Abstract
Advances in hematopoietic cell transplantation (HCT) techniques and supportive care strategies have led to dramatic improvements in relapse mortality in patients with high-risk hematological malignancies. These improvements, however, conversely increase the risk of late-occurring non-cancer competing causes, mostly cardiovascular disease (CVD). HCT recipients have a significantly increased risk of CVD-specific mortality, including elevated incidence of coronary artery disease (CAD), cerebrovascular disease, and heart failure (HF) compared to age-matched counterparts. Accordingly, there is an urgent need to identify techniques for the detection of early CVD in HCT patients to inform early prevention strategies. Aerobic training (AT) is established as the cornerstone of primary and secondary disease prevention in multiple clinical settings, and may confer similar benefits in HCT patients at high-risk of CVD. The potential benefits of AT either before, immediately after, or in the months / years following HCT have received limited attention. Here, we discuss the risk and extent of CVD in adult HCT patients, highlight novel tools for early detection of CVD, and review existing evidence in oncology and non-oncology populations supporting the efficacy of AT to attenuate HCT-induced CVD. This knowledge can be utilized to optimize treatment, while minimizing CVD risk in individuals with hematological malignancies undergoing HCT.
Keywords: cardiovascular disease, exercise, detection, hematopoietic stem cell transplantation
1.0 Introduction
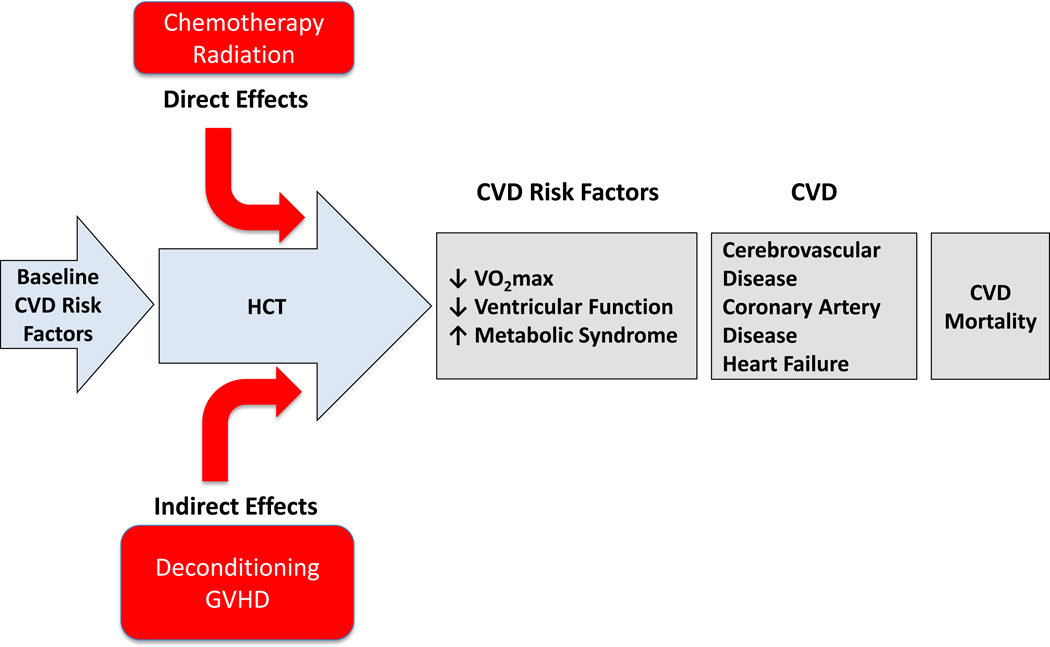
More than 60,000 individuals are expected to undergo allogeneic or autologous hematopoietic cell transplantation (HCT) annually worldwide for treatment of hematological malignancies.1 Advances in transplantation techniques and supportive care strategies have dramatically improved cancer specific survival rates in the past 30 years; 10-year survival rates now exceed 80% following HCT.1,2 However, with prolonged survival, the risk of treatment-induced late-occurring morbidity and mortality from competing (non-relapse mortality; NRM) causes has substantially increased. Specifically, in comparison with age-sex-matched counterparts from non-oncology settings, HCT recipients have a 2.3 to 4.0-fold increased risk of cardiovascular-specific mortality, a 0.6 to 5.6-fold increased risk of cardiovascular disease (CVD) including coronary artery disease (CAD), cerebrovascular disease, and heart failure (HF), and a 7.0 to 15.9-fold increased risk of CVD risk factors such as hypertension, diabetes, and dyslipidemia.3–11 This excess CVD risk23–26 is likely a consequence of acute direct (i.e., direct cytotoxic/radiation-induced injury) as well as indirect (i.e., impacts secondary to therapy such as deconditioning) effects of HCT therapy.12 A research agenda that comprehensively and systematically tackles the issues related to CVD prevalence, pathogenesis, detection, and treatment in HCT recipients is urgently required.
Current cardiovascular screening and monitoring guidelines for post-HCT adult survivors recommend yearly cardiovascular risk factor screening, with assessment of global cardiac function (left ventricular ejection fraction, LVEF), and resting electrocardiography (ECG) in patients at high-risk for cardiovascular complications.13 However, HCT-specific recommendations are based on retrospective studies that have identified cardiovascular complications in long-term survivors rather than optimal screening strategies developed by US Preventative Services Taskforce for the general population.13,14 Moreover, assessment of resting LVEF and ECG in high risk patients may fail to detect early signs of alterations in cardiovascular morphology, function, and coronary artery narrowing,15,16 suggesting that complementary stratification tools are required to fully evaluate CVD risk and identify those individuals at highest risk of future events.
Interventions that prevent and/or treat CVD risk factors and CVD in HCT patients will be of the utmost importance to mitigate CVD-specific mortality. In particular, an approach taking into account four intervention time points is needed:16 (1) primordial prevention (prophylactic therapy given before or during HCT to prevent anticipated injury), (2) primary prevention (therapy provided to selected patients with early signs of myocardial and/or coronary vascular damage to treat injury and prevent progression), (3) secondary prevention (therapy provided after the detection of LVEF decline or coronary artery calcification to treat impairment), and (4) tertiary treatment (therapy provided after detection of HF or CAD clinical symptoms). Aerobic training (AT) is established as the cornerstone of disease prevention and treatment in multiple clinical settings,17 and is well documented to improve insulin sensitivity, decrease lipids, and lower blood pressure with concomitant improvements in cardiovascular function and overall mortality in non-oncology settings.18–21 Similarly, promising data in the oncology setting indicates that AT is safe and is associated with significant improvements in CVD risk factors.22,23 AT may confer similar benefits in HCT patients at high risk of CVD; however, the potential cardioprotective properties of AT in the context of HCT have received limited attention.
Here, we briefly discuss the risk and extent of CVD in adult HCT recipients, highlight novel tools for early detection of CVD, and review existing evidence in oncology and non-oncology populations supporting the potential role of AT as a viable therapeutic modality to abate/attenuate HCT-associated CVD.
2.0 Accelerated CVD Following HCT: Current Evidence
For a comprehensive overview of CVD risk factors and CVD in HCT patients, the reader is referred to prior excellent reviews;4,15,24 a summary of CVD following HCT is provided in Table 1. In the following sections we briefly review the incidence of CVD risk factors, CVD, and CVD-specific mortality.
Table 1.
Incidence of CVD risk factors and overt CVD following HCT.
| Outcome | Incidence |
|---|---|
| CVD Risk Factors | |
| Hypertension | 28%–74%7,10,137,61,27,138 |
| Dyslipidemia | 33%–58%7,10,26,137,23 |
| Diabetes | 10%–41%7,10,26,137,23 |
| Obesity | 20–44%26,61,23 |
| Low exercise tolerance | 100%125 |
| Decreased LVEF | 5%–43%,139,140 |
| Overt CVD | |
| Arrhythmia | 2%–13%7,10,137 |
| Stroke | 0.2%–4.8%7,10,26,137,61 |
| Transient ischemic attack | 0.3%4 |
| Myocardial ischemia | 1%–6%7,10,77,137 |
| Heart failure | 1% to 9%3–5,61 |
2.1 Prevalence of CVD Risk Factors
The third National Cholesterol Education Program Adult Treatment Panel III (ATP III) report indicates that the age-adjusted prevalence of CVD risk factors such as hypertension, diabetes, and dyslipidemia in US adults is approximately 22%.25 Importantly, the same level and extent of CVD risk factor prevalence occurs at much earlier age following HCT. In a study that assessed the 10-year cumulative incidence, Armenian et al.7 found the prevalence of hypertension, diabetes, and dyslipidemia was 43.0%, 18.7%, and 48.3% respectively, in 1087 HCT recipients (median age of HCT: 44 years) compared to 34.6%, 8.5%, and 40.0% in the general population. Furthermore, Chow et al.26 found that compared to pre-HCT, use of antihypertensives and diabetes medications was significantly higher 1-year post-HCT (6.7% vs. 19.6% and 4.1% vs. 12.9%, respectively) in 1,379 HCT recipients (median age at time of HCT: 40 years), while Blaser et al.27 reported that a mean of two years following HCT, 73.4% and 72.5% had hypercholesterolemia and hypertryglyceridemia respectively in 761 HCT survivors (median age at transplantation: 49 years). These findings indicate there is a characteristic pattern of changes in CVD risk profiles early after HCT,26 which persist for up to 10 years.7 Importantly, there is no reason to expect that these rates will improve over time, likely making patients more susceptible to normal pathologies of aging.
2.2 Prevalence of CVD and CVD-Related Mortality
In the non-oncology setting, the presence of one or more comorbidities such as hypertension, diabetes, and dyslipidemia increases the risk of CVD by 29% to 67%.28,29 Thus, the heightened prevalence of CVD risk factors in HCT survivors likely increases risk of developing CVD. Indeed, in a cohort of 1244 HCT survivors (median age at HCT: 45; median follow-up 5 years), Armenian and colleagues8 reported that HCT survivors treated with high-dose anthracyclines are at a nearly 5-fold risk of HF when compared to age- and sex-matched individuals from the general population. The risk of HF increased substantially in patients with hypertension (OR: 35.3) or diabetes (OR: 26.8).8 The risk of CAD is up to 40% higher in HCT patients compared to matched counterparts.30–32 For example, Chow et al.26 examined the risk of developing CAD in 1379 HCT survivors (median age at HCT: 40 years; median follow up: 7 years); patients with hypertension or diabetes had 3.6-fold and 2.8-fold higher risk, respectively. Importantly, there appears to be premature onset of CVD in HCT survivors. Tichelli and colleagues33 reported that the median age of first CVD event (cerebral, coronary, or peripheral ischemic event) was 49 years in 265 HCT patients (median age of HCT: 27 years); almost 20 years earlier than the first CVD event reported in the general population from the Framingham Heart Study (67 years).28,34 A larger, multicenter study in 548 HCT patients (median age of HCT: 27 years; median follow-up: 9 years) also reported premature development of overt CVD (cerebral, coronary, or peripheral ischemic event) after HCT; the median age of the first CVD event was 54 years.30 Accordingly, evidence suggests that not only is there is a greater magnitude of CVD in HCT patients, but also that the occurrence of CVD occurs earlier.
Not surprisingly, the risk of premature CVD-related mortality is significantly higher in HCT recipients.5,35 The Bone Marrow Transplant Survivor Study35,36 evaluated mortality in 1479 HCT patients (median age at HCT: 26 years; median follow-up: 10 years); results indicated that there is a 2–4-fold increased risk of CVD death among HCT survivors compared with the general population. Chow et al.5 examined CVD-related mortality in 1491 HCT recipients (median age at HCT: 41 years), and found that transplant recipients experienced nearly a 4-fold increased risk of CVD death (adjusted incidence rate difference, 3.6 per 1000 person-years [95% CI, 1.7 to 5.5]) compared with an age-sex-matched population cohort. Together, these findings provide mounting credence to the notion of HCT as a model of ‘accelerated CVD phenotype’ and provide compelling rationale to examine HCT-related CVD sequelae.
3.0 Pathogenesis of HCT-Induced Accelerated CVD
3.1 ‘Direct’ Cardiovascular Injury
Cardiovascular injury may occur during treatment of primary malignancy (anthracycline or radiation exposure prior to relapse or during primary remission), or during HCT-associated therapeutic exposures (total-body irradiation (TBI) and/or high dose alkylating exposures to obtain immuno- and myelosuppression and to create space in the marrow to allow engraftment of transplanted cells).36 Radiation and/or chemotherapy causes direct cardiovascular injury contributing to the manifestation of two distinct but related forms of CVD morbidity and mortality: cardiomyopathy associated with HF, and CAD.37
3.1.1 Therapy-related HF
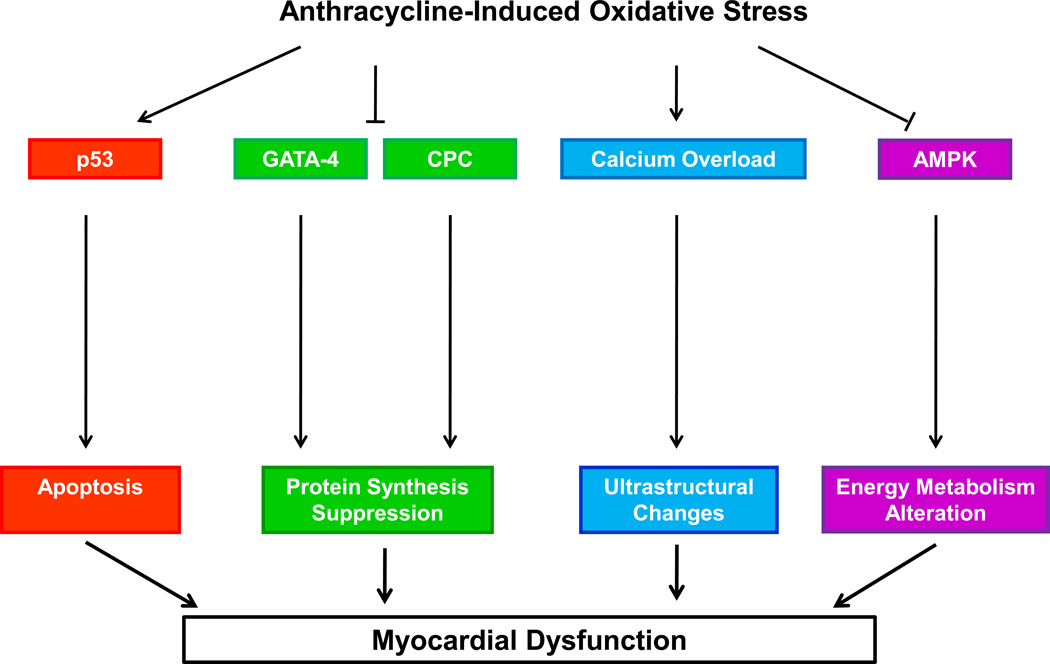
We have previously summarized the potential mechanisms underlying chemotherapy-induced cardiovascular alterations (Figure 1A; modified from38–40). In brief, anthracycline-induced generation of reactive oxygen species (ROS) are a primary contributor to cardiotoxicity via activation of multiple pathways including: the tumor suppressor protein, p53,41,42 and suppression of sarcomere protein synthesis through depletion of GATA-4 dependent gene expression43,44 and cardiac progenitor cells (CPCs).45,46 The resulting cardiac myocyte apoptosis ultimately contributes to impairments in LV systolic (contractile) and diastolic (lusitropic) function,47–49 and elevated afterload (increased wall stress).50
Figure 1. Mechanisms underlying ‘direct’ cardiovascular hits.
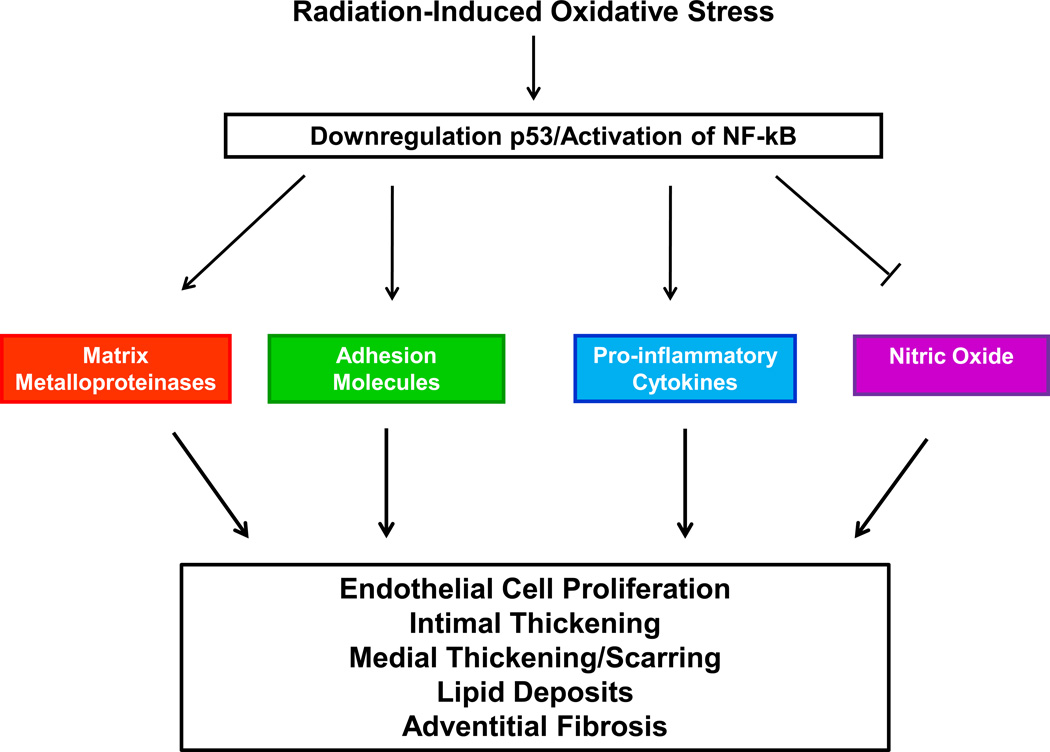
A) Anthracycline-induced generation of ROS is a central mediator of: 1) accelerated myofilament apoptosis via upregulation of p53 pathway, 2) suppression of myofilament protein synthesis via inhibition of CPCs and GATA-4, 3) alterations in cardiac energy metabolism via downregulation of AMPK, 4) ultrastructural changes to myocytes via calcium overload. These changes lead to myocardial and vascular dysfunction. B) Radiation-induced vascular injury occurs via downregulation of endothelial cell-specific p53/activation of nuclear transcription factor NF-κB, which ultimately up-regulates matrix metalloproteinases, adhesion molecules, pro-inflammatory cytokines, while inactivating vasculoprotective nitric oxide. Eventually, coronary vascular injury characterized by endothelial cell proliferation, intimal thickening, medial scarring, lipid deposits and adventitial fibrosis may occur. ROS, reactive oxygen species; mitogen activated protein kinases, MAPK; cardiac progenitor cells, CPCs; AMP-activated protein kinase, AMPK.
3.1.2 Therapy-related CAD
Oxidative stress and up regulation of pro-inflammatory molecules51,52 are key pathways thought to contribute to radiation-induced CAD (Figure 1B).53,54 Evidence suggests that activation of the nuclear transcription factor NF-κB55 or downregulation of endothelial cell-specific p5356 induce oxidative stress and chronic inflammation, which ultimately up-regulates numerous pathways pertinent to vascular disease, including matrix metalloproteinases, adhesion molecules, pro-inflammatory cytokines, while inactivating vasculoprotective nitric oxide.57–59 Eventually, coronary vascular injury characterized by endothelial cell proliferation, intimal thickening, medial scarring, lipid deposits and adventitial fibrosis may occur.60–63
3.2 ‘Indirect’ Cardiovascular Injury
Direct insults may occur in conjunction with indirect injury resulting from pre-existing CVD risk factors at diagnosis, lifestyle modifications during and following HCT, and HCT-specific complications such as graft versus host disease (GVHD) and type of transplantation (autologous vs. allogenic). Indeed, the incidence of pre-HCT comorbidities such as hypertension, diabetes, and hyperlipidemia are reported to be as high as 25%,11 5%,11 32%,27 respectively. Pre-HCT CVD risk profile is, in turn, a strong predictor of post-HCT CVD risk, with ≥2 of the following factors: obesity, dyslipidemia, hypertension, and diabetes associated with a 5.2-fold increased risk of CAD or cerebrovascular disease.10
3.2.1 Lifestyle Modifications
Acute and chronic alterations in lifestyle (e.g., deconditioning, weight gain) also contribute to increased CVD risk. Immediately following HCT, patients undergo ≥30 days of inpatient bed rest. The impact of acute inpatient bed rest on CVD sequelae has not been examined in HCT patients; however, the changes in cardiovascular structure and function during short- and long-term bed rest have been under investigation for many years. A landmark 1966 study by Saltin and colleagues64 found that 20 days of bed rest in healthy young males caused a 27% decrease in peak oxygen consumption (VO2peak), a 25% decrease in stroke volume, a 7% decrease in left ventricular mass, and a 20% increase in resting heart rate. Remarkably, a 30-year follow-up of subjects previously studied in 1966 established that 3 weeks of bed rest at 20 years of age had a more profound impact on VO2peak than did 3 decades of aging.65 Importantly, VO2peak is inversely correlated with cardiovascular and all-cause mortality in a broad range of adult populations,66–69 while Cooney et al.70 found that among 10,519 men and 11,334 women followed in a Finish population-based study, a 15 beat increase in resting heart rate was associated with a 24% and 32% increase in future cardiovascular death in men and women, respectively. Other acute disuse-induced cardiovascular alterations include significant declines in left ventricular systolic and diastolic function,71–73 marked conduit artery wall thickening,74 and endothelial dysfunction.75 Studies characterizing both the acute cardiovascular effects of bed rest and the long-term consequences of disuse-induced changes in cardiovascular structure and function are now required in HCT patients.
Epidemiological evidence indicates that long-term physical inactivity increases the relative risk of CAD, stroke, and hypertension, by 45%, 60%, and 30%, respectively.76 Initial evidence suggests that physical inactivity may also contribute to CVD risk in HCT patients. Tichelli and colleagues30 reported that among 548 HCT survivors, patients with an arterial event (cerebral, coronary, or peripheral ischemia) were more often sedentary (75% vs. 44%). Chow et al.77 compared 2362 HCT survivors (median age, 55.9 years; median 10.8 years since HCT) with a general population sample (National Health and Nutrition Examination Survey [NHANES]; n = 1192), and found that HCT survivors with CVD were less likely to be currently physically active (ORs, 1.7 to 3.1).
3.2.2 HCT-specific Complications
Graft versus host disease (GVHD) has been hypothesized to contribute to increased CVD risk, where an allo-immune response results in a sequential influx of lymphocytes, macrophages and neutrophils.78 Such an inflammatory environment promotes plaque instability, ultimately resulting in plaque rupture, thrombus formation, and infarction.79 Indeed, biomarkers of endothelial injury such as von Willebrand Factor (vWF) show a close relation to chronic GVHD, suggesting that an immunological mechanism may result in chronic endothelial dysfunction and accelerated atherosclerosis.80
After accounting for effects of active chronic GVHD, allogeneic HCT survivors appear to have an increased risk of developing hypertension, dyslipidemia, and diabetes which predispose towards more serious CVD compared with other cancer survivors or the general population.3,30,33 For example, Armenian and colleagues7 examined the incidence and predictors of CVD risk factors and subsequent CVD in 1885 1+year survivors of HCT (median age, 44.4 years; median 5.9 years since HCT), and reported that allogeneic HCT recipients were at a significantly higher risk of hypertension, diabetes, and dyslipidemia compared with autologous HCT recipients. Furthermore, in 1491 HCT recipients (median age at HCT: 41 years), Chow et al.5 found that although most outcomes did not markedly differ between patients who received allogeneic versus autologous grafts, the hazard of hypertension was increased after allogeneic HCT (HR,1.8 [CI, 1.3 to 2.5]). Although the mechanisms underlying HCT allogenic-specific CVD have not yet been well described, certain generalizations can be extrapolated from solid organ transplant recipients where immunosuppressive agents (including glucocorticoids, calcineurin inhibitors, and sirolimus) are well-known to contribute to CVD pathogenesis.81 82–84 For instance, dyslipidemia has been reported in up to 80% of solid-organ transplantation patients on immunosuppressive agents, and insulin resistance and hypertension are frequently encountered side-effects of immunosuppressive medications.7,11,85
3.3 ‘Multiple-Hit’ Hypothesis
As HCT patients progress through treatment regimens, they are subjected to multiple cardiovascular insults coupled with lifestyle perturbations that collectively leave patients with significantly elevated risk of CVD risk factors, overt CVD, and ultimately, CVD-related mortality (Figure 2). There is currently limited data available to support the contention of the “multiple hit” in HCT patients. Future, large-scale, prospective studies are urgently required to comprehensively evaluate CVD sequelae associated with HCT therapy.
Figure 2. Model of accelerated CVD phenotype.
At diagnosis, a significant proportion of HCT patients present with pre-existing or heightened CVD risk factors, which increase the risk of therapy-associated cardiovascular injury. Independently, total-body irradiation and/or high dose chemotherapy are associated with direct adverse effects on the cardiovascular system. These direct effects occur in the context of concomitant lifestyle perturbations (indirect effects: deconditioning, GVHD). Collectively, these direct and indirect insults enhance susceptibility to CVD risk factors, CVD, and premature CVD mortality. CVD, cardiovascular disease; HCT, hematopoietic cell transplantation; GVHD, graft versus host disease.
4.0 CVD Detection
The assumption behind cardiovascular screening is that detection of subclinical disease would result in interventions that may delay or even prevent the onset of clinically apparent disease; however, this notion has not been rigorously investigated in HCT recipients. Current HCT-specific recommendations for yearly fasting lipid and blood sugar assessment in all HCT patients, and evaluation of global cardiac function (LVEF) and ECG in symptomatic patients,13 are based on retrospective studies that have identified CVD risk factors and overt CVD in long-term survivors rather than screening strategies developed by US Preventative Services Taskforce for the general population.13,14 Accordingly, current HCT screening tools may fail to detect early signs of CVD pathogenesis when interventions would be most beneficial.15 Early identification of patients at high-risk of HCT-induced CVD may optimize long-term overall survival after HCT. To this end, novel assessment techniques incorporating exercise testing, blood and imaging markers could enable the detection of early CVD, and thus provide unique insight into both the type and timing of subsequent interventional approaches. For example, reduced strain and strain rate revealed impaired myocardial function prior to LVEF decline86,87 and heart failure symptoms 88 in patients treated with anthracycline-containing therapy. Optimal timing for subclinical cardiac assessments in HCT recipients remains undetermined, but emerging evidence suggests it has a potential role for predicting therapy-related CVD that merits further investigation. Indeed, in the contexts of CAD, HF, and diabetes, identification of cardiovascular phenotypes has initiated research into individualization and optimization of exercise training programs based on morphology and function 89–93 – concepts that could be readily applied in the HCT setting. Here, we briefly review evidence detailing novel methods of CVD detection.
4.1 Imaging-Based Approaches
4.1.1 Echocardiography
The use of more sensitive and specific echocardiographic techniques to evaluate LV function may address the current limitations of conventional cardiac imaging techniques employed in the oncology setting for detection of HF. Specifically, speckle tracking assessment of systolic function with strain and strain rate is more sensitive for detecting altered myocardial performance beyond LVEF94 and, in the case of strain rate, less susceptible to alterations in loading conditions compared to LVEF.95 In the clinical setting, reduced strain and strain rate revealed impaired myocardial function prior to LVEF decline86,87,96 and HF symptoms88 in conventionally treated cancer patients treated with anthracycline-containing therapy. Changes in LV torsion have also been shown to improve CVD risk prediction in several cardiac patient populations.97–99 Novel indices of diastolic function such as tissue Doppler imaging (TDI), flow propagation velocity (Vp), and diastolic strain and strain rate measures may also provide early markers of alterations in cardiac function. In anthracycline-induced cardiotoxicity, changes in diastolic function have been shown to precede systolic dysfunction.100–102 Optimal echocardiography assessment techniques remain undetermined in HCT patients; studies are needed to specifically define the potential role of novel echocardiography techniques for predicting therapy-related HF.
4.1.2 Computer Tomographic (CT)-based Imaging
In non-oncology populations, CT-based imaging (coronary artery calcium scoring, CT angiography [CTA]) has emerged as an accurate, non-invasive measure of CAD risk. Over the last decade, multiple retrospective cohort studies have demonstrated the strong independent prognostic value of coronary artery calcium in predicting CAD events.103 As a result, the recent American College of Cardiology Foundation/ American College of Cardiology Guidelines for assessment of cardiovascular risk in asymptomatic individuals contain an indication for evaluation of coronary artery calcium.103 The extent of coronary artery calcification provides valuable prognostic information regarding CAD risk; however, significant atherosclerosis may be present in the absence of calcium.79 Accordingly, CTA allows a more direct, yet still non-invasive, measurement of total plaque in the coronary arteries.104 Each of the 17 coronary segments are visually assessed and classified on the basis of stenosis severity, and each plaque is classified as calcified, non-calcified, or mixed. CTA therefore enables visualization of ulcerated lesions as well as accurate assessment of plaque morphology. There is a paucity of studies evaluating the utility of coronary artery calcium or CTA in assessment of coronary atherosclerosis in cancer survivors at high risk for CAD. In the only study to date that evaluated a CT-based approach to CAD detection, Jain and colleagues105 examined coronary calcium scoring with concomitant CTA in 20 (median age at study: 46 years; median follow-up: 6 years) HCT recipients. CAD was detected in 4 of 15 (26.6%) patients who would be considered ‘low risk’ by conventional Framingham Risk Score stratification;105 highlighting how conventional risk classification algorithms may not be adequate or appropriate for HCT patients. Large-scale prospective investigations are clearly required to examine the clinical use of these screening strategies in HCT recipients.
4.2 Blood-Based Approaches
4.2.1 Biomarkers
Biomarkers that individually or in aggregate predict risk of CVD could aid in developing targeted prevention strategies during the preclinical phase of CVD, when intervention may be more likely to alter disease progression. The advent of highly sensitive assays has made detection of extremely low concentrations of biomarkers possible, and provides prognostic information above and beyond that provided by traditional risk factors (Table 2).106 For example, a transient rise in cardiac troponin I has been demonstrated to predict the occurrence 107 as well as the magnitude of LVEF decline 108–110 in patients with hematologic and solid malignancies. The role of conventionally used CVD biomarkers in the HCT setting has yet to be determined.
Table 2.
CVD Specific Biomarkers.
| Cardiac Markers | Definition | CVD Outcomes |
|---|---|---|
| Ultra-sensitive cardiac troponin (hsTnI) |
Marker of proteolysis and turnover of myocardial contractile proteins.141 |
Associated with all-cause and cardiovascular mortality.106 |
| Soluble ST2 (sST2) | Marker of myocardial stress and myocyte stretch.142 |
Associated with the risk of CVD events 142 |
| Growth differentiation factor- 15 (GDF-15) |
Marker of myocardial143,144 and vascular145–147 inflammation and tissue injury. |
Associated with the risk of CVD events.106,148,149 |
| N-terminal-pro-B-type natriuretic peptide (NT- proBNP) |
Marker of cardiovascular remodeling.150 | Associated with the risk of CVD events and death.151 |
| High-sensitivity C-reactive protein (hsCRP) |
Marker of inflammation.79 | Associated with the risk of CVD events.152 |
| Homocysteine | Marker of oxidative stress and inflammation.153 |
Associated with endothelial dysfunction, atherosclerosis113,115,154,155 |
A single marker may not provide sufficient biological information for an accurate assessment of cardiac and vascular damage.111 In fact, several studies have reported that multiple biomarkers are superior to individual biomarkers in predicting subclinical and clinical CVD.112–115 Wang et al.115 measured 10 biomarkers in 3209 Framingham Heart Study participants and noted that persons with “multimarker” scores (based on regression coefficients of significant biomarkers) in the highest quintile as compared with those with scores in the lowest two quintiles had elevated risks of death (adjusted hazard ratio, 4.08; P<0.001) and major CVD events (adjusted hazard ratio, 1.84; P=0.02). In a subsequent study from the Framingham Heart Study (n=3428), a panel of high sensitivity biomarkers including soluble ST2 (sST2), growth differentiation factor-15 (GDF-15), and ultra-sensitive cardiac troponin (hsTnI), individuals with multimarker scores in the highest quartile had an elevated risk of future CVD events.106 Integration of comprehensive biomarkers may identify risk factors before the onset of overt disease, and thereby potentially lead to earlier and more accurate identification of HCT patients at high CVD risk.
4.2.2 High-Throughput ‘Omics’ – Metabolomics
Metabolites are closely linked to cellular and whole-body phenotypes, thus providing “proximal reports” of cellular states. Metabolomics, the systematic analysis of metabolites, has been established as a clinical diagnostic tool that can predict future diabetes,116 chronic kidney disease, and CVD.112,117–120 for example, Wang et al.97 performed a nested case-control study of 188 individuals in the Framingham Heart Study who developed diabetes and 188 propensity-matched controls, and found that individuals with the metabolite 2-aminoadipic acid concentration in the top quartile had a 4-fold higher risk of developing diabetes over a 12-year follow-up period compared with those in the lowest quartile (adjusted OR: 4.49, 95% CI, 1.86 to 10.89). To determine whether a metabolite score was related to functional consequences of CVD, Lewis and colleagues121 examined the relationship of a metabolomic amino acid score to exercise-induced myocardial ischemia in 166 subjects referred for diagnostic exercise stress testing. Of great interest, compared with the lowest quartile of the amino acid score, the top quartile of the score was associated with a nearly 5-fold risk (adjusted OR: 1.47–16.09) of inducible myocardial ischemia.122 Future studies will need to evaluate the predictive value of metabolomics for prediction of CVD in HCT survivors. Ultimately, integration of comprehensive biomarkers with metabolomic profiling may be an innovative way to unravel the etiology and pathophysiology of HCT therapy-induced CVD.
4.3 Exercise-Based Approaches
4.3.1 Incremental Exercise Testing
Resting cardiac function, in contrast to exercise-based measures such as cardiopulmonary exercise testing (CPET), does not provide assessment of the integrative nature of cardiovascular function, assess cardiovascular reserve, or reliably predict VO2peak. Our group has shown that CPET is a safe and feasible tool to provide an objective assessment of cardiovascular reserve and VO2peak in select cancer populations.68,69,123,124 In addition, these studies demonstrate that cancer patients have significant and marked reductions in VO2peak and submaximal [e.g., ventilatory threshold (VT), minute ventilation – carbon dioxide production relationship (VE/VCO2), oxygen uptake efficiency slope (OUES)] measures of exercise capacity across the entire survivorship continuum.69,123,124 Of particular importance, we explored the utility and prognostic value of CPET prior to allogeneic HCT in 21 patients (mean age 44 years) with high risk hematological malignancies. After 25 months of follow-up, CPET-derived peak and sub-maximal measures were strong independent predictors of NRM.125 Wood et al.126 also evaluated CPET in 29 patients (mean age 55 years) prior to HCT and found that patients with pre-HCT VO2peak <16 mL/kg/min had higher risk of mortality post HCT (HR: 9.1). CVD-specific mortality was not evaluated in these two preliminary studies; however, there is now strong rationale for further investigations into the utility of CPET to improve CVD risk stratification in HCT patients.
5.0 Aerobic Training to Attenuate HCT-induced Cardiovascular Disease
There is a wealth of observational data demonstrating that higher exposure to exercise is associated with substantial decreased incidence of CVD mortality in non-oncology settings.23,127–129 For example, in 44,452 men from the Health Professional Study, increased metabolic equivalent tasks (METs) was associated with a 42% risk reduction (RR, 0.58; 95% CI, 0.44–0.77) of myocardial infarction,130 while Mora et al.,131 in a prospective study of 27,055 women, found that the adjusted CVD rate ratio was 41% in the least active group compared with the most active group. Evidence from adult survivors of childhood cancer with a history of HCT indicates that weekly exercise time is also associated with decreased CVD risk. Specifically, Jones et al.132 examined the association between exercise exposure (MET hours/week) and risk of major CVD events in adults survivors of childhood Hodgkin lymphoma (n = 1,187; median age, 31.2 years, median follow up, 11.9 years). Compared with survivors reporting 0 MET hours/week, the adjusted rate ratio for any CVD event was 0.47 (95% CI, 0.23 to 0.95) for those in the highest exercise exposure quartile.132 Additionally, adherence to national exercise guidelines was associated with a 51% lower risk of any CVD event in comparison with not meeting the guidelines (< 9 MET hours/week).132 The above findings indicate that adoption of regular exercise consistent with national vigorous exercise recommendations could confer substantial cardiovascular benefits in HCT recipients. However, there are significant limitations -- such as reverse causality -- associated with observational studies. Indeed, it is not possible to delineate whether higher levels of exercise simply reflect lower CVD burden as opposed to a direct exercise-induced effect. Phase 2 trials wherein the dose of exercise is carefully quantified are required to inform the design of confirmatory randomized controlled trials (RCTs).
To examine the current evidence outlining the effects of AT on CVD sequelae in the HCT setting, we searched PubMed using the following MeSH terms and text words: hematological, malignancies, stem cell transplantation, exercise, exercise therapy, exercise training, aerobic training, exercise capacity, cardiorespiratory fitness, VO2peak, cardiac, CAD, CVD, CVD risk factors, myocardial infarction, stroke, HF, LV dysfunction, heart rate (HR), LVEF, LV mass, LV end diastolic volume, LV end systolic volume, hypertension, blood pressure, echocardiography, systolic function, diastolic function, body weight, body fat, vascular, endothelial function, biomarkers, tumor necrosis factor alpha (TNF-α), cholesterol, triglyceride, C- reactive protein (CRP), insulin, glucose, leptin, c peptide, interleukin-6 (IL-6), and hemoglobin. RCTs or single-arm (pre-post) of structured exercise training involving adults (≥18 years of age) with hematological malignancies undergoing HCT were included. Studies with a participant mean age <18 years, not written in English, review articles, and animal studies were excluded.
Study characteristics are presented in Table 3. In brief, studies consisted of 9 (82%) RCTs and 2 (18%) single-arm studies including a total of 820 patients (n=430, exercise training; n=390, usual care; n=479 male; n=313 female). Ten studies excluded patients with pre-existing documented CVD; one study did not report exclusion criteria. In terms of baseline (pre-intervention) CVD risk factor profile, only 1 study reported history of hypertension (prevalence of 29%) and hypercholesterolemia (prevalence of 30%); no studies reported history of type II diabetes. In general, exercise prescriptions followed the standard exercise guidelines for healthy individuals: 3–5 days per week for ≥30 min per session for moderate-intensity exercise or 3 days per week for ≥20 min per session for vigorous-intensity exercise.114,115 Eight studies (73%) prescribed intensity based on estimated peak HR or perceived exertion, while all studies (100%) used a conventional (linear) approach to exercise prescription which maintains a static intensity, frequency, and duration after an initial lead in period.133
Table 3.
Summary of Exercise Interventions Aimed at Attenuating HCT-Induced CVD.
| Author | N | Cohort/Design/Setting | Exercise | Outcomes |
|---|---|---|---|---|
| Battaglini et al. (2009)156 | 10 | Acute leukemia/intervention during treatment |
30min/d; 3d/wk; 40–50% estimated HRR; 3–5 weeks |
Total minutes on bicycle ergometer at 60% HRR: ↑ 88% Body weight: ↓ 4% |
| Coleman et al. (2003)157 | 14 | Multiple myeloma/RCT during treatment |
60min/d; 3d/wk; 12–15 Borg scale; 22 wks |
6-Minute Walk Test: ↓ 2% in AT; ↓ 2% in control |
| Coleman et al. (2008)135 | 60 | Multiple myeloma/RCT during treatment |
20min/d; 3d/wk; 11–13 Borg scale; 15 wks |
Hemoglobin: ↓ 7% in AT; ↓ 10% in control |
| Coleman et al. (2012)158 | 95 | Multiple myeloma/RCT during treatment |
30min/d; 5d/wk; 11–13 Borg scale; 15 wks |
Hemoglobin: ↓ 6% in AT; ↓ 5% in control |
| Courneya et al. (2009)159 | 60 | Lymphoma/RCT during treatment |
15–45min/d; 3d/wk; 60–75% peak power output; 12 wks |
Body weight: ↓ 0.4% in AT; ↓ 0.6% in control |
| Courneya et al. (2009)134 | 60 | Lymphoma/RCT during treatment |
15–45min/d; 3d/wk; 60–75% peak power output; 12 wks |
Measured VO2peak: ↑ 19% in AT; ↓ 1% in control |
|
Groeneveldt et al. (2013)160 |
28 | Multiple myeloma/Intervention post treatment |
15–30min/d; 3d/wk; 50–60% HRR; 24 wks |
Measured VO2peak: ↑ 1% |
| Jarden et al. (2009)161 | 21 | Allogeneic HCT/ RCT during treatment |
15–30min/d; 5d/wk; 45–75% estimated max HR; 4–6 wks |
Estimated VO2peak: ↑ 0.01% in AT; ↓ 28% in control |
| Oechsle et al. (2014)162 | 24 | Myeloablative chemotherapy/ RCT during treatment |
10–40min/d; 5d/wk; intensity NR; 4 wks |
Estimated VO2peak: ↑ 11% in AT; ↓ 26% in control |
| Shelton et al. (2009)163 | 30 | Allogeneic HCT/ RCT post treatment |
20–30min/d; 3d/wk; 60–75% estimated max HR; 4 wks |
6-Minute Walk Test: ↑ 12% in AT; ↑ 10% in control |
| Streckmann et al. (2014)164 | 28 | Lymphoma/RCT during treatment |
60min/d; 2d/wk; 60–80% estimated max HR; 36 wks |
Incremental step test: ↑ in AT; ↓ in control (values NR) |
Abbreviations: HCT, hematopoietic cell transplantation; HRR, heart rate reserve; RCT, randomized controlled trial; AT; aerobic training; HR; heart rate; NR, not reported.
Reported cardiovascular end points included estimated (n=5; 45%) or measured (n=2; 18%) VO2peak, body weight (n=2; 18%), and hemoglobin (n=2; 18%). Overall, findings indicate that AT has beneficial cardiovascular effects during and following HCT. For example Courneya et al.134 found that following a 12 week intervention during therapy, VO2peak increased 19% in AT patients, compared to a 1% decrease in sedentary controls, while Coleman and colleagues135 demonstrated that AT attenuated a decrease in hemoglobin compared to controls. These preliminary investigations indicate that AT is a promising strategy to prevent and/or treat HCT-induced CVD; however, further high quality research is clearly required. A more personalized approach incorporating the principles of training may be required for optimal mitigation of CVD-related morbidity and mortality in HCT patients, including individualization, specificity, progressive overload, and rest and recovery.133 An essential prerequisite in the design of all exercise training trials is the objective assessment of patients’ VO2peak and peak heart rate, as well as their submaximal cardiopulmonary responses to exercise, thus permitting precise tailoring of training to the individual patient. These individualized approaches are currently being used in an ongoing trial in breast cancer patients;136 future investigations should examine the efficacy of personalized AT in HCT patients. To this end, adequately powered multicenter RCTs with appropriate CVD endpoints are required to evaluate the efficacy of AT to prevent/treat HCT-induced CVD.
6.0 Conclusions
CVD is a frequent and devastating adverse complication of HCT leading to morbidity, poor quality of life, and premature mortality. As reviewed here, there is evidence indicating that HCT patients are subjected to direct and indirect cardiovascular injury that collectively leave patients with an increased prevalence of CVD risk factors, overt CVD, and CVD-related mortality. It is important to stress that the current evidence base is emergent with a small number of studies; many areas of HCT-induced CVD remain to be defined and addressed. A summary of future investigations needed in the HCT setting is provided in Table 4. To this end, we propose that in combination with continual advancements in anticancer therapy, expansion of screening and surveillance of CVD with multimodal techniques is required. Additionally, preliminary evidence indicates that AT may abate HCT-induced cardiovascular injury. These findings provide a sound rationale to test the efficacy of a new exercise paradigm that focuses on a personalized medicine approach to optimize health and longevity in the HCT setting by preventing and/or attenuating HCT-associated CVD.
Table 4.
Future Directions in HCT Research.
Underlying mechanisms of HCT-induced CVD
|
Detection of HCT-induced CVD
|
Clinical importance of HCT-induced CVD
|
Prevention/Management of HCT-induced CVD
|
Evaluation of HCT-induced injury to other organ systems
|
Acknowledgments
Funding / Support: LWJ is supported by research grants from the National Cancer Institute and from AKTIV Against Cancer Foundation.
Role of the Funding Source
Study sponsors had no involvement in the writing of the manuscript or in the decision to submit the manuscript for publication.
Biographies
Jessica Scott, PhD, is a Senior Scientist in the Exercise Physiology and Countermeasures Laboratory at NASA Johnson Space Center with expertise in cardiovascular ultrasound and exercise countermeasures in astronauts and cancer patients.
Lee Jones, PhD, the director of the Cardio-Oncology Program at Memorial Sloan Kettering Cancer Center (MSKCC), is a cardio-oncology and exercise-oncology specialist and leads numerous investigations examining the effects of exercise on cardiovascular health in cancer patients.
Saro Armenian, D.O., M.P.H., is a pediatric oncologist with expertise in pediatric cancer, epidemiology, and cancer survivorship, an associate professor in the Departments of Pediatrics and Population Sciences, and the Director of the Childhood Cancer Survivorship Program at City of Hope.
Sergio Giralt, MD, is the Chief of Bone Marrow Transplantation Services at MSKCC with over 20 years’ experience in hematology-oncology research and patient care.
Javid Moslehi, MD, is a cardiologist with expertise in therapy-induced injury to the heart in cancer patients. He is the Director of the Cardio-Oncology Program at Vanderbilt University.
Thomas Wang, MD, is a cardiologist with an extensive background in the use of biomarkers and metabolomics for disease risk prediction, and is the Chief of the Division of Cardiovascular Medicine at Vanderbilt University.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 3.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone marrow transplantation. 2012;47(5):619–625. doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 5.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. British journal of haematology. 2008;142(1):11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 7.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118(23):6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118(5):1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(8):1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116(8):1197–1204. doi: 10.1182/blood-2010-03-276576. [DOI] [PubMed] [Google Scholar]
- 12.Jones LW, Haykowsky M, Swartz J, Douglas PD, Mackey JR. Early breast cancer and cardiovascular injury. J Am Coll Cardiol. doi: 10.1016/j.jacc.2007.06.037. in press. [DOI] [PubMed] [Google Scholar]
- 13.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Hematology/oncology and stem cell therapy. 2012;5(1):1–30. doi: 10.5144/1658-3876.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Armenian SH, Chow EJ. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120(4):469–479. doi: 10.1002/cncr.28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122(12):1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 18.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbs S, Hollriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3(4):486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 20.Eisele JC, Schaefer IM, Randel Nyengaard J, et al. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol. 2008;103(1):12–21. doi: 10.1007/s00395-007-0684-x. [DOI] [PubMed] [Google Scholar]
- 21.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol. 2008;294(2):H928–H935. doi: 10.1152/ajpheart.01231.2007. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 23.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of cancer survivorship : research and practice. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 24.Baker KS, Armenian S, Bhatia S. Long-term consequences of hematopoietic stem cell transplantation: current state of the science. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(1 Suppl):S90–S96. doi: 10.1016/j.bbmt.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 26.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(6):794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaser BW, Kim HT, Alyea EP, 3rd, et al. Hyperlipidemia and statin use after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(4):575–583. doi: 10.1016/j.bbmt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Wilson PW, Larson MG, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94(1):20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 30.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 31.Peres E, Levine JE, Khaled YA, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone marrow transplantation. 2010;45(1):149–152. doi: 10.1038/bmt.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo XD, Xu LP, Liu DH, et al. Heart failure after allogeneic hematopoietic stem cell transplantation. Int J Cardiol. 2013;167(6):2502–2506. doi: 10.1016/j.ijcard.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aristei C, Tabilio A. Total-body irradiation in the conditioning regimens for autologous stem cell transplantation in lymphoproliferative diseases. Oncologist. 1999;4(5):386–397. [PubMed] [Google Scholar]
- 37.Nishimoto M, Nakamae H, Koh H, et al. Risk factors affecting cardiac left-ventricular hypertrophy and systolic and diastolic function in the chronic phase of allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2013;48(4):581–586. doi: 10.1038/bmt.2012.179. [DOI] [PubMed] [Google Scholar]
- 38.Scott JM, Lakoski S, Mackey JR, Douglas PS, Haykowsky MJ, Jones LW. The potential role of aerobic exercise to modulate cardiotoxicity of molecularly targeted cancer therapeutics. Oncologist. 2013;18(2):221–231. doi: 10.1634/theoncologist.2012-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JM, Koelwyn GJ, Hornsby WE, et al. Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin Oncol. 2013;40(2):218–228. doi: 10.1053/j.seminoncol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation. 2011;124(5):642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim CC, Zuppinger C, Guo X, et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279(9):8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 42.Vedam K, Nishijima Y, Druhan LJ, et al. Role of heat shock factor-1 activation in the doxorubicin-induced heart failure in mice. Am J Physiol Heart Circ Physiol. 2010;298(6):H1832–H1841. doi: 10.1152/ajpheart.01047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Ma AG, Kitta K, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Molecular pharmacology. 2003;63(2):368–377. doi: 10.1124/mol.63.2.368. [DOI] [PubMed] [Google Scholar]
- 44.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101(18):6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C, Zhang X, Ramil JM, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121(5):675–683. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Angelis A, Piegari E, Cappetta D, et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121(2):276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keung EC, Toll L, Ellis M, Jensen RA. L-type cardiac calcium channels in doxorubicin cardiomyopathy in rats morphological, biochemical, and functional correlations. J Clin Invest. 1991;87(6):2108–2113. doi: 10.1172/JCI115241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeki K, Obi I, Ogiku N, Shigekawa M, Imagawa T, Matsumoto T. Doxorubicin directly binds to the cardiac-type ryanodine receptor. Life Sci. 2002;70(20):2377–2389. doi: 10.1016/s0024-3205(02)01524-2. [DOI] [PubMed] [Google Scholar]
- 49.Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):114–121. doi: 10.1007/s12012-007-0005-5. [DOI] [PubMed] [Google Scholar]
- 50.Chaosuwannakit N, D’Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28(1):166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 52.Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275(43):33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 53.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67(1):10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 54.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. The British journal of radiology. 2007;80(1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 55.Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol. 2010;55(12):1227–1236. doi: 10.1016/j.jacc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 56.Lee CL, Moding EJ, Cuneo KC, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Science signaling. 2012;5(234):ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematology/oncology clinics of North America. 2008;22(2):305–318. vii. doi: 10.1016/j.hoc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35(10):612–623. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, Parry T. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. Journal of physiology and biochemistry. 2012 doi: 10.1007/s13105-012-0200-0. [DOI] [PubMed] [Google Scholar]
- 61.Matsuura C, Brunini TM, Carvalho LC, et al. Exercise training in doxorubicin-induced heart failure: effects on the L-arginine-NO pathway and vascular reactivity. J Am Soc Hypertens. 2010;4(1):7–13. doi: 10.1016/j.jash.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Duquaine D, Hirsch GA, Chakrabarti A, et al. Rapid-onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8(2):101–107. doi: 10.1191/1358863x03vm476oa. [DOI] [PubMed] [Google Scholar]
- 63.Kalabova H, Melichar B, Ungermann L, et al. Intima-media thickness, myocardial perfusion and laboratory risk factors of atherosclerosis in patients with breast cancer treated with anthracycline-based chemotherapy. Med Oncol. 2011;28(4):1281–1287. doi: 10.1007/s12032-010-9593-1. [DOI] [PubMed] [Google Scholar]
- 64.Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(5 Suppl):VII1–VII78. [PubMed] [Google Scholar]
- 65.McGuire DK, Levine BD, Williamson JW, et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation. 2001;104(12):1350–1357. [PubMed] [Google Scholar]
- 66.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 67.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 68.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159(4):612–619. e613. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 71.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest : “cardiovascular deconditioning” or hypovolemia? Circulation. 2001;103(14):1851–1857. doi: 10.1161/01.cir.103.14.1851. [DOI] [PubMed] [Google Scholar]
- 72.Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol (1985) 2010;108(5):1177–1186. doi: 10.1152/japplphysiol.01408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96(2):517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 74.van Duijnhoven NT, Green DJ, Felsenberg D, Belavy DL, Hopman MT, Thijssen DH. Impact of bed rest on conduit artery remodeling: effect of exercise countermeasures. Hypertension. 2010;56(2):240–246. doi: 10.1161/HYPERTENSIONAHA.110.152868. [DOI] [PubMed] [Google Scholar]
- 75.Demiot C, Dignat-George F, Fortrat JO, et al. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol. 2007;293(5):H3159–H3164. doi: 10.1152/ajpheart.00591.2007. [DOI] [PubMed] [Google Scholar]
- 76.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiological genomics. 2007;28(2):146–157. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 77.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32(3):191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nieder ML, McDonald GB, Kida A, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(11):1573–1584. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 80.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marini BL, Choi SW, Byersdorfer CA, Cronin S, Frame DG. Treatment of dyslipidemia in allogeneic hematopoietic stem cell transplant patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(5):809–820. doi: 10.1016/j.bbmt.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tichelli A, Gratwohl A. Vascular endothelium as ‘novel’ target of graft-versus-host disease. Best practice & research. Clinical haematology. 2008;21(2):139–148. doi: 10.1016/j.beha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Biedermann BC. Vascular endothelium and graft-versus-host disease. Best practice & research. Clinical haematology. 2008;21(2):129–138. doi: 10.1016/j.beha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359(9323):2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 85.Miller LW. Cardiovascular toxicities of immunosuppressive agents. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(9):807–818. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 86.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158(2):294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 87.Jurcut R, Wildiers H, Ganame J, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21(12):1283–1289. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Mercuro G, Cadeddu C, Piras A, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007;12(9):1124–1133. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 89.Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64(6):541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. J Appl Physiol (1985) 2014 doi: 10.1152/japplphysiol.00518.2014. jap 00518 02014. [DOI] [PubMed] [Google Scholar]
- 91.Warburton DE, McKenzie DC, Haykowsky MJ, et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95(9):1080–1084. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Fang F, Yip GW, et al. Quantification of left ventricular performance in different heart failure phenotypes by comprehensive ergometry stress echocardiography. Int J Cardiol. 2013;169(4):311–315. doi: 10.1016/j.ijcard.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49(24):2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 94.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101(12):1766–1771. doi: 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 95.Weidemann F, Jamal F, Sutherland GR, et al. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283(2):H792–H799. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 96.Ganame J, Claus P, Uyttebroeck A, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20(12):1351–1358. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Hansen DE, Daughters GT, 2nd, Alderman EL, Stinson EB, Baldwin JC, Miller DC. Effect of acute human cardiac allograft rejection on left ventricular systolic torsion and diastolic recoil measured by intramyocardial markers. Circulation. 1987;76(5):998–1008. doi: 10.1161/01.cir.76.5.998. [DOI] [PubMed] [Google Scholar]
- 98.Haberka M, Liszka J, Kozyra A, Finik M, Gasior Z. Two-dimensional speckle tracking echocardiography prognostic parameters in patients after acute myocardial infarction. Echocardiography. 2015;32(3):454–460. doi: 10.1111/echo.12666. [DOI] [PubMed] [Google Scholar]
- 99.Spinelli L, Morisco C, Assante di Panzillo E, Izzo R, Trimarco B. Reverse left ventricular remodeling after acute myocardial infarction: the prognostic impact of left ventricular global torsion. The international journal of cardiovascular imaging. 2013;29(4):787–795. doi: 10.1007/s10554-012-0159-5. [DOI] [PubMed] [Google Scholar]
- 100.Garot J, Derumeaux GA, Monin JL, et al. Quantitative systolic and diastolic transmyocardial velocity gradients assessed by M-mode colour Doppler tissue imaging as reliable indicators of regional left ventricular function after acute myocardial infarction. Eur Heart J. 1999;20(8):593–603. doi: 10.1053/euhj.1998.1335. [DOI] [PubMed] [Google Scholar]
- 101.Nagy AC, Cserep Z, Tolnay E, Nagykalnai T, Forster T. Early Diagnosis of Chemotherapy-induced Cardiomyopathy: a Prospective Tissue Doppler Imaging Study. Pathol Oncol Res. 2008;14(1):69–77. doi: 10.1007/s12253-008-9013-4. [DOI] [PubMed] [Google Scholar]
- 102.Tassan-Mangina S, Codorean D, Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7(2):141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 103.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. Jacc. 2011;4(5):537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Jain NA, Chen MY, Shanbhag S, et al. Contrast enhanced cardiac CT reveals coronary artery disease in 45% of asymptomatic allo-SCT long-term survivors. Bone marrow transplantation. 2014;49(3):451–452. doi: 10.1038/bmt.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126(13):1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 108.Cardinale D, Sandri MT, Martinoni A, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13(5):710–715. doi: 10.1093/annonc/mdf170. [DOI] [PubMed] [Google Scholar]
- 109.Sandri MT, Cardinale D, Zorzino L, et al. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin Chem. 2003;49(2):248–252. doi: 10.1373/49.2.248. [DOI] [PubMed] [Google Scholar]
- 110.Auner HW, Tinchon C, Linkesch W, et al. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol. 2003;82(4):218–222. doi: 10.1007/s00277-003-0615-3. [DOI] [PubMed] [Google Scholar]
- 111.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10):1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 112.Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. Journal of the American Society of Nephrology : JASN. 2013;24(8):1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim HC, Greenland P, Rossouw JE, et al. Multimarker prediction of coronary heart disease risk: the Women’s Health Initiative. J Am Coll Cardiol. 2010;55(19):2080–2091. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 114.Lieb W, Larson MG, Benjamin EJ, et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 2009;119(1):37–43. doi: 10.1161/CIRCULATIONAHA.108.816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 116.Wang TJ, Ngo D, Psychogios N, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123(10):4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ge Y, Wang TJ. Circulating, imaging, and genetic biomarkers in cardiovascular risk prediction. Trends in cardiovascular medicine. 2011;21(4):105–112. doi: 10.1016/j.tcm.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110–1120. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Science translational medicine. 2010;2(33):33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Magnusson M, Lewis GD, Ericson U, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ruden E, Reardon DA, Coan AD, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29(21):2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelsey CR, Scott JM, Lane A, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone marrow transplantation. 2014 doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wood WA, Deal AM, Reeve BB, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone marrow transplantation. 2013;48(10):1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 127.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 131.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. Journal of cachexia, sarcopenia and muscle. 2015;6(2):115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 135.Coleman EA, Coon SK, Kennedy RL, et al. Effects of exercise in combination with epoetin alfa during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncology nursing forum. 2008;35(3):E53–E61. doi: 10.1188/08.ONF.E53-E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones LW, Douglas PS, Eves ND, et al. Rationale and design of the Exercise Intensity Trial (EXCITE): A randomized trial comparing the effects of moderate versus moderate to high-intensity aerobic training in women with operable breast cancer. BMC Cancer. 2010;10:531. doi: 10.1186/1471-2407-10-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26(34):5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2009;43(1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hertenstein B, Stefanic M, Schmeiser T, et al. Cardiac toxicity of bone marrow transplantation: predictive value of cardiologic evaluation before transplant. J Clin Oncol. 1994;12(5):998–1004. doi: 10.1200/JCO.1994.12.5.998. [DOI] [PubMed] [Google Scholar]
- 140.Fujimaki K, Maruta A, Yoshida M, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone marrow transplantation. 2001;27(3):307–310. doi: 10.1038/sj.bmt.1702783. [DOI] [PubMed] [Google Scholar]
- 141.Wang TJ. Significance of circulating troponins in heart failure: if these walls could talk. Circulation. 2007;116(11):1217–1220. doi: 10.1161/CIRCULATIONAHA.107.721845. [DOI] [PubMed] [Google Scholar]
- 142.Weinberg EO, Shimpo M, De Keulenaer GW, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 144.Bonaca MP, Morrow DA, Braunwald E, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2011;31(1):203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 145.Bermudez B, Lopez S, Pacheco YM, et al. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008;79(2):294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 146.Ding Q, Mracek T, Gonzalez-Muniesa P, et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150(4):1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 147.Schlittenhardt D, Schober A, Strelau J, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell and tissue research. 2004;318(2):325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 148.Xanthakis V, Enserro DM, Murabito JM, et al. Ideal Cardiovascular Health: Associations with Biomarkers and Subclinical Disease, and Impact on Incidence of Cardiovascular Disease in the Framingham Offspring Study. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 149.Lind L, Wallentin L, Kempf T, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30(19):2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 150.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 151.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 152.Eapen DJ, Manocha P, Patel RS, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62(4):329–337. doi: 10.1016/j.jacc.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 154.Levy D, Hwang SJ, Kayalar A, et al. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation. 2007;115(24):3079–3085. doi: 10.1161/CIRCULATIONAHA.106.652842. [DOI] [PubMed] [Google Scholar]
- 155.McDermott MM, Liu K, Ferrucci L, et al. Circulating blood markers and functional impairment in peripheral arterial disease. Journal of the American Geriatrics Society. 2008;56(8):1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Battaglini CL, Hackney AC, Garcia R, Groff D, Evans E, Shea T. The effects of an exercise program in leukemia patients. Integr Cancer Ther. 2009;8(2):130–138. doi: 10.1177/1534735409334266. [DOI] [PubMed] [Google Scholar]
- 157.Coleman EA, Coon S, Hall-Barrow J, Richards K, Gaylor D, Stewart B. Feasibility of exercise during treatment for multiple myeloma. Cancer nursing. 2003;26(5):410–419. doi: 10.1097/00002820-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 158.Coleman EA, Goodwin JA, Kennedy R, et al. Effects of exercise on fatigue, sleep, and performance: a randomized trial. Oncology nursing forum. 2012;39(5):468–477. doi: 10.1188/12.ONF.468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Courneya KS, Sellar CM, Stevinson C, et al. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2600–2607. doi: 10.1158/1055-9965.EPI-09-0504. [DOI] [PubMed] [Google Scholar]
- 160.Groeneveldt L, Mein G, Garrod R, et al. A mixed exercise training programme is feasible and safe and may improve quality of life and muscle strength in multiple myeloma survivors. BMC Cancer. 2013;13:31. doi: 10.1186/1471-2407-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone marrow transplantation. 2009;43(9):725–737. doi: 10.1038/bmt.2009.27. [DOI] [PubMed] [Google Scholar]
- 162.Oechsle K, Aslan Z, Suesse Y, Jensen W, Bokemeyer C, de Wit M. Multimodal exercise training during myeloablative chemotherapy: a prospective randomized pilot trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(1):63–69. doi: 10.1007/s00520-013-1927-z. [DOI] [PubMed] [Google Scholar]
- 163.Shelton ML, Lee JQ, Morris GS, et al. A randomized control trial of a supervised versus a self-directed exercise program for allogeneic stem cell transplant patients. Psycho-oncology. 2009;18(4):353–359. doi: 10.1002/pon.1505. [DOI] [PubMed] [Google Scholar]
- 164.Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25(2):493–499. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 165.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(12):1261–1269. doi: 10.1016/j.bbmt.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(5):560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 168.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23(27):6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 169.Kovalszki A, Schumaker GL, Klein A, Terrin N, White AC. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone marrow transplantation. 2008;41(11):965–969. doi: 10.1038/bmt.2008.15. [DOI] [PubMed] [Google Scholar]
- 170.Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]