Introduction
Great progress has been made in many areas of pediatric oncology. However, tumors of the central nervous system (CNS) remain a significant challenge. A recent explosion of data has led to an opportunity to understand better the molecular basis of these diseases and is already providing a foundation for the pursuit of rationally chosen therapeutics targeting relevant molecular pathways. The molecular biology of pediatric brain tumors is shifting from a singular focus on basic scientific discovery to a platform upon which insights are being translated into therapies.
High Grade Glioma
Histopathology and Genetics
Pediatric high grade gliomas (pHGGs) are histologically indistinguishable from HGGs occurring in adults (aHGGs) and are graded according to the WHO classification of CNS tumors. High grade gliomas include WHO grade III and IV tumors1. Histologically, grade III glioma (anaplastic astrocytoma) is characterized by atypical nuclei, increased cellularity, and increased mitotic activity1. Grade IV glioma, also known as glioblastoma multiforme (GBM), is the most pathologically advanced and clinically aggressive2. These tumors are characterized by vascular proliferation and necrosis in addition to the characteristics of grade III glioma1. The distribution of sites within the CNS in which these high grade tumors occur varies amongst age groups: aHGGs typically occur in the cerebral cortex, while pHGGs are more widely distributed3. The highest grade of oligodendroglioma, referred to as anaplastic oligodendroglioma (WHO grade III), and mixed oligo-astrocytomas are observed, albeit rarely, in pHGG patients4.
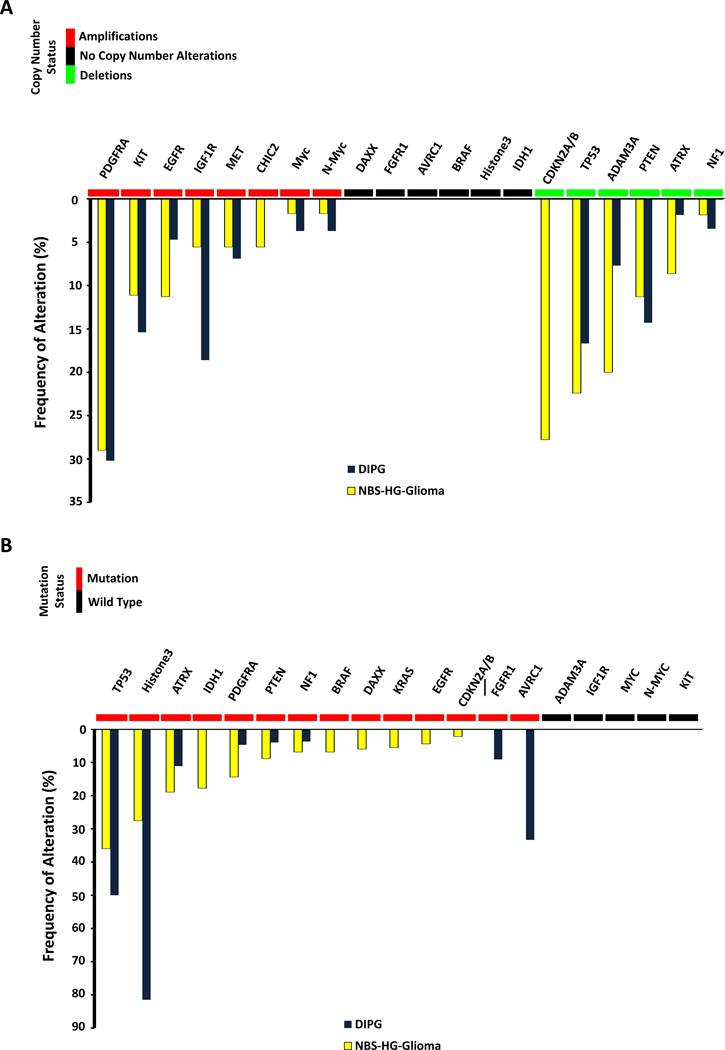
Molecularly, pHGGs are distinct from aHGG and are characterized by gene amplifications, deletions, and other types of mutations5–18. These are summarized in Figure 1. The most commonly amplified genes in pHGGs are receptor tyrosine kinases including PDGFRA, EGFR, KIT, IGF1R, and MET12,14,16,17 (Figure 1A). The most commonly deleted genes include CDKN2A, TP53, and ADAM3A6,8,9,12 (Figure 1A). Other mutations, both activating and inactivating, have been reported (Figure 1B), and amongst the inactivating mutations, homozygous inactivation of p53 and histone 3.3 (H3F3A) are the most common. While the genetic differences amongst gliomas occurring at different locations in the CNS have not been completely characterized, data available to date suggest that a K27M mutation in histone3.3 occurs at a much lower rate in non-brainstem HGG (NBS-HGG) than in DIPG3,18. Also, a G34R mutation in histone 3.3 is thought to be present exclusively in NBS-HGG18. The histone 3.3 K27M mutation acts as a dominant negative inhibitor of histone methylation,11 and patients whose HGG bear that mutation have distinctive DNA methylation patterns that alone are sufficient to define a subgroup of pHGG13. Gene rearrangements are also observed in glioma leading to fusion products that may serve as tumor specific drug targets. In pHGG, both the neurotrophic tyrosine kinase receptors (NTRKs)15 and PDGFRA19 have fusion variants that drive the transformation of normal CNS cells19. Differences in the genomic alterations between pediatric and adult high-grade gliomas have been identified. PDGFRA is amplified much more frequently in pHGG than in aHGG17 and EGFR is more commonly amplified than PDGFRA17 in aHGG. Additionally, histone 3.3 mutations are observed almost exclusively in pHGGs7.
Figure 1.
Genetic alterations observed in pHGG. (A) Copy number alterations and (B) mutations of the major genes with genetic alterations observed in non-brainstem high grade glioma (NBS-HGG) and DIPG5–18.
Current Therapy and Therapeutic Opportunities
Current treatment for pHGG occurring in the cerebrum typically includes initial surgery followed by radiation and chemotherapy20. There is widespread agreement that total resection of tumor tissue improves patient outcome. For patients treated on the Children’s Cancer Group study CCG945, patients with >90% resection had improved 5-year progression-free survival (35+/− 7% compared to 17+/− 4%)21. Focal radiation therapy has also become standard in the treatment of patients greater than 3 years of age with pHGG. However, the toxicities of radiation therapy in children can be particularly significant because of the increased sensitivity of the developing brain to irradiation22,23. Long-term side effects in survivors include cognitive deficits, cerebrovascular disease, and secondary tumors and can oftentimes reduce patients’ quality of life24. The role of chemotherapy in managing patients with pHGG is uncertain, and although evidence of efficacy is modest25,26, chemotherapeutic agents are often employed in the treatment of these patients27. While the results of a Children’s Oncology Group trial evaluating temozolomide for the treatment of pediatric patients with high grade glioma were disappointing28, the drug continues to be utilized in the treatment of these patients. Tolerability and ease of administration may be important factors in the choice of chemotherapy for pediatric patients. Regimens containing nitrosoureas are also employed20,27. Given the lack of effective therapies for pHGG, patients should be treated on a clinical trial if possible. When gliomas recur, the treatment approach depends on the therapy patients have received previously as the potential for repeated surgery or additional radiation is often limited by previous treatment.
Currently, novel agents targeting key pathologic pathways or the products of mutated genes are under investigation for the treatment of pHGG. Imatinib, an inhibitor of PDGFR activation and a prototypic targeted therapy, has been examined in a phase 1 clinical trial conducted in pHGG patients and a tolerable dose was determined29. However, no phase II results are available and a suspected link between Imatinib and increased hemorrhage in these patients has raised concerns of unacceptable toxicity29. Other inhibitors of PDGFR are also being evaluated in clinical trials (see Table 1).
TABLE 1.
Treatment trials targeting molecular abnormalities in pediatric brain tumors
| Clinical Trial Number |
Tumor Type |
Title | Agent | Target | Sponsor |
|---|---|---|---|---|---|
| NCT01902771 | HGG | Dendritic Cell Vaccine Therapy With In Situ Maturation in Pediatric Brain Tumors |
dendritic cell vaccine |
vaccine | University of Miami |
|
NCT00074334 (terminated) |
HGG | TP-38 Toxin in Treating Young Patients With Recurrent or Progressive Supratentorial High-Grade Glioma |
TP-38 (TGFa-PE38 immunotoxin) via local delivery |
PBTC | |
| NCT02031965 | HGG | Oncolytic HSV-1716 in Treating Younger Patients With Refractory or Recurrent High Grade Glioma That Can Be Removed By Surgery |
HSV-1716 (intratumoral) |
PBTC | |
| NCT01393912 | DIPG / HGG | PDGFR Inhibitor Crenolanib in Children/Young Adults with DIPG or recurrent HGG |
crenolanib | PDGFR | St. Jude Children’s Research Hospital |
| NCT01644773 | DIPG / HGG | Study of the Combination of Crizotinib and Dasatinib in Pediatric Research Participants With Diffuse Pontine Glioma and High-Grade Glioma |
crizotinib dasatinib |
c-Met / Alk PDGFR/src/c- kit |
St. Jude Children’s Research Hospital |
| NCT00890786 | DIPG / HGG | A study of bevacizumab therapy in patients with newly diagnosed HGG or DIPG |
bevacizumab | VEGF | Children’s Hospital Medical Center, Cincinnati |
| NCT00879437 | DIPG / HGG | Valproic Acid and Radiation Followed by Maintenance Valproic Acid and Bevacizumab in Children With High Grade Gliomas or Diffuse Intrinsic Pontine Glioma |
valproic acid bevacizumab |
HDAC VEGF |
Baylor College of Medicine |
| NCT02359565 | DIPG / HGG | Pembrolizumab in Treating Younger Patients With Recurrent, Progressive, or Refractory High-Grade Gliomas or Diffuse Intrinsic Pontine Gliomas |
pembrolizumab | PD-1 | PBTC |
| NCT01952769 | DIPG / HGG | Anti PD1 Antibody in Diffuse Intrinsic Pontine Glioma |
CT-011 (pidilizumab) |
PD-1 | Hadassah Medical Oganization, Jerusalem, Israel |
| NCT01400672 | DIPG | Imiquimod/Brain Tumor Initiating Cell (BTIC) Vaccine in Brain Stem Glioma |
imiquimod / vaccine from cell line GBM-6 |
Vaccine | Masonic Cancer Center, University of Minnesota |
| NCT01502917 | DIPG | Convection-Enhanced Delivery of 124I-8H9 for Patients With Non-Progressive Diffuse Pontine Gliomas Previously Treated With External Beam Radiation Therapy |
124I-8H9 | B7-H3 | MSKCC |
| NCT01182350 | DIPG | Molecularly Determined Treatment of Diffuse Intrinsic Pontine Gliomas (DIPG) |
bevacizumab erlotinib temozolomide |
VEGF EGFR |
Dana-Farber Cancer Institute |
| NCT02233049 | DIPG | Biological Medicine for Diffuse Intrinsic Pontine Glioma (DIPG) Eradication (BIOMEDE) |
erlotinib everolimus dasatinib |
EGFR mTOR PDGFR/src/c- kit |
Gustave Roussy, Cancer Campus, Grand Paris |
| NCT01165333 | DIPG | Cilengitide in Combination With Irradiation in Children With Diffuse Intrinsic Pontine Glioma |
cilengitide | αvβ3 and αvβ5 integrins |
Centre Oscar Lambret, Lille, France |
| NCT01922076 | DIPG | WEE1 Inhibitor MK-1775 and Local Radiation Therapy in Treating Younger Patients With Newly Diagnosed Diffuse Intrinsic Pontine Gliomas |
MK-1775 | Wee1 | COG phase 1 consortium |
| NCT01189266 | DIPG | Vorinostat and Radiation Therapy Followed by Maintenance Therapy With Vorinostat in Treating Younger Patients With Newly Diagnosed Diffuse Intrinsic Pontine Glioma |
vorinostat | HDAC | COG |
| NCT01514201 | DIPG | Veliparib, Radiation Therapy, and Temozolomide in Treating Younger Patients With Newly Diagnosed Diffuse Pontine Gliomas |
Velaparib (ABT-888) |
PARP | PBTC |
| NCT01884740 | glioma | Phase I/II Trial Of Super-Selective Intraarterial Infusion Of Erbitux and Bevacizumab For Treatment Of Relapsed/Refractory Intracranial Glioma In Patients Under 22 Years Of Age |
cetuxumab bevacizumab |
HER2 VEGF |
Weill Medical College of Cornell University |
| NCT01130077 | glioma | A Pilot Study of Glioma Associated Antigen Vaccines in Conjunction With Poly-ICLC in Pediatric Gliomas |
glioma antigen peptides vaccine |
vaccine | University of Pittsburgh |
| NCT01795313 | ependymoma | Immunotherapy for recurrent ependymomas in children Treatment for Recurrent Ependymomas Using HAL-A2 Restricted Tumor Antigen Peptides in Combination with Imiquimod |
imiquimod vaccine |
vaccine | University of Pittsburgh |
| NCT02125786 | ependymoma | A Trial of Surgery and Fractionated Re- Irradiation for Recurrent Ependymoma |
re-irradiation | St. Jude Children’s Research Hospital |
|
| NCT01188096 | LGG | A Trial of Poly-ICLC in the Management of Recurrent Pediatric Low Grade Gliomas |
poly-ICLC | Immune modulatory |
UCSD |
| NCT01887522 | LGG | Study of Vinblastine in Combination With Nilotinib in Children, Adolescents, and Young Adults (VINILO) |
nilotinib vinblastine |
PDGFR | Gustave Roussy, Cancer Campus, Grand Paris |
| NCT01734512 | LGG | PNOC 001: Phase II study of Everolimus for Recurrent or Progressive low-grade gliomas in Children |
everolimus | mTORR | PNOC |
| NCT01089101 | LGG | Selumetinib in treating young patients with recurrent or refractory low grade glioma |
selumetinib | MEK1 | PBTC |
| NCT02332889 | HGG Medullo- blastoma CNS PNET |
Phase I/II: Decitabine/Vaccine Therapy in Relapsed/Refractory Pediatric High Grade Gliomas/Medulloblastomas/CNS PNETs |
dendritic cell vaccine targeting NY-ESO-1; MAGE-A1, and MAGE-A3 |
vaccine | University of Louisville |
| NCT02255461 | CNS tumors | Palbociclib Isethionate in Treating Younger Patients With Recurrent, Progressive, or Refractory Central Nervous System Tumors |
palbociclib |
CDK4,6 | PBTC |
| NCT01677741 | Phase 1 | A Study to determine safety, tolerability and pharmacokinetics of oral Dabrafenib in children and adolescent subjects |
dabrafenib | BRAF | GlaxoSmithKline |
| NCT02124772 | Tumors with V600 mutation |
Study to investigate safety, Pharmakokinetic (PK), pharmacodynamics (PD) and clinical activity of Trametinib in subjects with cancer or plexiform neurofibromas and Trametinib in combination with Dabrafenib in subjects with cancers harboring V600 mutations |
dabrafenib trametinib |
BRAF MEK |
GlaxoSmithKline |
Invasion of normal tissue is a hallmark of HGG that typically makes complete tumor resection impossible and fuels recurrence after surgery30. Inhibiting pathways that regulate invasion may be advantageous to patients with HGG31. Cilengitide, an inhibitor of αvβ3 and αvβ5 integrins, has activity decreasing in vitro surrogates of tissue invasion and has been actively studied in multiple cancers32. In an early phase trial of cilengitide as a single agent in patients with relapsed pHGG, no effect on patient outcomes was noted33,34. Further studies of cilengitide combined with other agents in adults with GBM provided no evidence of anti-invasive activity and did not affect survival35,36.
A potential reason why targeted therapies have, to date, had limited success in the treatment of patients with pHGG is intra-tumoral variation in target gene expression.The widespread genetic heterogeneity of HGG suggests that cytotoxic agents with increased activity or combination therapy with agents targeting different genetic alterations within the same tumor could be especially advantageous37. Targeting mechanisms of tumor invasion to inhibit tumor spreading, while simultaneously targeting genetic drivers (such as PDGFRA or NRTK), might further enhance patient survival. Additional therapeutic strategies being explored in patients with pHGG include dendritic cell-based vaccination and angiogenesis inhibition; however, to date, their use has not provided a survival advantage38–41.
The presence of H3.3 mutations and aberrant methylation patterns in pHGG suggests that epigenetic modifiers may be effective therapies for these tumors. Panobinistat, a histone deacetylase inhibitor, has shown preclinical activity against pHGG and clinical trials with this agent are planned42. The combination of a histone demethylase inhibitor with panobinostat was synergistic in vitro and in xenograft models of DIPG43 and suggests that this drug combination could have activity in pHGG with H3.3 mutations.
Medulloblastoma
Histopathology and Genetics
Medulloblastoma accounts for 15–20% of pediatric brain tumors 44. While it can occur at any age from infancy through adulthood, it is most typically seen in children with bimodal incidence peaks between three and four and eight and nine years of age 45. It arises in the cerebellum and commonly invades the fourth ventricle with about one third of cases developing metastases throughout the neuraxis.
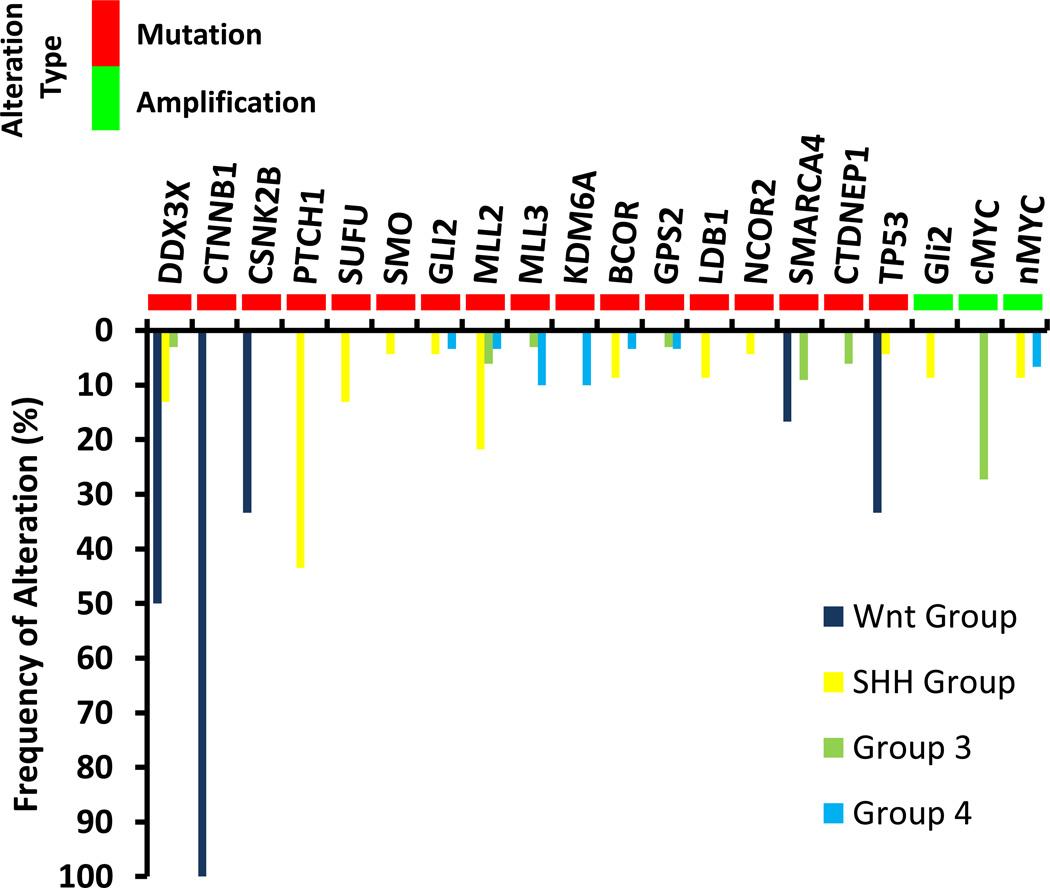
Medulloblastoma is a primitive embryonal tumor composed of densely packed, small, round, blue cells with irregular nuclei and appears undifferentiated or less commonly, with neuronal features2. Neuronal markers such as synaptophysin are often focally expressed 46. The 2007 WHO classification of tumors of the CNS describes five histologic variants of medulloblastoma including classic histology, anaplastic, large cell variant, desmoplastic/nodular, and medulloblastoma with extensivenodularity2. While these histologic variants have prognostic relevance, molecular analysis has allowed for the more precise classification of these tumors. There are four well-recognized molecular subgroups of medulloblastoma: WNT (wingless), SHH (Sonic Hedgehog), Group 3, and Group 447. Subtype specific genetic alterations in medulloblastoma that include mutations and amplifications are summarized in Figure 2 48,49. As suggested by the nomenclature, the molecular drivers of oncogenesis in the first two groups are better understood. WNT is a family of receptors involved in embryogenesis and cell cycle control50. This subgroup comprises approximately 10% of tumors and has the best prognosis with overall survival near 90%. It occurs predominantly in older children47,51. The most common mutations in the WNT subgroup occur in CTNNB1 encoding β-catenin, and this subtype of medulloblastoma can be identified by nuclear accumulation of β-catenin52. WNT medulloblastoma is also associated with losses of chromosome 653.
Figure 2.
The SHH group accounts for approximately 30% of medulloblastoma48. SHH signaling is physiologically initiated by the SHH ligand binding to the receptor Patched1 (PTCH1) which leads to the de-repression of smoothened (SMO) activity and activation of GLI transcription factors49. Mutations in this pathway most commonly occur in PTCH1, but alterations in SMO and suppressor of fused (SUFU) have also been described in SHH medulloblastoma54. Deletions of 9q are most commonly found in this group.
The molecular pathogenesis of Group 3 (25% of cases) and Group 4 (35% of cases) is not as well understood47,48. Group 3 tumors have a poor prognosis with approximately 50% overall survival at five years. Group 3 disease is characterized by an increased frequency of copy number alterations, including loss of chromosome 17p and gain of 17q to generate an isochromosome 17q. High levels of MYC expression are also seen in Group 3 tumors and a subset of these has amplification of MYCN. Group 3 tumors have the worst prognosis of the four molecular subgroups and patients frequently have metastatic disease at diagnosis. While targeted therapies for Group 3 medulloblastoma have not been identified, high throughput screening has identified the combination of gemcitabine and pemetrexed as a possible therapy for this subtype55. Group 4 also has a high degree of chromosomal copy number aberrations as well as isochrome17q. It is characterized by a neuronal molecular signature and amplification of MYCN, MLL2, and MLL3 and KDM6A, a histone demethylase.
The parsing of medulloblastoma into four distinct molecular groups has greatly advanced the field and additional subdivisions within each group are being uncovered. Recent work by Northcott and colleagues evaluated somatic copy number aberrations in 1087 unique medulloblastomas56. They identified the presence of tandem duplication of the Parkinson’s gene SNCAIP in a subgroup of Group 4 medulloblastomas. They also identified recurrent translocations of PVT1 restricted to Group 3 tumors 56. In a recent multicenter study prognostically relevant cytogenetic features were identified that may continue to further stratify medulloblastoma within the current four molecular groups 57.
Current Therapy and Therapeutic Opportunities
The current management of medulloblastoma in children greater than three to five years of age includes maximal safe resection of the tumor followed by a combination of radiation and chemotherapy 58–60. Disease is classified as “average risk” in patients with a total or near-total resection and no evidence of tumor dissemination at the time of diagnosis. These patients are treated with adjuvant craniospinal radiation and a boost to the tumor bed followed bychemotherapy. This therapeutic strategy leads to five-year event free survival in more than 80% of these patients 61. However, the toxicity of surgical resection of a posterior fossa lesion, neuraxis radiation and chemotherapy are considerable. Long-term sequelae include cerebellar mutism, hearing loss, endocrine abnormalities, and neurocognitive deficits 62.Recent clinical trials have begun to explore decreasing the dose of craniospinal radiation in an effort to decrease the late effects. Patients with disseminated disease at diagnosis or sub-totally resected tumors are considered to have “high-risk” disease63,64. Infants and young children with medulloblastoma present a particular therapeutic challenge. Because of the high risk of neurologic sequelae, radiation therapy has been either avoided or delayed in these patients and they have received upfront chemotherapy alone58,65.
The molecular characterization of medulloblastoma provides opportunities to improve patient care both through improving the stratification of disease and the identification of therapeutic targets. WNT tumors have an excellent prognosis using current therapies with ten-year event free survival rates above 95% 66–68. This information suggests a strategy of de-intensifying therapy for patients with this group of medulloblastoma in an attempt to decrease late effects69,70. While MYCN amplification or the fusion gene PVT1-MYC is known to occur in Group 3 tumors, these abnormalities have been difficult to target therapeutically. Early clinical trials targeting the SHH pathway have shown some efficacy in the treatment of medulloblastoma with alterations in the SHH pathway. In a phase 1 study of vismodegib, which represses the SHH pathway by inhibition of SMO, in children with recurrent medulloblastoma activity was seen in one out of 3 evaluable patients with SHH medulloblastoma and 0 of 10 patients with other medulloblastoma subtypes 71. Preclinical data using both xenograft and transgenic murine models have shown that tumors with abnormalities downstream of SMO such as SUFU or GLI2 are resistant to SMO inhibition 72,73, suggesting that further stratification of patients will be necessary in order to optimize the use of pharmacological inhibition at various points in the SHH pathway.
Ependymoma
Histopathology and Genetics
Ependymomas occur in both children and adults and can arise throughout the entire neuraxis. While spinal cord tumors are more common in adults, in pediatric patients approximately 70% of ependymomas arise in the posterior fossa2,74. The WHO divides ependymomas into three subtypes. WHO Grade I ependymomas include myxopapillary ependymomas, which typically occur in the spine, and subependymomas that can occur in any location of the neuraxis. WHO grade II lesions are characterized by perivascular pseudorosettes on light microscopy with grade III ependymoma showing features of anaplasia including cellular pleomorphism and frequent mitoses75. Grade II ependymoma have been divided into four subtypes that include cellular, clear cell, papillary, and tanycytic. These neoplasms are characterized by considerable histopathological variation between tumors as well as within tumors, and this feature has led to difficulty discriminating between WHO grade II and WHO grade III tumors74,76. Based on the inherent difficulties in histopathological classification of ependymoma, molecular classification of these diseases has been proposed77.
Molecular analysis has been used by multiple groups to subclassify ependymomas. Complex chromosomal rearrangements and copy number abnormalities due to chromothripsis are seen in supratentorial ependymomas. These alterations as well as mRNA and microRNA profiles were used to separate ependymomas into nine subgroups that correlate with tumor location78. The functional consequences of genomic abnormalities identified in this work have been investigated. Activation of ephrin-type B receptor 2 (EPHB2) in a subpopulation of neuronal stem cells with Cyclin-dependent kinase inhibitor 2A loss led to the development of supratentorial ependymoma in a murine model78. A majority of supratentorial ependymomas also contain fusion of the v-rel avian reticuloendothelialiosis viral oncogene homologue A (RELA) with the uncharacterized gene C11orf95 79. The C11orf95-RELA fusion protein drives aberrant NF-kB transcription, and expression of this fusion protein leads to the formation of tumors with characteristics of ependymoma in preclinical models79. An additional 84 candidate oncogenes and 39 candidate tumor suppressor genes identified in these studies have recently been explored. Their ability to transform mouse embryonic Cdkn2a–Cdkn2b−/− cerebral neural stem cells was tested in an in vivo system80. This approach identified ten oncogenes and eight tumor suppressor genes that could induce ependymoma formation. These genes are involved in a number of cellular processes including vesicle trafficking and DNA modification and repair80.
There are significant genomic differences between posterior fossa ependymoma and supratentorial ependymoma. Posterior fossa tumors have been divided into posterior fossa A and posterior fossa B subtypes81. Posterior fossa A tumors do not exhibit the dramatic DNA rearrangements seen in supratentorial tumors, but are instead characterized by lack of DNA copy number abnormalities and absence of recurrent DNA mutations82. These tumors have increased DNA methylation of CpG islands that leads to transcriptional silencing of targets of polycomb repressive complex 2 (PRC2)82. Treatment of posterior fossa A ependymoma cells in primary culture with drugs targeting PRC2 or with DNA demethylating agents impairs cell proliferation82. Unlike posterior fossa A tumors, the posterior fossa group B tumors have numerous large scale chromosomal abnormalities81.
Current Therapy and Therapeutic Opportunities
For subependymoma complete surgical resection of the tumor can be curative and in patients with subtotal resection observation can be considered83. Upfront therapy for myxopapillary ependymoma includes an attempt at complete en bloc resection84,85. While historically, radiation therapy was not done for completely resected myxopapillary ependymoma, some evidence suggests that post-operative radiation therapy may improve disease free survival for these patients84,86–88. For WHO grade II and WHO grade III ependymoma an initial attempt at maximal safe surgical resection should be made. The extent of resection is correlated with survival in these patients89,90 and survival rates are improved in cases where a gross total resection can be achieved91. Local post-operative radiation therapy is also employed in these cases. A review of children under 3 years of age treated for ependymoma showed that there was significantly better three year overall survival in patients who received post-operative radiation therapy (81%) compared to those that did not (58%)92. The preliminary results of a phase II trial of conformal radiation therapy reported an estimated 3-year progression free survival of approximately 75%93. If resection is incomplete a second look surgery can be considered94. The role of chemotherapy in the treatment of patients with ependymoma remains to be defined. Two completed studies did not find a survival advantage to the addition of chemotherapy to the treatment of ependymoma95. However, in the Children’s Cancer Group study CCG9942 patients with a sub-total resection of the primary tumor who received pre-irradiation chemotherapy had similar 5-year EFS to children who underwent gross total resection of the tumor96. Studies are currently underway to better define the role of chemotherapy in these patients. Children’s Oncology Group (COG) trial ACNS0121 evaluated the role of chemotherapy and second look surgery in pediatric patients with ependymoma. The trial is closed to enrollment, but results are not yet published. A trial examining the impact of chemotherapy in pediatric patients with ependymoma is also ongoing (ACNS0831).
Recurrent ependymoma continues to have a dismal prognosis97. Palliative local therapy including both surgery and radiation therapy modalities can be considered and may provide some benefit to these patients98–100. This strategy is being explored in a current trial (NCT02125786). Expression of ERBB2 and ERBB4 is seen in approximately 75% of pediatric ependymoma 101. Based on these data, lapatinib, a small molecule inhibitor of ERBB1, ERBB2, and ERBB4 was used in combination with bevacizumab for the treatment of pediatric patients with recurrent ependymoma, but proved ineffective in this patient population102. Other therapeutic options including everolimus and vaccine-based strategies are being evaluated for patients with recurrent disease (Table 1).
Diffuse Intrinsic Pontine Glioma
Histopathology and Genetics
Diffuse intrinsic pontine glioma (DIPG) is a leading cause of mortality in children with brain tumors. Even with administration of focal radiation therapy, median overall survival remains only 10–12 months103 73 104,105. The diagnosis of DIPG is currently based on characteristic radiographic findings106. This has led to a paucity of untreated tumor tissue, limiting efforts to determine the genomic and molecular alterations that are characteristic of DIPG. Despite this challenge, our understanding of DIPG has improved in part as a result of autopsy material becoming available 107 13 and in part because of new biopsy protocols 108,109. Diffuse intrinsic pontine glioma (DIPG) usually present with a histopathology similar to GBM3 but may have characteristics of lower grade tumors. Myriad clinical trials have failed to improve survival beyond that seen in patients receiving focal radiation. These trials have evaluated the intensification of chemotherapy, variations in radiotherapy, and multiple adjuvant chemotherapeutic combinations. 110 111 112
As the landscape of DIPG biology evolves, identification of molecular phenotypes may impact prognostication as well as provide the potential for therapeutic targeting113. Approximately three-quarters of pediatric DIPG samples contain histone mutations, primarily histone 3.1 or 3.3 18 (Figure 1) with some groups showing a worse prognosis for patients with these mutations113. Multiple investigators have emphasized the role of these frequent H3 gene mutations in the tumorigenesis of DIPG, highlighting the importance of epigenetic alterations15,114. Other frequently reported abnormalities in DIPG include mutations in TP53, MET, KRAS 106,115, CDK4 and ATRX (Figure 1), as well as increased expression of PDGFRA, MYC, EGFRv3, PARP, SHH, ERBB1, and ACVR 110–112. Genomic and expression profiles led one analysis to segregate DIPGs into two subgroups: a more aggressive PDGFRA-driven subtype and a mesenchymal subtype with increased STAT3 expression116. Based on these data, xenograft and transgenic mouse models have been generated 107,113, the latter caused by overexpression of PDGF-B with Ink4a-ARF loss, which should facilitate preclinical testing of therapeutic agents for DIPG117.
The absence of a single driver pathway in DIPG emphasizes the need to identify specific tumor subsets susceptible to a particular targeted approach. Further, the utility of upfront biopsies to define these tumors is under debate, as is the adequacy of small biopsies in the context of considerable intratumoral heterogeneity18,118.
Current Therapy and Therapeutic Opportunities
The current standard of care for patients with DIPG is fractionated external beam radiation therapy to a dose of 60Gy119,120. Although radiation intensification has been attempted without success in DIPG, some groups continue to attempt optimization of this sole effective treatment modality in DIPG. The checkpoint kinase WEE1 is expressed in DIPG and its inhibition enhances radiation sensitivity in preclinical models121. Studies include using hypofractionated irradiation and radiosensitization with a Wee1 inhibitor are ongoing (Table 1).
Additional efforts are underway to improve drug delivery to these tumors by overcoming the blood-brain barrier and increased intratumoral pressure. Strategies include intra-arterial infusion of chemotherapy, including melphalan, and convection-enhanced delivery (CED). CED is a process requiring neurosurgical placement of catheters through which therapeutic agents are continuously delivered under positive pressure directly into the tumor. CED-based delivery of drugs has been investigated for the treatment of DIPG. The delivery of 124I-8H9 (an antibody attached to a radioisotope) to the brain using CED has been studied in preclinical models122 and a clinical trial using this approach is currently underway (NCT01502917). IL13-PE38QQR (a fusion molecule of Pseudomonas exotoxin with human IL-13) has also been used in a phase 1 trial targeting DIPG (NCT00880061). Modification of the chemotherapeutic backbone of existing drug classes such as taxanes to improve CNS penetration is also being used as a strategy to increase drug delivery to DIPG123,124. This is exemplified by a study using a novel taxane, cabazitaxel, which has improved CNS penetrance for the treatment of DIPG and HGG (NCT01751308).
Several trials use molecular markers identified using pre-treatment biopsies to determine therapy. In one current study all patients receive an initial diagnostic biopsy followed by standard focal irradiation, which is combined with an EGFR inhibitor (erlotinib, if EGFR activation is noted), and/or temozolomide (if O6-methylguanine-DNA methyltransferase (MGMT) displays a methylated promoter). All patients also receive bevacizumab, an anti-VEGF agent (NCT01182350). Another biopsy-dependent treatment protocol will use varying combinations of erlotinib, dasatinib, and everolimus depending on the overexpression of EGFR and loss of PTEN in newly diagnosed DIPG (NCT02233049).
Other studies seek to leverage the recognition and tumor-control capacity of the immune system against DIPG. One such study utilizes Pidilizumab, an anti-PD-1 monoclonal antibody to block tumor-driven immunosuppression, while another uses lenalidomide, a derivative of thalidomide with antiangiogenic and immunomodulatory properties in combination with radiotherapy (NCT01952769).
Pediatric Low-Grade Glioma
Pediatric low-grade glioma (PLGG) is the most common type of pediatric astrocytoma and pediatric brain tumor in general 125,126. According to the World Health Organization (WHO), PLGG are Grade I or II and include pilocytic astrocytoma (PA), subependymal giant cell astrocytoma (SEGA), pilomyxoid astrocytoma (PMA), pleomorphic xanthoastrocytoma (PXA) and low-grade fibrillary astrocytoma or diffuse astrocytoma 125–128. Of these, the PA is the most common type of glioma in children 125,128.
Histopathology and Genetics
PA is composed of a biphasic pattern of tightly packed bipolar cells intermingled with hypocellular, microcystic areas of loosely packed astrocytes126. The tumor cells may also contain long, thin, hair-like cytoplasmic extensions 126. Rosenthal fibers, eosinophilic granular bodies (EGBs) with occasionally appearing oligodendroglial-resembling cells 129 and rare mitotic bodies are present in PA 126. In contrast to PA, the PMA lack Rosenthal fibers and EGBs130, but contain a characteristic myxoid/extracellular mucoid matrix with highly compacted monomorphic cells usually located around blood vessels 126,127,130. The PXA contains characteristic lipid laden xanthomatous astrocytes 127 and shows pleomorphic cells 126 and spindle shaped cells with EGBs 126. Copious amounts of extracellular reticulin with occasional lymphocytes are also seen in PXA 126. The diffuse astrocytomas are characterized by a lack of abundant mitotic figures or vascular proliferation but are diffusely infiltrative into the normal brain parenchyma 126,127.
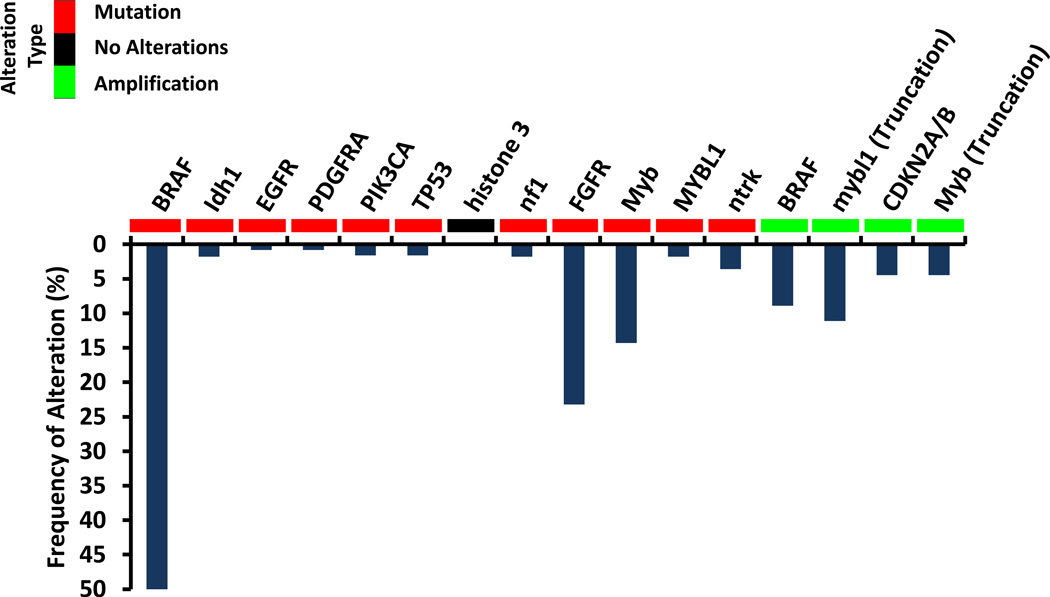
PLGG is characterized by numerous gene mutation and copy number alterations summarized in Figure 3131–135. The most common alterations in PLGG result in constitutive activation of the RAS/MAP signaling pathway 131–133. In patients with neurofibromatosis, inactivation of the RAS-GTPase activating protein neurofibromin leads to constitutive activation of RAS 134,135 most commonly resulting in tumors of the optic pathway 136. In non-NF1 associated PLGG, the most common somatic alteration is the fusion of a truncated BRAF protein with an uncharacterized protein KIAA1549 131–133,136,137. This fusion is caused when the kinase domain of BRAF fuses with the truncated KIAA1549, resulting in loss of BRAF regulation and activation of the MAP kinase pathway 131–133,136,137. A small number of PLGG harbor other fusion proteins involving BRAF 138. Approximately 11 percent of PLGG have an activating mutation, BRAFV600E 139, which is also found in melanoma, colon, and thyroid cancer 140–142. This substitution of valine for glutamic acid leads to constitutive activation of the BRAF143. In addition to neurofibromin and BRAF alterations, the MAP kinase and PI3K/mTOR pathways are activated in a subset of PLGG through intragenic duplication of the tyrosine kinase domain (TKD) of the fibroblast growth factor receptor 1 (FGFR1), resulting in auto-phosphorylation of FGFR1 138. Genomic alterations outside of the RAS/MAP pathway have been identified in a subclass of PLGG, diffusely infiltrating PLGG. These tumors have activating rearrangements of the MYB or MYBL1 genes, resulting in high levels of the MYB transcription factor 138,144.
Figure 3.
Current Therapies and Therapeutic Opportunities
Current therapies for low grade gliomas include carboplatin or TPCV (thioguanine, procarbazine, CCNU, vincristine) 145,146. Carboplatin can either be given alone on a monthly schedule 145 or weekly along with vincristine 147,148 as per the Children’s Oncology Group A9952 study 149. This large, multicenter trial found that patients treated with TPCV had no statistically significant difference in disease free survival compared to patients treated with carboplatin/vincristine 149. There was no increase in incidence of treatment related second malignancies in patients treated with TPCV. Other chemotherapeutic agents including single agent vinblastine or temozolomide as well as combinations of vinblastine plus carboplatin or actinomycin-D plus vincristine have activity against low-grade gliomas and are reasonable options for these patients150. Differences in the route of administration and logistics may lead to one regimen or another being chosen due to issues of patient adherence and convenience 149.
The common mutations in BRAF and the near-universal activation of the mitogen-activated protein (MAP) kinase pathway in PLGG have prompted clinical trials for recurrent/refractory PLGG evaluating the BRAF inhibitor dabrafenib as well as MAP kinase inhibitors selumetinib (AZD6244) and trametinib. Activation of mammalian target of rapamycin (mTOR) is also widely found in PLGG 126,151,152, and a trial of the mTOR inhibitor everolimus has completed enrollment and due to promising early responses, a follow-up clinical trial is ongoing.
Subependymal Giant Cell Astrocytoma
Subependymal giant cell astrocytoma (SEGA) is a WHO Grade I PLGG 127 and one of the spectrum of tumors associated almost exclusively with the genetic disorder tuberous sclerosis 126,153,154.
Histopathology and Genetics
SEGA grow under the ependymal surface and show gemistocytic-, spindled- and ganglion-like cells with rare mitotic figures or necrosis 126. They are usually not infiltrative but can have tumor cells with pleomorphic nuclei 126. Occasionally, the tumor cells can exhibit neuronal and glial differentiation, hence sometimes giving these tumors a designation of mixed glial-neuronal neoplasms 155–157.
The majority of patients who develop SEGA harbor inactivating mutations in either TSC1 (encoding the Hamartin protein) or TSC2 gene (encoding Tuberin protein) 158. These two proteins heterodimerize and are key negative regulators of mTOR and cell growth 158. Inactivation of either of the two genes in SEGA leads to increased mTOR activity, enabling cell growth and proliferation 159.
Current Treatments and Translational Opportunities
The standard therapy for SEGA has been complete surgical excision, which is recommended for tumors exhibiting growth or causing symptoms such as signs of intracranial hypertension160. Alterations in upstream regulators of mTOR lead to universal activation of this pathway in SEGA 126,159. Treatment with the mTOR inhibitor everolimus (a rapamycin analogue) is effective at reducing the growth of these tumors and decreasing the frequency of seizures 161. The use of mTOR inhibitors has been advocated in patients with tumors that are not amenable to surgical resection, where complete resection of the tumor is unlikely, or in tumors with more rapid regrowth following surgery160,162. The advent of newer, more potent mTOR inhibitors may lead to further improvements in care for patients with SEGA.
Conclusion
An evolving understanding of the molecular underpinnings of tumorigenesis in the pediatric CNS is changing the practice of pediatric neuro-oncology. Novel strategies targeting specific genetic and epigenetic abnormalities found in subgroups of tumors are being added to more established treatment regimens using cytotoxic drugs. Continued cooperation in the pediatric oncology community will be required to develop these new treatment strategies and identify groups of patients that will benefit from them.
Acknowledgments
This work is supported by the Hitchcock Foundation (GJR), the Jordan and Kyra Memorial Foundation (MAI), and the Theodora B. Betz Foundation (MAI). This work was supported, in part, by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (JG).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Dunn GP, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes & development. 2012;26:756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones C, Baker SJ. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nature Reviews Cancer. 2014 doi: 10.1038/nrc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. Journal of neurosurgery. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson RJ, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clinical cancer research. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 6.Zarghooni M, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor α and poly (ADP-ribose) polymerase as potential therapeutic targets. Journal of clinical oncology. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 7.Schwartzentruber J, et al. Driver mutations in histone H3. 3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, et al. IDH1 and IDH2 mutations in gliomas. New England Journal of Medicine. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrow J, et al. Homozygous loss of ADAM3A revealed by genome-wide analysis of pediatric high-grade glioma and diffuse intrinsic pontine gliomas. Neuro-oncology. 2011;13:212–222. doi: 10.1093/neuonc/noq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollack IF, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. Journal of Neurosurgery: Pediatrics. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 11.Bender S, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer cell. 2013;24:660–672. doi: 10.1016/j.ccr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman JD, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer research. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buczkowicz P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nature genetics. 2014;46:451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paugh BS, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. Journal of clinical oncology. 2011;29:3999–4006. doi: 10.1200/JCO.2011.35.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bax DA, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clinical cancer research. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 17.Paugh BS, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. Journal of clinical oncology. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paugh BS, et al. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer research. 2013;73:6219–6229. doi: 10.1158/0008-5472.CAN-13-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald T, Aguilera D, Kramm C. Treatment of high-grade glioma in children and adolescents. Neuro-oncology. 2011;13:1049–1058. doi: 10.1093/neuonc/nor092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay JL, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda A, et al. Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. Journal of neurochemistry. 2005;92:569–584. doi: 10.1111/j.1471-4159.2004.02894.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiehna EN, Mulhern RK, Li C, Xiong X, Merchant TE. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. Journal of clinical oncology. 2006;24:5283–5290. doi: 10.1200/JCO.2005.03.8547. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe KR, et al. Executive functions and social skills in survivors of pediatric brain tumor. Child Neuropsychology. 2013;19:370–384. doi: 10.1080/09297049.2012.669470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fangusaro J. Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol. 2012;2:105. doi: 10.3389/fonc.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cage TA, Mueller S, Haas-Kogan D, Gupta N. High-grade gliomas in children. Neurosurg Clin N Am. 2012;23:515–523. doi: 10.1016/j.nec.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Fangusaro J, Warren KE. Unclear standard of care for pediatric high grade glioma patients. J Neurooncol. 2013;113:341–342. doi: 10.1007/s11060-013-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen KJ, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollack IF, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro-oncology. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nature Reviews Neuroscience. 2014;15:455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahme G, Israel M. Id4 suppresses MMP2-mediated invasion of glioblastoma-derived cells by direct inactivation of Twist1 function. Oncogene. 2014 doi: 10.1038/onc.2013.531. [DOI] [PubMed] [Google Scholar]
- 32.Reardon DA, Cheresh D. Cilengitide A Prototypic Integrin Inhibitor for the Treatment of Glioblastoma and Other Malignancies. Genes & cancer. 2011;2:1159–1165. doi: 10.1177/1947601912450586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald TJ, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. Journal of clinical oncology. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald TJ, et al. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: A report from the Children's Oncology Group. Neuro-oncology. 2013;15:1438–1444. doi: 10.1093/neuonc/not058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stupp R, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated< i> MGMT</i> promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. The lancet oncology. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 36.Stupp R, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. Journal of clinical oncology. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 37.Snuderl M, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 38.LASKY JL, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer research. 2013;33:2047–2056. [PMC free article] [PubMed] [Google Scholar]
- 39.Xu LW, Chow KK, Lim M, Li G. Current Vaccine Trials in Glioblastoma: A Review. Journal of immunology research. 2014;2014 doi: 10.1155/2014/796856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert MR, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. New England Journal of Medicine. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinot OL, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. New England Journal of Medicine. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 42.Bagcchi S. Panobinostat active against diffuse intrinsic pontine glioma. Lancet Oncol. 2015;16:e267. doi: 10.1016/S1470-2045(15)70230-5. [DOI] [PubMed] [Google Scholar]
- 43.Grasso CS, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21:827. doi: 10.1038/nm0715-827a. [DOI] [PubMed] [Google Scholar]
- 44.Ostrom QT, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 46.Pfister S, Hartmann C, Korshunov A. Histology and molecular pathology of pediatric brain tumors. J Child Neurol. 2009;24:1375–1386. doi: 10.1177/0883073809339213. [DOI] [PubMed] [Google Scholar]
- 47.Taylor MD, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11:714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 49.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 50.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Ellison DW, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–218. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 53.Jones DT, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kool M, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morfouace M, et al. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. Cancer Cell. 2014;25:516–529. doi: 10.1016/j.ccr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Northcott PA, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shih DJ, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32:886–896. doi: 10.1200/JCO.2013.50.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerber NU, et al. Recent developments and current concepts in medulloblastoma. Cancer Treat Rev. 2014;40:356–365. doi: 10.1016/j.ctrv.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Martin AM, Raabe E, Eberhart C, Cohen KJ. Management of Pediatric and Adult Patients with Medulloblastoma. Curr Treat Options Oncol. 2014 doi: 10.1007/s11864-014-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adamski J, Ramaswamy V, Huang A, Bouffet E. Advances in managing medulloblastoma and intracranial primitive neuro-ectodermal tumors. F1000Prime Rep. 2014;6:56. doi: 10.12703/P6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Packer RJ, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 62.Mulhern RK, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 63.Dufour C, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer. 2014;61:1398–1402. doi: 10.1002/pbc.25009. [DOI] [PubMed] [Google Scholar]
- 64.Jakacki RI, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol. 2012;30:2648–2653. doi: 10.1200/JCO.2011.40.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutkowski S, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11:201–210. doi: 10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reeves CB, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 67.Ellison DW, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 68.Clifford SC, et al. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5:2666–2670. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 69.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- 70.Leary SE, Olson JM. The molecular classification of medulloblastoma: driving the next generation clinical trials. Curr Opin Pediatr. 2012;24:33–39. doi: 10.1097/MOP.0b013e32834ec106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gajjar A, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res. 2013;19:6305–6312. doi: 10.1158/1078-0432.CCR-13-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 73.He X, et al. The G protein alpha subunit Galphas is a tumor suppressor in Sonic hedgehog-driven medulloblastoma. Nat Med. 2014;20:1035–1042. doi: 10.1038/nm.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tihan T, et al. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children's Oncology Group study and a review of prognostic factors. Mod Pathol. 2008;21:165–177. doi: 10.1038/modpathol.3800999. [DOI] [PubMed] [Google Scholar]
- 75.Reni M, Gatta G, Mazza E, Vecht C. Ependymoma. Crit Rev Oncol Hematol. 2007;63:81–89. doi: 10.1016/j.critrevonc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Ellison DW, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pajtler KW, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson RA, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parker M, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506:451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohankumar KM, et al. An in vivo screen identifies ependymoma oncogenes and tumor-suppressor genes. Nat Genet. 2015;47:878–887. doi: 10.1038/ng.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Witt H, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20:143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mack SC, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain A, et al. Subependymoma: clinical features and surgical outcomes. Neurol Res. 2012;34:677–684. doi: 10.1179/1743132812Y.0000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akyurek S, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80:177–183. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 85.Bagley CA, et al. Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg Rev. 2009;32:321–334. doi: 10.1007/s10143-009-0190-8. discussion 334. [DOI] [PubMed] [Google Scholar]
- 86.Pica A, et al. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys. 2009;74:1114–1120. doi: 10.1016/j.ijrobp.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 87.Fassett DR, Pingree J, Kestle JR. The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients. Report of five cases and review of the literature. J Neurosurg. 2005;102:59–64. doi: 10.3171/ped.2005.102.1.0059. [DOI] [PubMed] [Google Scholar]
- 88.Agbahiwe HC, Wharam M, Batra S, Cohen K, Terezakis SA. Management of pediatric myxopapillary ependymoma: the role of adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85:421–427. doi: 10.1016/j.ijrobp.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merchant TE, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cage TA, et al. A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. J Neurosurg Pediatr. 2013;11:673–681. doi: 10.3171/2013.2.PEDS12345. [DOI] [PubMed] [Google Scholar]
- 91.Chintagumpala M, Gajjar A. Brain tumors. Pediatr Clin North Am. 2015;62:167–178. doi: 10.1016/j.pcl.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Koshy M, et al. Post-operative radiation improves survival in children younger than 3 years with intracranial ependymoma. J Neurooncol. 2011;105:583–590. doi: 10.1007/s11060-011-0624-3. [DOI] [PubMed] [Google Scholar]
- 93.Merchant TE, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 94.Massimino M, et al. Second-look surgery for ependymoma: the Italian experience. J Neurosurg Pediatr. 2011;8:246–250. doi: 10.3171/2011.6.PEDS1142. [DOI] [PubMed] [Google Scholar]
- 95.Lin FY, Chintagumpala M. Advances in Management of Pediatric Ependymomas. Curr Oncol Rep. 2015;17:47. doi: 10.1007/s11912-015-0470-0. [DOI] [PubMed] [Google Scholar]
- 96.Garvin JH, Jr, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children's Cancer Group protocol 9942: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:1183–1189. doi: 10.1002/pbc.24274. [DOI] [PubMed] [Google Scholar]
- 97.Zacharoulis S, et al. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26:905–911. doi: 10.1007/s00381-009-1067-4. [DOI] [PubMed] [Google Scholar]
- 98.Bouffet E, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys. 2012;83:1541–1548. doi: 10.1016/j.ijrobp.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 99.Hoffman LM, et al. Fractionated stereotactic radiosurgery for recurrent ependymoma in children. J Neurooncol. 2014;116:107–111. doi: 10.1007/s11060-013-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu AK, Foreman NK, Gaspar LE, Trinidad E, Handler MH. Maximally safe resection followed by hypofractionated re-irradiation for locally recurrent ependymoma in children. Pediatr Blood Cancer. 2009;52:804–807. doi: 10.1002/pbc.21982. [DOI] [PubMed] [Google Scholar]
- 101.Gilbertson RJ, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- 102.DeWire M, et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a collaborative ependymoma research network study (CERN) J Neurooncol. 2015;123:85–91. doi: 10.1007/s11060-015-1764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen KJ, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vallero SG, et al. Diffuse intrinsic pontine glioma in children and adolescents: a single-center experience. Childs Nerv Syst. 2014;30:1061–1066. doi: 10.1007/s00381-014-2359-x. [DOI] [PubMed] [Google Scholar]
- 105.Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38:27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 106.Kieran MW. Time to rethink the unthinkable: Upfront biopsy of children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25266. [DOI] [PubMed] [Google Scholar]
- 107.Monje M, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108:4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacDonald TJ. Diffuse intrinsic pontine glioma (DIPG): time to biopsy again? Pediatr Blood Cancer. 2012;58:487–488. doi: 10.1002/pbc.24090. [DOI] [PubMed] [Google Scholar]
- 109.Roujeau T, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107:1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 110.Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol. 2012;2:205. doi: 10.3389/fonc.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korones DN, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 112.Michalski A, et al. The addition of high-dose tamoxifen to standard radiotherapy does not improve the survival of patients with diffuse intrinsic pontine glioma. J Neurooncol. 2010;100:81–88. doi: 10.1007/s11060-010-0141-9. [DOI] [PubMed] [Google Scholar]
- 113.Khuong-Quang DA, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 115.Faury D, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 116.Puget S, et al. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7:e30313. doi: 10.1371/journal.pone.0030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Becher OJ, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70:2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Warren KE, et al. A phase II study of O6-benzylguanine and temozolomide in pediatric patients with recurrent or progressive high-grade gliomas and brainstem gliomas: a Pediatric Brain Tumor Consortium study. J Neurooncol. 2012;106:643–649. doi: 10.1007/s11060-011-0709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaye EC, Baker JN, Broniscer A. Management of diffuse intrinsic pontine glioma in children: current and future strategies for improving prognosis. CNS Oncol. 2014;3:421–431. doi: 10.2217/cns.14.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grimm SA, Chamberlain MC. Brainstem glioma: a review. Curr Neurol Neurosci Rep. 2013;13:346. doi: 10.1007/s11910-013-0346-3. [DOI] [PubMed] [Google Scholar]
- 121.Caretti V, et al. WEE1 kinase inhibition enhances the radiation response of diffuse intrinsic pontine gliomas. Mol Cancer Ther. 2013;12:141–150. doi: 10.1158/1535-7163.MCT-12-0735. [DOI] [PubMed] [Google Scholar]
- 122.Luther N, et al. The potential of theragnostic (1)(2)(4)I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro Oncol. 2014;16:800–806. doi: 10.1093/neuonc/not298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Semiond D, Sidhu SS, Bissery MC, Vrignaud P. Can taxanes provide benefit in patients with CNS tumors and in pediatric patients with tumors? An update on the preclinical development of cabazitaxel. Cancer Chemother Pharmacol. 2013;72:515–528. doi: 10.1007/s00280-013-2214-x. [DOI] [PubMed] [Google Scholar]
- 124.Girard E, et al. Efficacy of cabazitaxel in mouse models of pediatric brain tumors. Neuro Oncol. 2015;17:107–115. doi: 10.1093/neuonc/nou163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raabe E, Kieran MW, Cohen KJ. New strategies in pediatric gliomas: molecular advances in pediatric low-grade gliomas as a model. Clin Cancer Res. 2013;19:4553–4558. doi: 10.1158/1078-0432.CCR-13-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodriguez FJ, Lim KS, Bowers D, Eberhart CG. Pathological and molecular advances in pediatric low-grade astrocytoma. Annu Rev Pathol. 2013;8:361–379. doi: 10.1146/annurev-pathol-020712-164009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takei H, et al. Expression of oligodendroglial differentiation markers in pilocytic astrocytomas identifies two clinical subsets and shows a significant correlation with proliferation index and progression free survival. J Neurooncol. 2008;86:183–190. doi: 10.1007/s11060-007-9455-7. [DOI] [PubMed] [Google Scholar]
- 130.Tihan T, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58:1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 132.Jones DT, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pfister S, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gutmann DH, Donahoe J, Brown T, James CD, Perry A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol Appl Neurobiol. 2000;26:361–367. doi: 10.1046/j.1365-2990.2000.00258.x. [DOI] [PubMed] [Google Scholar]
- 135.Lau N, et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J Neuropathol Exp Neurol. 2000;59:759–767. doi: 10.1093/jnen/59.9.759. [DOI] [PubMed] [Google Scholar]
- 136.Chen YH, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2014;33:2019–2026. doi: 10.1038/onc.2013.148. [DOI] [PubMed] [Google Scholar]
- 137.Jones DT, Gronych J, Lichter P, Witt O, Pfister SM. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci. 2012;69:1799–1811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.MacConaill LE, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 141.Rajagopalan H, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 142.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 143.Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–394. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 144.Ramkissoon LA, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110:8188–8193. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gururangan S, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–2958. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- 146.Prados MD, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 147.Packer RJ, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 148.Packer RJ, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 149.Ater JL, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nageswara Rao AA, Packer RJ. Advances in the management of low-grade gliomas. Curr Oncol Rep. 2014;16:398. doi: 10.1007/s11912-014-0398-9. [DOI] [PubMed] [Google Scholar]
- 151.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 152.Hutt-Cabezas M, et al. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol. 2013;15:1604–1614. doi: 10.1093/neuonc/not132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujiwara S, Takaki T, Hikita T, Nishio S. Subependymal giant-cell astrocytoma associated with tuberous sclerosis. Do subependymal nodules grow? Childs Nerv Syst. 1989;5:43–44. doi: 10.1007/BF00706748. [DOI] [PubMed] [Google Scholar]
- 154.Grajkowska W, Kotulska K, Jurkiewicz E, Matyja E. Brain lesions in tuberous sclerosis complex. Review. Folia Neuropathol. 2010;48:139–149. [PubMed] [Google Scholar]
- 155.Buccoliero AM, et al. Subependymal giant cell astrocytoma (SEGA): Is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology. 2009;29:25–30. doi: 10.1111/j.1440-1789.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 156.Jozwiak J, Jozwiak S, Skopinski P. Immunohistochemical and microscopic studies on giant cells in tuberous sclerosis. Histol Histopathol. 2005;20:1321–1326. doi: 10.14670/HH-20.1321. [DOI] [PubMed] [Google Scholar]
- 157.Sharma MC, et al. Subependymal giant cell astrocytoma--a clinicopathological study of 23 cases with special emphasis on histogenesis. Pathol Oncol Res. 2004;10:219–224. doi: 10.1007/BF03033764. [DOI] [PubMed] [Google Scholar]
- 158.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 159.Chan JA, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–1242. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 160.Jozwiak S, Nabbout R, Curatolo P. Management of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): Clinical recommendations. Eur J Paediatr Neurol. 2013;17:348–352. doi: 10.1016/j.ejpn.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 161.Krueger DA, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 162.Wheless JW, Klimo P., Jr Subependymal giant cell astrocytomas in patients with tuberous sclerosis complex: considerations for surgical or pharmacotherapeutic intervention. J Child Neurol. 2014;29:1562–1571. doi: 10.1177/0883073813501870. [DOI] [PubMed] [Google Scholar]
- 163.Pugh TJ, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]