Abstract
Warfarin was the only oral anticoagulant available for the treatment of venous thromboembolism for about half a century until the recent approval of novel oral agents dabigatran, rivoraxaban and apixaban. This presents new classes of medications less cumbersome to use. They do not require frequent laboratory monitoring or have nurmerous drug interactions. On the other hand it also poses a challenge to the physicians deciding which agent to use in specific patient populations, how to predict the bleeding risk compared to warfarin and between the different novel agents and how to manage bleeding with relatively recent discovery of few potential antidotes. This review summarizes the major trials that led to the approval of these agents and their exclusion criteria helping physicians understand which patient types might not benefit from these agents. It provides clinical pearls invaluable in everyday practice such as transitioning between traditional and novel anticoagulants, dose adjustments for high risk populations, drug interactions and cost analysis. Futhermore, the review provides direct comparisons with warfarin and indirect comparisons among the novel agents in terms of efficacy and bleeding risk narrating the numbers of patients with intracranial, gastrointestinal and fatal hemorrhages in each of the major trials. We hope that this review will help the physicians inform their patients about the benefits and risks of these agents and enable them to make an informed selection of the most appropriate anticoagulant.
Keywords: Novel anticoagulants, dabigatran, rivaroxaban, apixaban, bleeding, hemorrhagic complications, warfarin, deep venous thrombosis (DVT), pulmonary embolism (PE), venous thromboembolism (VTE), vitamin K antagonist (VKA), idarucizumab, andexanet alfa
INTRODUCTION
Venous thromboembolism (VTE) is the leading cause of vascular death after cerebral vascular disease and myocardial infarction with a significant annual incidence of 1-2 adults per 1000 population [1]. The mortality from reported cases of acute DVT and PE is 9.0% and 30.1% in the first 3 months respectively [2]. Without adequate treatment, upto 50% of the patients with DVT may develop post thrombotic syndrome and upto 4% of the patient with PE may develop chronic pulmonary hypertension [3]. Therefore, this serious condition requires anticoagulation with reliable efficacy like heparin and warfarin. Heparin and fondaparinux (indirect factor Xa inhibitor) are FDA approved for VTE treatment and prophylaxis but they are dosed parenterally which limits their use to hospital setting or early outpatient period until the slower acting warfarin or other vitamin K antagonists (VKA) take effect.
VKA therapy poses significant challenges both for the treating physician and the patient requiring repetitive laboratory testing, frequent dose adjustments and numerous drug- drug and food interactions. In the EINSTEIN PE trial comparing rivaroxaban with warfarin in the treatment of pulmonary embolism, patients reported rivaroxaban treatment as less cumbersome when compared to VKA [4]. In spite of the ACCP guidelines recommending home INR monitoring over outpatient laboratory INR testing in motivated patients (ACCP Grade 2B) [5], warfarin use remains laborious. The ACCP 2012 guidelines were published before the novel anticoagulants received FDA approval for treatment of VTE. Recent landmark trials have led to the emergence of novel oral anticoagulants that can potentially replace VKA for the treatment and prophylaxis of VTE. The physicians of this era are still learning the properties of these medications, learning their pros and cons and assessing the management options for serious bleeding episodes. This review is dedicated to the trials leading to the FDA approval for these agents, their pharmokinetic properties, dosing regimens, dose adjustments in high risk populations, cost analysis, efficacy and bleeding risk assessment. At the end, there is a summary of the available treatment options for hemorrhagic complications with these agents.
Major Hemorrhage
Before discussing the landmark trials of VTE treatment, it is imperative to understand the meaning of major and non major clinically relevant bleeding. Major bleeding in non surgical patients in these trials of novel anticoagulants is defined by the International Society of Thrombosis and Haemostasis using the following criteria [6]:
Fatal bleeding
Symptomatic bleeding at a critical location (eg intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular, compartment syndrome)
Bleeding resulting in a fall in hemoglobin of atleast 2 g/dl or requiring a transfusion of atleast two units of packed red cells or whole blood
However the meaning of clinically relevant bleeding that does not satisfy the criteria for major bleeding used in these trials is less clearly defined. It includes non major hemorrhage which is considered clinically significant by the treating physician. It may require medical intervention, interruption of anticoagulation therapy or complete discontinuation. Examples include epistaxis or gingival bleeding lasting for more than 5 minutes, hematuria persisting for greater than 24 hours post procedure, melena, hemoptysis with more than a few drops of blood and spontaneus hematoma larger than 25 cm2 [1].
Rivaroxaban
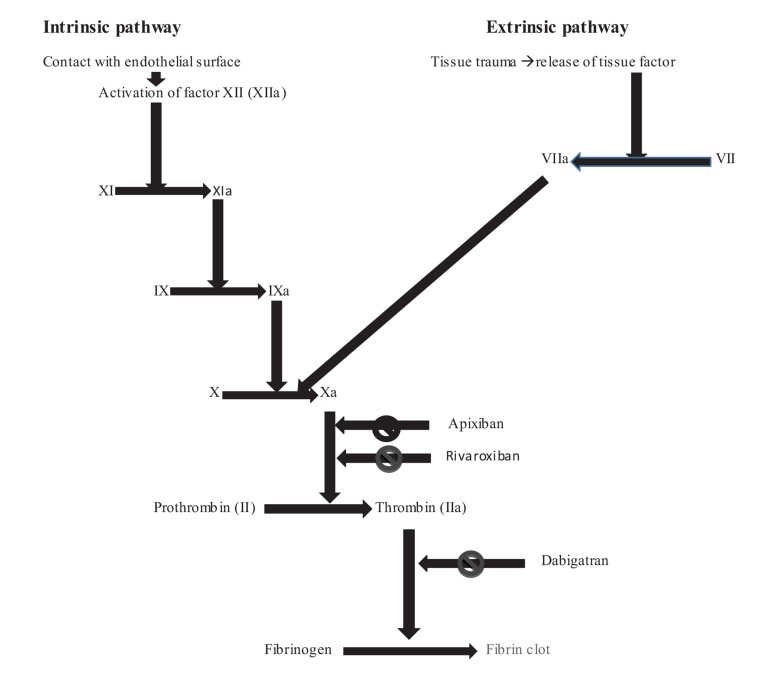
It is the first oral novel anticoagulant approved for the treatment of VTE approximatey 60 years after the approval of warfarin. It is a direct factor Xa inhibitor. The mechanism of action of novel anticoagulants is depicted in Fig. 1 [7-9]. The pharmacokinetic properties of novel oral anticoagulants and drug interactions are described in Table 1 [10-12].
Fig. (1).
Mechanism of action of novel anticoagulants on the coagulation cascade [7-9].
Rivaroxaban received FDA approval for prophylaxis of VTE after knee and hip arthroplasty in July 2011. It was approved for stroke prevention in atrial fibrillation in November 2011. Rivaroxaban received FDA approval for treatment of VTE in November 2012 due to the landmark EINTSTEIN trials undertaken and published in 2010 [1].
The EINSTEIN trials were a set of 3 trials comprising of 8282 patients designed to compare rivaroxaban to standard treatment (enoxaparin 1mg/kg twice daily followed by a VKA either wafarin or acenocoumarol) in patients with VTE over a period of upto one year. Rivaroxaban was found to be non inferior to standard therapy in efficacy with similar bleeding risk negating the need for initial heparinization and laboratory monitoring during treatment [1, 13]. However, it had a lower risk of major bleeding than standard therapy in the pooled analysis (1.0% vs 1.7%, hazard ratio 0.54; 95% CI 0.37- 0.79; p= 0.002) [13]. This is likely due to the inclusion of EINSTEIN- PE in the pooled analysis that showed non inferiority of rivaroxaban as well as statistically significant reduction in major bleeding risk. The composite of major and clinically relevant non major bleeding however was similar between rivaroxaban and standard therapy [14]. The details of these trials are summarized in Table 2, the demographic data of study population in Table 3 and the outcomes of VTE recurrence in Table 4. The incidence of major bleeding, clinically relevant non major bleeding, important bleeding sites (intracranial, gastrointestinal), fatal bleeding as well as mortality from VTE or any other cause in the major VTE trials is summarized in Table 5.
Table 2. Landmark trials in VTE treatment (summary of designs and sample size of the study/control groups).
| Trial | Design | Duration | Treatment (Rx) | N (Rx) |
Alternative/
Control Group |
N (Alternative) |
|---|---|---|---|---|---|---|
| EINSTEIN-DVT 2007- 2009 |
Open label randomized, event driven, noninferiority study Multicenter |
3, 6 and 12 months | Rivaroxaban 15 mg twice daily for 21 days followed by 20 mg once daily | 1731 | Enoxaparin 1 mg/kg twice daily followed by warfarin or acenocoumarol | 1718 |
| EINSTEIN- PE 2007-2011 |
Same as EINSTEIN- DVT trial | 3, 6 and 12 months | Rivaroxaban 15 mg twice daily for 21 days followed by 20 mg once daily | 2419 | Enoxaparin 1 mg/kg twice daily followed by warfarin or acenocoumarol | 2413 |
| EINTEIN-EXT 2007-2009 |
Double blind, randomized, event driven superiority study for secondary prevention; Multicenter | 6 to 12 months | Rivaroxaban 20 mg once daily | 602 | placebo | 594 |
| EINSTEIN-pooled | Prespecified pooled analysis of EINSTEIN DVT and PE trials | 3, 6 and 12 months | Same as EINSTEIN- DVT | 4151 | Same as EINSTEIN- DVT | 4131 |
| RECORD- pooled 2006-2007 |
Pooled analysis of RECORD 1-3 (double blind randomized) | ~5 wks in R1-2 ~2 wks in R-3 |
Rivaroxaban 10 mg | 4657 | enoxaparin | 4692 |
| RE-COVER 2006- 2008 |
Randomized controlled double blind, double dummy; multicenter | 6 months | Atleast 5 days of iv anticoagulant followed by dabigatran 150 mg twice daily + warfarin like placebo | 1273 | Atleast 5 days of iv anticoagulant + wafarin + dabigatran like placebo | 1266 |
| RE-COVER II 2008-2010 | Same as RE-COVER trial | 6 months | Same as RE-COVER trial | 1279 for efficacy; 1280 for safety analysis | Same as RE-COVER trial | 1289 for efficacy; 1288 for safety analysis |
| RE-COVER- pooled | Same as RE-COVER trial | 6 months | Same as RE-COVER trial | 2553 had dabigatran | Same as RE-COVER trial | 2554 had warfarin |
| RE-MEDY 2006-2010 | Double blind, For secondary prevention |
6-36 months | Dabigatran 150 mg twice daily + warfarin like placebo | 1430 | Warfarin + dabigatran like placebo | 1426 |
| RE-SONATE 2007-2010 | Double blind, Randomized |
12 months | Dabigatran 150 mg twice daily | 681 | Placebo | 662 |
| AMPLIFY 2008-2012 |
Double blind, Randomized |
6 months | Apixaban 10 mg twice daily X 7 days, then 5 mg twice daily for 6 months | 2691 | Enoxaparin + warfain | 2704 |
| AMPLIFY- EXT 2008- 2011 | Double blind, Randomized |
12 months | Apixaban 5 mg twice daily (AH) and apixaban 2.5 mg twice daily (AL) after 6-12 moths of pretreatment | AH 813 AL 840 |
Placebo | 829 |
Rx= Treatment group; N = Number of patients; AH = Apixaban high dose of 5 mg twice daily; AL = Apixaban low dose of 2.5 mg twice daily.
Table 3. Patient demographics/risk factors for VTE in the major trials.
| Trial |
Previous VTE;
N (%) |
Active Cancer;
N (%) |
Unprovoked VTE;
N (%) |
Recent Surgery/Trauma;
N (%) |
|---|---|---|---|---|
| EINSTEIN- DVT | 336 (19.4%) | 118 (6.8%) | 1055 (60.9%) | 338 (19.5%) |
| EINSTEIN- PE | 455 (18.8%) | 114 (4.7%) | 1566 (64.7%) | 415 (17.2%) |
| EINSTEIN-EXT | 108 (17.9%) | 28 (4.7%) | 440 (73.1%) | 21 (3.5%) |
| EINSTEIN- pooled | 791 (19.1%) | 232 (5.6%) | 2621 (63.1%) | 753 (18.1%) |
| RECORD- pooled | 105 (2.3%) | Not specified | Not specified | Peri operative time period for all participants |
| RE-COVER | 327 (25.7%) | 64 (5.0%); unclear if only active cancer included | Not specified | Not specified |
| RE-COVER II | D 247 (19.3%) W 203 (15.8%) Pvalue 0.02 |
50 (3.9%); unclear if only active cancer included | Not specified | Not specified |
| RE-COVER pooled | Not specified | Not specified | Not specified | Not specified |
| RE-MEDY | Not specified | 60 (4.2%) | Not specified | Not specified |
| RE-SONATE | Not specified | 1 | Not specified | Not specified |
| AMPLIFY | A 463 (17.2%) W 409 (15.1%) |
66 (2.5%) | 2416 (89.8%) | Not specified |
| AMPLIFY-EXT | AH 118 (14.5%) AL 99 (11.8%) Pb 99 (11.9%) |
AH 9 (1.1%) AL 15(1.8%) Pb 18 (2.2%) |
AH 737 (90.7%) AL 783 (93.2%) |
Not specified |
(Number of patients given only for study group/ novel anticoagulant due to very similar distribution of patients between study and control/ standard therapy groups. In cases where the distribution is different, both study and control group statistics are given.); N = number of subjects; A = Apixaban; AH = apixaban high dose of 5 mg twice daily; AL = apixaban low dose of 2.5 mg twice daily; D = dabigatran; W = warfarin; Pb = placebo.
Table 4. Efficacy outcome of recurrent VTE in major trials.
| Trial |
Efficacy Outcome
(Study Group) π N (Subjects) |
Efficacy Outcome (Control Group) | Hazard Ratio/ 95% CI/ p value |
|---|---|---|---|
| EINSTEIN-DVT | 36 (2.1%) | 51 (3.0%) | 0.68 (0.44-1.04) P< 0.001 (noninferiority margin 2) |
| EINSTEIN-PE | 50 (2.1%) | 44 (1.8%) | 1.12 (0.75- 1.68) P 0.003 (noninferiority margin 2) |
| EINSTEIN-EXT | 8 (1.3%) | 42 (7.1%); some patients with multiple events | 0.18 (0.09-0.39); p<0.001 |
| EINSTEIN-pooled | 86 (2.1%) | 95 (2.3%) | 0.89; (0.66-1.19) Pinferiority <0.001£ |
| RECORD pooled | 23 (0.5%) | 61 (1.3%) | Odds ratio 0.38 (0.22-0.62); p<0.001 |
| RE-COVER | 30 (2.4%) | 27 (2.1%) | 1.10 (0.65-1.84); p<0.001 |
| RE-COVER II | 30 (2.3%) | 28 (2.2%) | 1.08 (0.64-1.80); p<0.001 |
| RE-COVER pooled | 60 (2.4%) | 55 (2.2%) | 1.09 (0.76-1.57) |
| RE-MEDY | 26 (1.8%) | 18 (1.3%) | 1.44 (0.78-2.64); p 0.01, non inferiority margin 2.85 |
| RE-SONATE | 3 (0.4%)┴ | 37 (5.6%) | 0.08 (0.02-0.25); p< 0.001 |
| AMPLIFY | 59 (2.3%) | 71 (2.7%) | RR 0.84 (0.60-1.18); p<0.001 for non inferiority |
| AMPLIFY-EXT | AL 32 (3.8%) AH 34 (4.2%) |
96 (11.6%) | RR 0.36 (0.25-0.53) for AH vs Pb RR 0.33 (0.22-0.48) for AL vs Pb |
Pb = Placebo
£ Prespecified noninferiority margin 1.75
Π Efficacy outcome is recurrent fatal or non fatal DVT or PE
┴ recurrent VTE analysis includes all patients who received atleast one dose of the study drug and includes the entire period of the study even if study drug prematurely discontinued.
Table 5. Incidence of major bleeding, clinically relevant non major bleeding with distribution of bleeding based on sites (cranial and gastrointestinal), fatal bleeding, mortality from VTE and all cause mortalilty of novel anticoagulants in major VTE trials.
| Trial |
Fatal Bleeding
(Number of Subjects) |
Intracranial Bleeding
(Number of Subjects) |
GI Bleed
(Number of Subjects) |
Major Bleeding
N Subjects; HR; (95% CI) |
Clinically Relevant non Major Bleed (Number of Subjects) | Mortality from PE or Suspected PE (Number of Subjects) |
Mortality
(All Cause), HR; (95% CI) (Number of Subjects) |
|---|---|---|---|---|---|---|---|
| EINSTEIN-DVT | R 2 S 5 |
NS | NS | R 14 (0.8%) S 20 (1.2%) 0.65 (0.33-1.30) p 0.21 |
R 126 (7.3%) S 119 (7.0%) |
R 4 S 6 |
R 38 (2.2%) S 49 (2.9%) 0.67 (0.44-1.02) p 0.06 |
| EINSTEIN- PE | R 2 S 3 |
R 3 (2 fatal) S 12 (2 fatal) |
NS | R 26 (1.1%) S 52 (2.2%) 0.49 (0.31-0.79) p 0.003 |
R 228 (9.5%) S 235 (9.8%) |
R 11 S 7 |
R 58 (2.4%) S 50 (2.1%) 1.13 (0.77-1.65) p 0.53 |
| EINSTEIN- EXT | R 0 Pb 0 |
R 0 Pb 0 |
R 3 major + 1 minor Pb 0 |
R 4 (0.7%) Pb 0 p 0.11 |
R 32 (5.4%) Pb 7 (1.2%) |
R 1 (0.2%) Pb 1 (0.2%) |
R 1 (0.2%) Pb 2 (0.3%) |
| EINSTEIN- pooled | R 6 (0.1%) S 9 (0.2%) |
R 5 (2 fatal) S 13 (4 fatal) |
R 15 S 26 |
R 40 (1.0%) S 72 (1.7%) 0.54 (0.37-0.79) p 0.002 |
R 354 (8.6%) S 346 (8.4%) |
R 15 (0.4%) S 13 (0.3%) |
R 96 (2.3%) S 99 (2.4%) |
| RECORD pooled | R 1 (before receiving rivaroxaban) E NS |
R 0 E 0 |
NS | R 14 (0.3%) E 9 (0.2%) p 0.305 |
R 138 (3.0%) E 115 (2.5%) |
R 0 E 3 (<0.1%) |
R 7 (0.2%) E 19 (0.4%) p 0.03 |
| RE-COVER | D 1* W 1* |
D 0* W 3* |
D 53* W 35* |
D 20 (1.6%) W 24 (1.9%) 0.82 (0.45-1.48) |
NS DeductionΩ D 51 W 87 |
D 1 (0.1%) W 3 (0.2%) |
D 21 (1.6%) W 21 (1.7%) 0.98 (0.53-1.79) |
| RE-COVER II | D 0* W 1* |
D 2* W 2* |
D 48* W 33* |
D 15 (1.2%) W 22 (1.7%) 0.69 (0.36-1.32) |
NS DeductionΩ D 49 W 80 |
D 3 (0.2%)- 2 patients died before starting dabigatran W 0 |
D 25 (2.0%) W 25 (1.9%) 0.98 (0.56-1.71) |
| RE-COVER pooled | NS | D 2 (0.1%) W 5 (0.2%) Double dummy period only: D 2 (0.1%) W 4 (0.2%) |
NS | D 37 (1.4% W 51 (2.0%) 0.73 (0.48-1.11) Double dummy period only: D 24 (1.0%) W 40 (1.6%) 0.6 (0.36-0.99) |
NS DeductionΩ D 99 W 166 Double dummy period only: NS DeductionΩ D 85 W 149 |
D 2 (0.1%) W 3 (0.1%) |
D 46 (1.8%) W 46 (1.8%) 1 (0.67-1.51) |
| RE-MEDY | D 0* W 1* |
D 2* W 4* (3 cerebral, 1 subdural) |
D 5* W 8* |
D 13 (0.9%)* W 25 (1.8%)* 0.52 (0.27-1.02); p 0.06 |
NS DeductionΩ D 67* W 120* |
D 1 (0.1%) W 1 (0.1%) |
D 17 (1.2%) W 19 (1.3%) 0.90 (0.47-1.72) p 0.74 |
| RE-SONATE | D 0 Pb 0 |
D 0 Pb 0 |
D 2* Pb 0* |
D 2 (0.3%) * Pb 0* HR not estimable; p 1.0 |
NS DeductionΩ D 34* Pb 12* |
D 0 Pb 0 |
NS |
| AMPLIFY | A 1 (<0.1%); GI W 2 (0.1%); 1 GI, 1 intramuscular |
A 3 (0.1%) W 6 (0.2%) |
A 7 (0.3%) W 18 (0.7%) |
A 15 (0.6%) W 49 (1.8%) 0.31 (0.17-0.55); p<0.001 for superiority |
A 103 (3.8%) W 215 (8%) |
A 12 (0.4%) W 16 (0.6%) |
A 41 (1.5%) W 52 (1.9%) 0.79 (0.53-1.19) |
| AMPLIFY- EXT | AH 0 AL 0 Pb 0 |
AH 0 AL 0 Pb 1 (hemorrhagic transformation of infarct) |
AH 1 (0.1%) + 7 rectal bleeds AL 3 (0.4%) + 4 rectal Pb 2 (0.2%)+ 3 rectal |
AH 1 (0.1%) RR 0.25 (0.03-2.24) vs Pb AL 2 (0.2%) RR 0.49 (0.09-2.64) vs Pb RR 1.93 (0.18-21.25) vs AH Pb 4 (0.5%) |
AH 34 (4.2%) AL 25 (3%) Pb 19 (2.3%) RR 0.71 (0.43-1.18) for AL vs AH |
AH 3 (0.4%) AL 2 (0.2%) Pb 7 (0.8%) |
AH 4 (0.5%) AL 7 (0.8%) Pb 14 (1.7%) |
NS Not specified; GI = gastrointestinal; HR = hazard ratio; RR = relative risk
R = rivaroxaban; S = standard therapy; Pb = placebo; p = p value; E = enoxaparin; AH = apixaban high dose 5 mg twice daily; AL = apixaban low dose 2.5 mg twice daily; D = dabigatran; W = Warfarin; A = Apixaban; RR = Relative risk
deductionΩ = deduction from the table in the article (Number of subjects with clinically relevant non major bleeding = number of subjects with major or clinically relevant nonmajor bleeding events – number of subjects with major bleeding events)
*Number of bleeding events (rather than number of subjects with bleeding).
VTE treatment in April 2014 due to four landmark clinical trials (RE-COVER, RE-COVER II, RE-MEDY and RE-SONATE).
In fragile patients (age greater than 75 years, CrCl less than 50 ml/min or body weight less than 50 kg), major bleeding risk was 1.3% vs 4.5% favoring rivaroxaban (Hazard ratio 0.27; 95% CI 0.13- 0.54; NNT 31 to prevent one major bleed
compared to standard therapy) [13]. Interestingly, the use of aspirin upto 100 mg/day, clopidogrel 75 mg/day or both were allowed in the EINSTEIN-DVT and EINSTEIN- EXT trials based on physician discretion [1]. The efficacy and safety profile of rivaroxaban was similar to standard therapy in subgroup analysis of patients with malignancy, those with a high clot burden and those with recurrent VTE [13]. Non-hemorrhagic adverse effects of rivaroxaban in the EINSTEIN-EXT study were upper abdominal pain, dyspepsia, sinusitis, tooth ache, UTI, back pain and fatigue (all less than 5%) whereas post marketing voluntary reporting of adverse effects include anaphylaxis, angioedema, Stevens-Johnson syndrome, agranulocytosis, jaundice and hepatitis [10].
The EINSTEIN- PE trial was crucial in suggesting a lower risk of major bleeding in rivaroxaban treated patients compared to standard therapy with atleast similar efficacy in preventing recurrent VTE [14]. The investigators suggested further research to be done to verify or refute this finding. Subgroup analysis of fixed dose rivaroxaban revealed similar efficacy and overall bleeding risk irrespective of age, gender, BMI, renal function or amount of clot burden in PE [14].
The limitation of the EINSTEIN trials was the open label design of the studies which was compensated for partially by allowing an independent blinded committee to formulate outcomes. Patients with severe hepatic or renal impairment (CrCl <30 ml/min), uncontrolled hypertension and women of child bearing age without proper contraception were excluded which constitute a significant portion of patients with VTE in real life. Length of anticoagulation with rivaroxaban was approximately 193.6 to 216.3 days [13]. The bleeding risk for long term anticoagulation (for life) yet needs to be determined.
The EINSTEIN trials have important implications as they were large multicenter trials that included patients with active cancer and recurrent VTE. Patients with VTE who were diagnosed with cancer after randomization were reassigned to the cancer group in most cases with lower rate of bleeding and lower risk of recurrent VTE compared to standard therapy [13]. This raises the possiblity of long term rivaroxaban use in cancer associated VTE. However the standard therapy in the EINTEIN trials was long term warfarin whereas the standard of care in cancer patients with VTE is long term enoxaparin. Head to head trials of long term enoxaparin with rivaroxaban need to be undertaken to verify the findings of the EINSTEIN trials. The trials had a low rate of loss to follow-up.The time spent in the therapeutic INR range by the patients assigned to standard therapy was similar to other contemporary studies (54.1 to 66.4%) [1, 13].
The role of rivaroxaban in prophylaxis of VTE in patients undergoing total knee and hip arthroplasties was evaluated in the RECORD trials. This is a set of 4 trials (RECORD 1-2 in total hip arthoplasty; RECORD 3-4 in total knee arthroplasty). The pooled analysis of the RECORD trials 1-3 showed rivaroxaban 10 mg once daily is superior in efficacy and reduced all cause mortality compared to enoxaprin 40 mg subcutaneously once daily in VTE prevention both at 2 weeks and at the end of the study period (0.5% vs 1.3% in favor of rivaroxaban at the end of study period; Odds ratio 0.38; 95% CI 0.22-0.62; p<0.001) with similar risk of bleeding (0.3% vs 0.2% in favor of rivaroxaban) [15]. The rates of wound hemorrhage requiring re-operation and wound infections were similar between rivaoxaban and enoxaparin [15].
Dabigatran
Dabigatran is a direct thrombin inhibitor approved by the FDA for thromboembolic prophylaxis in non valvular atrial fibrillation in October 2010. It was approved by the FDA for
The RE-COVER trial showed that dabigtran 150 mg twice daily is non inferior in preventing recurrent VTE compared to warfarin. Major bleeding risk was similar in the two arms albeit a trend towards decreased non major bleeding events was seen in favor of dabigatran (Major bleed 1.6% vs 1.9%; hazard ratio 0.82; 95% CI 0.45- 1.48. Major or clinically relevant non major bleeding 5.6% vs 8.8% ; hazard ratio 0.63; 95% CI 0.47-0.84; p=0.002) [16]. There was however a trend towards increased gastrointestinal bleeding with dabigatran compared to warfarin (53 GI bleeding events with dabigatran vs 35 with warfarin) [16]. The RE-COVER trial had excellent blinding with the double dummy model ensuring patients taking warfarin took a dabigtran like placebo and vice versa. The study had a low rate of loss to follow-up and INR was therapeutic 60% of the time which is comparable to contemporary studies [16]. The limitations include a high preponderance of white race in the study population (95%), few patients with renal impairment (90% had CrCl greater than 50 ml/min) and a requirement for parenteral anticoagulation (either unfractionated heparin or enoxaparin) for a median of 9 days before starting dabigatran per study protocol.
The RE-COVER II trial was conducted subsequently by the RE-COVER trial investigators for better subgroup analysis [17]. An independent adjudication committee and safety monitoring board oversaw the efficacy and safety blinded to the assignments. There was more ethnic diversity in the RE-COVER II trial.The trial including upto 20% asian patients confirmed the results of the RE-COVER trial [17]. There was an absolute increased risk of 0.2% of having acute coronary syndrome with dabigatran compared to warfarin although it was statistically non significant [17]. The RE-COVER pooled analysis of the double dummy period (only the time when dabigatran was taken by the study group) revealed a reduction in major bleeding which almost reached statistical significance [17]. (RE-COVER and RE-COVER II had shown significant reduction in major and clinically relevant non major bleeding but not major bleeding alone) [16, 17]. There was no increased bleeding or increase in recurrent VTE observed in patients with prior bleeding, age over 75 years or CrCl 30- 49 ml/min, ethnicity, gender, BMI or prior VTE [17].
The RE-MEDY trial confirmed the non inferiority of dabigatran compared to warfarin although the inferiority margin was large at 2.85 allowing almost three times the risk of VTE to still be considered non inferior [18]. Dabigtran had a significantly lower risk of major and clinically relevant non major bleeding compared to warfarin (5.6% vs 10.2% in favor of dabigatran, HR 0.54, 95% CI 0.41-0.71, p<0.001), but not statistically significant if major bleeding considered alone (0.9% vs 1.8% in favor of dabigatran, HR 0.52, 95% CI 0.27-1.02; p 0.06) [18]. The trial included only 2% African-Americans and nearly 90% cacausians limiting the applicability of these results in treating non-caucasian patients [18].
Dabigatran was more efficacious than placebo in secondary prevention of VTE in the RE-SONATE trial with numbers similar to rivaroxaban or warfarin versus placebo (HR 0.08 for dabigatran, HR 0.18 for rivaroxaban, odds ratio 0.05 for warfarin, all values versus placebo) [18]. The risk of clinically relevant bleeding with dabigatran is higher than placebo (HR 2.9; 95% CI 1.5-5.6) but it is comparable to the bleeding risk of rivaroxaban versus placebo (HR 5.2; 95% CI 2.3- 11.7 in favor of placebo) [1, 18].
Non hemorrhagic adverse effects of dabigtran include gastrointestinal in 35% of patients (dyspepsia, GERD, abdominal pain, gastritis, peptic ulcer) and drug hypersensitivity reactions (anaphylaxis, urticaria, rash, pruritis) in less than 0.1% of patients [11].
Apixaban
Apixaban is the most recent additon to the anticoagulation arsenal. It is a direct factor Xa inhibitor appoved by FDA for VTE treatment in August 2014 [19]. The landmark AMPLIFY trial compared oral apixaban with standard treatment. Apixaban was non inferior to standard treatment for preventing recurrent VTE (2.3% vs 2.7% in favor of apixaban; relative risk 0.84; 95% CI 0.60-1.18; p<0.001) but also had lower rates of major bleeding that reached statistical significance (0.6% vs 1.8% in favor of apixaban; relative risk 0.31; 95% CI 0.17-0.55; p<0.001 for superiority) [20].
The strengths of the AMPLIFY trial include low rate of loss to follow-up and good adherence to treatment by participants (INR therapeutic 61% of the time) [20]. The subgroup analysis revealed similar efficacy and safety across fragile patient groups aged over 75 years and obese with body weight above 100 kg [20]. The limitations of the trial included few patients having cancer, low body weight or CrCl less than 50 ml/min [20]. There was an independent blinded committee adjucating the outcomes [20].
The AMPLIFY-EXT trial showed that continuation of apixaban (both doses 2.5 mg and 5 mg twice daily) for one year after initial VTE treatment is more effective than placebo in preventing VTE with acceptably low risk of major bleeding (NNT to prevent one VTE is 14, NNH for one major or clinically relevant non major bleeding is 200) [21]. The limitations of the trial include short term follow up of one year only and few patients with cancer, age above 75 years, low body weight below 60 kg or severe renal dysfunction [21].
The adverse effects of apixaban are primarily hemorrhagic without any significant adverse effects affecting other organ systems [12].
Indirect Comparison Between Novel Oral Anticoagulants
There are no head to head trials comparing the novel oral anticoagulants making it difficult to choose the most suitable agent for each indivisual patient with unique circumstances. Indirect comparison by Mantha et al. showed no statistically significant difference in recurrence of VTE or all cause mortality between apixaban, rivaroxaban and dabigatran [22]. However the major bleeding risk seems to be lower with apixaban compared to other novel agents [22]. It reaches statistical significance for major bleeding (apixaban vs dabigatran; RR 0.42; 95% CI 0.21-0.87, p = 0.02 in favor of apixaban) and composite outcome of major and clinically relevant non major bleeding (apixaban vs rivaroxaban, RR 0.47; 95% CI 0.37-0.61, p< 0.001 in favor of apixaban) [22].
Alotaibi et al. performed a network meta-analysis of the novel anticoagulants with similar conclusion of no significant difference between them in efficacy to prevent VTE or all cause mortality [23]. Their conclusion about the safety of the medications was different than Mantha et al. stating that there was no significant difference in the risk of major bleeding between apixaban (regardless of dose), rivaroxaban or dabigatran [23]. Clinically relevant non major bleeding was significantly less with either dose of apixaban when compared with rivaroxaban 20 mg daily (OR 0.23, 95% CI 0.08-0.62, p=0.004 in favor of apixaban 2.5 mg twice daily and OR 0.31, 95% CI 0.11-0.82, p=0.019 in favor of apixaban 5 mg twice daily) [23]. Only the low dose apixaban showed statistically signicant reduction in clinically relevant non major bleeding when compared with dabigatran 150 mg twice daily (OR 0.4, 95% CI 0.16-0.9, p=0.04) [23]. Rivaroxaban 20 mg daily and dabigatran 150 mg twice daily had similar bleeding risk profiles [23].
Hirschl et al. found similar efficacy of novel anticoagulants in VTE prevention in their systematic review when compared with VKA or indirectly among themselves [24]. Major bleeding appeared to be reduced significantly by apixaban and rivaroxaban with absolute risk reduction of 1% for each of them [24]. Regarding composite bleeding outcomes, apixaban did better than all the others and dabigatran did better than rivaroxaban [24].
Rollins et al. did not find any difference in recurrent VTE, mortality or clinically relevant non major bleeding between the novel agents [25]. Bleeding risk was somewhat higher with rivaroxaban but the wide intervals for rivaroxaban made the comparison less reliable [25]. Cui et al. suggest that prophylaxis of VTE in orthopedic surgery is superior with apixaban and rivaroxaban compared to dabigatran [26]. Rivaroxaban works as well as apixaban in VTE prophylaxis with higher bleeding risk than apixaban [26]. Head to head trials with direct comparison are needed to provide definitive information in the future.
Point of Care INR Testing Defect and its Implications for Novel Anticoagulants
The FDA issued a notice of Class I device recall in 2014 due to defective point of care INR testing in some patients with INR monitoring devices (INRatio and INRatio2 PT/INR Monitor system) by Alere Inc [27]. Recently, this has cast a doubt over the validity of the ROCKET- AF trial since the same devices were used for POC INR testing in the ROCKET-AF trial for the control group patients on warfarin [28]. The device may erroneously report a lower INR compared to plasma based lab INR testing in patients with certain conditions. These recall conditions are as follows [27]:
Anemia of any type with hematocrit less than 30%
Any conditions associated with elevated fibrinogen levels including acute or chronic inflammatory conditions, infections or chronically elevated fibrinogen for any reason
Hospitalized or advanced stage cancer or end stage renal disease patients requiring hemodialysis
Any bleeding or unusual bruising, clinically observed or reported by the patient
The clinical researchers of the ROCKET- AF trial denied knowledge of any defects in the INR monitoring system however the device manufacturers stated that they had received reports of defective INR testing in many instances since 2002, which is before the start of the ROCKET- AF trial [28].
Patel et al., who conducted the ROCKET- AF trial, published a recent post hoc analysis of the trial by dividing both the study and the control group into two subsets, one having a recall condition (thus may be having a false low INR) and the other without a recall condition. They have concluded that the subgroup analysis confirms the results of the original trial in terms of non inferiority and safety of rivaroxaban [29]. They also report that the risk of hemorrhage is slightly higher in the rivaroxaban group with a recall condition when compared to the warfarin group with a recall condition. This finding is contrary to the popular criticism that falsely lower INR values in the recall group taking warfarin may have led to warfarin dosage increments and a possibly higher risk of hemorrhage which could make rivaroxaban seem safer than what it truly is. Many have called for independent review and analysis of the ROCKET- AF trial data to have an unbiased conclusion about the validity of the results. There have been suggestions to publically disclose information about the specific devices and testing methods used in future trials especially those investigating novel anticoagulants. The FDA is considering the option of novel anticoagulant drug serum level testing in routine clinical practice to optimize risk – benefit ratio [28].
Dosing of Novel Anticoagulants
The treatment dose for acute VTE is 15 mg daily for 21 days followed by 20 mg daily for rivaroxaban, 150 mg twice daily for dabigatran (dabigatran requires LMWH or UFH for atleast 5 days prior to starting dabigatran) and 10 mg twice daily for 7 days followed by 5 mg twice daily for apixaban [10, 12, 16, 17].
The dose for secondary prophylaxis for patients requiring long term anticoagulation is rivaroxaban 20 mg daily, dabigatran 150 mg twice daily or apixaban 2.5 mg twice daily [10-12].
No laboratory monitoring is required for any of the novel anticoagulants. They can be used in an outpatient setting conveniently except dabigatran which requires at least 5 days of parenteral anticoagulant. Contraindications for all the three novel anticoagulants are the same; i.e active pathological bleeding and prior hypersensitivity reaction to the novel anticoagulant in question [10-12].
Transition Between Traditional and Novel Anticoagulants
The FDA recommendations for transitioning between traditional and novel anticoagulants is described in the Table 6 with emphasis on the fact that all three of the novel anticoagulants prolong the INR and make it less reliable especially when transitioning to warfarin [10-12].
Table 6. Transition between heparin, warfarin and novel anticoagulants per the FDA.
| Agent | Transition (Tr) from Warfarin | Tr to Warfarin | Transition from Anticoagulants (Ac) other than Warfarin | Tr to Ac other than Warfarin |
|---|---|---|---|---|
| Rivaroxaban | Start when INR is below 3.0 | no guidelines; may start parenteral Ac + warfarin at the time of next dose of rivaroxaban | -stop iv heparin and start rivaroxaban at the same time -start 0-2 hours prior to the evening dose of other Ac and skip the other Ac |
Ac to be given at the time rivaroxaban would be due and skip rivaroxaban |
| Dabigatran | Start when INR is below 2.0 | • For CrCl ≥50 mL/min, start warfarin 3 days before discontinuing dabigatran • For CrCl 30- 50 mL/min, start warfarin 2 days before discontinuing • For CrCl 15-30 mL/min, start warfarin 1 day before discontinuing • For CrCl <15 mL/min, no recommendations can be made |
-stop iv heparin and start rivaroxaban at the same time -start 0-2 hours prior to the evening dose of other Ac and skip the other Ac |
wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of dabigatran before initiating treatment with a parenteral anticoagulant |
| Apixaban | Start when INR is below 2.0 | may start parenteral Ac + warfarin at the time of next dose of apixaban | Discontinue Ac and begin apixaban at the next scheduled dose | Discontinue apixaban and begin the other Ac at the next scheduled dose |
Tr = Transition; Ac = Anticoagulant; iv = Intravenous; CrCl = Creatinine clearance.
Cost Analysis
The cost of the various anticoagulants and INR testing is compared in Table 7 [30].
Table 7. Cost of anticoagulants based on www.goodrx.com (accessed May 10, 2015).
| Anticoagulant | Monthly Cost (30 Day Supply) | Miscellaneous Cost |
|---|---|---|
| Warfarin | $ 4 | INR test (lab) = $ 6.19–145.70/test [30] Home INR testing machine costs $ 695 and up. € Testing strips for INR (50 strips) = $ 190 |
| Enoxaparinµ | $ 952.95 (once daily) $ 1,425.90 (twice daily) |
Approximately costs $1.1/ mg of enoxaparin; Once daily dose is more economical |
| Rivaroxaban | $ 325.95 (all strengths) | No testing cost |
| Dabigatran | $ 325.95 (all strengths) | No testing cost |
| Apixaban | $ 326.24 (all strengths) | No testing cost |
€ $ price of Coag-Sense PT/INR Self Test (Home User) System Monitor available online from Wilburnmedicalusa.com (The lowest online price found by the author)
µ Price estimated for 80 kg person for 1.5 mg/kg dose daily (120 mg daily) and 1 mg/kg twice daily dose (80 mg twice daily).
Venous Thromboembolism Prophylaxis
Venous thromboembolism prophylaxis is of two types. Primary prophylaxis is directed at reducing the rate of VTE in hospitalized patients or perioperatively especially in cases of orthopedic surgery. Secondary prophylaxis is for patients with prior VTE who are deemed candidates for long term anticoagulation to prevent recurrence.
Hospitalized patients on medical units require prophylaxis only if deemed to be at high risk of VTE using LMWH or unfractionated heparin (ACCP 1B) [31]. For orthopedic surgery, low molecular weight heparin started 12 hours before or 12 hours after hip or knee replacement surgery is preferred by the ACCP over other anticoagulants (2B for dabigatran, apixaban, rivaroxaban stating lack of long term data as the reason for preferring LMWH; 2C for warfarin, aspirin due to lack of efficacy and/or increased bleeding risk) [32].
Rivaroxaban should be started 6-10 hours after surgery and dosed at 10 mg daily for 35 days post hip arthroplasty and 12 days post knee arthroplasty [10]. Dabigatran should be used at a dose of 150 or 220 mg once daily starting 4 hours after surgery [32]. A cochrane review in 2010 found dabigatran to be as effective as LMWH or warfarin in VTE prophylaxis after orthopedic surgery [33]. However dabigatran was associated with increased bleeding risk (OR 1.40; 95% CI 1.06-1.85) and increased all cause mortality compared to LMWH (OR 2.06; 95% CI 1.10-3.87) [33]. Apixaban should be used at a dose of 2.5 mg twice daily starting 12-24 hours post orthopedic surgery and may be more efficacious in preventing VTE than LMWH without increased hemorrhagic complications [34]. The duration of prophylaxis is similar for all novel anticoagulants i.e 35 days for major orthopedic surgery (such as total hip replacent or hip fracure surgery) and atleast 10-14 days for other orthopedic surgeries including total knee replacement surgery [32].
Perioperative Management of Anticoagulation
The use of novel anticoagulants removes the need for perioperative bridging with unfractionated intravenous heparin and/or enoxaparin making them a convenient therapeutic option for both the patients and their physicians.
There is uncertainity about the optimal timing of novel anticoagulant discontinuation before surgery and restarting post operatively. Physicians should consider stopping noval anticoagulant therapy 24 hours before surgery and restart 24 hours later if low risk surgery, extending to 48 hours before and after surgery for moderate risk of bleeding and 5 days before surgery if additional factors (liver, renal dysfunction) are present [10, 12, 35]. The optimal timing of restarting novel anticoagulants after surgery is at the discretion of the surgeon and the condition of hemostasis. Anderson et al. showed that starting novel anticoagulants carried similar bleeding risk as warfarin with enoxaparin bridge for atrial fibrillation after coronary artery bypass when started on or around post operative day 4 [36]. Allowing 18 hours before and 6 hours after epidural catheter removal is advisable to prevent intraspinal hemorrhage with risk of permanent neurologic sequelae. [15].
Drug Interactions of Novel Anticoagulants
Significant drug interactions of novel anti- coagulants are described in Table 1. Medications that strongly inhibit both CYP3A4 and P glycoprotein (P-gp) include Itraconazole, ketoconazole, clarithromycin, lopinavir, ritonavir, indinavir and conivaptan. Moderate dual inhibitors include Verapamil, diltiazem, dronedarone and erythromycin. Weak dual inhibitors include azithromycin, amiodarone, ranolazine, felodipine and quinidine. Strong CYP3A4 inhibitors also include grapefruit juice, Boceprevir, telaprevir, voriconazole, posaconazole and nefazodone. Strong P-gp inhibitors include captopril, carvedilol and cyclosporine, These medications can increase the levels of novel anticoagulants in the body with increased risk of toxicity.
On the other hand medications that strongly induce both CYP34A and P-gp are phenytoin, carbamazepine, rifampin, St. John’s wort. Weaker CYP3A4 inducers are prednisone, nafcillin, efavirenz and many others. These medications can decrease the level of novel anticoagulants in the body. There is no clinically significant interaction between food and any of the novel anticoagulants [37].
The FDA recommends against the concomittant use of strong dual CYP3A4 or P-gp inhibitors or inducers with rivaroxaban [10]. For patients taking apixaban 5 mg twice daily while on strong dual CYP3A4 and P-gp inhibitors, the dose of apixaban can be reduced to 2.5 mg twice daily or completely discontinued [12]. Moreover patients on apixaban 2.5 mg twice daily should avoid using strong dual inhibitors. Strong inducers of both CYPA34 and P-gp should be avoided in all patients with apixaban [12].
Dabigatran use should be avoided with P-gp inducers. In patients with CrCl 30-50 mL/min who are taking a P-gp inhibitor consider reducing dabigatran dose or avoid completely [38]. Dabigatran use on P-gp inhibitors in patients with CrCl <30 mL/min is not recommended [38].
Anticoagulant Dosing in High Risk Patients
There are certain populations of patients that may be at increased risk of adverse effects particularly hemorrhagic complications from anticoagulants. This is particularly important in case of novel anticoagulants due to few and relatively new antidotes. The patients with CrCl less than 30 ml/min, liver dysfunction (acute or chronic) with transaminase levels 2-3 x upper normal limit of the testing laboratory, massive PE, thrombolytic use, vena cava filter placement, uncontrolled hyper- tension, pregnany, lactation, life expectancy less than 3-6 months and other indications for warfarin use were mostly excluded from the trials. Patients with active bleeding, head trauma, major surgery, peptic ulcer, gastrointestinal bleeding, intracranial arteriovenous malformations or tumors within a few weeks to few months of the VTE diagnosis were also excuded. It is therefore difficult to guage the risk benefit ratio in these patients and the use of novel anticoagulants is generally avoided. Table 8 describes the dose adjustments of novel anti- coagulants in some high risk patient populations approved for use in the USA. [1, 10, 12, 38, 39]. The dose adjustments in high risk patients with atrial fibrillation for stroke prevention are different and may not be applicable to patients with VTE due to different therapeutic targets, demographics and co-morbidities.
Table 8. Dose adjustment recommendations by the FDA in high risk patient populations.
| Risk Factors | Rivaroxaban | Dabigatran | Apixaban |
|---|---|---|---|
| CrCl < 30 ml/min | Avoid use. No dose reduction as long as CrCl >30 ml/min | No dose reduction as long as CrCl > 30 ml/min; no dosing recommendation for CrCl< 30 ml/min. Avoid concomitant use with P-gp inhibitors if CrCl < 50 ml/min |
No dose adjustment recommended. Trials excluded patients with CrCl < 25 ml/min or serum creatinine of 2.5 mg/dl of higher |
| Liver disease | Avoid in Child-Pugh B and C. Trials excluded transaminase levels higher than 3 X ULN | No recommendations, Trials excluded transaminase level higher than 2 X ULN, active hepatitis and cirrhosis | No dose adjustment for mild hepative impairment. No recommendation for moderate impairment. Avoid use in severe impairment. Trials excluded transaminase levels > 2 X ULN or active liver disease and total bilirubin > 1.5 X ULN except Gilbert’s syndrome |
| BMI | No dose adjustment for extremes of BMI | No dose adjustments recommended. May exercise caution if weight < 50 kg | No dose adjustments recommended |
| Age | No dose adjustment for age > 75 years | No dose adjustment for age > 75 years however may exercise caution as dabigatran levels are found to be higher in this population |
No dose adjustments for age recommended |
| Atrial Fibrillation/ Stroke prevention dose (for comparison) | If CrCl 15-50 ml/min, reduce dose to 15 mg daily. Avoid use in CrCl< 15 ml/min | If CrCr 15-30 ml/min, reduce dose to 75 mg twice daily. Avoid use in CrCl<15 ml/min or dialysis. 110 mg twice daily may be used if CrCl 30-50 ml/min, age 75-80 years or severe esophagitis/gastritis according to the European guidelines. 110 mg twice daily dose is not approved in the USA. |
In patients with at least two of the following risk factors, the recommended dose is 2.5 mg orally twice daily: age ≥80 years body weight ≤60 kg serum creatinine ≥1.5 mg/dL Patients undergoing hemodialysis receive 5 mg twice daily except if they are older than 80 years or have body weight ≤60 kg (then receive 2.5 mg twice daily) |
Patients with a malignancy were about 5% of the study population in each of the landmark trials except for AMPLIFY trials that had 1-2.5%. The incidence of VTE and hemorrhagic complications was similar to non cancer patients in these trials but they were not designed to study cancer associated VTE. A recent meta-analysis of 1,132 patients revealed that novel anticoagulants are as effective and safe as standard treatment, however all the studies included in the meta-analysis had parenteral anticoagulant followed shortly by a vitamin K antagonist as the standard therapy for cancer patients rather than long term enoxaparin [40]. A cochrane review in 2014 found enoxaparin to be more effective than warfarin in preventing VTE with no impact on mortality in cancer patients (HR 0.47; 95% CI 0.32 to 0.71), however it did not include any studies with significant novel anticoagulant use [41].
Management of Hemorrhagic Complications
There are few clinical trials and case reports assessing the reversal of novel anticoagulants. This presents a huge challenge to the treating physicians who have to balance the advantages of simple, hassle free anticoagulation with the risk of potentially fatal hemorrhagic complications without time tested and reliable antidotes. Fresh frozen plasma (FFP) has not been studied in humans for reversal of novel anticoagulants [42]. The reversal options include prothrombin complex concentrates (PCCs) which could contain 3 factors (3F-PCC with factors II, IX, X) or 4 factors (4F-PCC with factors II, VII, IX, X), recombinant activated factor VII (rFVIIa) and idarucizumab. The 3F-PCC products are Bebulin and Profilnine containing factors II, IX and X [36]. The 4F-PCC products are Beriplex, Kcentra, Cofact and Octaplex containing significant amounts of factor VII in addition to the three factors listed above [43]. Kcenta is the only 4F-PCC (non activated) approved by FDA for warfarin reversal in April 2013. Prior to that, only 3F-PCC was available and approved in the USA. ACCP 2012 guidelines recommend reversal of warfarin induced major bleeding with 4F-PCC plus intravenous vitamin K rather than FFP due to faster and more complete reversal with PCC (Grade 2C) [5].
The 4F-PCC can be activated or non activated based on whether the factors in the product are active or inactive. The activated PCC is marketed as FEIBA (factor VIII inhibitor bypassing activity) and contains active form of many factors including factor X and prothrombin [44]. Its FDA approved indication is bleeding in hemophilia with factor VIII inhibitor. It has been tried for novel anticoagulant induced bleeding with some success [43]. Some of these products contain varying amounts of heparin, antithrombin III, protein C and protein S.
Both dabigatran and rivaroxaban can cause prolongation of prothrombin time (PT), activated partial thromboplastin time (aPTT) and numerous other laboratory parameters but these parameters do not consistently correlate with clinical bleeding and hemostasis [35]. There is a need for developing coagulation parameters that could reliably test for in vivo hemostasis after a hemorrhagic event or in case of emergent surgery. Dedicated tests under consideration include ecarin clotting time, hemoclot thrombin inhibitor and anti-FXa assays [35].
The most widely studied agent for reversal is dabigatran. Zhou et al. showed that 100 U/kg of 4F-PCC (Beriplex) was effective in preventing intracerebral hemorrhage expansion in dabigatran treated murine model in a dose dependant manner [45]. Fresh frozen plasma was less effective and lacked consistent results whereas rFVIIa (Novoseven) was ineffective [45]. rFVIIa is a viable option for dabigatran toxicity but may be less effective for dabigtran reversal when compared to its action on rivaroxaban and apixaban [46]. Dabigatran can be removed by dialysis [11].
An antidote for dabigatran, Idarucizumab has recently been granted accelerated approval by the FDA in October 2015. Idarucizumab is a human monoclonal antibody fragment which binds with dabigatran and therefore prevents dabigatran from binding to thrombin. Pollack CV et al. showed in the REVERSE AD trial that idarucizumab was able to effectively reverse dabigatran activity within minutes of administration of the antidote in patients with serious bleeding and those requiring urgent surgery [47]. The REVERSE AD trial prospectively studied two cohorts of patients on dabigatran therapy, group A (51 patients) with serious bleeding and group B (39 patients) requiring urgent surgical procedure. The primary endpoint was percentage reversal of thrombin time or ecarin clotting time within 4 hours of idarucizumab use. Normalization of thrombin time and ecarin clotting time were achieved in 88 to 98% of the patients. The secondary end point of clinical hemostasis was achieved at a median time of 11.4 hours in 35 patients in group A and 33 patients in group B. Unfortunately all patients could not be assessed for the status of hemostasis. The strengths of the trial are inclusion of a significant number of high risk patients (eg intracranial hemorrhage) in the study and few exclusion criteria. The limitations of the trial are lack of a control group.
Idarucizumab should be given as a 5 mg intravenous infusion for serious hemorrhagic events and/or prior to urgent surgical procedures per the FDA recommendations [48]. It has a half life of 47 minutes and a terminal half life of 10.3 hours. About one third of the dose is excreted in urine by the kidney in the first 24 hours of intake. The remaining drug undergoes proteolytic meta- bolism mainly in the kidneys. Potential adverse effects include hypersensitivity reaction, headache, delirium, fever, pneumonia, hypokalemia and constipation. It should be used with caution in patients with hereditary fructose intolerance due to the presence of sortibol.
Rivaraxaban is not dialyzable because it is 92-95% protein bound in the plasma [10]. In a randomized, double-blind, placebo controlled study, 50 IU/kg of 4F-PCC (Cofact) completely reversed the laboratory parameter of anti- coagulation in 6 healthy volunteers taking rivaroxaban, however no effect was seen in case of dabigatran (6 volunteers) [49]. Unfortunately neither PCC nor rFVIIa was able to fully reverse rivaroxaban induced bleeding in rabbits although they improved the laboratory coagulation assays in vitro [50]. rFVIIa is in general less effective than PCC in reversing novel anticoagulants but can be used for rivaroxaban induced bleeding [35]. rFVIIa has not been studied in humans for reversal of novel anticoagulants [42]. FEIBA (activated PCC) is another option for rivaroxaban reversal [35]. The PCC and rFVII have been associated with increased risk of arterial thrombosis with the risk of both activated and non activated PCC approaching 1-3% [42]. Similarly there is a risk of thrombosis and no difference in mortality with rFVIIa use in patients without haemophilia (RR 1.45; 95% CI 1.02-2.05) [51]. Some recommend using non activated PCC before the activated PCC or rFVIIa use due to lower prothrombotic risk [52]. PCC were associated with DIC initially but that does not seem to be true about the new PCC products [53].
Andexanet Alfa is a potential antidote undergoing clinical trials to reverse the anticoagulant effects of Factor Xa inhibitors (Apixiban and Rivaroxiban). Andexanet alfa is recombinant human protein that directly binds to activated factor Xa inhibitors and activated antithrombin III [54]. Siegal D et al. studied adexanet alfa in reversing factor Xa activity of rivaroxaban and apixaban in a total of 101 healthy volunteers aged 50- 75 years in a randomized, double blind and placebo controlled trial (ANNEXA-A and ANNEXA-R trials) [55]. Andexanet alfa administered as intravenous bolus followed by an intravenous infusion for 1 to 2 hours reduced factor Xa activity by 92- 94% within a few minutes of the bolus dose without any significant adverse effects or thrombotic events.
A new phase 3 multicenter open label single arm study (NCT02329327) is under way to assess the efficacy of andexanet alfa in patients taking rivaroxaban, apixaban, edoxaban or enoxaparin who present with major bleeding [56].
PER977 (arapazine, ciraparantag) is another potential antidote for novel anticoagulants and is administered as an intravenous infusion. It binds to factor Xa inhibitors, direct thrombin inhibitors and heparin (both unfractionated and low molecular weight heparin) through non covalent bonds and electrical charge interactions between molecules. PER977 reduced hemorrhage by greater than 90% within 30 minutes in rats given supraphysiologic doses of rivaroxaban, apixaban and dabigatran [57]. PER977 was found to decrease clotting time from 37% to 10% above baseline within a few minutes in healthy human subjects treated with edoxaban without any prothrombotic adverse effects [58].
Apixaban is the least studied for potential reversal agents. Andexanet alfa is an experimental agent that binds to direct factor Xa inhibitors thus inhibiting their activity and is under investigation in healthy volunteers taking apixaban [44]. This presents a hope for the future to meet the dire need of a definitive antidote for factor Xa inhibitors.
Conclusion
The physicians of the new era have oral anticoagulants like dabigatran, rivaroxaban and apixaban added to their arsenal for the treatment and prophylaxis of VTE. The efficacy of these agents has been proven in non inferiority trials in comparison with warfarin. Risk of bleeding has been assessed to some extent. There is a dire need to establish evidence based guidelines for reliable and consistent reversal of these novel agents. There is extensive ongoing research in this field that hopes to discover the much awaited antidotes and establish the safety and reliability of the currently available antidotes. This will allow the patients and their physicians in the modern world to comfortably use these novel anticoagulants.
Table 1. Anticoagulants for VTE-pharmacokinetics and drug interactions [10-12].
| Agent | Half Life Hours (h) | Time to Reach Cmax (h) | Mode of Excretion (Estimated Values) | Drug Interactions |
|---|---|---|---|---|
| Rivaroxaban | 5-9 11-13 in elderly |
2-4 | Renal scretion 36% Renal excretion of inactive metabolites 30% Total renal elimination 66% Fecal excretion 28% |
Not recommended to use with strong CYP3A4 and P-gp (P glycoprotein) inhibitors or inducers |
| Apixaban | 12 | 3-4 | Hepatobiliary excretion (major route) Renal scretion 27% |
Not recommended to use with strong CYP3A4 and P-gp inducers. May reduce apixaban dose from 5 mg to 2.5 mg twice daily with strong CYP3A4 and P-gp inhibitors. Avoid use with strong CYP3A4 and P-gp inhibitors if on apixaban 2.5 mg twice daily |
| Dabigatran | 12-17 | 1 h (fasting state); delayed by 2 h with high fat meal | Renal elimination 80% | Not recommended to use with strong P-gp inhibitors or inducers |
| Warfarin | 20-60 (mean 40) | Within 4 h | Eliminated by metabolism, mostly in the urine (92%) | Multiple interactions |
Cmax = Maximum concentration in plasma, h = Hour(s). Warfarin information accessed on Feb 28th 2015 from http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009218s107lbl.pdf.
ACKNOWLEDGEMENTs
Declared none.
ABBREVIATIONS
- A
Apixaban
- Ac
Anticoagulant
- AH
Apixaban high dose (5 mg twice daily)
- AL
Apixaban low dose (2.5 mg twice daily)
- AVM
Arteriovenous malformation
- CI
Confidence interval
- Cmax
Maximum concentration in plasma
- CrCl
creatinine clearance
- CVA
Cerebrovascular accident
- D
Dabigatran
- DVT
Deep venous thrombosis
- E
Enoxaparin
- GI
Gastrointestinal
- H
Hour(s)
- HepA
Hepatitis A
- HepB
Hepatitis B
- HepC
Hepatitis C
- IO
In investigator’s Opinion
- Iv
Intravenous
- MI
Myocardial infarction
- N
Number (of patients)
- NS
Not specified
- p
p value
- Pb
Placebo
- PE
Pulmonary embolism
- P-gp
P glycoprotein
- R
Rivaroxaban
- RR
Relative risk
- Rx
Treatment
- S
Standard therapy
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
- Tr
Transition
- UFH
Unfractionated heparin
- UNL
Upper normal limit
- V
VTE
- VKA
Vitamin K antagonist
- W
Warfarin
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bauersachs R., Berkowitz S.D., Brenner B., et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 2.Galanis T., Merli G.J. Contemporary treatment of venous thromboembolic disease. Cardiol. Clin. 2015;33(1):49–57. doi: 10.1016/j.ccl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Walter R.J., Moores L.K., Jimenez D. Pulmonary embolism: current and new treatment options. Curr. Med. Res. Opin. 2014;30(10):1975–1989. doi: 10.1185/03007995.2014.936931. [DOI] [PubMed] [Google Scholar]
- 4.Prins M.H., Bamber L., Cano S.J., et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb. Res. 2015;135(2):281–288. doi: 10.1016/j.thromres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Holbrook A., Schulman S., Witt D., et al. Antithrombotic therapy and prevention of thrombosis. 9th. American college of chest physicians evidence-based clinical practice guidelines Chest; 2012. pp. 152–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulman S., Angeras U., Bergqvist D., Eriksson B., Lassen M.R., Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010;8(1):202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 7.Madan S., Shah S., Partovi S., et al. Use of novel oral anticoagulant agents in atrial fibrillation: current evidence and future perspective. Cardiovasc. Diagn. Ther. 2014;4(4):314–323. doi: 10.3978/j.issn.2223-3652.2014.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabral K.P. Pharmacology of the new target-specific oral anticoagulants. J. Thromb. Thrombolysis. 2013;36(2):133–140. doi: 10.1007/s11239-013-0929-5. [DOI] [PubMed] [Google Scholar]
- 9.Saraf K., Morris P., Garg P., et al. Non-vitamin K antago¬nist oral anticoagulants (NOACs): clinical evidence and therapeutic considerations. Postgrad. Med. J. 2014;90(1067):520–528. doi: 10.1136/postgradmedj-2014-132605. [DOI] [PubMed] [Google Scholar]
- 10.FDA rivaroxaban[cited: Feb 28th 2015). Available from:http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/ 022406s004lbl.pdf. 2015.
- 11.FDA dabigatran[cited: Feb 28th 2015]. Available from:http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/ 022512s007lbl.pdf.
- 12.FDA apixaban.[cited: April 5th 2015]. Available from:http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/ 202155s006lbl.pdf.
- 13.Prins M.H., Lensing A.W., Bauersachs R., et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb. J. 2013;11(1):21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The EINSTEIN-PE investigators Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson B.I., Kakkar A.K., Turpie A.G., et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J. Bone Joint Surg. Br. 2009;91(5):636–644. doi: 10.1302/0301-620X.91B5.21691. [DOI] [PubMed] [Google Scholar]
- 16.Schulman S., Kearon C., Kakkar A.K., et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S., Kakkar A.K., Goldhaber S.Z., et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S., Kearon C., Kakkar A.K., et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N. Engl. J. Med. 2013;368(8):709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 19.FDA approval apixaban[cited: Feb 22th 2015]. Available from:http://www.accessdata.fda.gov/drugsatfda_docs/appletter/ 2014/202155Orig1s006ltr.pdf .
- 20.Agnelli G., Buller H.R., Cohen A., et al. Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 21.Agnelli G., Buller H.R., Cohen A., et al. Apixaban for extended treatment of venous thromboembolism. N. Engl. J. Med. 2013;368(8):699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 22.Mantha S., Ansell J. Indirect comparison of dabigatran, rivaroxaban, apixaban and endoxaban for the treatment of acute venous thromboembolism. J. Thromb. Thrombolysis. 2015;39(2):155–165. doi: 10.1007/s11239-014-1102-5. [DOI] [PubMed] [Google Scholar]
- 23.Alotaibi G., Alsaleh K., Wu C., Mcmurtry M.S. Dabigatran, rivaroxaban and apixaban for extended venous thromboembolism treatment: network meta-analysis. Int. Angiol. 2014;33(4):301–308. [PubMed] [Google Scholar]
- 24.Hirschl M., Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism – a systematic review with indirect comparisons. Vasa. 2014;43(5):353–364. doi: 10.1024/0301-1526/a000373. [DOI] [PubMed] [Google Scholar]
- 25.Rollins B.M., Silva M.A., Donovan J.L., Kanaan A.O. Evaluation of oral anticoagulants for the extended treatment of venous thromboembolism using a mixed-treatment comparison, meta-analytic approach. Clin. Ther. 2014;36(10):1454–1464. doi: 10.1016/j.clinthera.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Cui J., Wu B., Liu C., Li Z. A systematic review and adjusted indirect comparison of oral anticoagulants. Orthopedics. 2014;37(11):763–771. doi: 10.3928/01477447-20141023-07. [DOI] [PubMed] [Google Scholar]
- 27.FDA notice (Cited Feb 27th 2016) available at:http://www. fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsf orHumanMedicalProducts/ucm396324.htm . 2016.
- 28.Cohen D. Rivaroxaban: can we trust the evidence? BMJ. 2016;352:i575. doi: 10.1136/bmj.i575. [DOI] [PubMed] [Google Scholar]
- 29.Patel M. Point-of-Care Warfarin Monitoring in the ROCKET AF Trial. N. Engl. J. Med. 2016;374:785–788. doi: 10.1056/NEJMc1515842. [DOI] [PubMed] [Google Scholar]
- 30.Chambers S., Chadda S., Plumb J.M. How much does international normalized ratio monitoring cost during oral anticoagulation with a vitamin K antagonist? A systematic review. Int. J. Lab. Hematol. 2010;32(4):427–442. doi: 10.1111/j.1751-553X.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 31.Kahn S.R., Lim W., Dunn A.S., et al. 9th ed: Chest: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falck-Ytter Y., Francis C., Johanson N., et al. 9th ed: Chest: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.; 2012. Prevention of VTE in Orthopedic Surgery Patients; Antithrombotic Therapy and Prevention of thrombosis; pp. e278S–e325S.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar C.A., Malaga G., Malasquez G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database Syst. Rev. 2010;(4):CD005981. doi: 10.1002/14651858.CD005981.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raskob G.E., Gallus A.S., Pineo G.F., et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J. Bone Joint Surg. Br. 2012;94(2):257–264. doi: 10.1302/0301-620X.94B2.27850. [DOI] [PubMed] [Google Scholar]
- 35.Miebach W., Seifried E. New direct oral anticoagulants – current therapeutic options and treatment recommendations for bleeding complications. Thromb. Haemost. 2012;108(4):625–632. doi: 10.1160/TH12-05-0319. [DOI] [PubMed] [Google Scholar]
- 36.Anderson E., Johnke K., Leedahl D., et al. Novel oral anticoagulants vs warfarin for the management of postoperative atrial fibrillation: clinical outcomes and cost analysis. Am. J. Surg. 2015;210(6):1095–1103. doi: 10.1016/j.amjsurg.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Levy J.H., Spyropoulos A.C., Samama C.M., Douketis J. Direct oral anticoagulants. JACC Cardiovasc. Interv. 2014;7(12):1333–1351. doi: 10.1016/j.jcin.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Boehringer Ingelheim Pharma GmbH & Co. KG. PRADAXA(dabigatran etexilate mesylate) capsules for oral use.[cited: May 10th 2015]. Available from:http://us. boehringer-ingelheim.com/news_events/press_releases/ press_release_archive/2014/04-07-14-fda-approves-pradaxadabigatran- etexilate-mesylate-treatment-reduction-risk-ofrecurrence- deep-venous-thrombosis-pulmonary-embolism. html.
- 39.Greig S., McKeage K. Dabigatran Etexilate: A Review of Its Use in the Treatment of Acute Venous Thromboembolism and Prevention of Venous Thromboembolism Recurrence. Drugs. 2014;74(15):1785–1800. doi: 10.1007/s40265-014-0304-7. [DOI] [PubMed] [Google Scholar]
- 40.Vedovati M.C., Germini F., Agnelli G., Becattini C. Direct Oral Anticoagulants in Patients With VTE and Cancer: A Systematic Review and Meta-analysis. Chest. 2015;147(2):475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 41.Akl E.A., Kahale L., Barba M., et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst. Rev. 2014;7:CD006650. doi: 10.1002/14651858.CD006650.pub4. [DOI] [PubMed] [Google Scholar]
- 42.Levy J.H., Faraoni D., Spring J.L., Douketis J.D., Samama C.M. Managing new oral anticoagulants in the perioperative and Intensive care setting. Anesthesiology. 2013;118(6):1466–1474. doi: 10.1097/ALN.0b013e318289bcba. [DOI] [PubMed] [Google Scholar]
- 43.Babilonia K., Trujillo T. The role of prothrombin complex concentrates in reversal of target specific anticoagulants. Thromb. J. 2014;12:8. doi: 10.1186/1477-9560-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebright J., Mousa S.A. Oral anticoagulants and status of antidotes for the reversal of bleeding risk. Clin. Appl. Thromb. Hemost. 2015;21(2):105–114. doi: 10.1177/1076029614545211. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W., Schwarting S., Illanes S., et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with direct thrombin inhibitor dabigatran. Stroke. 2011;42(12):3594–3599. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 46.Hobl E., Jilma B. Towards the development of specific antidotes: Idarucizumab for reversal of dabigatran effects. Thromb. Haemost. 2015;113(6):1162–1163. doi: 10.1160/TH15-04-0324. [DOI] [PubMed] [Google Scholar]
- 47.Pollack C.V., Jr, Reilly P.A., Eikelboom J., et al. Idarucizumab for Dabigatran Reversal. N. Engl. J. Med. 2015;373(6):511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 48.FDA Idarucizumab[cited: February 20th 2016]. Available from:http://www.accessdata.fda.gov/drugsatfda_docs/label/ 2015/761025lbl.pdf.
- 49.Eerenberg E.S., Kamphuisen P.W., Sijpkens M.K., Meijers J.C., Buller H.R., Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 50.Godier A., Miclot A., Le Bonniec B., et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabit model. Anesthesiology. 2012;116(1):94–102. doi: 10.1097/ALN.0b013e318238c036. [DOI] [PubMed] [Google Scholar]
- 51.Simpson E., Lin Y., Stanworth S., Birchall J., Doree C., Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia. Cochrane Database Syst. Rev. 2012;3:CD005011. doi: 10.1002/14651858.CD005011.pub4. [DOI] [PubMed] [Google Scholar]
- 52.Turpie A.G., Kreutz R., Llau J., Norrving B., Haas S. Management consensus guidance for the use of rivaroxaban -an oral, direct factor Xa inhibitor. Thromb. Haemost. 2012;108(5):876–886. doi: 10.1160/TH12-03-0209. [DOI] [PubMed] [Google Scholar]
- 53.Levi M.M., Eerenberg E., Lowenberg E., Kamphuisen P.W. Bleeding in patients using new anticoagulants or antiplatelet agents: risk factors and management. Neth. J. Med. 2010;68(2):68–76. [PubMed] [Google Scholar]
- 54.Ebright J., Mousa S.A. Oral anticoagulants and status of antidotes for the reversal of bleeding risk. Clin. Appl. Thromb. Hemost. 2015;21:5–14. doi: 10.1177/1076029614545211. [DOI] [PubMed] [Google Scholar]
- 55.Siegal D., Curnutte J., Connolly S., et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N. Engl. J. Med. 2015;373:2413–2424. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 56.Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding. ClinicalTrials.gov Identifier: NCT02329327. https://clinicaltrials.gov/ct2/show/NCT02329327 .
- 57.Laulicht B., Bakhru S., Lee C., et al. Small molecule antidote for anticoagulants. Circulation. 2012;126:A11395. [Abstract]. [Google Scholar]
- 58.Ansell J.E., Bakhru S.H., Laulicht B.E., et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N. Engl. J. Med. 2014;371(22):2141–2142. doi: 10.1056/NEJMc1411800. [DOI] [PubMed] [Google Scholar]