Abstract
A protein identified as the 158-amino acid product of the greA gene was isolated from Escherichia coli. When added to a halted ternary transcription complex, the GreA protein induced cleavage and removal of the 3' proximal dinucleotide from the nascent RNA. The new 3' terminus generated by the cleavage could be extended into longer transcripts. GreA-mediated cleavage of a transcript appears to permit a ternary complex to resume transcription from a state of indefinite elongation arrest induced by a specific DNA site. The GreA protein tended to interact with RNA polymerase during purification and recycled between RNA polymerase molecules in the course of the in vitro cleavage reaction. Similar biochemical activities have been reported in eukaryotic RNA polymerases, indicating that transcript cleavage and restart of elongation may be a general transcriptional mechanism.
Full text
PDF
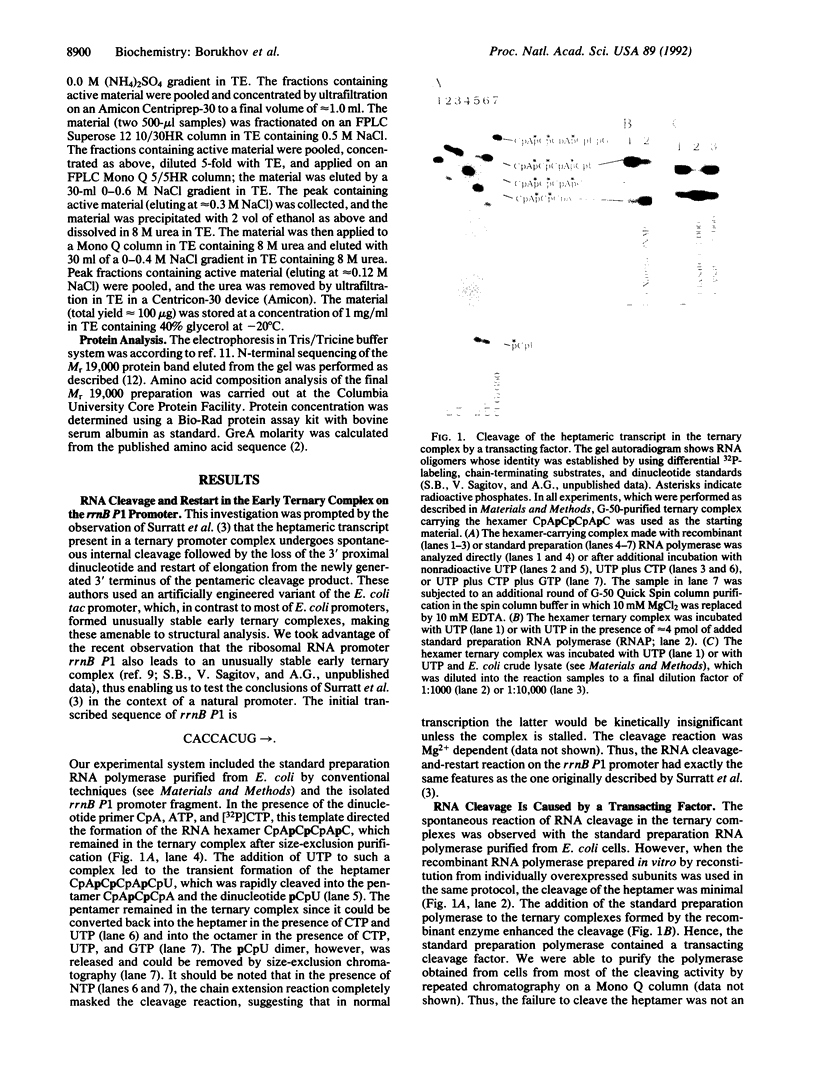

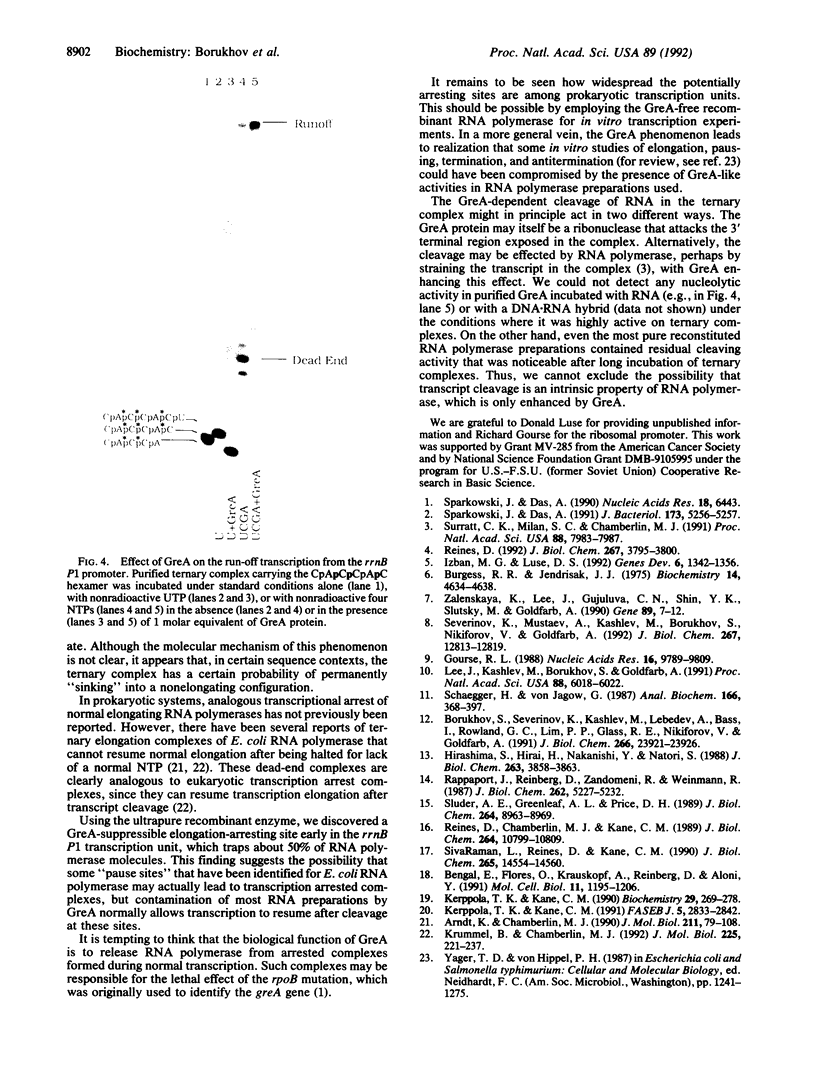
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt K. M., Chamberlin M. J. RNA chain elongation by Escherichia coli RNA polymerase. Factors affecting the stability of elongating ternary complexes. J Mol Biol. 1990 May 5;213(1):79–108. doi: 10.1016/S0022-2836(05)80123-8. [DOI] [PubMed] [Google Scholar]
- Bengal E., Flores O., Krauskopf A., Reinberg D., Aloni Y. Role of the mammalian transcription factors IIF, IIS, and IIX during elongation by RNA polymerase II. Mol Cell Biol. 1991 Mar;11(3):1195–1206. doi: 10.1128/mcb.11.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S., Severinov K., Kashlev M., Lebedev A., Bass I., Rowland G. C., Lim P. P., Glass R. E., Nikiforov V., Goldfarb A. Mapping of trypsin cleavage and antibody-binding sites and delineation of a dispensable domain in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1991 Dec 15;266(35):23921–23926. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Gourse R. L. Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988 Oct 25;16(20):9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima S., Hirai H., Nakanishi Y., Natori S. Molecular cloning and characterization of cDNA for eukaryotic transcription factor S-II. J Biol Chem. 1988 Mar 15;263(8):3858–3863. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. The RNA polymerase II ternary complex cleaves the nascent transcript in a 3'----5' direction in the presence of elongation factor SII. Genes Dev. 1992 Jul;6(7):1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. Analysis of the signals for transcription termination by purified RNA polymerase II. Biochemistry. 1990 Jan 9;29(1):269–278. doi: 10.1021/bi00453a037. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. RNA polymerase: regulation of transcript elongation and termination. FASEB J. 1991 Oct;5(13):2833–2842. doi: 10.1096/fasebj.5.13.1916107. [DOI] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. Structural analysis of ternary complexes of Escherichia coli RNA polymerase. Individual complexes halted along different transcription units have distinct and unexpected biochemical properties. J Mol Biol. 1992 May 20;225(2):221–237. doi: 10.1016/0022-2836(92)90917-9. [DOI] [PubMed] [Google Scholar]
- Lee J., Kashlev M., Borukhov S., Goldfarb A. A beta subunit mutation disrupting the catalytic function of Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6018–6022. doi: 10.1073/pnas.88.14.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J., Reinberg D., Zandomeni R., Weinmann R. Purification and functional characterization of transcription factor SII from calf thymus. Role in RNA polymerase II elongation. J Biol Chem. 1987 Apr 15;262(11):5227–5232. [PubMed] [Google Scholar]
- Reines D., Chamberlin M. J., Kane C. M. Transcription elongation factor SII (TFIIS) enables RNA polymerase II to elongate through a block to transcription in a human gene in vitro. J Biol Chem. 1989 Jun 25;264(18):10799–10809. [PubMed] [Google Scholar]
- Reines D. Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J Biol Chem. 1992 Feb 25;267(6):3795–3800. [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Severinov K., Mustaev A., Kashlev M., Borukhov S., Nikiforov V., Goldfarb A. Dissection of the beta subunit in the Escherichia coli RNA polymerase into domains by proteolytic cleavage. J Biol Chem. 1992 Jun 25;267(18):12813–12819. [PubMed] [Google Scholar]
- SivaRaman L., Reines D., Kane C. M. Purified elongation factor SII is sufficient to promote read-through by purified RNA polymerase II at specific termination sites in the human histone H3.3 gene. J Biol Chem. 1990 Aug 25;265(24):14554–14560. [PubMed] [Google Scholar]
- Sluder A. E., Greenleaf A. L., Price D. H. Properties of a Drosophila RNA polymerase II elongation factor. J Biol Chem. 1989 May 25;264(15):8963–8969. [PubMed] [Google Scholar]
- Sparkowski J., Das A. Location of a new gene, greA, on the Escherichia coli chromosome. J Bacteriol. 1991 Sep;173(17):5256–5257. doi: 10.1128/jb.173.17.5256-5257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkowski J., Das A. The nucleotide sequence of greA, a suppressor gene that restores growth of an Escherichia coli RNA polymerase mutant at high temperature. Nucleic Acids Res. 1990 Nov 11;18(21):6443–6443. doi: 10.1093/nar/18.21.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt C. K., Milan S. C., Chamberlin M. J. Spontaneous cleavage of RNA in ternary complexes of Escherichia coli RNA polymerase and its significance for the mechanism of transcription. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7983–7987. doi: 10.1073/pnas.88.18.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalenskaya K., Lee J., Gujuluva C. N., Shin Y. K., Slutsky M., Goldfarb A. Recombinant RNA polymerase: inducible overexpression, purification and assembly of Escherichia coli rpo gene products. Gene. 1990 Apr 30;89(1):7–12. doi: 10.1016/0378-1119(90)90199-2. [DOI] [PubMed] [Google Scholar]