Abstract
Background
This meta-analysis investigated the correlation of ABCA1 R219K and CRP +1059G/C gene polymorphisms with susceptibility to coronary heart disease (CHD).
Material/Methods
We searched PubMed, Springer link, Wiley, EBSCO, Ovid, Wanfang database, VIP database, and China National Knowledge Infrastructure (CNKI) databases to retrieve published studies by keyword. Searches were filtered using our stringent inclusion and exclusion criteria. Resultant high-quality data collected from the final selected studies were analyzed using Comprehensive Meta-analysis 2.0 software. Eleven case-control studies involving 3053 CHD patients and 3403 healthy controls met our inclusion criteria. Seven studies were conducted in Asian populations, 3 studies were done in Caucasian populations, and 1 was in an African population.
Results
Our major finding was that ABCA1 R219K polymorphism increased susceptibility to CHD in allele model (OR=0.729, 95% CI=0.559~0.949, P=0.019) and dominant model (OR=0.698, 95% CI=0.507~0.961, P=0.027). By contrast, we were unable to find any significant association between the CRP +1059G/C polymorphism and susceptibility to CHD (allele model: OR=1.170, 95% CI=0.782~1.751, P=0.444; dominant model: OR=1.175, 95% CI=0.768~1.797, P=0.457).
Conclusions
This meta-analysis provides convincing evidence that polymorphism of ABCA1 R219K is associated with susceptibility to CHD while the CRP +1059G/C polymorphism appears to have no correlation with susceptibility to CHD.
MeSH Keywords: Coronary Disease, Disease Susceptibility, Odds Ratio
Background
Coronary heart disease (CHD), including its severe sequelae, myocardial infarction (MI), is the leading cause of disease-related morbidity and mortality worldwide, and accounts for approximately 7 million deaths each year [1,2]. CHD is a multifactorial disease, with both environmental and genetic factors involved [3–5]. The key risk factors for CHD are smoking, family history, hypertension, obesity, diabetes, sedentary behavior, hyperlipidemia, and low-density lipoprotein cholesterol (LDL-C) [6–8]. With advancing age, inflammatory deposits or atherosclerotic plaques coat the walls of arteries supplying blood and oxygen to the heart and consequently severely restrict blood supply, causing angina, and shortness of breath; plaques rupture or break, causing heart attack and myocardial infarction, which severely diminishes heart function and is often fatal [9,10]. Atherosclerotic inflammation is the critical factor in formation of coronary plaques and the progression of plaques to an unstable state, resulting in MI [11]. In the past decade, the influence of genetic factors in the development of CHD has been widely investigated [12].
Adenosine triphosphate-binding cassette transporter A1 (ABCA1) belongs to the ATP-binding cassette super family, and single-nucleotide polymorphisms (SNPs) have been discovered in this gene that are relevant to human disease conditions [13,14]. In particular, mutations in this gene lead to heritable high-density lipoprotein (HDL) disorder and Tangier disease, both of which are associated with increased risk of early-onset CHD [4,15,16]. Among the ABSC1 SNPs, R219K polymorphism occurs in the coding region, leading to an amino acid substitution from arginine to lysine in exon 7 (rs2230806), and is widely studied for its association with susceptibility to CHD [17]. The C-reactive protein gene (CRP) is mapped to chromosome 1q21 to 1q23 within a conserved genetic region spanning approximately 1.9 kb [18,19]. Elevated levels of serum CRP reflect an inflammatory state and CRP itself participates in inflammation responses; and its circulating concentration is a sensitive marker for cardiovascular disease risk [20]. Previous studies reported that SNPs in CRP alter CRP levels in plasma and, since CRP protein is present in atherosclerotic plaques, where it elicits pro-inflammatory and atherogenic outcomes, SNPs in CRP are of direct relevance to CHD risk [20–22]. Although the precise role of genetic factors in susceptibility to CHD remains unclear, the role of CRP and ABCA1 gene polymorphisms in CHD susceptibility is of significant interest due to their high clinical value. However, a few previous studies noted no significant association of CRP gene polymorphisms with the susceptibility to CHD [23,24], but other studies showed a significant association of CRP and ABCA1 gene polymorphisms with CHD susceptibility [20,21]. To address the conflicting data and to investigate the role of CRP and ABCA1 gene polymorphisms in CHD susceptibility, we studied 1059G >C SNP in exon 2 of CRP and R219K (rs2230806) polymorphism of ABCA1in this meta-analysis.
Material and Methods
Data sources and keywords
To identify relevant published studies, the electronic databases PubMed, Springer link, Wiley, EBSCO, Ovid, Wanfang database, VIP database, and China National Knowledge Infrastructure (CNKI) were searched exhaustively (last updated search in October, 2014) by applying a sensitive search strategy using search terms: (“coronary heart disease” or “myocardial infarction” or “MI” or “acute myocardial infarction” or “AMI”) and (“ABCA1” or “Adenosine Triphosphate-binding cassette transporter A1” or “CRP” or “CRP C-reactive protein” or “genetic polymorphism”). Manual searches were conducted to retrieve other cross-references.
Inclusion and exclusion criteria
Published studies retrieved met these inclusion criteria: (1) research topic: correlations between ABCA1 R219K (rs2230806) or CRP +1059G/C (rs1800947) gene polymorphisms and susceptibility to CHD; (2) study type: case-control studies; (3) subject investigated: CHD patients and normal controls; (4) relevant indicators: allele and genotype frequency; and (5) studies published in Chinese or English. Only the latest complete study was considered when the extracted studies were published by the same authors. The exclusion criteria were: (1) insufficient data; (2) animal studies; (3) duplicate publications; and (4) absence of data integrity.
Data extraction and quality assessment
Two reviewers screened each study independently to determine whether it met the inclusion criteria. Disagreements between the investigators were resolved by discussion with a third investigator. Collected information included: the first author, publication year, country, age, sex, gene, number of case and controls, ethnicity, language, genotyping method, detection method, and SNP site. The study quality was assessed by 2 or more investigators independently using the Critical Appraisal Skill Program (CASP) criteria (http://www.casp-uk.net/).
Statistical analysis
Comprehensive Meta-analysis 2.0 (Biostat Inc., Englewood, New Jersey, USA) was used for data analysis. The differences in allele and genotype frequency of ABCA1 R219K (rs2230806) and CRP +1059G/C (rs1800947) polymorphisms were estimated by odds ratio (OR) with 95% confidence intervals (95%CI). The Z test was conducted to assess the overall effect values [25]. Forest plots were used to reflect the comparisons of ORs with 95%CI between the 2 groups. The heterogeneity was determined using the Cochran’s Q-statistic (P<0.05 was considered significant) and I2 test (0%, no heterogeneity; 100%, maximal heterogeneity) [26,27]. The random-effects model was used in case of significant heterogeneity (P<0.05 or I2 test exhibited >50%); otherwise, the fixed-effects model was used [28]. The potential sources of heterogeneity were evaluated by univariate and multivariate meta-regression analysis, confirmed by Monte Carlo method [26,29]. Sensitivity analysis was conducted to assess whether a study had significant influences on the overall results by deleting each study sequentially. Funnel plots, classic fail-safe N, and Egger’s linear regression test were used to define the publication bias [30,31]. All tests were 2-sided and P<0.05 indicated a statistically significant difference.
Results
Baseline characteristics
Our search identified 139 published records, from which we excluded duplicates (n=17), letters, reviews, or meta-analysis (n=4), non-human studies (n=4), and studies in languages other than Chinese or English (n=3). The remaining 111 studies were reviewed and a total of 78 studies were removed because they were not case-control (n=10), they were unpublished studies (n=2), or they were irrelevant to ABCA1 and CRP gene (n=35) or irrelevant to CHD (n = 31). After further assessment, 22 studies were removed for not containing enough information. Following this multi-step screening process, 11 studies with 3053 CHD patients and 3403 healthy controls were finally selected after a full-text analysis [20,23,32–40]. The 11 studies were published from 2005 to 2013, with sample sizes ranging from 121 to 1900. The gene loci of ABCA1 R219K (rs2230806) and CRP +1059G/C (rs1800947) are presented in Figure 1. Among the 11 studies, 3 studies were performed in Caucasians, 7 studies were performed in Asians, and 1 study was performed in Africans. Polymerase chain reaction with restriction fragment length polymorphism (PCR-RFLP) was a common genotyping method among the studies. The baseline characteristics and selection procedure for the 11 studies are presented in Table 1 and Figure 2, respectively.
Figure 1.
The gene loci on ABCA1 R219K (rs2230806) and CRP +1059G/C (rs1800947).
Table 1.
The baseline characteristics of 11 eligible studies in present meta-analysis investigating the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with the susceptibility of coronary heart disease.
| First author | Year | Country | Ethnicity | Disease | Gender (M/F) | Age (years) | Gene | SNP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||
| Li Y | 2005 | China | Asians | CHD | 237/159 | 248/169 | 60.1±8.8 | 59.4±7.6 | ABCA1 | R219K (rs2230806) |
| Balistreri CR | 2006 | Italy | Caucasians | MI | 106/0 | 120/0 | 41 (20–46) | 39 (20–50) | CRP | +1059G/C(rs1800947) |
| Martin M | 2006 | Spain | Caucasians | MI | 170/0 | NR | 43±5 | NR | ABCA1 | R219K (rs2230806) |
| Zhao BC | 2006 | China | Asians | MI | 99/52 | 40/40 | 44–75 | 42–76 | CRP | +1059G/C(rs1800947) |
| Pai JK | 2008 | USA | Caucasians | CHD | 266/249 | 531/498 | 62.75±0.11 | 62.7±0.08 | CRP | +1059G/C(rs1800948) |
| Yu B | 2008 | China | Asians | MI | 49/0 | 72/0 | 55.8±3.8 | 51.4±4.0 | ABCA1 | R219K (rs2230806) |
| Li J | 2009 | China | Asians | CHD | 176/189 | NR | 63±14 | 61±13 | ABCA1 | R219K (rs2230806) |
| Sun DL | 2011 | China | Asians | CHD | 81/17 | 99/26 | 55.69±11.48 | 49.97±11.64 | CRP | +1059G/C(rs1800947) |
| Akbarzadeh Najar R | 2012 | Iran | Asians | MI | 478/472 | 475/475 | 52.96±1.89 | 49.85±0.36 | CRP | +1059G/C(rs1800947) |
| Ghattas MH | 2012 | Egypt | Africans | MI | 90/60 | 93/62 | 47.7±4.85 | 49.10±10.30 | CRP | +1059G/C(rs1800947) |
| Wang JR | 2013 | China | Asians | MI | 59/44 | 67/47 | 65.9±13.1 | 63.8±11.9 | ABCA1 | R219K (rs2230806) |
MI – myocardial infarction; CHD – coronary heart disease; M – male; F – female; PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism; NR – no reference.
Figure 2.

Flow chart shows the study selection procedure. Eleven studies were included.
Meta-analysis of correlation between ABCA1 R219K (rs2230806) polymorphism and susceptibility to CHD
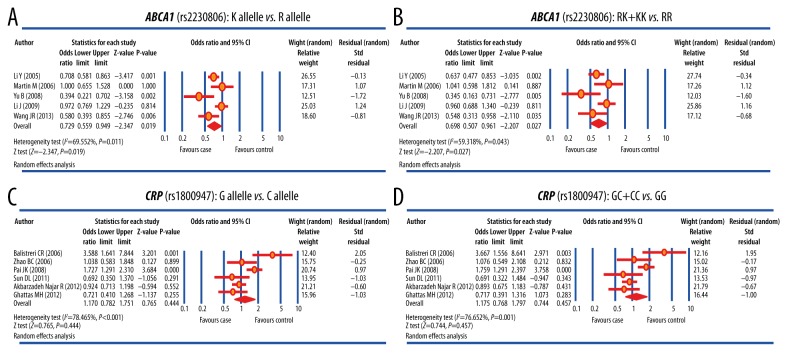
Five studies reported the correlation between ABCA1 R219K (rs2230806) polymorphism and susceptibility to CHD. Considering the significant heterogeneity among studies, the random-effects model was used (allele model: I2=69.552%, P=0.011; dominant model: I2=59.318%, P=0.043). As shown in Figure 3A, 3B, and Table 2, the ABCA1 R219K (rs2230806) polymorphism significantly increased the susceptibility to CHD (allele model: OR=0.729, 95% CI=0.559~0.949, P=0.019; dominant model: OR=0.698, 95% CI=0.507~0.961, P=0.027). Meta-regression analysis showed that publication year, ethnicity, and sample size were neither the heterogeneous sources nor the key factors that influenced the overall effect size (both P> 0.05) (Figure 4A–4C, Table 3A).
Figure 3.
Forest plots for the differences of genotype and allele frequencies in the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with susceptibility to coronary heart disease.
Table 2.
Comparisons of genotype and allele frequencies between the case and the control groups in present meta-analysis investigating the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with the susceptibility to coronary heart disease.
| Gene Model | ABCA1 (rs2230806) | CRP (rs1800947) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| M allele vs. W allele (Allele model) | 0.729 | 0.559–0.949 | 0.019 | 1.170 | 0.782–1.751 | 0.444 |
| WM + MM vs. WW (Dominant model) | 0.698 | 0.507–0.961 | 0.027 | 1.175 | 0.768–1.797 | 0.457 |
| MM vs. WW (Homozygous model) | 0.552 | 0.331–0.921 | 0.023 | 1.265 | 0.738–2.169 | 0.392 |
| WM vs. MM (Heterozygous model) | 1.288 | 0.991–1.676 | 0.059 | 0.841 | 0.475–1.488 | 0.552 |
| MM vs. WW + WM (Recessive model) | 0.695 | 0.543–0.890 | 0.004 | 1.252 | 0.731–2.145 | 0.413 |
OR – odds ratio; 95% CI – 95% confidential intervals.
Figure 4.
Meta-regression analysis for the differences of genotype and allele frequencies in the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with susceptibility to coronary heart disease.
Table 3A.
Meta-regression analyses of potential sources of heterogeneity for ABCA1 R219K by analyzing publication year, ethnicity and sample size.
| Heterogeneity factors | Coefficient | SE | t | P (Adjusted) | 95% CI | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Year | 0.117 | 0.081 | 1.46 | 0.545 | −0.906 | 1.140 |
| Ethnicity | −1.190 | 0.546 | −2.18 | 0.355 | −8.133 | 5.753 |
| Sample size | 0.002 | 0.001 | 1.92 | 0.431 | −0.009 | 0.013 |
Meta-analysis of correlation between CRP +1059G/C (rs1800947) polymorphism and susceptibility to CHD
A total of 6 studies reported the correlation between CRP +1059G/C (rs1800947) polymorphism and susceptibility to CHD. The random-effects model was used due to significant heterogeneity (allele model: I2=78.465%, P<0.001; dominant model: I2=76.652%, P=0.001). The results of the present meta-analysis failed to show any significant correlation between the polymorphism of CRP +1059G/C (rs1800947) and susceptibility to CHD (allele model: OR=1.170, 95% CI=0.782~1.751, P=0.444; dominant model: OR=1.175, 95% CI=0.768~1.797, P=0.457) (Figure 3C, 3D, Table 2). Meta-regression analysis suggested that publication year, ethnicity and sample size are not heterogeneous sources or key factors influencing the overall effect size (both P>0.05) (Figure 4D–4F, Table 3B).
Table 3B.
Meta-regression analyses of potential sources of heterogeneity for CRP +1059G/C by publication year, ethnicity and sample size.
| Heterogeneity factors | Coefficient | SE | t | P (Adjusted) | 95% CI | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Year | −0.049 | 0.129 | −0.38 | 0.914 | −0.604 | 0.506 |
| Ethnicity | −0.492 | 0.459 | −1.08 | 0.472 | −2.449 | 1.465 |
| Sample size | −0.001 | 0.001 | −0.20 | 0.982 | −0.001 | 0.001 |
SE – standard error; LL – lower limit; UL – upper limit.
Sensitivity analysis and publication bias
The sensitivity analysis showed that no single study significantly affected the pooled ORs of correlations between CRP +1059G/C (rs1800947) polymorphism and susceptibility to CHD. Except for the studies by Li et al. 2005, Yu et al. 2008 and Wang et al. 2013, no single study affected the pooled ORs of correlations between ABCA1 R219K (rs2230806) polymorphism and susceptibility to CHD (Figure 5). The shape of funnel plots did not reveal any evidence of funnel plot asymmetry and the statistical results did not show any publication bias. Classic fail-safe N and Egger’s linear regression test confirmed that there was no significant publication bias (all P>0.05) (Figure 6).
Figure 5.
Sensitivity analysis of the summary odds ratio coefficients for the differences of genotype and allele frequencies in the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with susceptibility to coronary heart disease.
Figure 6.
Publication biases for genotype and allele frequencies in the correlation of ABCA1 R219K and CRP +1059G/C polymorphisms with susceptibility to coronary heart disease.
Discussion
To investigate the associations of CRP and ABCA1 gene polymorphisms with CHD susceptibility, we selected 2 polymorphisms, 1059G >C (rs1800947) of CRP and R219K (rs2230806) of ABCA1, and performed a comprehensive meta-analysis of the available data. The results of the present meta-analysis suggest that R219K (rs2230806) polymorphism of ABCA1 is associated with a significantly increased risk of CHD. The influence of ABCA1 on plasma lipid levels may be a potential mechanism by which ABCA1 R219K polymorphism is involved with the risk of CHD [4]. Disorders of lipid homeostasis are important in the development and progression of CHD and hyperlipidemia is a major risk factor for CHD [8]. Atherosclerosis, the major cause of CHD, is characterized by accumulation of unbalanced lipid in the arterial wall, resulting in narrowing of the vessel lumen [10]. The major pathogenesis of atherosclerosis is reverse cholesterol transport (RCT) mediated by HDL-C, which facilitates cholesterol efflux from peripheral cells [41]. ABCA1 stimulates cholesterol efflux to lipid-poor HDL apolipoproteins, the initial step in reverse cholesterol transport [4,42]. High plasma LDL-C concentration contributes to the development of atherosclerotic plaques, whose break-up or rupture result in angiemphraxis and ischemic cardiac events [12]. Another probable mechanism is that R219K polymorphism enhances ABCA1 activity, leading to mediation of cholesterol efflux, independent of plasma HDL-C levels [4]. Several case-control studies investigated the association between ABCA1 R219K polymorphism and the susceptibility to CHD, but the findings were conflicting [4,17]. Consistent with our results, the R219K variant was shown to modulate the HDL-C response to CHD medication in patients with CHD, suggesting a possible association between R219K gene polymorphism and CHD [24,33]. Studies investigating the association between polymorphisms of the CRP gene and atherosclerosis suggest that variations in CRP might be involved in the pathogenesis of CHD and could be helpful in predicting CHD [18,32,43]. In the present meta-analysis, we found no significant association between CRP 1059G >C (rs1800947) polymorphism and CHD susceptibility. In accordance with our result, a previous study also found that CRP 1059G/C gene variation resulted in higher plasma CRP levels but was not associated with risk for AMI and CHD [20].
Some limitations should be noted while interpreting the results of the present meta-analysis. First, inter-study heterogeneity still existed in this meta-analysis even though we minimized its likelihood by performing a sensitive search strategy. Second, as a retrospective study, the present meta-analysis may have recall or selection bias, possibly influencing the stability of our results. Third, the limited access to the original data from some studies restrained our further investigation of the potential interactions between other factors and CHD risks, such as gene-environment and gene-gene interactions. Fourth, most of the 11 eligible studies were performed in Asians and only 1 study was performed in Africans. Finally, the language of included studies was limited to English and Chinese, and studies published in other languages were excluded.
Conclusions
In summary, our meta-analysis shows that ABCA1 R219K (rs2230806) polymorphism is associated with susceptibility to CHD, but CRP +1059G/C (rs1800947) is not correlated with CHD risk. Furthermore, gene-to-gene and gene-to-environment interactions should also be investigated in future studies. A better understanding of the mechanism of CHD pathogenesis will be beneficial in future studies on prevent CHD progression.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
Source of support: Departmental sources
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricke AD, Swiryn S, Bauernfeind RA, et al. Improved pacemaker pulse detection: Clinical evaluation of a new high-bandwidth electrocardiographic system. J Electrocardiol. 2011;44:265–74. doi: 10.1016/j.jelectrocard.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Yin YW, Hu AM, Sun QQ, et al. Association between interleukin-6 gene –174 G/C polymorphism and the risk of coronary heart disease: a meta-analysis of 20 studies including 9619 cases and 10,919 controls. Gene. 2012;503:25–30. doi: 10.1016/j.gene.2012.04.075. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Tang K, Zhou K, et al. Quantitative assessment of the effect of ABCA1 R219K polymorphism on the risk of coronary heart disease. Mol Biol Rep. 2012;39:1809–13. doi: 10.1007/s11033-011-0922-z. [DOI] [PubMed] [Google Scholar]
- 5.Shirai K, Iso H, Ohira T, et al. Perceived level of life enjoyment and risks of cardiovascular disease incidence and mortality: The Japan public health center-based study. Circulation. 2009;120:956–63. doi: 10.1161/CIRCULATIONAHA.108.834176. [DOI] [PubMed] [Google Scholar]
- 6.Kivimaki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: A collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–97. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, He HW, Wang ZM, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira DM, da Silva RL, Vieira JL, et al. Role of vascular inflammation in coronary artery disease: Potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc Drugs. 2015;15:1–11. doi: 10.1007/s40256-014-0094-z. [DOI] [PubMed] [Google Scholar]
- 11.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow DI, Holmes MV, Harrison S, Humphries SE. The genetics of coronary heart disease. Br Med Bull. 2012;102:59–77. doi: 10.1093/bmb/lds009. [DOI] [PubMed] [Google Scholar]
- 13.Brunham LR, Singaraja RR, Hayden MR. Variations on a gene: Rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu Rev Nutr. 2006;26:105–29. doi: 10.1146/annurev.nutr.26.061505.111214. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzi I, von Eckardstein A, Cavelier C, et al. Apolipoprotein A-I but not high-density lipoproteins are internalised by RAW macrophages: Roles of ATP-binding cassette transporter A1 and scavenger receptor BI. J Mol Med. 2008;86:171–83. doi: 10.1007/s00109-007-0267-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Gulshan K, Brubaker G, et al. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2013;33:1197–205. doi: 10.1161/ATVBAHA.112.301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunham LR, Kang MH, Van Karnebeek C, et al. Clinical, biochemical, and molecular characterization of novel mutations in ABCA1 in Families with Tangier Disease. JIMD Rep. 2015;18:51–62. doi: 10.1007/8904_2014_348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu N, Hou M, Ren W, et al. The R219K polymorphism on ATP-binding cassette transporter A1 gene is associated with coronary heart disease risk in Asia population: Evidence from a meta-analysis. Cell Biochem Biophys. 2015;71:49–55. doi: 10.1007/s12013-014-0161-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Liu T, He H, et al. C-reactive protein gene polymorphisms and myocardial infarction risk: A meta-analysis and meta-regression. Genet Test Mol Biomarkers. 2013;17:873–80. doi: 10.1089/gtmb.2013.0340. [DOI] [PubMed] [Google Scholar]
- 19.Vatay A, Bene L, Kovacs A, et al. Relationship between the tumor necrosis factor alpha polymorphism and the serum C-reactive protein levels in inflammatory bowel disease. Immunogenetics. 2003;55:247–52. doi: 10.1007/s00251-003-0575-8. [DOI] [PubMed] [Google Scholar]
- 20.Ghattas MH, Abo-Elmatty DM, El-Eraki AZ. C-reactive protein 1059G/C gene polymorphism, C-reactive protein levels and acute myocardial infarction. J Cardiovasc Med. 2012;13:716–19. doi: 10.2459/JCM.0b013e3283577170. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Singh M, Nagpal HS, et al. A novel haplotype within C-reactive protein gene influences CRP levels and coronary heart disease risk in Northwest Indians. Mol Biol Rep. 2014;41:5851–62. doi: 10.1007/s11033-014-3459-0. [DOI] [PubMed] [Google Scholar]
- 22.Emerging Risk Factors C. Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbarzadeh Najar R, Ghaderian SM, Tabatabaei Panah AS. C-reactive protein (CRP) gene polymorphisms: implication in CRP plasma levels and susceptibility to acute myocardial infarction. Mol Biol Rep. 2012;39:3705–12. doi: 10.1007/s11033-011-1145-z. [DOI] [PubMed] [Google Scholar]
- 24.Doosti M, Najafi M, Reza JZ, Nikzamir A. The role of ATP-binding-cassette-transporter-A1 (ABCA1) gene polymorphism on coronary artery disease risk. Transl Res. 2010;155:185–90. doi: 10.1016/j.trsl.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Manning AK, Dupuis J. A method of moments estimator for random effect multivariate meta-analysis. Biometrics. 2012;68:1278–84. doi: 10.1111/j.1541-0420.2012.01761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 28.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 29.Huizenga HM, Visser I, Dolan CV. Testing overall and moderator effects in random effects meta-regression. Br J Math Stat Psychol. 2011;64:1–19. doi: 10.1348/000711010X522687. [DOI] [PubMed] [Google Scholar]
- 30.Wikstrom EA, Naik S, Lodha N, Cauraugh JH. Balance capabilities after lateral ankle trauma and intervention: a meta-analysis. Med Sci Sports Exerc. 2009;41:1287–95. doi: 10.1249/MSS.0b013e318196cbc6. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balistreri CR, Vasto S, Listi F, et al. Association between +1059G/C CRP polymorphism and acute myocardial infarction in a cohort of patients from Sicily: a pilot study. Ann NY Acad Sci. 2006;1067:276–81. doi: 10.1196/annals.1354.036. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Wang LF, Li ZQ, Pan W. Effect of R219K polymorphism of the ABCA1 gene on the lipid-lowering effect of pravastatin in Chinese patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36:567–70. doi: 10.1111/j.1440-1681.2008.05119.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhang SZ, Ma YX, et al. [Relationship between the R219K polymorphism of ATP-binding cassette transporter 1 gene and coronary heart disease]. Yi Chuan. 2005;27:549–22. [in Chinese] [PubMed] [Google Scholar]
- 35.Martin M, Gonzalez P, Reguero JJ, et al. ABCA1 polymorphisms and prognosis after myocardial infarction in young patients. Int J Cardiol. 2006;110:267–68. doi: 10.1016/j.ijcard.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Pai JK, Mukamal KJ, Rexrode KM, Rimm EB. C-reactive protein (CRP) gene polymorphisms, CRP levels, and risk of incident coronary heart disease in two nested case-control studies. PLoS One. 2008;3:e1395. doi: 10.1371/journal.pone.0001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun DL, Zhong LJ, Liu Y, Zhang Q. [Relationship of C-reactive protein genetic polymorphisms with chronic periodontitis and coronary heart disease]. Journal of Chinese Practical Diagnosis and Therapy. 2011;25:219–22. [in Chinese] [Google Scholar]
- 38.Wang JR, Zhang YL. Association between ATP-binding cassette transporter A1 R219K gene polymorphisms and acute mocardial infarction. Academic Journal of Chinese PLA Medical School. 2013:777–78. [Google Scholar]
- 39.Yu B, Deng B. Correlation of polymorphism of R219K in ABCA1 with lipid metabolism and the risk of AMI. Journal of Tongji University(Medical Science) 2008;29:64–67. [Google Scholar]
- 40.Zhao BC, Dai XH, Yang RX, Li JX. Influence of the C-reactive protein level and gene polymorphism on the prognosis of the acute myocardial infarction. Chinese General Practice. 2006;9:807–8. [Google Scholar]
- 41.Li YY, Zhang H, Qin XY, et al. ATP-binding cassette transporter A1 R219K polymorphism and coronary artery disease in Chinese population: A meta-analysis of 5,388 participants. Mol Biol Rep. 2012;39:11031–39. doi: 10.1007/s11033-012-2006-0. [DOI] [PubMed] [Google Scholar]
- 42.Oram JF. HDL apolipoproteins and ABCA1: Partners in the removal of excess cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–27. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- 43.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]