumes with FD > 0.3 mm or DVARS > 0.3%, as well as the 2 preceding and 2 following volumes, were discarded from the timeseries. Only participants with at least 120 volumes remaining after scrubbing were included in the group analyses, resulting in 54 young and 34 older adults for this analysis. Finally, for both analyses, the volumetric time series was then smoothed with a 6 mm FWHM Guassian kernel (3dBlurInMask), then normalized to the AFNI MNI_152 atlas space and resampled at 3 mm isotropic (3dAllineate).
Task-related FC
The preprocessed autobiographical planning data from Experiment 1 were analyzed with seed partial least squares (PLS, Krishnan, Williams, McIntosh, & Abdi, 2011; McIntosh, 1999). Seed PLS is a data-driven multivariate statistical technique that reveals functional activity across the entire brain that correlates with activity in a seed region. The covariance between activity in the seed and all other brain voxels is decomposed into latent variables (LVs) that can identify multiple patterns of FC. The advantage of block seed PLS is that potential movement confounds associated with age are substantially reduced. Furthermore, the decomposition and associated resampling techniques consider all voxels simultaneously, thus avoiding the problem of multiple statistical comparisons. Because of its ability to identify groups of brain regions with covarying FC, this technique is methodologically suited to the investigation of large-scale brain networks. In two seed PLS analyses, activity was extracted from two regions of interest (ROI: peak voxel plus 26 neighboring voxels) centered on the location of peak activation within the MPFC and PCC (MNI coordinates: −4, 62, 14 and −10, −48, 36 respectively) during autobiographical planning (Figure 1) and correlated across participants with all other brain voxels; PLS then was used to identify patterns of correlation that differed between young and older adults. Significance of the LVs was determined by 2500 permutation tests, using resampling without replacement. Reliability of each voxel’s contribution to a LV across participants was calculated by a bootstrap procedure that resampled the data 500 times, with replacement, to estimate the standard error of the weight of each voxel on the LV. A bootstrap ratio, calculated as the ratio of each weight to its standard error, was thresholded at ±1.96, equivalent to p < 0.05. For each participant, a composite brain score was calculated that provides an index of how strongly each participant expresses the pattern of activity identified by that LV. To examine differences in connectivity across groups, the correlation between these scores from each significant LV and the seed values was calculated. Confidence intervals (95%) were calculated from the bootstrap, and differences between groups were determined via a lack of overlap in these confidence intervals. In a set of auxiliary analyses, we found that the observed pattern of functional connectivity was not related to measures of grey and white matter atrophy (See supplemental material).
In a complementary ROI-based analysis, we next extracted BOLD signal from 6 ROIs of the dorsal attention network and 6 ROIs of the default network defined in an independent study delineating the brain’s large-scale networks in 1000 adults (Yeo et al., 2011). For each group, the between-subjects cross-correlation matrix was determined. See Table 1 for all seed region coordinates.
Table 1.
Age by network connectivity interactions
| GSR+Scrubbing | ANATICOR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Network | ROI | Coordinates (x, y, z) | Interaction | Crossover | F-stat | p-value | Interaction | Crossover | F-stat | p-value |
| Both | All | 32.85 | < 0.001 | 16.45 | < 0.001 | |||||
| Dorsal Attention | All | 24.43 | < 0.001 | 9.50 | < 0.01 | |||||
| Default | All | 23.63 | < 0.001 | 15.37 | < 0.001 | |||||
| Dorsal Attention | FEF | −22, −8, 54 | 13.99 | < 0.001 | 4.59 | < 0.05 | ||||
| IPS | −34, −38, 44 | 21.88 | < 0.001 | 8.26 | < 0.01 | |||||
| SPL7a | −18, −69, 51 | 12.30 | < 0.001 | 4.76 | < 0.05 | |||||
| MT+ | −51, −64, −2 | 12.90 | < 0.001 | 3.69 | n.s. | |||||
| SPL7p | −8, −63, 57 | 2.41 | n.s. | 0.68 | n.s. | |||||
| PrCv | −49, 3, 34 | 26.42 | < 0.001 | 12.30 | < 0.001 | |||||
| Default | PFCdp | −27, 23, 48 | 16.24 | < 0.001 | 10.91 | < 0.001 | ||||
| IPL | −41, −60, 29 | 16.37 | < 0.001 | 9.84 | < 0.01 | |||||
| STS | −64, −20, −9 | 0.85 | n.s. | 1.22 | n.s. | |||||
| MPFC | −7, 49, 18 | 26.82 | < 0.001 | 19.73 | < 0.001 | |||||
| PCC | −7, −52, 26 | 18.62 | < 0.001 | 11.43 | < 0.001 | |||||
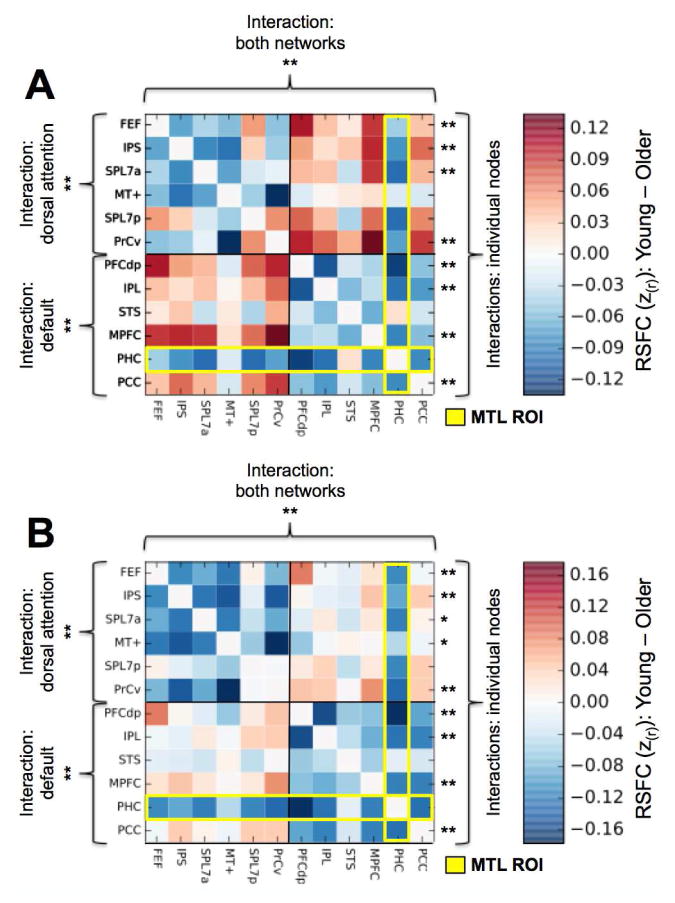
Interactions: significant age (young vs. older) × differentiation (within- vs. between-network RSFC) interaction; crossover, significant crossover interaction; FEF, frontal eye fields; IPS, inferior parietal sulcus, SPL7a, anterior superior parietal lobule; MT+, middle temporal area complex; SPL7p, posterior superior parietal lobule; PrCv, ventral precentral sulcus, PFCdp, posterior dorsolateral prefrontal cortex; IPL, inferior parietal lobule; STS, superior temporal sulcus; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; n.s., not significant.
Intrinsic FC
To analyze whole-brain patterns of intrinsic low-frequency BOLD correlations and assess their differences by age, the mean BOLD signal time-course was extracted from spherical seed ROIs with 6 mm radii centered on the location of peak activation within the MPFC and PCC during autobiographical planning (Figure 1). The correlation coefficient for each seed’s time-course with the time-course for every other voxel in the brain was computed using Pearson’s product-moment formula. These values were then converted to z-values using Fisher’s r-to-z transformation. The whole-brain voxel-wise z-map was then subjected to random-effects analyses to asses statistical significance across participants at the group level using t-tests (threshold: q < 0.05, FDR corrected). ROI-based analyses were then used to further explore age-related differences in within- and between-network RSFC among a set of critical nodes of both the dorsal attention and default networks. For each of 6 nodes within each network (Table 1), a spherical ROI with a radius of 6 mm was centered on the a priori coordinate based on previous literature as discussed above (Yeo et al., 2011). The pairwise RSFC of each node with every other node, both within and between the two networks, was calculated, and random effects analyses were used to assess age-related differences in within- and between-network FC.
Results
Whole-brain task-related FC
The task-related FC analysis, using the MPFC as a seed region, yielded two statistically significant LVs depicting qualitatively different FC patterns that differentiated the two age groups. One LV showed significant and reliable MPFC connectivity in the young, but not the older adults, accounting for 43.9% of the variance in the data (p < .05). We observed positive connectivity of the MPFC with the default network, and conversely, negative connectivity with the dorsal attention network (Figure 2A), demonstrating the expected pattern of anticorrelation. The other LV showed significant and reliable MPFC connectivity in the older (r = .84) but not the young (r = −.14) adults, accounting for 56.1% of the variance in the data (p < .05). We observed diffuse positive connectivity of the MPFC with the default network, key regions of the dorsal attention network, and other regions (Figure 2B). Multivariate patterns of connectivity were not significant for the PCC (p = .18).
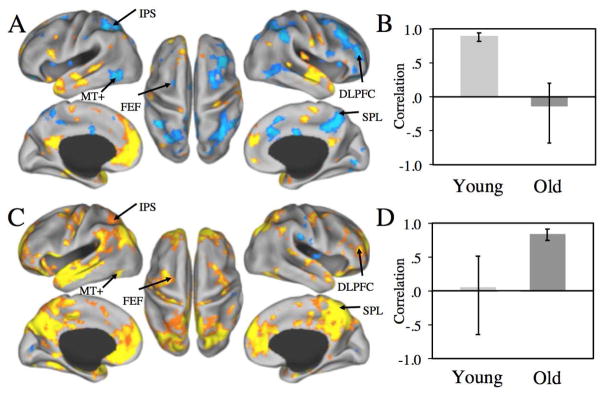
Figure 2.
Patterns of positive and negative correlations with the MPFC. A) Regions functionally connected with the MPFC during autobiographical planning in young adults only. C) Regions functionally connected with the MPFC in the older adults only. Correlations (B, D) represent the association between the MPFC seed activity and a composite brain activity score across participants within each group. The magnitude of correlation between MPFC and the activity represented in (A) was higher in the young than older adults (the correlation in the older adults did not differ from zero). The magnitude of correlation between the MPFC and the activity represented in (D) was higher in the older than young adults (the correlation in the young adults did not differ from zero). The pattern of functional connectivity in young adults demonstrates high within network and negative between network correlations, consistent with default-dorsal attention network anticorrelation. However, task-related functional connectivity in the older adults shows a qualitatively different pattern, with positive connectivity in FEF, MT+, IPS, SPL, and DLPFC – a network of regions comprising the dorsal attention network and anticorrelated with the default network in the young. Warm colors indicate positive correlations; Cool colors depict negative correlations.
ROI-based task-related FC
We next investigated between-subject correlations among critical nodes of the dorsal attention and default networks based on coordinates from previous literature (Yeo et al., 2011). Correlation patterns were consistent with the MPFC whole-brain connectivity results. Younger adults showed high positive within network connectivity, and negative between network connectivity, consistent with the predicted anticorrelation pattern between these networks (Figure 3). A notable exception is the PrCv connectivity with the default network, likely due to the motor demands of the autobiographical planning task (button press response). In contrast, task-based FC for the older adults showed a dedifferentiated correlation pattern (Figure 3).
Figure 3.
Node-wise task-related FC of the MPFC in young vs. older adults. In young adults (Top row), activity in the MPFC is coupled with other regions of the default network (warm colors) during autobiographical planning, and anticorrelated with only dorsal attention network regions (cool colors). In older adults (bottom row), the MPFC shows undifferentiated coupling with most nodes of both the default and dorsal attention networks (warm colors). See Table 1 node labels and coordinates.
Whole-brain intrinsic FC
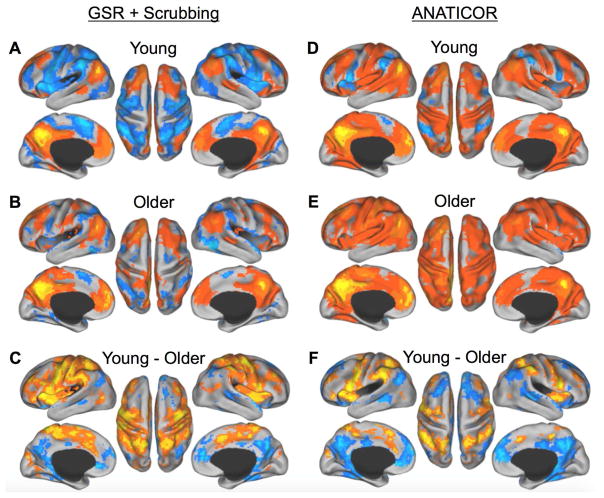
First, the GSR+Scrubbing RSFC analysis revealed the expected pattern of anticorrelations between the default and dorsal attention networks broadly in both young and older adults, when seeding both the MPFC and PCC, with older adults showing decreased within network and increased between network RSFC relative to young adults (Fig. 4A–C, 5A–C). However, as discussed previously, GSR can introduce spurious negative correlations and distort RSFC data in other ways (Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012), thus, our interpretations are based on our primary analysis which used the ANATICOR procudure without GRS+Scrubbing. As expected, anticorrelations between the dorsal attention and default networks were reduced in magnitude and more circumscribed. Nevertheless, this analysis provided a consistent and convergent pattern of results with the task driven analysis. The MPFC showed strong positive RSFC with the default network broadly, and negative correlations with the dorsal attention network, including the FEF, PrCv, MT+, IPS, SPL, and DLPFC in the young adults (Figure 4A). The older adults showed a similar pattern of MPFC RSFC, but with robustly attenuated anticorrelation with dorsal attention network regions (Figure 4E). A direct comparison between the young and older adults (Figure 4F) demonstrated reduced RSFC of the MPFC with other default network regions (PCC, IPL, MTL, lateral temporal cortex, including the superior temporal sulcus (STS), and superior frontal gyrus) and reduced anticorrelation with dorsal attention network regions (PrCv, IPS, SPL, and right DLPFC). RSFC analyses of the PCC showed a highly similar pattern of results as for the MPFC (Fig. 5), with the older adults showing substantially reduced RSFC with the majority of the default network (MPFC, STS, superior frontal gyrus, IPL, and MTL), and reduced anticorrelation with all nodes of the dorsal attention network (FEF, PrCv, MT+, IPS, SPL, and DLPFC) (Fig. 5F). All RSFC maps are displayed at a threshold of q < 0.05, FDR corrected.
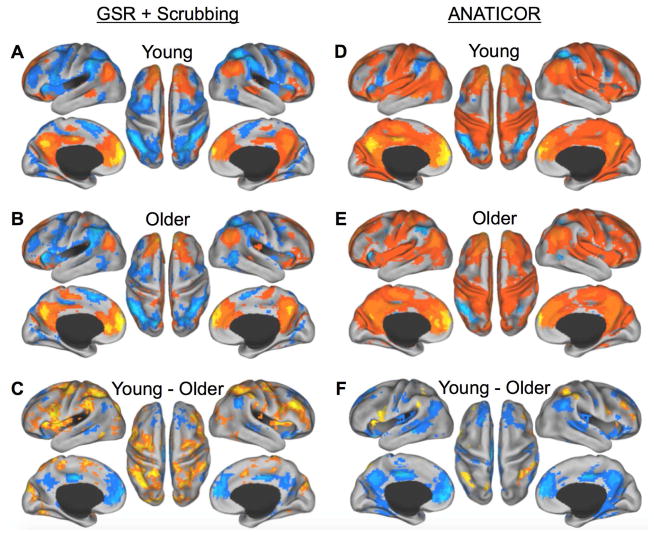
Figure 4.
RSFC of the MPFC in young vs. older adults using GRS+Scrubbing (A-C) and ANATICOR (D-F). A and D) In young adults, the MPFC shows positive RSFC with other regions of the default network (warm colors), and negative RSFC with only dorsal attention network regions (cool colors). B and E) In older adults, the MPFC shows reduced RSFC with other regions of the default network (warm colors) and reduced anticorrelations with dorsal attention network regions (cool colors). C and F) Contrast of young vs. older adults. In older adults, the MPFC shows reduced RSFC with other regions of the default network (cool colors), but increased RSFC with regions of the dorsal attention network (warm colors), relative to young adults. p < 0.05; FDR corrected.
Figure 5.
RSFC of the PCC in young vs. older adults using GRS+Scrubbing (A-C) and ANATICOR (D-F). A and D) In young adults, the PCC shows positive RSFC with other regions of the default network (warm colors), and negative RSFC with only dorsal attention network regions (cool colors). B and E) In older adults, the PCC shows reduced RSFC with other regions of the default network (warm colors) and reduced anticorrelations with dorsal attention network regions (cool colors). C and F) Contrast of young vs. older adults. In older adults, the PCC shows reduced RSFC with other regions of the default network (cool colors), but increased RSFC with regions of the dorsal attention network (warm colors), relative to young adults. p < 0.05; FDR corrected.
ROI-based intrinsic FC
To further explore age-related differences in RSFC within and between the default and dorsal attention networks, we analyzed RSFC between pairs and sets of critical nodes of these two networks as described in the task-based ROI analysis above. Figure 6 displays the contrast between the young vs. older adults’ full correlation matrices of every node of the default and dorsal attention networks with every other node for both the GSR+Scrubbing and ANATICOR analyses. Older adults showed a robust and consistent pattern of network dedifferentiation, with reduced within-network RSFC (cool colors) of both the default network (lower-right quadrant) and dorsal attention network (upper-left quadrant), and conversely, increased RSFC (warm colors) between multiple nodes only across these two networks (lower-left/upper-right quadrants). Moreover, the older adults showed reduced RSFC of the MTL node with all other nodes of both the default and dorsal attention networks (yellow outline). To quantify these differences in the ANATICOR data first, a series of ANOVAs were conducted: age (young vs. older) was a between-subjects factor; differentiation (within- vs. between-network RSFC) and network (default vs. dorsal attention) were within-subject factors. Because the MTL node showed a unique pattern of RSFC, and because its fundamental status as a default network region is questionable (see above), the MTL node was excluded from the network-level analyses. First, a 3-way ANOVA with age (young vs. older) as a between-subjects factor, differentiation (within- vs. between-network RSFC), and network (default vs. dorsal attention) as within-subjects factors revealed a significant main effect of age (F1,113 = 12.42, p < 0.001), differentiation (F1,113 = 528.39, p < 0.001), and importantly, a significant age by differentiation crossover interaction (F1,113 = 32.49, p < 0.001). Notably, there was no significant 3-way interaction (age × differentiation × network: F1,113 = 0.12, p = 0.73), indicating that this age-related dedifferentiation was not significantly different between the two networks. Analyses of each network and each node individually demonstrated that this pattern of age-related dedifferentiation characterized both networks individually, with significant main effects of age (default: F1,113 = 11.40, p < 0.001; dorsal attention: F1,113 = 9.12, p < 0.005), differentiation (default: F1,113 = 354.90, p < 0.001; dorsal attention: F1,113 = 431.47, p < 0.001), and age by differentiation crossover interactions (default: F1,113 = 23.63, p < 0.001; dorsal attention: F1,113 = 24.43, p < 0.001); and most of the individual nodes of both networks as well (see Table 1 for a full list of all significant interactions). The same analyses with the GSR+Scrubbing data showed a highly consistent pattern of results (see Table 1). Finally, a 2-way ANOVA demonstrated that the MTL ROI showed reduced RSFC across both networks, with a main effect of age (F1,113 = 178.18, p < 0.001), but notably, no age by differentiation interaction (F1,113 = 0.70, p = 0.40), indicating that the MTL showed a parallel reduction in RSFC with both the default and dorsal attention networks in the older adults.
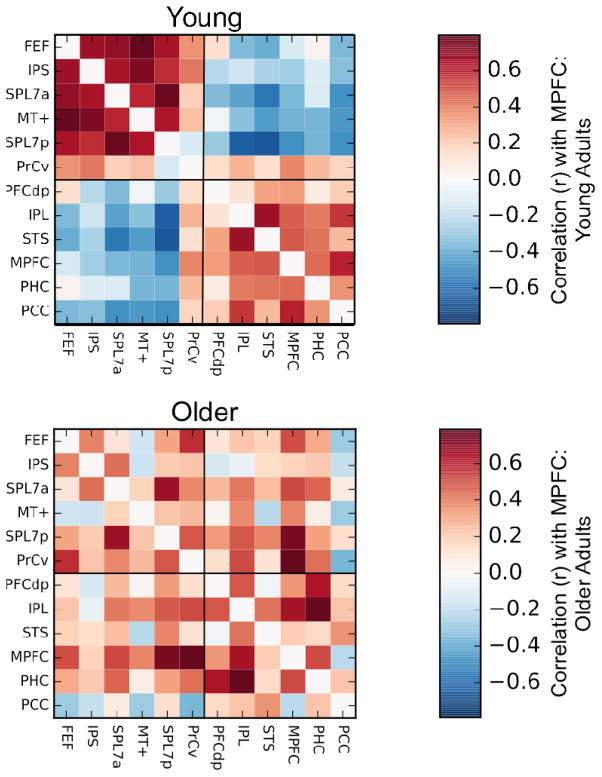
Figure 6.
Dedifferentiation of the default and dorsal attention networks in older adults. (A) GSR + Scrubbing. (B) ANATICOR. Contrasting the full correlation matrix of all ROIs of the default and dorsal attention networks for young vs. older adults demonstrates reduced (cool colors) within-network RSFC of both the default and dorsal attention networks, and increased (warm colors) between-network RSFC in older adults. Significant age (young vs. older) × differentiation (within- vs. between-network RSFC) interactions were observed for RSFC when collapsed across both networks (top), for each network individually (left), and for the majority of individual ROIs of both networks (right). The MTL ROI (PHC; yellow outline) showed reduced RSFC with all other ROIs across both networks in older relative to young adults. ** significant crossover interaction; * significant interaction. The findings are consistent across preprocessing procedures. See Table 1 for F-statistics, p-values, node labels, and node coordinates.
Discussion
Anticorrelation between low frequency BOLD signal fluctuations in the dorsal attention and default networks is a robust feature of the functional network architecture of the brain (Chai, Castanon, Ongur, & Whitfield-Gabrieli, 2012; Fox et al., 2005; Fox, Zhang, Snyder, & Raichle, 2009; Fransson, 2005; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008; Kundu et al., 2013). Here we were able to replicate this pattern in young adults during a task known to engage the default network, and during rest. Critically, we also demonstrated that anticorrelation between the dorsal attention and default networks is attenuated in older adults. This pattern is consistent across both task and resting-state conditions. During an autobiographical planning task, activity within the MPFC, a critical default network node, was positively correlated with other default network regions, and anticorrelated with regions of the dorsal attention network in younger adults. In older adults, task-related MPFC activity was functionally coupled with regions of both the default network, and key regions of the dorsal attention network, including FEF, MT+, IPS, SPL, and DLPFC (Figure 2).
RSFC analyses, which critically did not involve GSR, revealed an age-related pattern of reduced anticorrelation that closely overlapped with the task-based results. RSFC of MPFC and PCC seed regions revealed a more robust pattern of connectivity with other default network regions for younger versus older adults, consistent with previous reports (e.g., Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Further, default network regions showed reliable anticorrelations with dorsal attention regions, including FEF, PrCv, MT+, IPS, SPL, and DLPFC, in young. In contrast, older adults demonstrated greater correlations between default and dorsal attention network regions. Interregional RSFC between robustly validated network ROIs comprising the default and dorsal attention networks replicated this pattern. Age-related decreases within both the dorsal attention and default networks were observed in the context of increased between-network connectivity for older adults. These findings provide strong evidence, converging across both task and rest, that reduced anticorrelation between default and dorsal attention networks is a core feature of age-related functional brain change. Further, the MTL – a critical region known to show age-related declines in structure and function which may underlie age-related declines in memory (Grady, 2012) – had reduced intrinsic FC with both the dorsal attention and default networks in older adults, potentially reflecting a fractionation of the MTL memory system from other large-scale cortical networks in aging.
Age-related changes in functional network architecture
Although few studies have directly investigated age-related changes in network anticorrelations (see below), reductions in whole-brain functional segregation, or network modularity, have been reported (Betzel et al., 2014; Chan et al., 2014; Geerligs, Maurits, Renken, & Lorist, 2014; Geerligs et al., 2015; Meunier et al., 2009; Onoda & Yamaguchi, 2013; Song et al., 2014). Age-related changes have also been investigated within specific functional networks. Grady and colleagues (2016) showed that increased FC between the frontoparietal control and default networks predicted reduced connectivity within the default network, and better performance on cognitive measures. Spreng and Schacter (2012) reported reduced flexibility in the coupling of default and frontoparietal control network regions during shifts from internally- to externally-directed attention in older relative to young adults. With greater task challenge on a Tower of London planning task, greater lateral PFC coupling with the default network was observed in older adults (Turner & Spreng, 2015).
There is increasing evidence that altered network dynamics, whether at the level of the whole-brain, or among specific networks, is a central feature of brain aging. Yet few studies have investigated age-related changes in the pattern of anticorrelation between default and dorsal attention networks, one of the most robust features of the brain’s functional architecture in young adults. Increased correlations, and reduced anticorrelations have been reported for both whole brain (Betzel et al., 2014) and in more targeted analyses (Wu et al., 2011). However, these studies incorporated GSR as part of their RSFC data preprocessing, making it difficult to reliably interpret patterns of negative and positive correlations (Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012). Chan and colleagues (2014) observed increased FC between default and dorsal attention brain regions with increasing age, although they excluded negative correlations from their analyses owing to concerns with GSR. More recently, reduced anticorrelations, limited to regions of medial and lateral prefrontal cortex, were observed in older versus younger adults during rest (Keller et al., 2015). Greater anticorrelation between MPFC and lateral PFC brain regions was associated with better cognitive performance in young adults; however, no behavioral associations were observed in older adults leaving the question open as to whether changes to the anticorrelation dynamic indicate age-related cognitive decline.
Our results extend these earlier findings in three critical aspects. First, we demonstrate convergent patterns of reduced default-dorsal attention anticorrelations during both task and rest. Second, we show reduced anticorrelation during a task that is known to activate the default network similarly in both young and older adults, enabling us to distinguish age-related differences in connectivity in the context of matched levels of task-related activation, as opposed to in resting-state. Further, we show reduced anticorrelation during the resting-state without employing GSR, thereby avoiding the introduction of spurious negative correlations. Finally, we show a striking reduction of intrinsic FC of the MTL with both the dorsal attention and default networks.
Reduced anticorrelations during both task and rest
The patterns of age-related reductions in anticorrelation between dorsal attention and default networks observed during task and rest are strikingly consistent (see Figures 3 & 6). The correspondence across analysis methods provides strong support for our prediction that reduced anticorrelation between default and dorsal attention brain regions is a fundamental component of network dedifferentiation in aging (cf. Chan et al., 2014; Geerligs et al., 2015). Strong coherence in functional network architecture between task and rest has been reported previously (Smith et al., 2009; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013). From this perspective, age-related changes in the brain’s intrinsic network architecture, including the increased default and dorsal attention coupling observed here, may represent a continuous sculpting of these network interactions across the lifespan in response to, or perhaps leading to, lifespan changes in cognitive functioning (see Stevens & Spreng, 2014, for a review). Increased functional coupling between default and dorsal attention network regions in older adults may be attributable to sustained task effects, with older adults attending more to externally-presented stimuli during autobiographical planning. Reduced anticorrelation, or increased connectivity between the default and dorsal attention networks in older adulthood, is consistent with poor modulation of attentional processes and their neural substrates in response to shifting cognitive demands in older adults (Clapp, Rubens, Sabharwal, & Gazzaley, 2011; Turner & Spreng, 2012).
Reduced default-dorsal attention network anticorrelation during task
Task by age-group interactions have been widely observed within the dorsal attention network using fMRI (also referred to as the “task-positive” network – reviewed by Spreng, Wojtowicz, et al., 2010) and the default network (reviewed by Hafkemeijer et al., 2012). However, few studies have examined changes in default network activity during internally-oriented tasks such as the autobiographical task used here. In a previous report, we observed no age differences in the activation of the default network during autobiographical planning, although the design was sufficiently powered to detect differences in Tower of London planning task performance in the same sample (Spreng & Schacter, 2012, Figure 1). Here, we did observe an age-related difference in connectivity patterns between a key region of the default network, MPFC, and dorsal attention network regions during autobiographical planning (Figure 2). The autobiographical planning task enabled us to observe FC changes in older adults during a task known to engage the default network, thereby eliminating the potential confound of age-differences in task-based activations.
Reduced default-dorsal attention network anticorrelation during rest
Numerous prior studies have shown reduced connectivity within the default network during rest (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Grady, Grigg, & Ng, 2012; Hampson et al., 2012; Meier et al., 2012; Mevel et al., 2013; Onoda, Ishihara, & Yamaguchi, 2012; Sala-Llonch et al., 2012; Saverino, Grigg, Churchill, & Grady, 2015). As reviewed above, studies also report altered connectivity between default and other brain networks (Betzel et al., 2014; Chan et al., 2014; Geerligs et al., 2015; Keller et al., 2015; Meunier et al., 2009; Wu et al., 2011). Few studies have investigated anticorrelations, as negative correlations are often discarded from analysis due to confounds related to GSR (e.g. Chan et al., 2014). The biological meaning of anticorrelations is still unclear. In healthy younger adults, the magnitude of anticorrelation has been linked to individual differences in task performance (Hampson et al., 2010; Keller et al., 2015; Kelly et al., 2008). However, the behavioral implications of reduced anticorrelation for older adults remain unclear. Future work is required to elucidate the cognitive implications of the lifespan changes in interactions amongst large-scale functional brain networks (Onoda et al., 2012). Changes in the competitive relationship between the default and dorsal attention networks from younger to older adulthood, as we describe here, will need to be further explored in tandem with sufficient de-noising approaches (e.g. Chai et al., 2012; Jo et al., 2013; Kundu, Inati, Evans, Luh, & Bandettini, 2012) that allow for the valid interpretation of negative correlation differences between groups (Gotts et al., 2013; Saad et al., 2012). Alternate methods are emerging, which allow for an unconfounded assessment of anticorrelations, including anatomical CompCor (Behzadi, Restom, Liau, & Liu, 2007), temporal CompCor (Behzadi et al., 2007), or the median angle shift approach (He & Liu, 2012).
A striking finding of this study was the substantially reduced RSFC of the MTL with almost all critical nodes of both the dorsal attention and default networks. Previous work provides evidence that the MTL memory system may have a unique functional relationship with the default network: First, sub-regions of the MTL show dissociable patterns of FC with different cortical networks (Kahn et al., 2008; Vincent et al., 2006), which are differentially affected by aging (Das et al., 2013; Das et al., 2015). Second, using two different frequencies of transcranial magnetic stimulation to the same default network node in the IPL, Eldaief et al. (2011) demonstrated distinct changes in FC strength among default network nodes, such that low frequency stimulation modulates FC of IPL with the MTL but no other default network nodes, and conversely, high frequency simulation altered FC of the IPL with other default network nodes but not the MTL. These results demonstrate that at least two distinct sub-systems exist, dissociating the MTL from other default network regions. Finally, Ward et al. (2014) demonstrated that the MTL memory system is indirectly functionally connected to the default network via the posterior parahippocampal gyrus – the same MTL default network region investigated in the current study – demonstrating that this default network node may be the critical connection point linking the MTL memory system to the default network, a critical interaction required for episodic memory and prospection (Addis, Wong, & Schacter, 2007; Benoit & Schacter, 2015; Schacter, Addis, & Buckner, 2007). Whereas a previous study failed to find age-related differences in FC of the MTL sub-system of the default network, despite changes in all other default network subsystems (Campbell et al., 2013), here we find a robust decline in FC of the MTL with all other nodes of the default network investigated. Moreover, the age-related decline in FC was not limited to the default network, but was also apparent for FC with the dorsal attention network. Given the critical role of the posterior PHC in linking the MTL memory system with the default network, these results suggest a potential fractionation of the MTL memory system from other large-scale networks in typical non-pathological aging. This observation is consistent with a recent report of reduced MTL-cortical connectivity where the hippocampus becomes more functionally isolated (Salami, Pudas & Nyberg, 2014). Future research will be need to explore this intriguing possibility further, including FC with other brain networks as well.
Conclusion
The functional network architecture of the human brain undergoes significant changes across the adult lifespan, transitioning from a highly modular structure to a less segregated, or dedifferentiated, network architecture. A central feature of functional segregation in younger adults is a robust pattern of anticorrelated brain activity between dorsal attention and default network brain regions. Yet little was known about how this anticorrelated pattern of brain activity might change with age. Here, we demonstrate that anticorrelations between default and dorsal attention networks are reduced in older adults. Further, this pattern is highly consistent both during task performance and at rest. As anticorrelation between these networks is associated with cognitive function in young, mapping the trajectory of age-related changes in anticorrelations between dorsal attention and default networks may provide an important marker of age-related changes to cognition. Finally, our results suggest a fractionation of the MTL memory system from other large-scale brain networks, which may underlie episodic memory decline, a hallmark of both typical healthy and pathological neurocognitive aging.
Supplementary Material
Highlights.
Anticorrelation is positive within and negative between network correlations
The default and dorsal attention networks are anticorrelated in the young
In both task and rest, anticorrelation is reduced in healthy older adults
Connectivity of MTL with default and dorsal attention networks is reduced in aging
Acknowledgments
This work was supported by NIA AG008441 and NIMH MH060941 grants to DLS and an Alzheimer’s Association grant (NIRG-14-320049) to R. N. S. We thank Gary Turner for comments on the manuscript as well as Gagan Wig and Adrian Gilmore for assistance with data collection.
Footnotes
Disclosure Statement: The authors report no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–457. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goni J, Zuo XN, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(Pt 2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 2013;5:73. doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111(46):E4997–5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108(17):7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Orozco S, Dickerson BC, et al. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus. 2013;23(1):1–6. doi: 10.1002/hipo.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Yushkevich PA, Wolk DA. Anterior and posterior MTL networks in aging and MCI. Neurobiol Aging. 2015;36(Suppl 1):S141–150. S150 e141. doi: 10.1016/j.neurobiolaging.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 2014;35(1):319–330. doi: 10.1002/hbm.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb Cortex. 2015;25(7):1987–1999. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46(2):540–553. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiology of Aging. 2016;41:159–172. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Grady CL, Grigg O, Ng C. Age differences in default and reward networks during processing of personally relevant information. Neuropsychologia. 2012;50(7):1682–1697. doi: 10.1016/j.neuropsychologia.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochim Biophys Acta. 2012;1822(3):431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28(8):1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Shen X, Scheinost D, Papademetris X, Constable RT. Intrinsic brain connectivity related to age in young and middle aged adults. PLoS One. 2012;7(9):e44067. doi: 10.1371/journal.pone.0044067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Liu TT. A geometric view of global signal confounds in resting-state functional MRI. Neuroimage. 2012;59(3):2339–2348. doi: 10.1016/j.neuroimage.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Pelak VS, Cordes D. Aberrant default mode network in subjects with amnestic mild cognitive impairment using resting-state functional MRI. Magn Reson Imaging. 2012;30(1):48–61. doi: 10.1016/j.mri.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. Journal of Applied Mathematics, 2013 / 935154. 2013 doi: 10.1155/2013/935154. (935154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52(2):571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(1):129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JB, Hedden T, Thompson TW, Anteraper SA, Gabrieli JD, Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U S A. 2013;110(40):16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60(3):1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7(5–6):523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- Meier TB, Desphande AS, Vergun S, Nair VA, Song J, Biswal BB, et al. Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage. 2012;60(1):601–613. doi: 10.1016/j.neuroimage.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44(3):715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, La Joie R, Villain N, Mézenge F, Perrotin A, Eustache F, Desgranges B, Chételat G. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol Aging. 2013;34(4):1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Mevel K, Chételat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer’s disease. Int J Alzheimers Dis. 2011:535816. doi: 10.4061/2011/535816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24(11):2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Onoda K, Yamaguchi S. Small-worldness and modularity of the resting-state functional brain network decrease with aging. Neurosci Lett. 2013;556:104–108. doi: 10.1016/j.neulet.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Arenaza-Urquijo EM, Valls-Pedret C, Vidal-Pineiro D, Bargallo N, Junque C, et al. Dynamic functional reorganizations and relationship with working memory performance in healthy aging. Front Hum Neurosci. 2012;6:152. doi: 10.3389/fnhum.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci U S A. 2014;111(49):17654–17659. doi: 10.1073/pnas.1410233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverino C, Grigg O, Churchill NW, Grady CL. Age differences in the default network at rest and the relation to self-referential processing. Soc Cogn Affect Neurosci. 2015;10(2):231–239. doi: 10.1093/scan/nsu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, et al. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 2014;4(9):662–676. doi: 10.1089/brain.2014.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default Network Modulation and Large-Scale Network Interactivity in Healthy Young and Old Adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34(8):1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Hasher L, Chiew KS, Grady CL. A neural mechanism underlying memory failure in older adults. J Neurosci. 2008;28(48):12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Spreng RN. Resting-state functional connectivity MRI reveals active processes central to cognition. Wiley Interdiscip Rev Cogn Sci. 2014;5(2):233–245. doi: 10.1002/wcs.1275. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Tessler MH, Peng CS, Martin A. Functional connectivity constrains the category-related organization of human ventral occipitotemporal cortex. Hum Brain Mapp. 2015;36(6):2187–2206. doi: 10.1002/hbm.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol Aging. 2012;33(4):826 e821–813. doi: 10.1016/j.neurobiolaging.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Prefrontal Engagement and Reduced Default Network Suppression Co-occur and Are Dynamically Coupled in Older Adults: The Default-Executive Coupling Hypothesis of Aging. J Cogn Neurosci. 2015;27(12):2462–2476. doi: 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35(3):1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wu JT, Wu HZ, Yan CG, Chen WX, Zhang HY, He Y, et al. Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci Lett. 2011;504(1):62–67. doi: 10.1016/j.neulet.2011.08.059. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.