Abstract
Vaccines are increasingly targeted toward women of reproductive age, and vaccines to prevent influenza and pertussis are recommended during pregnancy. Prelicensure clinical trials typically have not included pregnant women, and when they are included, trials cannot detect rare events. Thus, postmarketing vaccine safety assessments are necessary. However, analysis of observational data requires detailed assessment of potential biases. Using data from 8 Vaccine Safety Datalink sites in the United States, we analyzed the association of monovalent H1N1 influenza vaccine (MIV) during pregnancy with preterm birth (<37 weeks) and small-for-gestational-age birth (birth weight < 10th percentile). The cohort included 46,549 pregnancies during 2009–2010 (40% of participants received the MIV). We found potential biases in the vaccine–birth outcome association that might occur due to variable access to vaccines, the time-dependent nature of exposure to vaccination within pregnancy (immortal time bias), and confounding from baseline differences between vaccinated and unvaccinated women. We found a strong protective effect of vaccination on preterm birth (relative risk = 0.79, 95% confidence interval: 0.74, 0.85) when we ignored potential biases and no effect when accounted for them (relative risk = 0.91; 95% confidence interval: 0.83, 1.0). In contrast, we found no important biases in the association of MIV with small-for-gestational-age birth. Investigators conducting studies to evaluate birth outcomes after maternal vaccination should use statistical approaches to minimize potential biases.
Keywords: biases, birth outcomes, monovalent H1N1 influenza vaccine safety, pregnancy, preterm delivery, small for gestational age
Editor's Note:An invited commentary on this article appears on page 187.
Large, linked electronic health care data provide the opportunity to study associations between exposures (e.g., medications, vaccinations, medical conditions) and pregnancy complications (e.g., acute reactions, maternal complications, birth outcomes) within large populations without conducting clinical trials. Observational data have been used specifically to study the safety of vaccines administered during pregnancy (1–12). Using electronic health care data from the Vaccine Safety Datalink (VSD), we evaluated risks of acute reactions, maternal medical conditions, and adverse birth outcomes after maternal vaccination (7, 10–13).
Previous observational postlicensure studies of vaccine safety or vaccine effectiveness using retrospective data have faced challenges. For example, influenza vaccination was found to be effective in reducing risk of pneumonia hospitalization and death in elderly patients. However, further work revealed that this observation was biased because of differences in health-seeking behaviors (14, 15).
In the present paper, we discuss the potential biases in the associations of maternal MIV vaccination with preterm and small-for-gestational-age (SGA) births. These biases may occur because of limited access to vaccines during the influenza season, referred as cohort truncation bias (16, 17); time-dependent exposure of vaccination, referred to as immortal time bias (18); and differences in baseline risk factors according to vaccination status. All of these biases have not been fully addressed in recent publications (2–5, 8, 19), and Savitz et al. (17) recommended re-analyzing data from existing observational studies. We present examples of when these biases occur in the VSD pregnancy cohort, propose analytical strategies, and demonstrate the effect of ignoring them. Results are presented for the full cohort and by trimester of vaccination.
METHODS
Study population
This study was conducted as part of the VSD, a collaboration between the Centers for Disease Control and Prevention's Immunization Safety Office and several integrated health care delivery systems. The VSD includes data on 9.5 million subjects, comprising 3% of the US population (20). Data for the present study came from 8 VSD sites: Group Health Cooperative (Washington), HealthPartners (Minnesota), Kaiser Permanente Colorado (Colorado), Kaiser Permanente Northwest (Oregon and Washington), Kaiser Permanente Northern California (California), Kaiser Permanente Southern California (California), Kaiser Permanente Georgia (Georgia), and Kaiser Permanente Hawaii (Hawaii).
Pregnant women enrolled in VSD sites with a pregnancy end date in 2009–2010 were identified using a validated algorithm developed by Hornbrook et al. (21) and adapted and validated for use in the VSD by Naleway et al. (22). The algorithm sets the pregnancy start date as the date of the woman's estimated last menstrual period using data from birth registries or electronic health records. In order to ensure availability of birth outcome data, only pregnancies linked to a livebirth were included. For these analyses, women were selected if they were 14–49 years of age at delivery and had a livebirth from January 1, 2009, to December 31, 2010. To ensure that all pregnancies in 2010 would be captured irrespective of their gestational age at delivery, pregnancies with a start date on or after the seventh study week of 2010 were excluded. In addition, women were required to have continuous enrollment, with no more than a 31-day administrative gap from 6 months before pregnancy started through 2 months postpartum and with at least 1 outpatient medical claim during pregnancy. We also excluded 1) women who received the MIV within 2 weeks of their pregnancy start date or within 1 week of the end of the pregnancy because of uncertainty about whether vaccination occurred during pregnancy; 2) women who received a live vaccine, which is contraindicated during pregnancy; and 3) women whose pregnancies resulted in a gestational duration less than 22 weeks or a birth weight below 500 g, which likely representing fetal deaths. Detailed information on exclusions is reported in Supplementary Data (available at http://aje.oxfordjournals.org/).
Identification of exposure
We used the VSD vaccine files to identify whether women had received inactivated MIV, trivalent influenza vaccine (TIV), or other vaccines during pregnancy. The period of observation included 1 season of H1N1 (2009–2010) and 3 seasons of seasonal influenza (2008–2009, 2009–2010, and 2010–2011). The data sources for these files include claims- and site-based vaccine registries (20). Workplace or pharmacy vaccination were available when manually entered by a health care provider based on patient report or when site-based registries were supplemented by state vaccine registries. The recorded date of vaccination is accurate (20) except when vaccination was administered during a hospitalization, when it may be assigned to the admission date. Timing of vaccination was stratified by pregnancy trimester: first trimester was defined as less than 14 weeks’ gestation, second trimester was defined as 14–28 weeks’ gestation, and third trimester was defined as 28 weeks’ gestation or later. Women who were vaccinated at 37 weeks or later were classified as unvaccinated because they were no longer at risk of having a preterm birth.
Outcomes
The 2 primary outcomes were preterm birth and SGA birth. Preterm birth was defined as delivery from 22 to 37 weeks’ gestation, and SGA birth was defined as a birth weight less than the 10th percentile for a given gestational age, based on national averages. Gestational age corresponds to the clinical estimate of gestational age. Weights for gestational age percentiles were obtained from Oken et al. (23). Their work provides comprehensive reference values for distributions of birth weights at 22–44 completed weeks of gestation that were derived from broadly based nationwide data and stratified by sex. Previous work has shown data captured in electronic health records and birth registries to be adequate: Through chart review, 94% were confirmed to have gestational ages within 14 days of the reference values, and 99% were confirmed as having low birth weight (less than 2,500 g) (24).
Baseline risk factors
Markers for high-risk pregnancies included pre-existing hypertension, diabetes, cardiovascular disease, and renal disease. These conditions were identified from inpatient and outpatient International Classification of Diseases, Ninth Revision, codes in electronic health care data starting 6 months before pregnancy and continuing through the end of the pregnancy. Health care utilization variables included receipt of medical care in the first trimester, the Kotelchuck Adequacy of Prenatal Care Utilization Index derived from VSD data (25, 26), and the number of hospitalizations during the first 20 weeks of pregnancy. Sociodemographic variables included race/ethnicity, maternal age at date of delivery, and census tract poverty level, which was defined for each subject as the percent of families in their census tract with an income below 150% of the federal poverty level. When a maternal address was missing (7%), data on poverty were imputed using the expectation maximization model algorithm that included health care utilization variables (27). Periods of influenza circulation were derived from FluNet data for 2009–2010 (28).
Analysis
We performed 3 analyses. First, we identified periods during 2009 and 2010 when pregnant women had access to vaccination or were exposed to influenza circulation. Second, we evaluated whether baseline risk factors during pregnancy were associated with vaccination. Third, we performed a sensitivity analysis in which we incorporated several strategies to account for potential biases in the vaccine–birth outcome associations.
To identify the period when women had no access to vaccination (MIV or TIV), we plotted the distribution of pregnancies according to study week of vaccination, pregnancy start week, and delivery week according to vaccination status and pregnancy trimester of vaccination. Weeks of the 2 calendar years 2009 and 2010 were combined and counted sequentially, and the weeks of the study were numbered. For example, the week of January 1, 2009, corresponded to week 0, and the week of February 13, 2010, corresponded to week 58.
To evaluate whether baseline characteristics were associated with vaccine exposure, we estimated the standardized difference (difference between means divided by the pooled standard deviation). This measure is insensitive to sample size. A standardized difference of 0.2 or greater in absolute value is considered a large imbalance, and above 0.1 is considered meaningful (29). Data on other potential confounders, such as parity, smoking, and alcohol use, were not available in VSD files.
We used a propensity adjustment approach to evaluate the potential confounding from baseline characteristics in the association between MIV receipt and birth outcome. To construct the propensity score, the following covariates were included: sociodemographic variables, VSD site, presence of medical conditions, and health utilization variables. We used a generalized additive model with a smooth parameter for maternal age and study week of last menstrual period to adequately capture the nonlinear associations. Model fit was evaluated using the C statistic and Hosmer-Lemeshow goodness-of-fit test.
To demonstrate the effect of ignoring potential biases when evaluating maternal vaccination and birth outcomes, we performed a sensitivity analysis. Analyses were performed for the full cohort and stratified by trimester of vaccination. We used 5 stepwise approaches to evaluate associations between maternal vaccination and birth outcomes. In the first, we ignored all potential biases (naïve approach) and used a Cox regression model to estimate the associations. In the case of the vaccine–preterm birth association in the first trimester or vaccine–SGA birth association during any point in pregnancy, this method is equivalent to a noncensored approach, and the estimates correspond to risk ratios. In the second, we accounted for time-dependent vaccine exposure within pregnancy (immortal time bias) using a time-dependent covariate Cox model. In the third, in addition to step 2, we excluded pregnancies having no access to MIV vaccination. 4) In addition to the factors in model 3, we added the propensity score to the model to account for potential imbalance of baseline risk factors. Finally, the fifth model, we used model 4 and added H1N1 circulation as a time-dependent confounder. Measures of association are presented with 95% confidence intervals. Analyses were performed using SAS/STAT, version 9.3 (SAS Institute, Inc., Cary, North Carolina). This study was approved by the institutional review boards at all participating sites and the Centers for Disease Control and Prevention.
RESULTS
Figure 1 presents histograms of study week during which vaccination occurred according to trimester of vaccination and vaccine type (MIV or TIV). These histograms show that access to MIV and TIV vaccination differed by pregnancy trimester and that the likelihood of vaccination depended on the study week. In all pregnancy trimesters, MIV was more likely to be given in the first weeks of the vaccination season. The distribution of study week for TIV reflects the 3 vaccination seasons captured in our cohort (2008–2009, 2009–2010, and 2010–11). Vaccination periods for TIV and MIV and H1N1 circulating periods are available in Supplementary Data.
Figure 1.
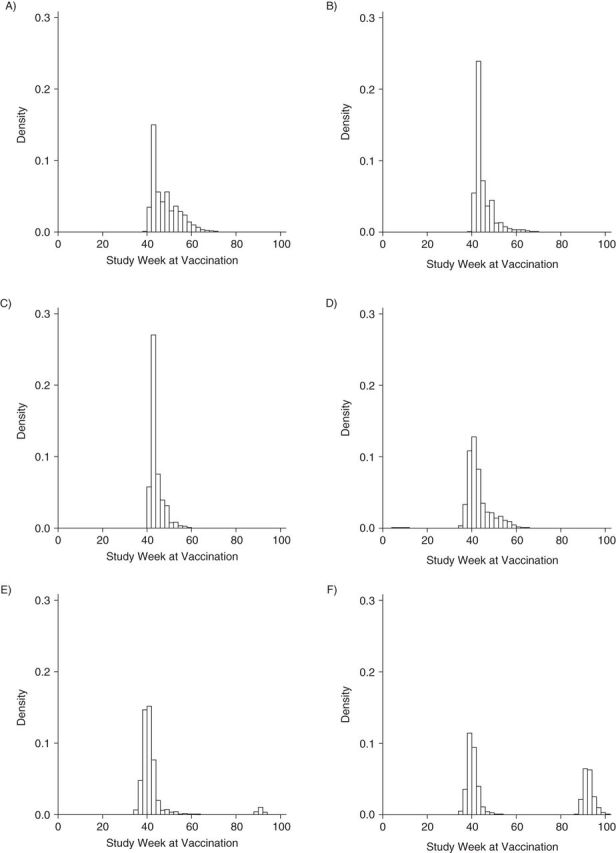
Histograms of study week during which vaccination occurred according to trimester of vaccination and type of vaccine (monovalent or trivalent), Vaccine Safety Datalink Cohort, 2009–2010. A) Monovalent influenza vaccine (MIV) in the first trimester; B) MIV in the second trimester; C) MIV in the third trimester; D) trivalent influenza vaccine (TIV) in the first trimester; E) TIV in the second trimester; and F) TIV in the third trimester.
Figure 2 presents histograms of study week at pregnancy start date according to vaccination status, trimester of vaccination, and type of vaccine (MIV or TIV). The distribution of study week at pregnancy start date in unvaccinated women was relatively uniform; women received MIV only in the first trimester when the pregnancy start date occurred after study week 25. Similarly, women received MIV in the second trimester when the pregnancy start date occurred after study week 11.
Figure 2.
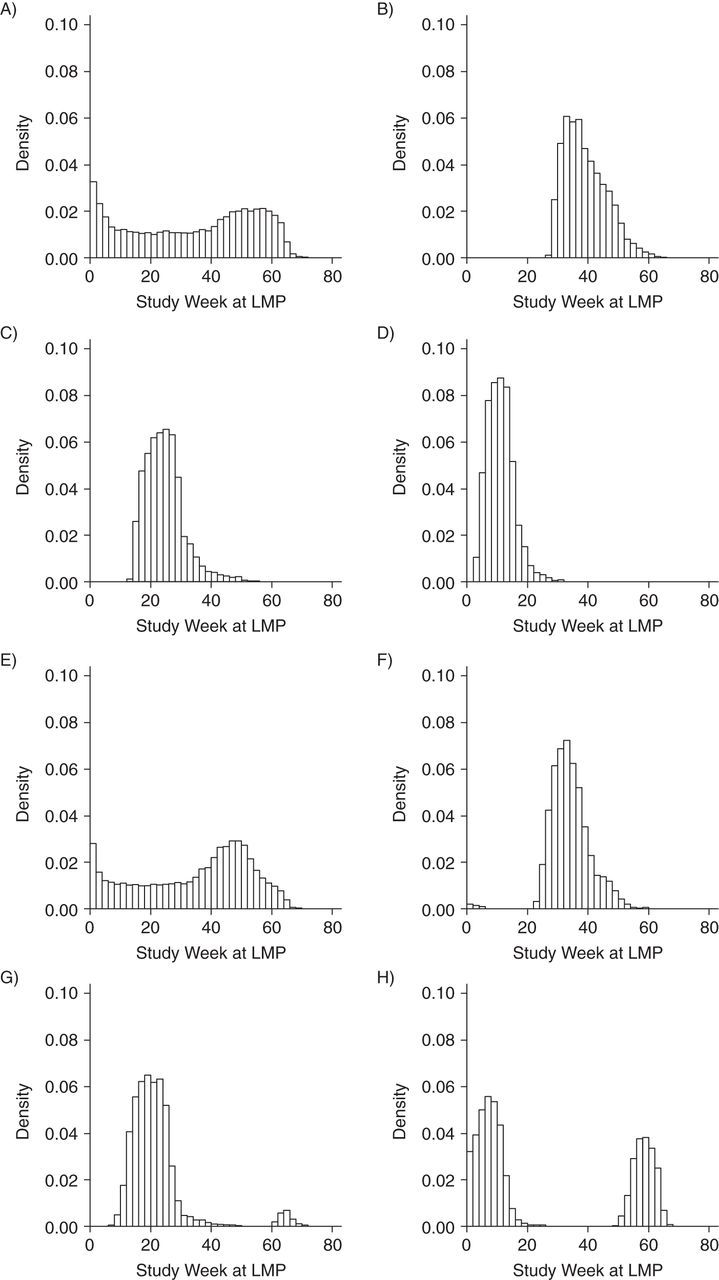
Histograms of study week of last menstrual period (LMP) date according to vaccination status, trimester of vaccination, and type of influenza vaccine (monovalent or trivalent), Vaccine Safety Datalink Cohort, 2009–2010. A) Not vaccinated with monovalent influenza vaccine (MIV); B) MIV in first the trimester; C) MIV in second the trimester; D) MIV in third the trimester; E) not vaccinated with trivalent influenza vaccine (TIV); F) TIV in first the trimester; G) TIV in second the trimester; and H) TIV in third the trimester.
Figure 3 presents histograms of study week at pregnancy end date according to vaccination status, trimester of vaccination, and type of vaccine (MIV or TIV). In this case, because MIV was available after study week 37, women who had pregnancies with an end date before study week 38 had no access to vaccine during pregnancy. Thus, in pregnancies with a start date that occurred in early 2009, vaccination was more likely for those with longer gestational period. We classified the period for having no access to vaccination as a pregnancy start date before study week 12. This group represented 20% of the cohort.
Figure 3.
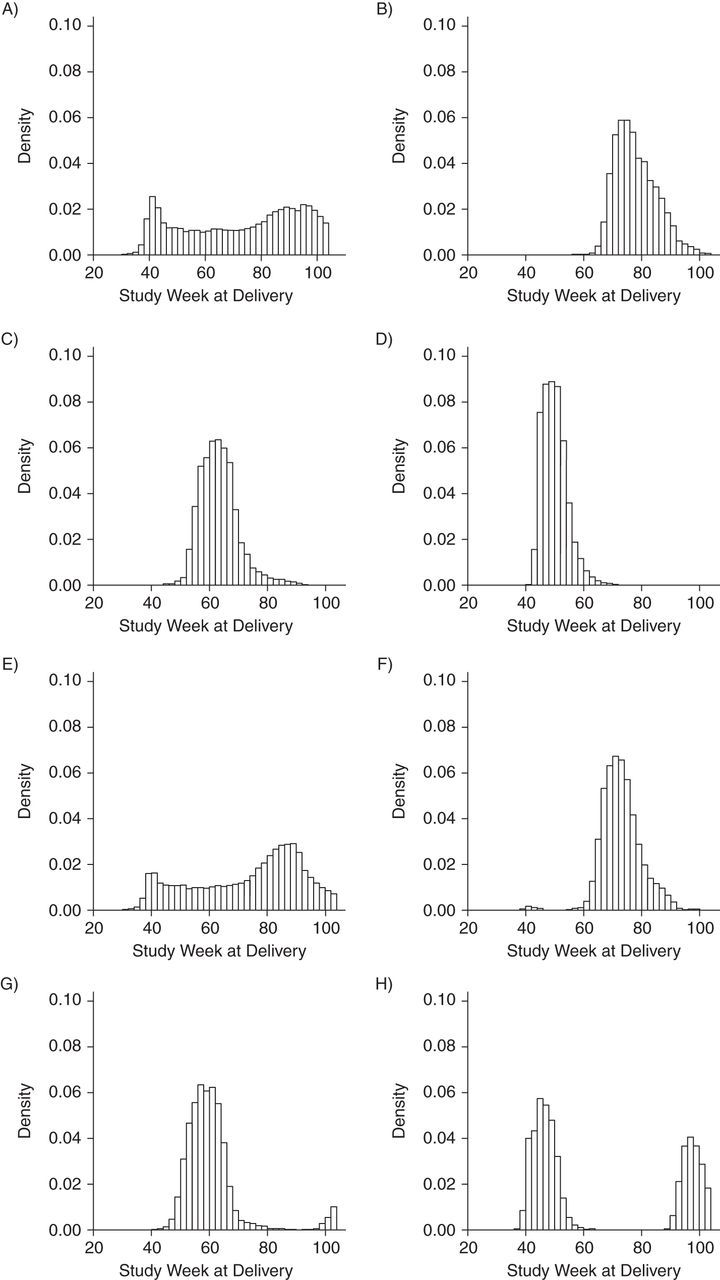
Histograms of study week during which delivery occurred according to vaccination status, trimester of vaccination, and type of influenza vaccine (monovalent or trivalent), Vaccine Safety Datalink Cohort, 2009–2010. A) Not vaccinated with monovalent influenza vaccine (MIV); B) MIV in first the trimester; C) MIV in second the trimester; D) MIV in third the trimester; E) not vaccinated with trivalent influenza vaccine (TIV); F) TIV in first the trimester; G) TIV in second the trimester; and H) TIV in third the trimester.
Likelihood of vaccination due to baseline risk factors
Moderate imbalances in health care utilization, race/ethnicity, and residing in areas with an elevated poverty level (standardized difference > 0.1) by vaccination status were observed. In addition, there was a large imbalance for receipt of TIV and study week at last menstrual period (standardized difference > 0.2) (Table 1).
Table 1.
Baseline Characteristics of Pregnant Women (n = 46,549) by Monovalent Influenza Vaccine Status, Vaccine Safety Datalink Cohort, 2009–2010
| Characteristic | No MIV (n =
28,080) |
MIV (n =
18,469) |
SD | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Pregnancy trimester of vaccination | |||||
| First | 6,796 | 36.8 | |||
| Second | 7,103 | 38.5 | |||
| Third | 4,570 | 24.7 | |||
| Prenatal visit in first trimestera | 26,733 | 95.2 | 18,061 | 97.8 | 0.12 |
| Adequacy of Prenatal Care Utilization Indexa | |||||
| Adequate/plus | 21,247 | 75.7 | 14,313 | 77.5 | 0.04 |
| Intermediate | 6,021 | 21.4 | 3,960 | 21.4 | 0.00 |
| Inadequate | 812 | 2.9 | 196 | 1.1 | −0.11 |
| Hospitalization before 20 weeks’ gestation | 966 | 3.4 | 610 | 3.3 | |
| Received TIVb | 7,676 | 27.3 | 14,060 | 76.1 | 1.10 |
| Same trimester as MIV | 10,974 | 59.4 | |||
| Different trimester than MIV | 3,086 | 16.7 | |||
| No TIV | 20,404 | 72.7 | 4,409 | 23.9 | |
| Age at end of pregnancy, years | |||||
| <20 | 1,089 | 3.9 | 493 | 2.7 | −0.06 |
| 20–34 | 20,339 | 72.4 | 13,038 | 70.6 | −0.04 |
| 35–55 | 6,652 | 23.7 | 4,938 | 26.7 | 0.07 |
| Residency in Census tract with >20% of households with an income below 150% of the federal poverty levela | 7,635 | 27.2 | 4,093 | 22.2 | −0.11 |
| Race/ethnicitya | |||||
| Black | 2,383 | 8.5 | 935 | 5.1 | −0.12 |
| Asian | 1,617 | 5.8 | 1,207 | 6.5 | 0.03 |
| Hispanic | 5,395 | 19.2 | 3,213 | 17.4 | −0.05 |
| Other | 1,792 | 6.4 | 1,083 | 5.9 | −0.02 |
| White | 16,893 | 60.2 | 12,031 | 65.1 | 0.10 |
| Medical conditions | |||||
| Diabetes (excluding gestational) | 490 | 1.7 | 331 | 1.8 | 0.01 |
| Hypertension (excluding hypertensive disorders during pregnancy) | 1,048 | 3.7 | 685 | 3.7 | 0.00 |
| Cardiovascular disease | 499 | 1.8 | 341 | 1.8 | 0.00 |
| Renal disease | 516 | 1.8 | 315 | 1.7 | −0.01 |
| Health care organizationa | |||||
| Kaiser Permanente Northern California | 14,739 | 52.5 | 9,827 | 53.2 | 0.01 |
| Kaiser Permanente Colorado | 876 | 3.1 | 1,009 | 5.5 | 0.14 |
| Kaiser Permanente Georgia | 469 | 1.7 | 248 | 1.3 | −0.03 |
| HealthPartners Minnesota | 235 | 0.8 | 106 | 0.6 | −0.02 |
| Kaiser Permanente Northwest | 432 | 1.5 | 162 | 0.9 | −0.05 |
| Kaiser Permanente Hawaii | 232 | 0.8 | 17 | 0.1 | −0.08 |
| Kaiser Permanente Southern California | 10,869 | 38.7 | 6,817 | 36.9 | −0.04 |
| Group Health Cooperative Washington | 228 | 0.8 | 283 | 1.5 | 0.08 |
| Study week at LMPb | |||||
| 1–8 | 5,613 | 20.0 | 452 | 2.4 | −0.44 |
| 9–16 | 3,141 | 11.2 | 3,459 | 18.7 | 0.24 |
| 17–24 | 2,548 | 9.1 | 3,748 | 20.3 | 0.39 |
| 25–32 | 2,715 | 9.7 | 3,875 | 21.0 | 0.38 |
| 33–40 | 2,785 | 9.9 | 3,752 | 20.3 | 0.35 |
| 41–48 | 3,931 | 14.0 | 2,170 | 11.7 | −0.07 |
| 49–58 | 7,347 | 26.2 | 1,013 | 5.5 | −0.47 |
| Pregnant during H1N1 circulationb | 20,733 | 73.8 | 17,456 | 94.5 | −0.21 |
Abbreviations: LMP, last menstrual period; MIV, monovalent influenza vaccine; SD, standardized difference; TIV, trivalent influenza vaccine.
a SD with an absolute value greater than 0.1 is considered a meaningful value.
b SD with an absolute value greater than 0.2 is considered a large imbalance.
Model fit of the propensity score had a C statistic = 0.77 and a P value < 0.00001 (8 df) for the Hosmer-Lemeshow test. Nonlinear associations were corroborated by the smoothing component (partial prediction) plots for maternal age at the end of pregnancy and pregnancy start week (Supplementary Data). When ignoring study week during which pregnancy started, propensity score properties were poor (C statistic = 0.58), confirming that most of the likelihood of MIV receipt is driven by the temporal availability of the vaccine.
Sensitivity analyses to evaluate potential bias of the MIV–birth outcome association
The crude prevalence rates of preterm birth (less than 37 weeks) and SGA birth (<10th percentile) by receipt of MIV and trimester of vaccination are presented in Table 2. SGA birth rates had small variations ranging from 7.5 to 8.9. In contrast, rates of preterm birth varied widely, from 8.2 for those who did not receive MIV to 4.2 in those who were vaccinated in the third trimester.
Table 2.
Crude Prevalence Rates (per 100 Births) of Preterm and Small-for-Gestational-Age Births Among Study Participants (n = 46,549) by Monovalent Influenza Vaccine Status, Vaccine Safety Datalink Cohort, 2009–2010
| MIV Status | No. of Pregnancies | SGA Birth (<10th Percentile) | Preterm Birth (<37 Weeks) |
|---|---|---|---|
| No MIV | 27,392 | 8.6 | 8.2 |
| Received MIV | |||
| Any time during pregnancy | 19,157 | 8.3 | 6.1 |
| First trimester | 6,788 | 7.5 | 6.8 |
| Second trimester | 7,096 | 8.9 | 6.9 |
| Third trimester | 5,244 | 8.5 | 4.2 |
Abbreviations: MIV, monovalent influenza vaccine; SGA, small-for-gestational-age.
Table 3 presents the results of sensitivity analyses of the MIV–birth outcome associations for the full cohort and by trimester of vaccination. For the MIV–preterm birth association in the full cohort, the naïve model indicated a protective effect (relative risk (RR) = 0.79; 95% confidence interval (CI): 0.74, 0.85; model 1). This protective effect was diluted when accounting for the time-dependent MIV exposure (RR = 0.88; 95% CI: 0.82, 0.94; model 2). After pregnancies in which the mothers had no access to vaccination were excluded, the relative risk was 0.91 (95% CI: 0.84, 0.98; model 3). Further adjustment by propensity score and accounting for H1N1 circulation resulted in a null association (RR = 0.91; 95% CI: 0.83, 1.0; model 5). Analyses specific to first and second trimester vaccination showed a protective effect when we ignored all potential biases (in model 1, for the first trimester, RR = 0.85, 95% CI: 0.77, 0.93; for the second trimester, RR = 0.87, 95% CI: 0.79, 0.95). When women with no access to vaccination were removed from the analyses, the protective effect was diluted (in model 3, for the first trimester, RR = 0.88, 95% CI: 0.79, 0.98; for the second trimester, RR = 0.90; 95% CI: 0.80, 1.01). Further adjustment for propensity to vaccination or H1N1 circulation did not affect the results (models 4 and 5). For third trimester vaccination, the time-dependent nature of exposure and access to vaccination affected the association estimate (in model 1, RR = 0.63, 95% CI: 0.55, 0.72; in model 3, RR = 0.97, 95% CI: 0.82, 1.14). Adjustment for propensity to be vaccinated and H1N1 circulation did not modify the association (in model 5, RR = 1.0, 95% CI: 0.83, 1.20). MIV–preterm birth associations were consistent in all 3 trimesters when we accounted for all potential biases.
Table 3.
Risk Ratios for Preterm and Small-for-Gestational-Age Births After Monovalent Influenza Vaccine Receipt Among Study Participants (n = 46,549), by Timing of Vaccination and Model Specification, Vaccine Safety Datalink Cohort, 2009–2010
| Modela | Outcome by Period of
Vaccination |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preterm Birth (<37 Weeks) |
SGA Birth (<10th Percentile) |
|||||||||||||||
| Any Time During Pregnancy |
First Trimester |
Second Trimester |
Third Trimester |
Any Time During Pregnancy |
First Trimester |
Second Trimester |
Third Trimester |
|||||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| 1 | 0.79 | 0.74, 0.85 | 0.85 | 0.77, 0.93 | 0.87 | 0.79, 0.95 | 0.63 | 0.55, 0.72 | 0.97 | 0.91, 1.03 | 0.88 | 0.80, 0.96 | 1.03 | 0.95, 1.12 | 0.99 | 0.89, 1.10 |
| 2 | 0.88 | 0.82, 0.94 | 0.84 | 0.76, 0.93 | 0.87 | 0.79, 0.96 | 0.83 | 0.73, 0.96 | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | 0.91 | 0.84, 0.98 | 0.87 | 0.78, 0.97 | 0.90 | 0.81, 1.00 | 0.97 | 0.82, 1.14 | 0.99 | 0.93, 1.06 | 0.88 | 0.80, 0.96 | 1.03 | 0.95, 1.12 | 1.10 | 0.96, 1.25 |
| 4 | 0.92 | 0.84, 1.00 | 0.88 | 0.79, 0.98 | 0.90 | 0.80, 1.01 | 1.0 | 0.85, 1.20 | 1.0 | 0.93, 1.08 | 0.90 | 0.82, 0.99 | 1.08 | 0.98, 1.20 | 1.11 | 0.96, 1.28 |
| 5 | 0.91 | 0.83, 1.00 | 0.87 | 0.78, 0.97 | 0.89 | 0.79, 1.00 | 1.0 | 0.83, 1.20 | 1.0 | 0.93, 1.08 | 0.90 | 0.82, 0.99 | 1.08 | 0.98, 1.20 | 1.11 | 0.96, 1.27 |
Abbreviations: CI, confidence interval; NA, not applicable; RR, relative risk; SGA, small-for-gestational-age.
a Model 1 was the naïve model. Model 2 was adjusted for the time-dependent monovalent vaccine exposure. Model 3 was adjusted for the factors in model 2 and for temporal access to vaccination. Model 4 was adjusted for the factors in model 3 and for propensity of vaccination. Model 5 was adjusted for the factors in model 4 and H1N1 circulation.
The MIV–SGA birth associations were consistent across all approaches. The risk of SGA birth was not higher after MIV vaccination during any point in pregnancy (in model 1, RR = 0.97, 95% CI: 0.91, 1.03; in model 5, RR = 1.0, 95% CI: 0.93, 1.08). Null results were observed for vaccination in the second and third trimesters. A weak protective effect was observed for first trimester vaccination (in model 5, RR = 0.90, 95% CI: 0.82, 0.99).
DISCUSSION
In the present retrospective cohort study, we demonstrated that ignoring potential biases can strongly affect the observed MIV–preterm birth associations but not MIV–SGA birth associations. Sources of bias in the MIV–preterm birth association depended strongly on the seasonality or timing of the start of pregnancy and the immortal time bias. When women were stratified according to vaccination status, the baseline characteristics addressed in these analyses were similar or had only small imbalances, except for pregnancy start week. Our results that were based on the fully adjusted models did not indicate an association of maternal receipt of MIV with preterm birth; only first trimester vaccination showed an association. An MIV–SGA association was not observed for the full cohort, but again, an association was observed in the first trimester.
Our results are consistent with those from previous studies (7, 30). In a meta-analysis of seasonal and H1N1 vaccines, no evidence of harmful effects with regard to preterm birth was found (7, 30). Seven studies (1, 2, 4, 5, 8, 19, 31) indicated a statistically strong protective effect for preterm birth, defined as less than 37 weeks gestation, after vaccination. The protective effect ranged from a relative risk of 0.40 to a relative risk of 0.86. There were no studies in which maternal vaccination was found to be associated with SGA birth. Of the studies in which results were presented by trimester of vaccination, there were none in which investigators found an association with preterm birth in the first trimester; however, a protective association in the third trimester was found in 1 study (8).
Our study complements the work presented by Fell et al. (30). Fell et al. reviewed the design heterogeneity (study design and analytical approach) of published articles on the association between vaccinations and preterm birth. In the present study, we addressed several of the potential biases that can be introduced when analyzing that association. For example, focusing on conventional methods, such as multivariable adjustment, propensity methods, matching, or exclusion of women who have rare exposure to risk factors, may not be sufficient to address other potential biases, such as access to vaccination, seasonal confounding, and immortal time bias.
There have been a number of studies in which researchers have addressed access to vaccination resulting in a cohort truncation bias (16, 17). The approaches used included having access to vaccination in the third trimester (2); having access to vaccination for each trimester (6, 7, 31); having a prenatal care visit during the vaccination period (33); or matching or stratifying by date of birth (1, 34, 35). However, methods that accounted only for the date of birth may not fully control for differential access to vaccination for preterm birth, because a longer pregnancy length is associated with having more opportunities to be vaccinated. Our present study included detailed analyses to identify calendar periods during which access to vaccination posed a threat to the validity of the results. Only pregnant women with longer gestational age would have been vaccinated in the early weeks of the vaccination campaign in 2009, which would have primarily affected maternal vaccination in the third trimester.
Seasonal confounding has been identified as a potential source of bias in studies of the associations of exposures with birth outcomes, with the rate of prematurity and birth weight being dependent on season of conception (36, 37), although this potential bias may be only an artifact of the cohort truncation bias. Therefore, grouping pregnancies by pregnancy start date, care date, or trimester of vaccination may account for the 2 potential biases simultaneously. Xu et al. (38) suggested the incorporation of other time-dependent confounders through a time-dependent Cox model. In our analysis, in addition to addressing the cohort truncation bias, we incorporated H1N1 circulation as a time-dependent covariate and found that our results were not affected. In our analysis, we did not adjust for receipt of TIV because the differential access to TIV observed in our study period may have introduced further bias, as there was little overlap in the periods when both vaccines were available.
Bias introduced by time-dependent exposure in analyses of preterm birth outcomes has been widely documented (18, 39–41). Time-dependent exposure was addressed in several but not all prior studies. Several studies lacked the date of vaccination (3, 4), had incomplete capture of vaccination date (8, 42), or used self-reported data (5, 19). In most studies in which the vaccination dates were available, investigators censored the vaccination exposure (6–8, 31, 42, 43), and only in a few studies was a time-dependent covariate method used to analyze the vaccine–preterm birth association (5, 34, 43). In other studies, researchers used stratified methods to compare with unvaccinated women who had not given birth (8, 44, 45) or included an interaction term for date of delivery (31).
Methods used to limit the effect of potential confounding because of imbalance of risk factors included exclusions of some subgroups and adjustment methods. Exclusions identified are multiple livebirths, not receiving prenatal care, exposure to H1N1, or having received treatment for influenza. Prior studies have included sociodemographic factors, the presence of comorbid conditions, behavioral factors, and other pregnancy-related characteristics as potential confounders. In terms of methods used for adjustment, studies have included multivariable adjustment (1, 2, 5, 19, 31, 33, 43, 45, 46), stratified approaches (8), propensity score adjustment (32, 34, 42), propensity score matching (2, 3, 6, 7), or other matching strategies (47). In most cases, when studies results were reported as crude and adjusted associations, results were similar (31, 33). In studies in which results based on different adjustment approaches were compared, no differences in associations were found (2, 5). In the present study, we found few covariates that were associated with MIV status. The only variable driving the performance of the propensity score was calendar date of last menstrual period. Thus, our results are consistent with other studies, indicating a minor concern for confounding by indication, which likely reflects the routine recommendation of vaccination during pregnancy during the period of observation (48).
Our study had some limitations. We did not have access to data on several potential confounders, such as smoking, parity, and prior history of preterm birth. We incorporated a broad number of conditions into the propensity to vaccination variable, some of which had a weak association with the birth outcomes presented (49). However, propensity approaches are appropriate for handling a larger number of potential confounders (50). Our cohort was limited to women with continuous insurance coverage and at least 1 outpatient encounter. Thus, women with no insurance or interrupted coverage were underrepresented. Our cohort excluded pregnancies for which no birth outcomes were available. Most of these pregnancies correspond to the 1-year lag in birth registries. Data on vaccination date have been shown to be accurate (20), but women who were vaccinated at alternate sites might have been misclassified in our cohort. Although our data relied heavily on automated electronic health record data and birth registries for assigning gestational age at delivery and birth weight, these sources of data in our systems have been found to be valid (24). Accuracy of clinical estimate of gestational age in this population may be explained by the wide access to ultrasound data in addition to the results of newborn examination (51). Finally, our analyses were restricted to livebirths, and thus we were unable to evaluate whether the exclusion of stillbirth affected the observed maternal MIV–birth outcome associations.
Conclusions
Our results are consistent with those from other studies in which investigators found no increased risk of preterm or SGA birth after maternal H1N1 vaccination. Our analyses demonstrated that the apparent protective effects observed in several studies of the association between influenza vaccination and preterm birth were attenuated when we accounted for temporal confounders. Further studies using retrospective cohorts analyzing vaccine–birth outcome associations may benefit by incorporating the analytical techniques that we utilized to minimize these potential biases.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: HealthPartners Institute, Bloomington, Minnesota (Gabriela Vazquez-Benitez, Elyse O. Kharbanda, James D. Nordin); Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon (Allison L. Naleway); Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale University, New Haven, Connecticut, (Heather Lipkind); Centers for Disease Control and Prevention, Atlanta, Georgia (Lakshmi Sukumaran); Division of Pediatric Infectious Diseases, Emory University, Atlanta, Georgia (Lakshmi Sukumaran); Centers for Disease Control and Prevention, Atlanta, Georgia (Natalie L. McCarthy); Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Saad B. Omer); Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California (Lei Qian); The Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado (Stanley Xu); Group Health Research Institute, Seattle, Washington (Michael L. Jackson); Center for Health Research, Kaiser Permanente Hawaii, Honolulu, Hawaii (Vinutha Vijayadev); and Division of Research, Kaiser Permanente Northern California, Oakland, California (Nicola P. Klein).
This work was supported by a subcontract with America's Health Insurance Plans under contract 200-2002-00732 and contract 200-2012-53526 from the Centers for Disease Control and Prevention.
We thank the Vaccine Safety Datalink site collaborators for their contributions, Avalow Y. Olsen and Beth A. Molitor for assisting with data preparation, and Leslie C. Kuckler for project management.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: A.L.N. has received research support from GlaxoSmithKline, and N.K.P. has received support from Sanofi Pasteur, GlaxoSmithKline, Novartis Medlmmune, Protein Science, Pfizer, Merck & Co., and Nuron Biotech for unrelated studies. The other authors report no conflicts.
REFERENCES
- 1. Omer SB, Goodman D, Steinhoff MC et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;85:e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards JL, Hansen C, Bredfeldt C et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;569:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fell DB, Sprague AE, Liu N et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;1026:e33–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodds L, Macdonald N, Scott J et al. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstet Gynaecol Can. 2012;348:714–720. [DOI] [PubMed] [Google Scholar]
- 5. Heikkinen T, Young J, van Beek E et al. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol. 2012;2073:177.e1–177.e8. [DOI] [PubMed] [Google Scholar]
- 6. Pasternak B, Svanstrom H, Molgaard-Nielsen D et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;3082:165–174. [DOI] [PubMed] [Google Scholar]
- 7. Nordin JD, Kharbanda EO, Vazquez Benitez G et al. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014;1645:1051–1057. [DOI] [PubMed] [Google Scholar]
- 8. Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix® during pregnancy and delivery outcome: a Swedish register study. BJOG. 2012;11913:1583–1590. [DOI] [PubMed] [Google Scholar]
- 9. Moro PL, Museru OI, Broder K et al. Safety of influenza A (H1N1) 2009 live attenuated monovalent vaccine in pregnant women. Obstet Gynecol. 2013;1226:1271–1278. [DOI] [PubMed] [Google Scholar]
- 10. Kharbanda EO, Vazquez-Benitez G, Lipkind HS et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;31218:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kharbanda EO, Vazquez-Benitez G, Lipkind H et al. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;1223:659–667. [DOI] [PubMed] [Google Scholar]
- 12. Nordin JD, Kharbanda EO, Benitez GV et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;1213:519–525. [DOI] [PubMed] [Google Scholar]
- 13. Kharbanda EO, Parker ED, Nordin JD et al. Influenza and pertussis vaccination coverage among privately insured women of reproductive age. Matern Child Health J. 2013;179:1631–1637. [DOI] [PubMed] [Google Scholar]
- 14. Fireman B, Lee J, Lewis N et al. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;1705:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hottes TS, Skowronski DM, Hiebert B et al. Influenza vaccine effectiveness in the elderly based on administrative databases: change in immunization habit as a marker for bias. PLoS One. 2011;67:e22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savitz D, Fell D, Ortiz J et al. Does influenza vaccination improve pregnancy outcome? Methodological issues and research needs. Vaccine. 2015;3347:6430–6435. [DOI] [PubMed] [Google Scholar]
- 18. Daniel S, Koren G, Lunenfeld E et al. Immortal time bias in drug safety cohort studies: spontaneous abortion following nonsteroidal antiinflammatory drug exposure. Am J Obstet Gynecol. 2015;2123:307.e1–307.e6. [DOI] [PubMed] [Google Scholar]
- 19. Rubinstein F, Micone P, Bonotti A et al. Influenza A/H1N1 MF59 adjuvanted vaccine in pregnant women and adverse perinatal outcomes: multicentre study. BMJ. 2013;346:f393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baggs J, Gee J, Lewis E et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(suppl 1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 21. Hornbrook MC, Whitlock EP, Berg CJ et al. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res. 2007;422:908–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naleway AL, Gold R, Kurosky S et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013;3127:2898–2903. [DOI] [PubMed] [Google Scholar]
- 23. Oken E, Kleinman KP, Rich-Edwards J et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrade SE, Scott PE, Davis RL et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf. 2013;221:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health. 1994;849:1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health. 1994;849:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Little R, Rubin DB. Statistical Analysis with Missing Data, 2nd ed New York, NY: John Wiley; 2002. [Google Scholar]
- 28. World Health Organization. FluNet. Geneva, Switzerland: World Health Organization; http://www.who.int/influenza/gisrs_laboratory/flunet/en/. [Google Scholar]
- 29. Mamdani M, Sykora K, Li P et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;3307497:960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fell DB, Platt RW, Lanes A et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG. 2015;1221:17–26. [DOI] [PubMed] [Google Scholar]
- 31. Legge A, Dodds L, MacDonald NE et al. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. Can Med Assoc J. 2014;1864:E157–E164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Louik C, Ahrens K, Kerr S et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine. 2013;3144:5033–5040. [DOI] [PubMed] [Google Scholar]
- 33. Cantu J, Biggio J, Jauk V et al. Selective uptake of influenza vaccine and pregnancy outcomes. J Matern Fetal Neonatal Med. 2013;2612:1207–1211. [DOI] [PubMed] [Google Scholar]
- 34. Oppermann M, Fritzsche J, Weber-Schoendorfer C et al. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;3030:4445–4452. [DOI] [PubMed] [Google Scholar]
- 35. Munoz FM. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol. 2012;207(3 suppl):S33–S37. [DOI] [PubMed] [Google Scholar]
- 36. Källén B. The problem of confounding in studies of the effect of maternal drug use on pregnancy outcome. Obstet Gynecol Int. 2012;2012:148616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee SJ, Steer PJ, Filippi V. Seasonal patterns and preterm birth: a systematic review of the literature and an analysis in a London-based cohort. BJOG. 2006;11311:1280–1288. [DOI] [PubMed] [Google Scholar]
- 38. Xu R, Luo Y, Glynn R et al. Time-dependent propensity score for assessing the effect of vaccine exposure on pregnancy outcomes through pregnancy exposure cohort studies. Int J Environ Res Public Health. 2014;113:3074–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savitz DA, Hertz-Picciotto I, Poole C et al. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol Rev. 2002;242:91–101. [DOI] [PubMed] [Google Scholar]
- 40. Kramer MS, Zhang X, Platt RW. Analyzing risks of adverse pregnancy outcomes. Am J Epidemiol. 2014;1793: 361–367. [DOI] [PubMed] [Google Scholar]
- 41. S O'Neill M, Hertz-Picciotto I, Pastore LM et al. Have studies of urinary tract infection and preterm delivery used the most appropriate methods? Paediatr Perinat Epidemiol. 2003;173:226–233. [DOI] [PubMed] [Google Scholar]
- 42. Chambers CD, Johnson D, Xu R et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine. 2013;3144: 5026–5032. [DOI] [PubMed] [Google Scholar]
- 43. Håberg SE, Trogstad L, Gunnes N et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349:g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ludvigsson JF, Zugna D, Cnattingius S et al. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol. 2013;287:579–588. [DOI] [PubMed] [Google Scholar]
- 46. Dodds L, McNeil SA, Fell DB et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;1764:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Munoz FM, Greisinger AJ, Wehmanen OA et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;1924:1098–1106. [DOI] [PubMed] [Google Scholar]
- 48. Hubka TA, Wisner KP, Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention. Vaccinations recommended during pregnancy and breastfeeding. J Am Osteopath Assoc. 2011;111(10 suppl 6):S23–S30. [PubMed] [Google Scholar]
- 49. Ovesen PG, Jensen DM, Damm P et al. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. a nation-wide study. J Matern Fetal Neonatal Med. 2015;2814:1720–1724. [DOI] [PubMed] [Google Scholar]
- 50. Toh S, García Rodríguez LA, Hernán MA. Confounding adjustment via a semi-automated high-dimensional propensity score algorithm: an application to electronic medical records. Pharmacoepidemiol Drug Saf. 2011;208:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mustafa G, David RJ. Comparative accuracy of clinical estimate versus menstrual gestational age in computerized birth certificates. Public Health Rep. 2001;1161:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


