Abstract
LPL hydrolyzes triglycerides in triglyceride-rich lipoproteins along the capillaries of heart, skeletal muscle, and adipose tissue. The activity of LPL is repressed by angiopoietin-like 4 (ANGPTL4) but the underlying mechanisms have not been fully elucidated. Our objective was to study the cellular location and mechanism for LPL inhibition by ANGPTL4. We performed studies in transfected cells, ex vivo studies, and in vivo studies with Angptl4−/− mice. Cotransfection of CHO pgsA-745 cells with ANGPTL4 and LPL reduced intracellular LPL protein levels, suggesting that ANGPTL4 promotes LPL degradation. This conclusion was supported by studies of primary adipocytes and adipose tissue explants from wild-type and Angptl4−/− mice. Absence of ANGPTL4 resulted in accumulation of the mature-glycosylated form of LPL and increased secretion of LPL. Blocking endoplasmic reticulum (ER)-Golgi transport abolished differences in LPL abundance between wild-type and Angptl4−/− adipocytes, suggesting that ANGPTL4 acts upon LPL after LPL processing in the ER. Finally, physiological changes in adipose tissue ANGPTL4 expression during fasting and cold resulted in inverse changes in the amount of mature-glycosylated LPL in wild-type mice, but not Angptl4−/− mice. We conclude that ANGPTL4 promotes loss of intracellular LPL by stimulating LPL degradation after LPL processing in the ER.
Keywords: lipid metabolism, adipose tissue, vascular biology, intracellular processing
Circulating triglyceride-rich lipoproteins, such as chylomicrons and VLDLs, supply tissues with lipid nutrients for storage or oxidation. Hydrolysis of circulating triglycerides is mediated by LPL, a glycoprotein found in multiple tissues, including adipose tissue, brain, heart, and skeletal muscle (1–4). LPL is produced by parenchymal cells and then transported to the luminal surface of capillaries by an endothelial cell protein, glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1) (5).
A critical step in the maturation of LPL is asparagine-linked glycosylation (6–8). LPL is glycosylated within the endoplasmic reticulum (ER) via cotranslational transfer of oligosaccharide chains high in mannose residues (6–9). After being translocated to the Golgi apparatus, the high-mannose oligosaccharides are trimmed and replaced by more complex oligosaccharides (6–9). Endoglycosidase H (EndoH) cleaves high-mannose oligosaccharides from proteins, but not complex oligosaccharides (6, 10), making it possible to distinguish LPL within the ER and Golgi compartments.
The catalytic activity of LPL along the capillary endothelium is considered rate-limiting for the lipolytic processing of plasma triglycerides and for the subsequent uptake of fatty acids by surrounding tissues (1, 4, 11). To ensure that supply of fatty acids to tissues matches metabolic demand, LPL activity is under tight regulatory control. Although it is possible to identify small fluctuations in Lpl mRNA levels, LPL activity appears to be regulated mainly at the posttranslational level (1, 4). An important physiological regulator of LPL is angiopoietin-like 4 (ANGPTL4). ANGPTL4 potently inhibits LPL activity in multiple tissues and regulates LPL activity during a variety of physiological conditions, including fasting, cold, and exercise (12–14). For example, changes in the expression of ANGPTL4 (originally called fasting-induced adipose factor) allow for swift changes in adipose tissue LPL activity during fasting (13, 15). Expression of ANGPTL4 is induced by fatty acids via PPARs as part of a feedback mechanism aimed at preventing lipid overload within cells (16, 17).
Genetic studies have strongly supported a role for ANGPTL4 in determining plasma triglyceride levels in humans and have linked inactivating variants in the ANGPTL4 gene to a reduced risk of coronary heart disease (18–20). However, cross-sectional studies have not revealed a clear correlation between the plasma levels of ANGPTL4 and triglycerides (21–24). These observations suggest that the plasma pool of ANGPTL4 may not be primarily responsible for regulating plasma triglyceride levels and that the inhibitory effects of ANGPTL4 on LPL activity may not occur exclusively on the surface of capillaries. Indeed, Robciuc et al. (25) proposed that ANGPTL4 could inhibit LPL activity not only at the cell surface, but also intracellularly, partly based on microscopy studies showing colocalization of LPL and ANGPTL4 within cells. At the same time, recent studies have raised the possibility that ANGPTL4 regulation of LPL might occur within the subendothelial spaces rather than along the capillary lumen (26–28). Studies of 3T3-L1 adipocytes suggested that inhibition of LPL activity by ANGPTL4 begins only after these proteins arrive at the cell surface (29). However, the extent to which the cultured cell studies are relevant to LPL activity in adipose tissue in vivo is uncertain. Accordingly, our objective in the current studies was to investigate the cellular location and mechanism for LPL inhibition by ANGPTL4 in adipose tissue. To address this objective, we used a combination of cell culture studies, and ex vivo and in vivo studies of adipocytes and adipose tissue from wild-type and Angptl4−/− mice.
MATERIALS AND METHODS
All animal experiments were performed in accordance with Directive 2010/63/EU from the European Union. All animal studies were reviewed and approved by the Animal Ethics Committee of Wageningen University.
Cell culture
CHO pgsA-745 cells were cultured as described previously (30, 31). CHO pgsA-745 cells were electroporated with an expression vector for Flag-tagged human ANGPTL4 (hANGPTL4) (either the full-length protein hANGPTL4[1–406] or the N-terminal domain of hANGPTL4[1–160]) or a vector for V5-tagged human LPL (or empty vector). After the cell transfections, the cells were mixed and coplated in the same well of a 24-well plate. After 24 h, the cell culture medium was collected for a LPL activity assay. After 48 h, the cell culture medium was collected and cell lysates were prepared for SDS-PAGE and Western blotting. ANGPTL4 and LPL expression vectors were also cotransfected into CHO pgsA-745 cells. For those studies, the cells were co-electroporated with a vector for Flag-tagged hANGPTL4 (either hANGPTL4[1–406] or hANGPTL4[1–160]) and a vector for V5-tagged human LPL (hLPL) (or empty vector). After 24 h, the cell culture medium was collected for a LPL activity assay. After 48 h, the cell culture medium was collected and cell lysates were prepared for SDS-PAGE and Western blotting.
Isolation and differentiation of stromal vascular fraction
Inguinal or gonadal white adipose tissue (gWAT) was removed from Angptl4−/− and wild-type mice and placed in DMEM (Lonza) supplemented with 1% penicillin/streptomycin (P/S) and 1% BSA (Sigma-Aldrich, Houten, The Netherlands). Fat pads from 2–3 mice were pooled, minced with scissors, and digested for 1 h at 37°C in collagenase-containing medium [DMEM with 3.2 mM CaCl2, 1.5 mg/ml collagenase type II (C6885; Sigma-Aldrich), 10% FCS, 0.5% BSA, and 15 mM HEPES]. Next, the cell suspension was filtered through a 100 μm cell strainer (Falcon) and centrifuged at 1,600 rpm for 10 min. The supernatant fluid was removed, and the pellet containing the stromal vascular fraction was resuspended in erythrocyte lysis buffer (155 mM NH4Cl, 12 mM NaHCO3, 0.1 mM EDTA) and incubated for 2–3 min at room temperature. After neutralization, cells were centrifuged at 1,200 rpm for 5 min. Pelleted cells were resuspended in DMEM containing 10% FCS and 1% P/S and plated. After reaching confluency, cells were differentiated according to standard protocol for 3T3-L1 cells with addition of 1 μM rosiglitazone (32).
Mouse studies
Tissue samples from ANGPTL4 knockout mice (Angptl4−/−), wild-type mice, and Angptl4-Tg mice from previously published studies were used for analyses of LPL glycosylation and mass (13, 14, 33–35). Angptl4−/− mice, wild-type mice, and Angptl4-Tg mice were on a C56BL/6J background for >10 generations. Wild-type and Angptl4-Tg mice were littermates. In Angptl4−/− mice, part of the Angptl4 gene was deleted by homologous recombination in embryonic stem cells, resulting in a nonfunctional ANGPTL4 protein (33, 34). Angptl4-Tg mice overexpress the Angptl4 gene in various tissues under the endogenous promoter (35). Brown adipose tissue samples from Angptl4−/− and wild-type mice exposed to cold or thermoneutral temperature for 10 days were from a study by Dijk et al. (14). gWAT samples from fed, fasted, and refed Angptl4−/− and wild-type mice were from a study described by Kroupa et al. (13). Hearts from fed and overnight-fasted Angptl4−/− and wild-type mice were collected and snap-frozen in liquid nitrogen. For “post-heparin” tissues, wild-type and Angptl4−/− mice were fed or fasted for 16 h. The mice were then anesthetized with isoflurane and injected with heparin (100 IU/kg in 0.9% NaCl) through the jugular vein. After 5 min, the mice were euthanized by cervical dislocation. gWAT was dissected, frozen immediately in liquid nitrogen, and stored at −80°C for further analyses.
LPL activity measurements
LPL activity levels in culture medium and cell lysates of transfected cells was assessed in duplicate with a [3H]triolein substrate (PerkinElmer) as previously described (36).
WAT explants
gWAT was harvested from Angptl4−/− and wild-type mice and placed in DMEM supplemented with 1% P/S and 1% BSA. Fat pads were minced into small pieces, which were further divided to make small mounds of WAT (∼50–100 mg of tissue). WAT explants were placed into wells containing medium (DMEM with 1% P/S and 10% FCS) with or without heparin (50 IU/ml). Explants were incubated for different times (as indicated in the figure legends), after which the medium was harvested and explant weights were determined. Explants were immediately lysed to prepare protein extracts. Protein concentrations in explants were determined and equal amounts of protein were used for SDS-PAGE and Western blotting studies.
NP40 solubilization of adipocytes
Adipocytes that had been differentiated from the stromal vascular fractions of Angptl4−/− and wild-type mice were prepared as described earlier. Upon differentiation, adipocytes were lysed in NP40 lysis buffer [50 mM Tris-HCl (pH 8.0), 0.5% NP40, 150 mM NaCl, 5 mM MgCl2] supplemented with protease and phosphatase inhibitors (Roche). After centrifugation, the supernatant fluid (NP40S) was mixed with 2× Laemmli sample buffer and heated at 65°C for 15 min. The pellet was resuspended in PBS and 2× Laemmli sample buffer and heated at 95°C for 30 min (NP40P). NP40S and NP40P fractions were then loaded onto SDS-PAGE for further analyses by Western blotting.
Western blots
Fat pads, explants, and differentiated adipocytes were lysed in a mild RIPA-like lysis buffer [25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol; Thermo Scientific, Landsmeer, The Netherlands] containing protease and phosphatase inhibitors (Roche). Lysates were centrifuged 2–3 times at 13,000 rpm for 10 min to remove fat droplets. Protein lysates (10–30 μg protein per lane), medium (10–15 μl), or plasma (0.75 μl) samples were diluted in Laemmli sample buffer, cooked for 5 min at 95°C, and loaded onto precast TGX gels (Bio-Rad, Veenendaal, The Netherlands). For Native PAGE, protein lysates were diluted in a SDS-free and DTT-free sample buffer, directly loaded onto precast TGX gels, and run in a Tris-glycine running buffer without SDS. The separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane with a Transblot Turbo system (Bio-Rad). Membranes were probed with a goat anti-mouse LPL antibody (37), a rabbit anti-mouse HSP90 antibody (Cell Signaling Technology; #4874), a rabbit anti-mouse H2A antibody (Abcam; #ab18255), a rat anti-mouse ANGPTL4 antibody (Adipogen; #Kairos 142-2), or a rabbit anti-mouse ADIPOQ antibody (ThermoScientific, #PAI-054) at 1:5,000 (LPL), 1:2,000 (HSP90, ANGPTL4), or 1:1,000 (H2A, ADIPOQ) dilution. All incubations were performed in TBS (pH 7.5), 0.1% Tween-20, and 5% nonfat dry milk. Membranes were washed in the TBS/Tween-20 solution. Antibody signals were detected with the ChemiDoc MP System (Bio-Rad) and Clarity ECL substrate (Bio-Rad). Equal loading of medium and plasma samples was verified by Coomassie blue staining, H2A, or HSP90 expression.
Proteins in culture supernatants and cell lysates from transfected CHO pgsA-745 cells were separated on 12% Bis-Tris SDS-polyacrylamide gels (Life Technologies) and transferred to a nitrocellulose membrane for Western blotting. The antibody dilutions were 1:500 for an IRdye800-conjugated mouse monoclonal against the V5-tag; 1:500 for an IRdye680-conjugated mouse monoclonal against the Myc-tag; 1:500 for IRdye680-conjugated mouse monoclonal 4-1a against hLPL (38); 1:500 for a mouse monoclonal against the Flag-tag (Sigma-Aldrich); 1:2,000 for IRdye680-conjugated donkey anti-mouse IgG (LI-COR Biosciences); 2 μg/ml for a goat polyclonal against β-actin (Santa Cruz Biotechnology); 1:2,000 for IRdye680-conjugated donkey anti-goat IgG (LI-COR); 2 μg/ml for a polyclonal antibody against human SLURP1 (Novus Biologicals); 1:2,000 for IRdye800-conjugated donkey anti-mouse IgG (LI-COR).
EndoH and PNGase digestions
Glycosylation of proteins was analyzed by Western blotting after digestion of 10–20 μg of protein with EndoH or PNGase (New England BioLabs) according to manufacturer’s instructions.
RESULTS
Secreted ANGPTL4 inactivates LPL outside of the cell
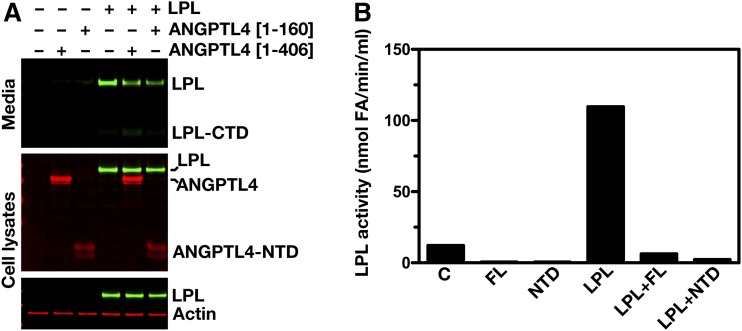
To assess to what extent secreted ANGPTL4 affects the amount of LPL and LPL activity in cells and cell culture medium, CHO pgsA-745 cells were transfected with either hANGPTL4 or hLPL expression vectors. The ANGPTL4-transfected cells were then mixed with the LPL-transfected cells and coplated. Coplating of hANGPTL4-expressing cells with hLPL-expressing cells did not change the amount of LPL in the cell extracts, but coplating did reduce the amount of LPL in cell culture medium (Fig. 1A). A similar reduction in the amount of LPL in cell culture medium was found when hLPL-transfected cells were coplated with cells expressing the N-terminal 160 residues of hANGPTL4 (Fig. 1A). As expected, coplating of hLPL-expressing cells with hANGPTL4-expressing cells resulted in a striking reduction in LPL activity levels in the cell culture medium (Fig. 1B). Thus, the presence of ANGPTL4 in the cell culture medium reduces the amount of LPL protein in the medium and markedly inhibits LPL activity in the medium. However, coplating of hLPL- and hANGPTL4-expressing cells had no effect on intracellular levels of LPL.
Fig. 1.
ANGPTL4 inactivates LPL outside of the cell. A: Western blots of cell culture media and cell lysates of CHO pgsA-745 cells. Cells were transfected with either a Flag-tagged ANGPTL4 expression vector or a vector for V5-tagged hLPL. The ANGPTL4- and LPL-transfected cells were then mixed and coplated. A full-length ANGPTL4 expression vector (hANGPTL4[1–406]) or a vector encoding the N-terminal domain of ANGPTL4 (hANGPTL4[1–160]) was used. Western blots were probed with an anti-Flag antibody to detect ANGPTL4 (red), an anti-V5 antibody to detect LPL (green), and an anti-actin antibody (red) (as a loading control). LPL-CTD, C-terminal domain of LPL; ANGPTL4-NTD, N-terminal domain of ANGPTL4. B: Bar graph representing LPL activity levels in the media of CHO pgsA-745 cells that had been transfected with empty vector (C), full-length ANGPTL4 alone (FL), the N-terminal domain of ANGPTL4 alone (NTD), hLPL alone (LPL), a mixture of ANGPTL4 (full-length)-transfected cells and LPL-transfected cells (LPL+FL), or a mixture of ANGPTL4 (N-terminal domain)-transfected cells and LPL-transfected cells (LPL+NTD).
Co-expression of ANGPTL4 and LPL inactivates LPL within cells
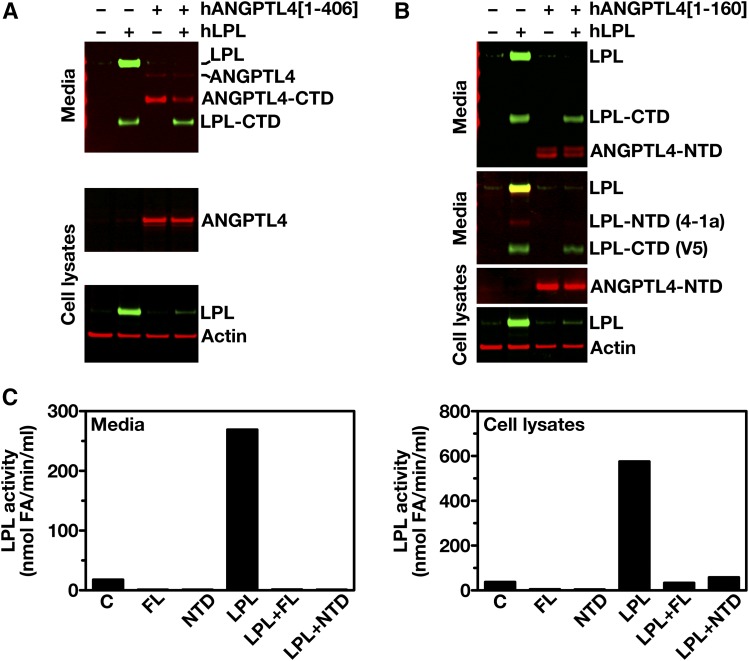
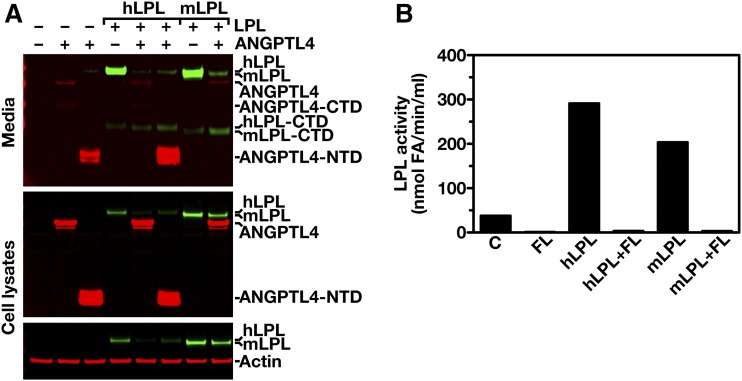
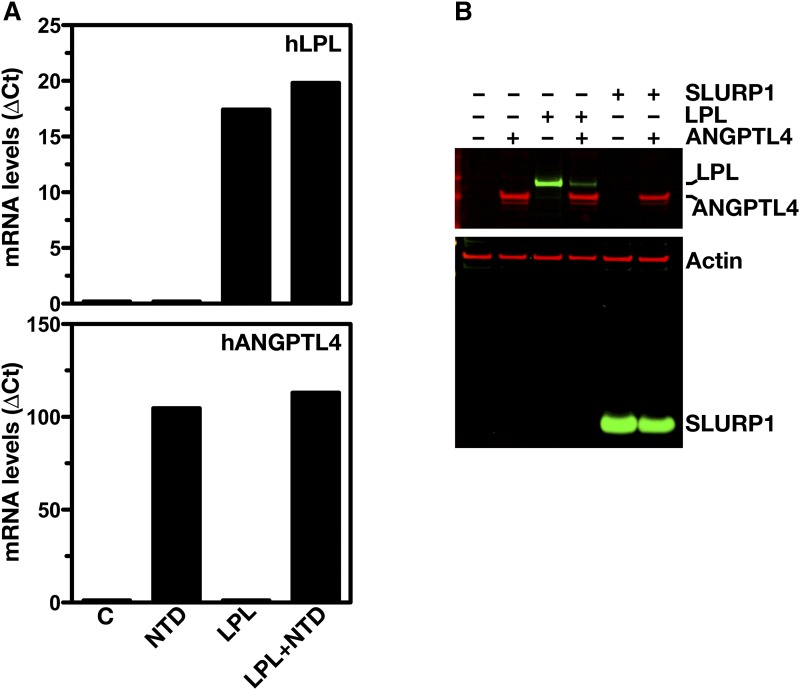
ANGPTL4 and LPL are both secreted glycoproteins, and are expected to follow a similar route to the cell surface (6, 8, 39). To determine whether ANGPTL4 expression affects the levels of LPL protein inside cells, CHO pgsA-745 cells were simultaneously transfected with hLPL and hANGPTL4 expression vectors. Cotransfection of hLPL and hANGPTL4 markedly reduced the amount of LPL in both the cell extracts and the cell culture medium (Fig. 2A). When we performed analogous studies with the N-terminal portion of hANGPTL4 (residues 1–160), a similar reduction in the amounts of LPL in the medium and inside cells was found (Fig. 2B). We also observed markedly reduced amounts of LPL catalytic activity, both within cells and in the cell culture medium (Fig. 2C). Similar to hLPL, cotransfection of mouse (m)LPL with hANGPTL4 reduced the amount and activity of mLPL within cells and in the cell culture medium (Fig. 3A, B). The reduced amounts of intracellular LPL in the cotransfection studies could not be explained by lower levels of LPL transcripts (Fig. 4A). Likewise, the reduced amounts of intracellular LPL in the ANGPTL4 co-expression studies could not be explained by a global decrease in secreted proteins inside cells; co-expression of ANGPTL4 with SLURP1, another secreted protein, did not result in reduced amounts of intracellular SLURP1 (Fig. 4B).
Fig. 2.
ANGPTL4 inactivates LPL inside cells. A: Western blots of cell culture media and cell lysates of CHO pgsA-745 cells that had been cotransfected with an expression vector for Flag-tagged hANGPTL4 (hANGPTL4[1–406]) and a vector for V5-tagged hLPL (or empty vector). Western blots were probed with an anti-Flag antibody to detect ANGPTL4 (red), an anti-V5 antibody to detect LPL (green), and an anti-actin antibody (red) (as a loading control). LPL-CTD, C-terminal domain of LPL; ANGPTL4-CTD, C-terminal domain of ANGPTL4. B: Western blots of cell culture media and cell lysates of CHO pgsA-745 cells cotransfected with an expression vector for myc-tagged hANGPTL4 (hANGPTL4[1–160]) and a vector for V5-tagged hLPL (or empty vector). Western blots were probed with an anti-Myc antibody to detect ANGPTL4 (red), an anti-V5 tag antibody (green), or Mab 4-1a (red) to detect LPL, and an anti-actin antibody (red) (as a loading control). LPL-CTD, C-terminal domain of LPL; LPL-NTD, N-terminal domain of LPL; ANGPTL4-NTD, N-terminal domain of ANGPTL4. C: LPL activity levels in the culture media and cell lysates of CHO pgsA-745 cells that had been transfected with an empty vector alone (C), full-length hANGPTL4 alone (FL), N-terminal domain of hANGPTL4 alone (NTD), full-length ANGPTL4 and LPL (LPL+FL), the N-terminal domain of ANGPTL4 and LPL (LPL+NTD).
Fig. 3.
hANGPTL4 inactivates mouse LPL inside cells. A: CHO pgsA-745 cells were cotransfected with an expression vector for Flag-tagged hANGPTL4 (hANGPTL4[1–406]) or the amino-terminus domain of hANGPTL4 (hANGPTL4[1–160] and a vector for V5-tagged hLPL or mLPL, or empty vector. Forty-eight hours later, cell culture media and cell lysates were examined by SDS-PAGE and Western blotting. Western blots were probed with an anti-Flag antibody to detect ANGPTL4 (red); an anti-V5 tag antibody was used to detect hLPL and mLPL (green). Actin (red) was used as a loading control. LPL-CTD, C-terminal domain of LPL; ANGPTL4-CTD, C-terminal domain of ANGPTL4; ANGPTL4-NTD, N-terminal domain of ANGPTL4. B: Bar graph representing LPL activity levels in cell culture media of CHO pgsA-745 cells that were cotransfected with an expression vector for Flag-tagged hANGPTL4 (hANGPTL4[1–406]) and a vector for V5-tagged hLPL or mLPL, or empty vector. C, empty vector control; FL, full-length ANGPTL4.
Fig. 4.
ANGPTL4 does not affect transcription of LPL or secretion of SLURP1. A: Transcript levels for LPL and ANGPTL4 in CHO pgsA-745 cells that had been transfected with empty control vector (C), an expression vector for the Flag-tagged N-terminal domain of hANGPTL4 (NTD), a vector for V5-tagged hLPL (LPL), or N-terminal hANGPTL4 and V5-tagged hLPL (LPL+NTD). B: Western blots of cell lysates of CHO pgsA-745 cells that had been cotransfected with an expression vector for Flag-tagged full-length hANGPTL4 and either a vector for V5-tagged hLPL or a vector for hSLURP1. Western blots were probed with an anti-Flag antibody to detect ANGPTL4 (red), an anti-SLURP1 antiserum (green), an anti-V5 tag antibody to detect LPL (green), and an anti-actin antibody (red) (as a loading control).
Deletion of ANGPTL4 causes accumulation of EndoH-resistant LPL in adipocytes
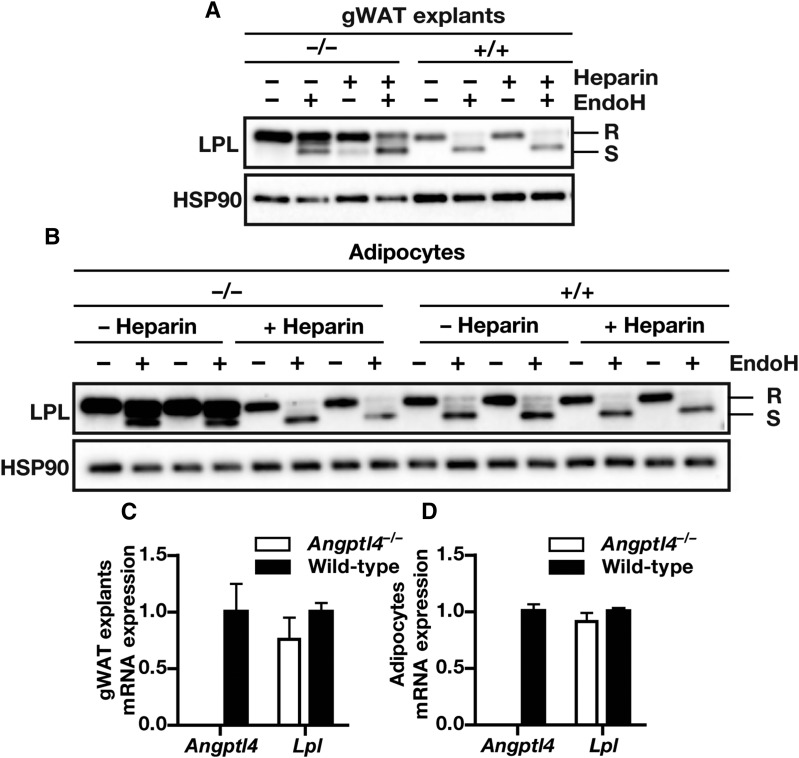
Adipose tissue displays robust expression of both ANGPTL4 and LPL (14). To determine whether ANGPTL4 expression affects intracellular LPL levels in adipose tissue, we studied the effects of ANGPTL4 deficiency on LPL protein levels in mouse adipose tissue. A deficiency of ANGPTL4 resulted in a marked accumulation of full-length LPL in adipose tissue explants and adipocytes from Angptl4−/− mice (Fig. 5A, B). The accumulation of LPL could not be attributed to higher Lpl expression levels because Lpl mRNA levels were similar in the explants and adipocytes of wild-type and Angptl4−/− mice (Fig. 5C, D).
Fig. 5.
ANGPTL4 lowers the amount of LPL on the adipocyte cell surface. A: Western blot of cell lysates of gWAT explants from Angptl4−/− and wild-type mice incubated in the absence or presence of heparin (50 IU/ml) for 3 h. Western blots were probed with antibodies against LPL and HSP90 (as loading control). B: Western blot of cell lysates of adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice and incubated in the absence or presence of heparin (10 IU/ml) for 20 min. Western blots were probed with antibodies against LPL and HSP90 (as loading control). C: Angptl4 and Lpl mRNA levels in gWAT explants from Angptl4−/− and wild-type mice. D: Angptl4 and Lpl mRNA levels in adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R; EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
To determine the location of LPL accumulation in adipose tissue from Angptl4−/− mice, adipose tissue lysates were treated with EndoH. These studies revealed that the accumulation of LPL in Angptl4−/− explants and adipocytes was mainly EndoH-resistant LPL (LPL in the Golgi or on the cell surface) rather than EndoH-sensitive LPL (LPL within the ER) (Fig. 5A, B). Further studies revealed that the EndoH-resistant LPL could be removed from cells by heparin, suggesting that the EndoH-resistant LPL in Angptl4−/− adipose tissue explants and Angptl4−/− adipocytes represented LPL attached to the cell surface by heparan-sulfate proteoglycans (HSPGs) or LPL present in rapidly-releasable secretory vesicles (40, 41) (Fig. 5A, B).
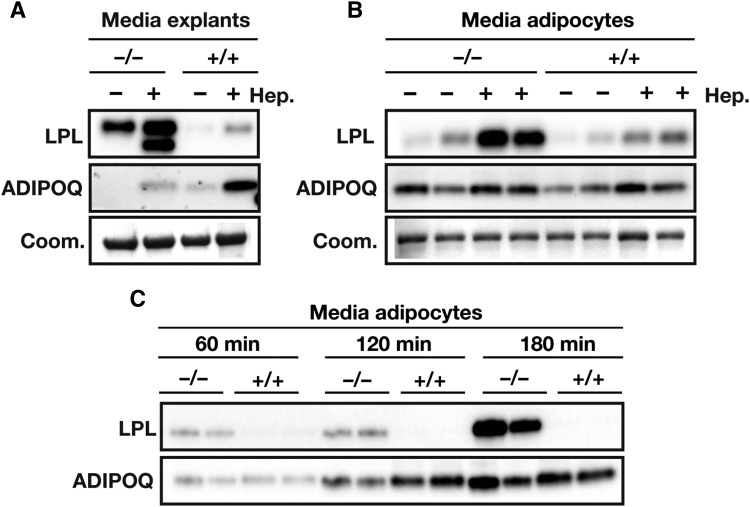
The accumulation of heparin-releasable EndoH-resistant LPL in Angptl4−/− adipose tissue was accompanied by a constitutively higher release of LPL from gWAT explants and adipocytes (Fig. 6A, B). Treatment with heparin magnified these differences; significantly higher amounts of heparin-releasable LPL were detected in the medium of gWAT explants and adipocytes from Angptl4−/− mice as compared with wild-type mice (Fig. 6A, B). By contrast, secretion of ADIPOQ was not altered in Angptl4−/− adipocytes, implying that a deficiency of ANGPTL4 did not have a general effect on the secretion of adipocyte proteins (Fig. 6A, B).
Fig. 6.
ANGPTL4 lowers LPL secretion. A: Western blot of media of gWAT explants from Angptl4−/− and wild-type mice treated in the absence or presence of heparin (50 IU/ml) for 3 h. Western blots were probed with antibodies against LPL and ADIPOQ. Coomassie blue staining was used to assess loading. B: Western blot of media of adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice and incubated in the absence or presence of heparin (Hep.) (10 IU/ml) for 20 min. Western blots were probed with antibodies against LPL and ADIPOQ. Coomassie blue staining was used to assess loading. C: Western blot of media of adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. Cells were pretreated for 20 min with heparin (10 IU/ml), washed with PBS, and incubated with heparin (10 IU/ml) for the indicated times. Western blots were probed with antibodies against LPL and ADIPOQ. EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R; EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
Mechanism of ANGPTL4-mediated loss of LPL
To assess whether ANGPTL4 affects LPL present on the cell surface and/or intracellularly, we treated differentiated adipocytes from Angptl4−/− and wild-type mice with heparin to remove cell-surface LPL. The cells were then washed and incubated for up to 180 min in the presence of heparin, followed by measurement of LPL levels in medium and cells. Heparin was included to prevent the sequestration of LPL on cell-surface HSPGs and to prevent its degradation in the medium (42). The accumulation of LPL in the medium was much greater in Angptl4−/− adipocytes than in wild-type adipocytes, implying increased LPL secretion from Angptl4−/− adipocytes (Fig. 6C). These data suggest that ANGPTL4 promotes intracellular LPL degradation in adipocytes, leading to reduced LPL secretion.
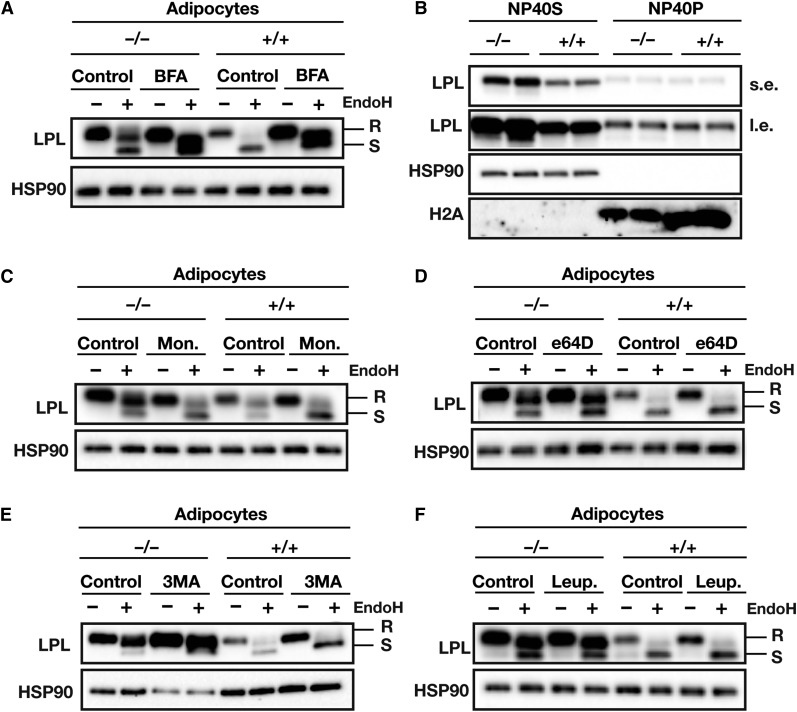
We next explored the mechanism accounting for the effect of ANGPTL4 on specifically EndoH-resistant LPL in adipocytes. Earlier studies revealed an accumulation of EndoH-sensitive LPL in cld/cld (Lmf1−/−) and Sel1L−/− mice, characterized by aggregated LPL in the ER that was targeted for ER-associated degradation (6, 10, 43–45). To determine whether LPL in ANGPTL4-expressing cells is targeted for degradation in the ER, we incubated primary adipocytes with brefeldin A, a fungal lactone antibiotic that is known to block protein transport from the ER and induce translocation of Golgi proteins to the ER. Brefeldin A-mediated inhibition of ER-Golgi transport abolished differences in the amounts of total LPL and EndoH-resistant LPL between wild-type and Angptl4−/− adipocytes, suggesting that ANGPTL4-mediated LPL degradation occurs in a post-ER compartment (Fig. 7A). In support of this notion, no differences in ER-associated degradation-associated LPL aggregation (NP40P) were observed between adipocytes of wild-type and Angptl4−/− mice (Fig. 7B). Also, incubation of adipocytes with monensin, an inhibitor of medial to trans-Golgi protein transport, abolished differences between wild-type and Angptl4−/− adipocytes (Fig. 7C).
Fig. 7.
ANGPTL4-mediated loss of LPL protein occurs in a post-ER compartment. A: Western blot of EndoH-treated cell lysates of adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. Cells were treated with 5 μg/ml brefeldin A for 2 h. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). B: Western blot of cell lysates of adipocytes that had been differentiated from stromal vascular fractions from WAT of Angptl4−/− and wild-type mice. Cells were lysed in NP-40 lysis buffer. Western blots were probed with antibodies against LPL, HSP90 (as a loading control), and H2A (as a loading control). NP40S, NP40-soluble LPL; NP40P, NP40-precipitated LPL; s.e., short exposure; l.e., long exposure. C: Western blot of EndoH-treated cell lysates of adipocytes that had been differentiated from the stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. Cells were treated with 10 μM monensin (Mon.) for 3 h. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). D: Western blot of EndoH-treated cell lysates from adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. The cells were treated with 20 μM E64D for 24 h. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). E: Western blot of EndoH-treated cell lysates of adipocytes that had been differentiated from stromal vascular fractions of WAT from Angptl4−/− and wild-type mice. Cells were treated with 5 mM 3MA for 10 h. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). F: Western blot of EndoH-treated cell lysates of adipocytes that had been differentiated from stromal vascular fractions from WAT of Angptl4−/− and wild-type mice. The cells had been treated with 10 mM leupeptin (Leup.) for 10 h. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R; EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
A potential explanation for the effect of ANGPTL4 on EndoH-resistant LPL is that ANGPTL4 targets LPL from the trans-Golgi toward lysosomal degradation, which has been postulated to account for the removal of up to 80% of basal LPL production (41, 42). Accordingly, we treated differentiated adipocytes from Angptl4−/− and wild-type mice with different inhibitors of autophagosomal and lysosomal degradation. Treatment of cells with 3-methyladenine (3MA), a PI3K inhibitor that blocks autophagy, as well as with the inhibitors of the lysosomal proteases, leupeptin and e64D, resulted in a pronounced accumulation of LPL, but did not eliminate the differences in the amounts of EndoH-resistant LPL between Angptl4−/− and wild-type adipocytes (Fig. 7D–F). Of note, 3MA consistently affected levels of the loading control HSP90 specifically in Angptl4−/− adipocytes, despite equal protein loading (data not shown). These data suggest that LPL is degraded by lysosomes, but that inhibition of lysosomal degradation with e64D, 3MA, or leupeptin cannot prevent ANGPTL4-mediated reduction in EndoH-resistant LPL.
Physiological regulation of ANGPTL4 affects LPL quantity and glycosylation in vivo
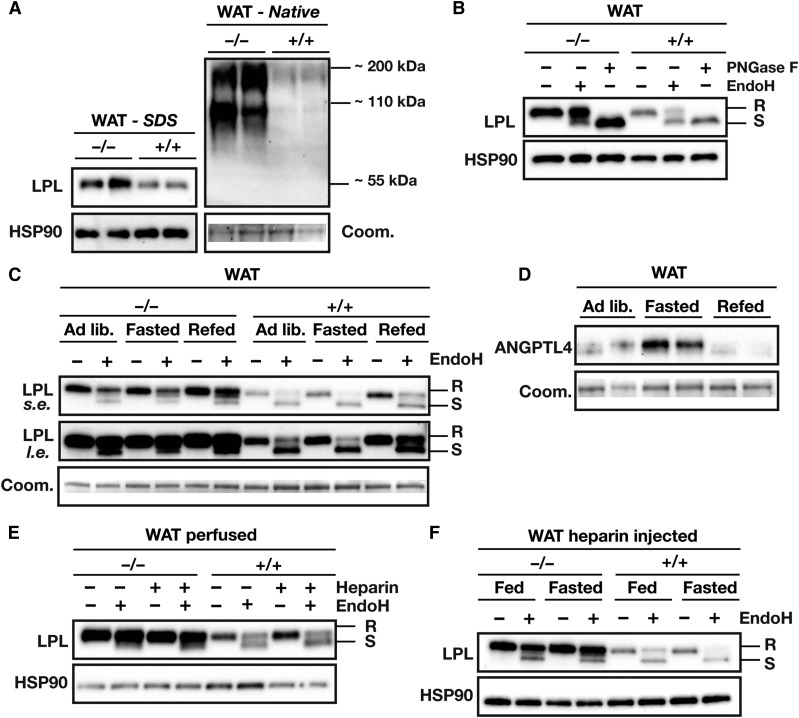
To determine whether the physiological regulation of Angptl4 expression affects intracellular degradation of LPL in vivo, we examined LPL quantity and LPL glycosylation in adipose tissue of fed and fasted wild-type and Angptl4−/− mice. Consistent with the ex vivo experimental data, the total LPL protein levels and levels of EndoH-resistant LPL were noticeably higher in Angptl4−/− adipose tissue than in wild-type adipose tissue (Fig. 8A, B). Accumulation of LPL in Angptl4−/− adipose tissue was observed for dimeric LPL (∼110 kDa) and multimeric LPL (∼220 kDa), as determined by native PAGE (Fig. 8A). Interestingly, while total LPL protein in WAT of wild-type mice mirrored previously published Lpl mRNA levels during fasting and refeeding (13), the amount of EndoH-resistant LPL decreased with fasting and increased with refeeding (Fig. 8C). These changes are in accordance with increased ANGPTL4 mRNA and protein levels upon fasting and decreased ANGPTL4 mRNA and protein levels upon refeeding (Fig. 8D) (13). By contrast, the amount of EndoH-resistant LPL in WAT of Angptl4−/− mice was not regulated by fasting and slightly increased by refeeding (Fig. 8C). Together, these data imply that the reciprocal regulation of EndoH-resistant LPL levels upon fasting and feeding in wild-type mice is likely mediated by ANGPTL4.
Fig. 8.
Physiological regulation of ANGPTL4 expression affects levels of EndoH-resistant LPL in vivo. A: Western blot of gWAT lysates of Angptl4−/− and wild-type mice, as analyzed by SDS-PAGE and native PAGE. Western blots were probed with antibodies against LPL and HSP90. Coomassie blue staining was used to assess loading. B: Western blot of EndoH-treated and PNGase-treated gWAT lysates of Angptl4−/− and wild-type mice. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). C: Western blot of EndoH-treated gWAT lysates prepared from ad libitum-fed, overnight-fasted, or refed Angptl4−/− and wild-type mice. The tissue samples were taken from an experiment described earlier (13). Western blots were probed with an antibody against LPL; Coomassie blue staining was used to assess loading. D: Western blot of gWAT lysates prepared from ad libitum fed, overnight-fasted, or refed wild-type mice. The tissue samples were taken from an experiment described earlier (13). Western blots were probed with an antibody against ANGPTL4; Coomassie blue staining was used to assess loading. E: Western blot of EndoH-treated gWAT lysates from Angptl4−/− and wild-type mice that were either not perfused or perfused with PBS containing heparin (50 IU/ml). Western blots were probed with antibodies against LPL and HSP90 (as a loading control). F: Western blot of EndoH-treated gWAT lysates prepared from fed and overnight-fasted Angptl4−/− and wild-type mice that had been given an intravenous injection of heparin (100 IU/kg). Western blots were probed with antibodies against LPL and HSP90 (as a loading control). EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R, EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
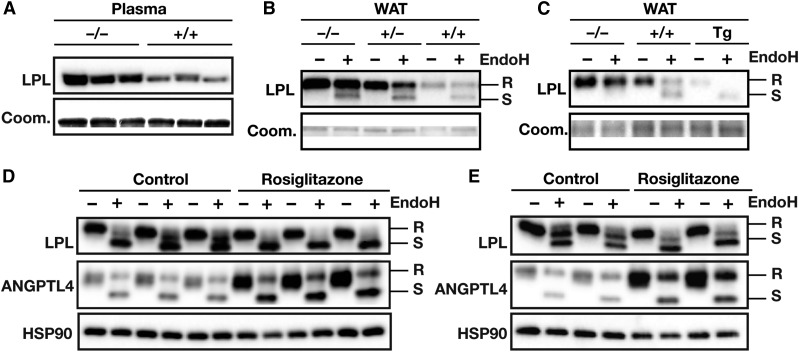
To determine whether the increased amount of EndoH-resistant LPL in adipose tissue of Angptl4−/− mice reflects the absence of ANGPTL4-mediated inhibition of LPL in capillaries, we detached LPL from the endothelium by perfusing tissues with heparin and injecting heparin intravenously. As expected, heparin reduced amounts of EndoH-resistant LPL in WAT lysates, but did not eliminate the differences in EndoH-resistant LPL between wild-type and Angptl4−/− mice (Fig. 8E, F). Together, these data suggest that EndoH-resistant LPL was inside cells or was within the interstitial spaces (possibly on the surface of adipocytes). Interestingly, the levels of LPL in the preheparin plasma were higher in Angptl4−/− mice than in wild-type mice, suggesting that some of the LPL that accumulates in the setting of Angptl4 deficiency ends up in plasma (Fig. 9A).
Fig. 9.
Levels of EndoH-resistant LPL are inversely related to Angptl4 expression. A: Western blot on 0.75 μl of plasma from Angptl4−/− and wild-type mice. Western blots were probed with an antibody against LPL; Coomassie blue staining was used to assess loading. B: Western blot of EndoH-treated WAT lysates prepared from Angptl4−/−, Angptl4+/−, and wild-type mice. Western blots were probed with an antibody against LPL; Coomassie blue staining was used to assess loading. C: Western blot of EndoH-treated WAT lysates prepared from Angptl4−/−, wild-type, and Angptl4-Tg mice. Western blots were probed with an antibody against LPL; Coomassie blue staining was used to assess loading. D: Western blot of EndoH-treated lysates of 3T3-F442a adipocytes that had been treated with rosiglitazone (10 μM) or DMSO control for 6 h. Western blots were probed with antibodies against LPL, ANGPTL4, and HSP90 (as a loading control). E: Western blot of EndoH-treated lysates of 3T3-L1 adipocytes that had been treated with rosiglitazone (10 μM) or DMSO control for 6 h. Western blots were probed with antibodies against LPL, ANGPTL4, and HSP90 (as a loading control). EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R, EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
To exclude the possibility that the regulation of LPL by ANGPTL4 in adipocytes is an artifact related to the use of Angptl4−/− adipocytes (where ANGPTL4 is completely absent), we assessed total and EndoH-resistant LPL levels in mouse adipose tissue from Angptl4−/−, Angptl4+/−, wild-type, and Angptl4-Tg mice. A clear dose-dependent reduction of EndoH-resistant LPL was observed with increasing ANGPTL4 levels (Fig. 9B, C). In addition, we tested whether inducing ANGPTL4 expression in 3T3-F442a and 3T3-L1 adipocytes with rosiglitazone leads to reduced amounts of EndoH-resistant LPL. In rosiglitazone-treated adipocytes, ANGPTL4 expression was increased concomitant with a reduction in levels of EndoH-resistant LPL (Fig. 9D, E).
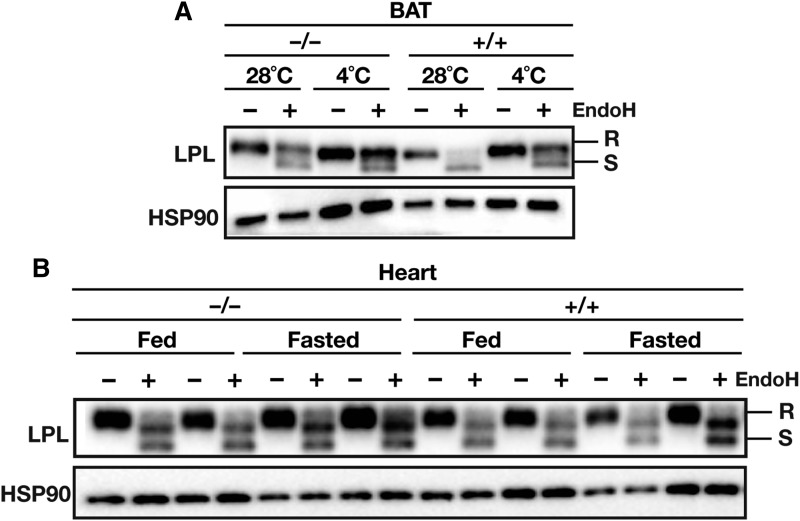
A final question was whether physiological regulation of ANGPTL4 expression affects LPL abundance and LPL glycosylation in other tissues. Prolonged cold exposure markedly increases LPL activity in brown adipose tissue (BAT) as a result of reduced ANGPTL4 expression (14). As in WAT, the amount of EndoH-resistant LPL in BAT was noticeably higher in Angptl4−/− mice than in wild-type mice, both at thermoneutral and cold temperatures (Fig. 10A). Cold exposure mildly affected levels of multiple loading controls, despite an equal loading of protein (Fig. 10A; data not shown). Consistent with reduced ANGPTL4 expression after prolonged cold exposure, the amount of EndoH-resistant LPL in BAT was markedly increased in wild-type mice, but less so in Angptl4−/− mice (Fig. 10B). In contrast, the amounts of total and EndoH-sensitive LPL in hearts of Angptl4−/− and wild-type mice during basal and fasting conditions were not different (Fig. 10B). Thus, it appears that ANGPTL4-mediated loss of EndoH-resistant LPL in vivo requires a high basal level of ANGPTL4 expression, which is characteristic of BAT and WAT, but not heart (14).
Fig. 10.
ANGPTL4 regulates levels of EndoH-resistant LPL in adipose tissue, but not heart. A: Western blot of EndoH-treated BAT lysates from Angptl4−/− and wild-type mice exposed to cold (4°C) or thermoneutral temperature (28°C) for 10 days. The tissue samples were taken from an experiment described earlier (14). Western blots were probed with antibodies against LPL and HSP90 (as a loading control). B: Western blot on EndoH-treated heart lysates from Angptl4−/− and wild-type mice fed ad libitum or fasted overnight. Western blots were probed with antibodies against LPL and HSP90 (as a loading control). EndoH-resistant LPL (high-mannose oligosaccharides, ER LPL) is indicated with R, EndoH-sensitive LPL (complex oligosaccharides; Golgi and cell surface LPL) is indicated with S.
DISCUSSION
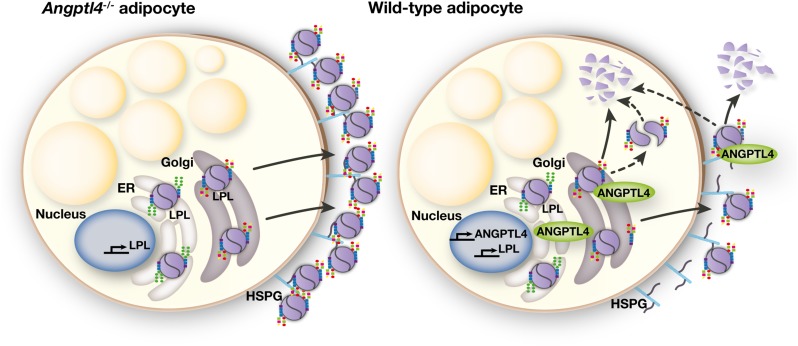
ANGPTL4 is known to inhibit LPL catalytic activity in a variety of tissues, including skeletal muscle and adipose tissue (17, 46). Here we show that ANGPTL4 reduces LPL protein in adipocytes by promoting intracellular degradation. Cotransfecting CHO pgsA-745 cells with ANGPTL4 and LPL expression vectors, but not coplating of independently transfected cells, markedly reduced the amount of LPL within cells. Conversely, the absence of ANGPTL4 expression in adipocytes led to an accumulation of heparin-releasable EndoH-resistant LPL. The suppressive effect of ANGPTL4 on total and EndoH-resistant LPL in adipose tissue was confirmed in adipose tissue in vivo. Together, our findings demonstrate that ANGPTL4 reduces the amount of LPL protein within adipocytes by promoting LPL degradation after its posttranslational processing in the ER (see Fig. 11 for a schematic model).
Fig. 11.
Schematic model. Within the ER, LPL acquires oligosaccharide side chains via cotranslational transfer of oligosaccharides high in mannose residues. Upon translocation to the Golgi apparatus, the high-mannose oligosaccharides are trimmed and replaced by more complex oligosaccharides. In the absence of ANGPTL4 (i.e., Angptl4−/− adipocytes), LPL with complex oligosaccharide side chains is packaged in secretory vesicles and secreted. Secreted LPL accumulates on the cell surface of adipocytes, mostly bound to HSPGs. In ANGPTL4-expressing adipocytes (i.e., wild-type adipocytes), ANGPTL4 interacts with LPL in a post-ER compartment. This interaction leads, potentially via converting LPL homodimers to monomers, to degradation of LPL. This results in a specific reduction of LPL with complex oligosaccharide side chains in wild-type adipocytes versus Angptl4−/− adipocytes. Once secreted, ANGPTL4 also reduces levels and activity of secreted LPL.
Inactivating variants in the human ANGPTL4 gene have been repeatedly linked to low plasma triglycerides and elevated HDL cholesterol, and have been associated with a reduced risk of coronary heart disease (18–20). Given the marked impact of ANGPTL4-inactivating mutants on plasma triglycerides, it is somewhat surprising that no clear correlation between plasma ANGPTL4 and plasma triglyceride levels has been observed (21–24, 47–49). A possible explanation for this apparent discrepancy is that ANGPTL4 regulates LPL via a local mechanism rather than via an endocrine mechanism, so as to match the uptake of fatty acids to the metabolic needs of underlying tissue. Our data strongly suggest that ANGPTL4 impacts LPL locally by acting on LPL intracellularly along the secretory pathway (25). An important action of ANGPTL4 inside the cell and in the subendothelial space, rather than intravascularly, is consistent with the observation that both circulating triglyceride-rich lipoproteins and GPIHBP1 protect LPL from inhibition by ANGPTL4 (27, 28). It should be noted that our findings per se do not argue against the ability of ANGPTL4 to act on extracellular and intravascular LPL. Indeed, secreted ANGPTL4, recombinant ANGPTL4, and anti-ANGPTL4 antibodies have all been shown to be able to impact extracellular LPL activity (25, 28, 50). In addition, liver-specific Angptl4 overexpression and injection of mice with recombinant ANGPTL4 effectively lower plasma triglycerides and plasma LPL activity levels, implying that circulating ANGPTL4 is able to target and inhibit intravascular LPL (33, 51). Similarly, monoclonal antibodies against ANGPTL4, which are expected to primarily affect intravascular ANGPTL4 and to a lesser extent subendothelial ANGPTL4, have been shown to potently lower plasma triglyceride levels in hyperlipidemic mice and monkeys (19, 52, 53). Future studies are necessary to assess the relative importance of intracellular, subendothelial, and intravascular inhibition of LPL by ANGPTL4. It can be speculated that the importance of the three routes may differ between tissues and may depend on the physiological state of the organism.
Our experiments show that EndoH-resistant LPL could be rapidly released by heparin, suggesting that the accumulated EndoH-resistant LPL in Angptl4−/− mice is either bound to HSPGs on the cell surface or located in rapidly-releasable secretory vesicles (40, 41). The precise location for the interaction of ANGPTL4 and LPL within cells remains to be determined. However, both ANGPTL4 and LPL are secreted proteins that consequently are expected to be found at similar locations within the cell, which limits the applicability of immunofluorescence studies. Makoveichuk and coworkers found that ANGPTL4-producing cells have lower LPL activity in cell culture medium, but concluded that the inhibition of LPL by ANGPTL4 occurred after both proteins arrived at the cell surface (29, 50). Our data suggest that ANGPTL4-mediated removal of LPL starts within cells, before LPL is secreted. In the presence of heparin, which is known to stabilize LPL (42), the secretion of LPL by Angptl4−/− adipocytes is higher than by wild-type adipocytes. Similarly, co-expression of ANGPTL4 and LPL in CHO pgsA-745 cells, which are deficient in cell-surface HSPGs (54), markedly reduced amounts of LPL within cells. Presently, we cannot fully exclude the possibility that the elimination of LPL occurs (partly) on the cell surface, as secreted LPL may be inactivated by ANGPTL4 on the cell surface and directly taken up by the cell for degradation. However, our data strongly suggest that ANGPTL4 eliminates the majority of LPL before the enzyme reaches the cell surface. ANGPTL4 could mediate intracellular degradation of LPL by stimulating endosomal transport of LPL from the (trans-)Golgi or cell surface to lysosomes (42, 55–58). Pulse-chase experiments have shown that ∼80% of newly synthesized LPL in adipocytes is degraded intracellularly, primarily by lysosomes (41, 42). Our data confirmed that inhibition of the lysosomal pathway reduces intracellular degradation of LPL. However, inhibition of autophagosomal or lysosomal degradation did not abolish differences in the intracellular LPL levels between Angptl4−/− and wild-type adipocytes. It is possible that the use of chemical inhibitors may not permit us to detect the effect of ANGPTL4 on lysosomal degradation of LPL or that ANGPTL4-induced removal of EndoH-resistant LPL occurs, at least in part, in a compartment other than the lysosomes. One possibility is that ANGPTL4 alters LPL protein conformation and stability or interferes with the addition of complex oligosaccharides. Biochemical studies have indicated that ANGPTL4 inactivates LPL by converting the catalytically active LPL dimers into catalytically inactive monomers (59). Accordingly, the degradation of LPL could be secondary to an effect of ANGPTL4 on dimer-monomer conversion and/or LPL protein stability. The exact mechanism by which ANGPTL4 promotes intracellular LPL degradation will be the subject of further investigation.
The fact that LPL might be regulated before secretion is not surprising given that other key players in lipid metabolism, illustrated by apolipoprotein B and the LDL receptor, are regulated along the secretory pathway (60–65). We propose that a multilevel regulation of LPL by ANGPTL4, along with tight regulation of ANGPTL4 expression, permits for rapid adjustments in local LPL activity in accordance with the requirements of the tissue. To assure the appropriate distribution of lipid nutrients, ANGPTL4 may collaborate with its family members ANGPTL3 and ANGPTL8 to regulate LPL activity levels during different physiological conditions (66). Although ANGPTL3 and ANGPTL8 have been proposed to mainly exert their actions via an endocrine mechanism, it would be of interest to study whether ANGPTL3 and ANGPTL8 may also regulate LPL intracellularly and promote LPL degradation (66). In this context, it is interesting to mention that homozygous carriers of an inactivating mutation in the ANGPTL3 gene have markedly higher levels of postheparin LPL mass and activity as compared with noncarriers (67).
In conclusion, our data indicate that ANGPTL4 interacts with LPL inside cells, both in CHO pgsA-745 cells and in adipocytes ex vivo and in vivo. ANGPTL4 promotes intracellular degradation of LPL within adipocytes after LPL processing within the ER. Together, our studies reveal a novel site and mechanism for ANGPTL4-mediated regulation of LPL activity.
Acknowledgments
The authors thank Loren Fong for careful proofreading of the final manuscript and Anja Köster (Eli Lilly) for donating the Angptl4−/− mice.
Footnotes
Abbreviations:
- ANGPTL4
- angiopoietin-like 4
- BAT
- brown adipose tissue
- EndoH
- endoglycosidase H
- ER
- endoplasmic reticulum
- GPIHBP1
- glycosylphosphatidylinositol-anchored HDL binding protein 1
- gWAT
- gonadal white adipose tissue
- hANGPTL4
- human angiopoietin-like 4
- hLPL
- human LPL
- HSPG
- heparan-sulfate proteoglycan
- mLPL
- mouse LPL
- 3MA
- 3-methyladenine
- P/S
- penicillin/streptomycin
- WAT
- white adipose tissue
This study was supported by Fondation Leducq Grant 12CVD04 and Office of Extramural Research, National Institutes of Health Grants HL090533 and HL087228. All authors have no potential conflicts of interest relevant to this study to report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Kersten S. 2014. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta. 1841: 919–933. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg I. J., Eckel R. H., and Abumrad N.. 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 50(Suppl): S86–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., and Eckel R. H.. 2012. Lipoprotein lipase in the brain and nervous system. Annu. Rev. Nutr. 32: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young S. G., and Zechner R.. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27: 459–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies B. S. J., Beigneux A. P., Barnes R. H., Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Zeev O., Doolittle M. H., Davis R. C., Elovson J., and Schotz M. C.. 1992. Maturation of lipoprotein lipase. Expression of full catalytic activity requires glucose trimming but not translocation to the cis-Golgi compartment. J. Biol. Chem. 267: 6219–6227. [PubMed] [Google Scholar]
- 7.Simsolo R. B., Ong J. M., and Kern P. A.. 1992. Characterization of lipoprotein lipase activity, secretion, and degradation at different sites of post-translational processing in primary cultures of rat adipocytes. J. Lipid Res. 33: 1777–1784. [PubMed] [Google Scholar]
- 8.Semenkovich C. F., Luo C. C., Nakanishi M. K., Chen S. H., Smith L. C., and Chan L.. 1990. In vitro expression and site-specific mutagenesis of the cloned human lipoprotein lipase gene. Potential N-linked glycosylation site asparagine 43 is important for both enzyme activity and secretion. J. Biol. Chem. 265: 5429–5433. [PubMed] [Google Scholar]
- 9.Ben-Zeev O., Stahnke G., Liu G., Davis R. C., and Doolittle M. H.. 1994. Lipoprotein lipase and hepatic lipase: the role of asparagine-linked glycosylation in the expression of a functional enzyme. J. Lipid Res. 35: 1511–1523. [PubMed] [Google Scholar]
- 10.Davis R. C., Ben-Zeev O., Martin D., and Doolittle M. H.. 1990. Combined lipase deficiency in the mouse: evidence of impaired lipase processing and secretion. J. Biol. Chem. 265: 17960–17966. [PubMed] [Google Scholar]
- 11.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 12.Catoire M., Alex S., Paraskevopulos N., Mattijssen F., Evers-van Gogh I., Schaart G., Jeppesen J., Kneppers A., Mensink M., Voshol P. J., et al. 2014. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proc. Natl. Acad. Sci. USA. 111: E1043–E1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroupa O., Vorrsjö E., Stienstra R., Mattijssen F., Nilsson S. K., Sukonina V., Kersten S., Olivecrona G., and Olivecrona T.. 2012. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiol. 12: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijk W., Heine M., Vergnes L., Boon M. R., Schaart G., Hesselink M. K., Reue K., van Marken Lichtenbelt W. D., Olivecrona G., Rensen P. C., et al. 2015. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. eLife. 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., and Wahli W.. 2000. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275: 28488–28493. [DOI] [PubMed] [Google Scholar]
- 16.Georgiadi A., Lichtenstein L., Degenhardt T., Boekschoten M. V., van Bilsen M., Desvergne B., Müller M., and Kersten S.. 2010. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ. Res. 106: 1712–1721. [DOI] [PubMed] [Google Scholar]
- 17.Dijk W., and Kersten S.. 2014. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol. Metab. 25: 146–155. [DOI] [PubMed] [Google Scholar]
- 18.Romeo S., Pennacchio L. A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H. H., and Cohen J. C.. 2007. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39: 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewey F. E., Gusarova V., O’Dushlaine C., Gottesman O., Trejos J., Hunt C., Van Hout C. V., Habegger L., Buckler D., Lai K-M. V., et al. 2016. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 374: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. 2016. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N. Engl. J. Med. 374: 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robciuc M. R., Naukkarinen J., Ortega-Alonso A., Tyynismaa H., Raivio T., Rissanen A., Kaprio J., Ehnholm C., Jauhiainen M., and Pietiläinen K. H.. 2011. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 52: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smart-Halajko M. C., Robciuc M. R., Cooper J. A., Jauhiainen M., Kumari M., Kivimaki M., Khaw K-T., Boekholdt S. M., Wareham N. J., Gaunt T. R., et al. 2010. The relationship between plasma angiopoietin-like protein 4 levels, angiopoietin-like protein 4 genotype, and coronary heart disease risk. Arterioscler. Thromb. Vasc. Biol. 30: 2277–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robciuc M. R., Tahvanainen E., Jauhiainen M., and Ehnholm C.. 2010. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 51: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta N., Qamar A., Qu L., Qasim A. N., Mehta N. N., Reilly M. P., and Rader D. J.. 2014. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arterioscler. Thromb. Vasc. Biol. 34: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robciuc M. R., Skrobuk P., Anisimov A., Olkkonen V. M., Alitalo K., Eckel R. H., Koistinen H. A., Jauhiainen M., and Ehnholm C.. 2012. Angiopoietin-like 4 mediates PPAR delta effect on lipoprotein lipase-dependent fatty acid uptake but not on beta-oxidation in myotubes. PLoS One. 7: e46212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies B. S. J., Goulbourne C. N., Barnes R. H., Turlo K. A., Gin P., Vaughan S., Vaux D. J., Bensadoun A., Beigneux A. P., Fong L. G., et al. 2012. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J. Lipid Res. 53: 2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi X., Shetty S. K., Shows H. W., Hjelmaas A. J., Malcolm E. K., and Davies B. S. J.. 2015. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. J. Biol. Chem. 290: 11865–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson S. K., Anderson F., Ericsson M., Larsson M., Makoveichuk E., Lookene A., Heeren J., and Olivecrona G.. 2012. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim. Biophys. Acta. 1821: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 29.Makoveichuk E., Vorrsjö E., Olivecrona T., and Olivecrona G.. 2013. Inactivation of lipoprotein lipase in 3T3-L1 adipocytes by angiopoietin-like protein 4 requires that both proteins have reached the cell surface. Biochem. Biophys. Res. Commun. 441: 941–946. [DOI] [PubMed] [Google Scholar]
- 30.Voss C. V., Davies B. S. J., Tat S., Gin P., Fong L. G., Pelletier C., Mottler C. D., Bensadoun A., Beigneux A. P., and Young S. G.. 2011. Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1. Proc. Natl. Acad. Sci. USA. 108: 7980–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beigneux A. P., Davies B. S. J., Tat S., Chen J., Gin P., Voss C. V., Weinstein M. M., Bensadoun A., Pullinger C. R., Fong L. G., et al. 2011. Assessing the role of the glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) three-finger domain in binding lipoprotein lipase. J. Biol. Chem. 286: 19735–19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alex S., Lange K., Amolo T., Grinstead J. S., Haakonsson A. K., Szalowska E., Koppen A., Mudde K., Haenen D., Al-Lahham S., et al. 2013. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 33: 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein L., Mattijssen F., De Wit N. J., Georgiadi A., Hooiveld G. J., Van Der Meer R., He Y., Qi L., Köster A., Tamsma J. T., et al. 2010. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 12: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandard S., Zandbergen F., Van Straten E., Wahli W., Kuipers F., Müller M., and Kersten S.. 2006. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281: 934–944. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson-Olivecrona G., and Olivecrona T.. 1992. Assay of lipoprotein lipase and hepatic lipase. In Lipoprotein Analysis: A Practical Approach. R. E. Skinner and C. A. Converse, editors. Oxford University Press, Oxford, UK. 169–185. [Google Scholar]
- 37.Weinstein M. M., Yin L., Beigneux A. P., Davies B. S. J., Gin P., Estrada K., Melford K., Bishop J. R., Esko J. D., Dallinga-Thie G. M., et al. 2008. Abnormal patterns of lipoprotein lipase release into the plasma in GPIHBP1-deficient mice. J. Biol. Chem. 283: 34511–34518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensadoun A., Mottler C. D., Pelletier C., Wu D., Seo J. J., Leung C. S., Adeyo O., Goulbourne C. N., Gin P., Fong L. G., et al. 2014. A new monoclonal antibody, 4-1a, that binds to the amino terminus of human lipoprotein lipase. Biochim. Biophys. Acta. 1841: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge H., Yang G., Huang L., Motola D. L., Pourbahrami T., and Li C.. 2004. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 279: 2038–2045. [DOI] [PubMed] [Google Scholar]
- 40.Braun J. E., and Severson D. L.. 1992. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem. J. 287: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannier C., and Ailhaud G.. 1989. Biosynthesis of lipoprotein lipase in cultured mouse adipocytes. II. Processing, subunit assembly, and intracellular transport. J. Biol. Chem. 264: 13206–13216. [PubMed] [Google Scholar]
- 42.Cupp M., Bensadoun A., and Melford K.. 1987. Heparin decreases the degradation rate of lipoprotein lipase in adipocytes. J. Biol. Chem. 262: 6383–6388. [PubMed] [Google Scholar]
- 43.Sha H., Sun S., Francisco A. B., Ehrhardt N., Xue Z., Liu L., Lawrence P., Mattijssen F., Guber R. D., Panhwar M. S., et al. 2014. The ER-associated degradation adaptor protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab. 20: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J. W., Blanchette-Mackie E. J., and Scow R. O.. 1996. Brefeldin A enables synthesis of active lipoprotein lipase in cld/cld and castanospermine-treated mouse brown adipocytes via translocation of Golgi components to endoplasmic reticulum. Biochem. J. 317: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Zeev O., Mao H. Z., and Doolittle M. H.. 2002. Maturation of lipoprotein lipase in the endoplasmic reticulum. Concurrent formation of functional dimers and inactive aggregates. J. Biol. Chem. 277: 10727–10738. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., and Eckel R. H.. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297: E271–E288. [DOI] [PubMed] [Google Scholar]
- 47.Brands M., Sauerwein H. P., Ackermans M. T., Kersten S., and Serlie M. J.. 2013. Omega-3 long-chain fatty acids strongly induce angiopoietin-like 4 in humans. J. Lipid Res. 54: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Raalte D. H., Brands M., Serlie M. J., Mudde K., Stienstra R., Sauerwein H. P., Kersten S., and Diamant M.. 2012. Angiopoietin-like protein 4 is differentially regulated by glucocorticoids and insulin in vitro and in vivo in healthy humans. Exp. Clin. Endocrinol. Diabetes. 120: 598–603. [DOI] [PubMed] [Google Scholar]
- 49.Jonker J. T., Smit J. W. A., Hammer S., Snel M., Van Der Meer R. W., Lamb H. J., Mattijssen F., Mudde K., Jazet I. M., Dekkers O. M., et al. 2013. Dietary modulation of plasma angiopoietin-like protein 4 concentrations in healthy volunteers and in patients with type 2 diabetes. Am. J. Clin. Nutr. 97: 255–260. [DOI] [PubMed] [Google Scholar]
- 50.Makoveichuk E., Sukonina V., Kroupa O., Thulin P., Ehrenborg E., Olivecrona T., and Olivecrona G.. 2012. Inactivation of lipoprotein lipase occurs on the surface of THP-1 macrophages where oligomers of angiopoietin-like protein 4 are formed. Biochem. Biophys. Res. Commun. 425: 138–143. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida K., Shimizugawa T., Ono M., and Furukawa H.. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 52.Shah D. K., and Betts A. M.. 2013. Antibody biodistribution coefficients: Inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs. 5: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desai U., Lee E-C., Chung K., Gao C., Gay J., Key B., Hansen G., Machajewski D., Platt K. A., Sands A. T., et al. 2007. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc. Natl. Acad. Sci. USA. 104: 11766–11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esko J. D., Stewart T. E., and Taylor W. H.. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA. 82: 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klinger S. C., Glerup S., Raarup M. K., Mari M. C., Nyegaard M., Koster G., Prabakaran T., Nilsson S. K., Kjaergaard M. M., Bakke O., et al. 2011. SorLA regulates the activity of lipoprotein lipase by intracellular trafficking. J. Cell Sci. 124: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 56.Chappell D. A., Fry G. L., Waknitz M. A., Iverius P. H., Williams S. E., and Strickland D. K.. 1992. The low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor binds and mediates catabolism of bovine milk lipoprotein lipase. J. Biol. Chem. 267: 25764–25767. [PubMed] [Google Scholar]
- 57.Obunike J. C., Sivaram P., Paka L., Low M. G., and Goldberg I. J.. 1996. Lipoprotein lipase degradation by adipocytes: receptor-associated protein (RAP)-sensitive and proteoglycan-mediated pathways. J. Lipid Res. 37: 2439–2449. [PubMed] [Google Scholar]
- 58.Cisar L. A., Hoogewerf A. J., Cupp M., Rapport C. A., and Bensadoun A.. 1989. Secretion and degradation of lipoprotein lipase in cultured adipocytes. Binding of lipoprotein lipase to membrane heparan sulfate proteoglycans is necessary for degradation. J. Biol. Chem. 264: 1767–1774. [PubMed] [Google Scholar]
- 59.Sukonina V., Lookene A., Olivecrona T., and Olivecrona G.. 2006. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 103: 17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maxwell K. N., Fisher E. A., and Breslow J. L.. 2005. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA. 102: 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poirier S., and Mayer G.. 2013. The biology of PCSK9 from the endoplasmic reticulum to lysosomes: New and emerging therapeutics to control low-density lipoprotein cholesterol. Drug Des. Devel. Ther. 7: 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poirier S., Mayer G., Poupon V., McPherson P. S., Desjardins R., Ly K., Asselin M. C., Dy R., Duclos F. J., Witmer M., et al. 2009. Dissection of the endogenous cellular pathways of PCSK9-induced low density Lipoprotein receptor degradation. Evidence for an intracellular route. J. Biol. Chem. 284: 28856–28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan M., Maitin V., Parathath S., Andreo U., Lin S. X., St Germain C., Yao Z., Maxfield F. R., Williams K. J., and Fisher E. A.. 2008. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc. Natl. Acad. Sci. USA. 105: 5862–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fisher E. A., Pan M., Chen X., Wu X., Wang H., Jamil H., Sparks J. D., and Williams K. J.. 2001. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276: 27855–27863. [DOI] [PubMed] [Google Scholar]
- 65.Strong A., Ding Q., Edmondson A. C., Millar J. S., Sachs K. V., Li X., Kumaravel A., Wang M. Y., Ai D., Guo L., et al. 2012. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J. Clin. Invest. 122: 2807–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dijk W., and Kersten S.. 2016. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 27. In press. [DOI] [PubMed] [Google Scholar]
- 67.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Öörni K., Oörni K., Metso J., Minicocci I., Ciociola E., et al. 2013. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33: 1706–1713. [Erratum. 2013. Arterioscler. Thromb. Vasc. Biol 33: e124.] [DOI] [PubMed] [Google Scholar]