Abstract
Carotenoids affect a rich variety of physiological functions in nature and are beneficial for human health. However, knowledge about their biological action and the consequences of their dietary accumulation in mammals is limited. Progress in this research field is limited by the expeditious metabolism of carotenoids in rodents and the confounding production of apocarotenoid signaling molecules. Herein, we established a mouse model lacking the enzymes responsible for carotenoid catabolism and apocarotenoid production, fed on either a β-carotene- or a zeaxanthin-enriched diet. Applying a genome wide microarray analysis, we assessed the effects of the parent carotenoids on the liver transcriptome. Our analysis documented changes in pathways for liver lipid metabolism and mitochondrial respiration. We biochemically defined these effects, and observed that β-carotene accumulation resulted in an elevation of liver triglycerides and liver cholesterol, while zeaxanthin accumulation increased serum cholesterol levels. We further show that carotenoids were predominantly transported within HDL particles in the serum of mice. Finally, we provide evidence that carotenoid accumulation influenced whole-body respiration and energy expenditure. Thus, we observed that accumulation of parent carotenoids interacts with lipid metabolism and that structurally related carotenoids display distinct biological functions in mammals.
Keywords: liver; cholesterol; microarray; triglyceride; lipoproteins; β-carotene; zeaxanthin; β,β-carotene-15,15′-oxygenase; β,β-carotene-9′,10′-oxygenase
Carotenoids are a chemically diverse group of isoprenoid pigments produced by plants, bacteria, and fungi. The initial steps of their biosynthesis follow a common scheme that is similar to other isoprenoids, such as tocopherols, phylloquinones, and sterols (1, 2). The synthesis of these tetraterpenoids (C40) involves the condensation of eight isoprenoid units (C5), desaturation of double bonds, and introduction of terminal hexyl rings. The enormous diversity of carotenoids is achieved by modulation in length of the polyene chromophore, shifts of the conjugated double bonds, and the addition of functional groups (3, 4).
Animals are unable to synthesize carotenoids from endogenous precursors and acquire them from the diet. The biological actions of carotenoids within man are thought to be manifold and complex. The inherent physical and chemical properties of carotenoids make them amenable to act as light filters and antioxidants in blood and tissues (5–8). Additionally, evidence has been provided that carotenoids may interact with other lipids and their transporters, binding proteins, and metabolizing enzymes (9). In these processes, individual carotenoids display distinct health benefits. For instance, epidemiological observations indicate that lycopene is beneficial for prostate health (10, 11), whereas lutein and zeaxanthin are beneficial for eye health (12). In addition to these functions, carotenoids are the metabolic precursor for apocartenoids, including retinoids (vitamin A and its metabolites) (13, 14). These vital compounds play critical roles as visual chromophores (15) and ligands of transcription factors including retinoic acid receptors, which influence many aspects of cell signaling during the mammalian life cycle (16, 17).
Despite the panoply of health effects ascribed to parent carotenoids, our knowledge about their mode of action in mammals is still limited. Progress in this research field has been hampered by the dual role of these lipids as parent carotenoids and precursors for apocarotenoid signaling molecules in animal physiology. Over the past years, key molecular players in carotenoid biochemistry have been characterized (18). Among them, two carotenoid metabolizing enzymes have been identified, denoted as β-carotene-15,15′-dioxygenase (BCO1; also annotated as BCMO1) and β-carotene-9′,10′-dioxygenase (BCO2, also annotated as BCDO2) (19, 20). BCO1 converts a limited number of provitamin A carotenoids to retinaldehyde, from which all naturally occurring retinoids can be synthesized by endogenous pathways (21–23). BCO2 displays broad substrate specificity and catalyzes oxidative cleavage of the C9,C10 double bond of a large variety of carotenoids (24, 25).
We have established mouse models deficient for each carotenoid-dioxygenase allowing for the accumulation of carotenoids in a tunable fashion (25, 26). This breakthrough now allows for the dissection of the effects of parent carotenoids apart from their apocarotenoid signaling molecules in a small animal model with striking similarity to humans in anatomy, physiology, and genetics. We here subjected female compound-knockout mice for BCO1 and BCO2 (DKO mice) to supplementation with carotenoids or carotenoid-free diet. We applied genomic and biochemical approaches to examine the roles of parent carotenoids in this animal model focusing on liver physiology. For this investigation, we chose two distinct, but chemically related, carotenoids [β,β-carotene and 3,3′-dihydroxy-β,β-carotene (zeaxanthin)]. Our analysis reveals genomic and nongenomic actions of these carotenoids and demonstrates the usefulness of this small animal model in understanding the impact of carotenoids on mammalian biology.
MATERIALS AND METHODS
Animals, husbandry, and experimental diets
Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. In all experiments, mice were maintained at 24°C in a 12 h light-dark cycle and had free access to food and water. The generation of Bco1−/− Bco2−/− (DKO) mice has been described elsewhere (22). DKO mice had a C57/BL6;129Sv mixed genetic background. During breeding and weaning periods (up to 5 weeks of age), all DKO mice were maintained on breeder chow containing 29,000 IU vitamin A per kilogram of diet (Prolab RMH 3000; LabDiet, St. Louis, MO).
For all experiments described herein, 5-week-old female DKO mice were fed for 10 weeks a vitamin A-deficient pelleted diet based on the AIN-93G formulation, containing 0.15 mg/g zeaxanthin (zeaxanthin diet), 0.15 mg/g β-carotene (β-carotene diet), or the same diet without carotenoids (control diet). The vitamin A-deficient diets were prepared by Research Diets, Inc. (New Brunswick, NJ) by cold extrusion. Carotenoids were incorporated with the addition of water-soluble formulation of beadlets (DSM Ltd., Sisseln, Switzerland). The control diet contained beadlets without carotenoids. During the experimental period, animals were monitored for any indications of vitamin A deficiency or carotenoid toxicity, such as weight or fur loss. After dietary intervention, mice were anesthetized by intraperitoneal injection of a mixture containing ketamine 16.5 mg/ml and xylazine 1.65 mg/ml in saline, at a dose of 0.25 ml/25 g of mouse. Blood was collected directly from the heart by cardiac puncture under deep anesthesia. Mice were perfused with 10 ml of PBS and euthanized by cervical dislocation before tissue collection. After dissection, tissues were snap-frozen in liquid nitrogen until further usage.
HPLC analyses
Lipophilic compounds were extracted by adding 200 μl of water, 400 μl of acetone, 400 μl of diethyl ether, and 100 μl of petroleum ether to homogenized sample material. This mixture was vortexed for 30 s and centrifuged for 2 min at 5,000 g at room temperature. The organic phase was collected and dried down in a SpeedVac (Eppendorf, Hamburg, Germany). Extracted lipids were then resuspended in 200 μl of hexane and separated by an 1100 Agilent HPLC series instrument equipped with a diode array detector and a normal-phase Zorbax Sil (5 μm, 4.6 × 250 mm) column (Agilent, Santa Clara, CA). Chromatographic separation was achieved by isocratic flow of 20% ethyl acetate/hexane at a flow rate of 1.4 ml/min. For quantification of molar amounts of carotenoids, the HPLC was scaled with the synthetic standards of zeaxanthin and β-carotene (Wild, Eppelheim, Germany). Owing to a lack of 3,3′-didehydrozeaxanthin standard, the molar amount of this carotenoid was calculated based on the zeaxanthin standard.
For MS/MS analysis of the unknown compounds, initial separation was achieved from the previously mentioned HPLC analysis. The eluate was collected when the unknown compound was detected by spectral absorbance and retention time. This eluate was then injected onto a reverse phase HPLC Zorbax C-18 (5 μm, 4.6 × 250 mm) column (Agilent) and eluted in a gradient of acetonitrile in water at a flow rate of 0.5 ml/min. The eluate was directed into a LXQ linear ion trap mass spectrometer (Thermo Scientific, Waltham, MA) through an electrospray ionization source working in the positive mode. To ensure optimal sensitivity, the instrument parameters were tuned with apocarotenoids and parent carotenoids.
Microarray and pathway analysis
RNA was collected using TRIzol reagent (Thermo Fisher, San Diego, CA) from the livers of 5-week-old DKO mice fed either a zeaxanthin diet (n = 5, control = 4) or β-carotene (n = 5, control = 6) for 10 weeks. cRNA was generated using an Illumina Total Prep 96 amplification kit (Illumina, San Diego, CA). The cRNA concentration was assessed according to 260 nm absorbance measured with a Nanodrop spectrophotometer and adjusted to 1,500 ng for a six-sample array in a total volume of 10 μl or to a concentration of 750 ng for an eight-sample array in a total volume of 5 μl. The volume of each sample was adjusted to 20 μl or 10 μl using Illumina hybridization buffer for a six- or eight-sample array, respectively. All samples were treated in a heating block at 65°C for 5 min. Following, 30 μl of sample was loaded on a six-sample or 15 μl of sample and was loaded on an eight-sample Mouse WG-6 v2.0 BeadChip (Illumina). The Illumina hybridization chamber was assembled with the loaded array chips and incubated at 58°C in an Illumina hybridization oven overnight for at least 16 h, but no more than 24 h. The next day, arrays were washed using Illumina E1BC wash buffer, 100% ethanol, and Illumina Block E1 buffer. Arrays were stained using Cy3-streptavidin dye (1 mg/ml) for 10 min at room temperature. Then beadchips were washed with E1BC buffer and dried in a centrifuge for 4 min. All beadchips were scanned in Illumina iScan for gene expression. BeadChip preparation and measurements were carried out by a trained specialist at the Genomics Core of Cleveland Clinic’s Lerner Research Institute.
Results were analyzed using Genome Studio software (Illumina). Comparative analysis was considered significant if there was a statistically significant difference between the two groups (P < 0.05), a fold change greater than 1.5 in either direction, and if signal values were greater than three times that of the background. For pathway analysis, all previous mouse samples [zeaxanthin diet (n = 5, control = 4) or β-carotene (n = 5, control = 6)] were included. Genes that met the statistical difference and signal over noise cut-off were analyzed with the use of QIAGEN’s Ingenuity Pathway Analysis (Redwood City, CA).
Real-time PCR
RNA was isolated from mouse liver using TRIzol (Thermo Fisher, San Diego, CA) and was reverse transcribed to cDNA with the use of a high-capacity RNA-to-cDNA kit (Thermo Fisher). This cDNA was then used for quantitative real-time PCR using TaqMan Gene Expression Master Mix (Thermo Fisher). Measurements in relative levels of Bcdo2 (Mm00460051_m1), Scarb1 (Mm00450234_m1), Prkaa2 (Mm01264789_m1), Spon2 (Mm00513596_m1), Arrdc3 (Mm01352845_g1), and Elovl2 (Mm00517086_m1) mRNA levels were normalized to levels of 18S rRNA (4319413E) and were measured as biological and technical triplicates. All real-time experiments were performed with the ABI Step-One Plus qRT-PCR machine (Thermo Fisher).
HDL and LDL separation
Lipoproteins were fractionated using the LDL/VLDL and HDL purification kit (Cell Biolabs, Inc, San Diego, CA) following the manufacturer’s protocol. The protocol relies on precipitation of lipoprotein complexes with the use of dextran sulfate, and purification through further precipitation and low-speed differential centrifugation. Purity of fractions was assessed by separating samples by SDS-PAGE, staining with Coomassie blue dye, and observing bands corresponding to weights of ApoB48 (250 kDa) for LDL fractions and ApoA1 (27 kDa) for HDL fractions.
Total triglyceride measurement
Triglyceride content was measured using a Triglyceride Colorimetric Assay kit (Caymen Chemical Co., Ann Arbor, MI) following the protocol provided by the manufacturer. Briefly, triglycerides were subjected to lipoprotein lipase treatment. The liberated glycerol was then phosphorylated by glycerol kinase, which was oxidized by glycerol phosphate oxidase, which produced hydrogen peroxide. The hydrogen peroxide was detected with 4-aminoantipyrine, N-ethyl-N-(3-sulfopropyl)-m-anisidine, and peroxidase to form quinoneimine dye. Absorbance at 545 nm was then measured within a FlexStation 3 96-well plate reader (Molecular Devices, Sunnyvale, CA). A standard solution of a known amount of triglycerides was used to establish a standard curve to calculate triglyceride content of the samples.
Total cholesterol measurement
Cholesterol content was measured using a Cholesterol Fluorometric Assay kit (Caymen Chemical) following the protocol provided by the manufacturer. Briefly, samples were homogenized in 100 mM potassium phosphate buffer [50 mM NaCl, and 5 mM cholic acid (pH 7.4)]. Cholesterol esterase was added to convert cholesterol esters to cholesterol, followed by cholesterol oxidase to produce hydrogen peroxide. Horseradish peroxidase was then used to oxidize 10-acetyl-3,7-dihydroxyphenoxazine to the fluorescent compound, resorufin. A standard curve of cholesterol was used to calculate concentrations of cholesterol content in the samples using a FlexStation 3 96-well plate reader (Molecular Devices).
Measurement of energy expenditure by indirect calorimetry
Metabolic rates were measured in live mice using an eight-chamber open-circuit Oxymax system (CLAMS; Columbus Instruments, Columbus, OH) at the Mouse Metabolic Phenotyping Center at Case Western Reserve University. Briefly, female mice (n = 3 per group) were acclimated to the experimental room for 1 week prior to the experiment. The mice were individually housed in acrylic calorimeter chambers and acclimated for 3 days before data were collected. These chambers have air with a known O2 concentration passed through them at a constant flow rate. The system automatically withdrew gas samples from each chamber hourly for 6 h. The system then calculated the volume of O2 consumed (VO2) and the volume of CO2 generated (VCO2) by each mouse in 15 min intervals. The RQ, which is the ratio of VCO2 to VO2, is then directly calculated. Energy expenditure (EE) was calculated by rearrangement of the Weir formula; multiplying the calorific value [caloric value = 3.815 + (1.232 × RQ)] by the observed VO2 (EE = CV × VO2). Measurements were carried out during the light cycle and fed condition. Mice were maintained at 25°C and had free access to water and food.
RESULTS
Zeaxanthin and β-carotene metabolites exist in DKO mice
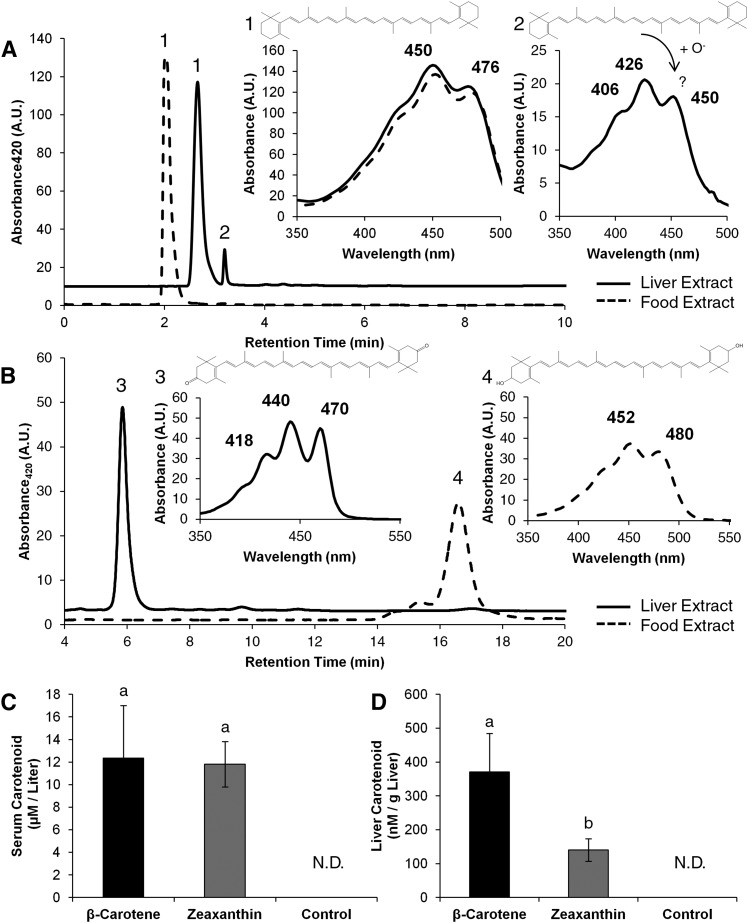
To examine the actions of carotenoids on hepatic physiology, we employed female mice that were deficient for both carotenoid-dioxygenases (DKO). We raised these mice on standard breeder chow, which is rich in vitamin A. Five-week-old DKO siblings were then randomly subjected to feeding on a purified diet with either zeaxanthin or β-carotene (0.15 mg carotenoid per gram diet) supplementation. DKO siblings subjected to feeding with the same diet without carotenoid supplementation served as controls in these experiments. All diets were vitamin A-free to avoid interactions of dietary carotenoids and retinoids at the level of intestinal absorption (27, 28). After 10 weeks of dietary intervention, animals were euthanized and blood and tissues were collected for further analyses. Initial normal-phase HPLC analysis of lipid extracts from liver and serum samples demonstrated that animals accumulated significant amounts of carotenoids (Fig. 1). While most of the β-carotene accumulated as parent compound, we observed small amounts of a more polar β-carotene metabolite, which did not exist in the diet (Fig. 1A, peak 2). This metabolite had an increased retention time and its absorption spectrum was blue-light shifted when compared with parent β-carotene (Fig. 1A, insert 2). Previously, it has been reported that β-apocarotenals can be formed by carotenoid-oxygenase-independent pathways (13). To test this possibility, we intended to chemically convert the metabolite to the corresponding oxime. However, treatment of the liver extracts with hydroxylamine did not change the retention time or spectral characteristics of the metabolite, as would be expected for an apocarotenal (data not shown). Thus, we separated and collected the purified metabolite by normal-phase HPLC separation. We then subjected the collected fraction to reverse-phase LC-MS/MS analysis (supplemental Fig. S1A). This analysis detected two dominant species having molecular masses of 534.6 and 552.4 Da (m/z 535.6 and 553.4, respectively, [MH]+) (supplemental Fig. S1B, C). HPLC-MS/MS analysis of the 552.4 Da molecule resulted in the formation of a 534 Da fragmentation product (m/z 535, [MH]+) (supplemental Fig. S1C, insert). The increase of the metabolite’s molecular mass by 16 Da suggested that it was an oxidized β-carotene derivative, e.g., an epoxide. However, positive identification was not possible due a lack of standards for these compounds.
Fig. 1.
Carotenoids are modified in the absence of carotenoid-oxygenases. Five-week-old Bco1−/− Bco2−/− (DKO) female mice were supplemented on either β-carotene (n = 4), zeaxanthin (n = 4), or a control carotenoid-free diet (n = 4) for 10 weeks. Carotenoid content was measured in mouse liver and serum, as well as food. A: HPLC trace at 420 nm of lipids isolated from β-carotene-fed mice (solid line) and their food (dashed line). Inserts: spectral characteristics of β-carotene (peak 1) and a β-carotene metabolite (peak 2). B: HPLC trace at 420 nm of lipids isolated from zeaxanthin-fed mice (solid line) and their food (dashed line). Inserts: spectral characteristics of zeaxanthin (peak 4) found in food, and the previously described oxidative modification of zeaxanthin (peak 3). Molar quantification of carotenoids present in serum (C) and liver (D). Values are means, error bars represent standard deviation; values which do not share the same letter are statistically significant (P < 0.05, by two-tailed t-test). N.D., not detected.
Biochemical analyses of zeaxanthin-supplemented animals revealed that the majority of this xanthophyll accumulated as an oxidized metabolite. This metabolite displayed a shorter retention time on the HPLC system and altered spectral characteristics when compared with the parent zeaxanthin found in the food of these animals (Fig. 1B; peaks 3, 4). This metabolic conversion of zeaxanthin to a less polar metabolite has been previously described in mice by us (25) and relies on an oxidation of the 3-hydroxy-groups to the corresponding keto-groups at both terminal β-ionone rings (structures shown in Fig. 1B).
Quantification of the molar amounts of carotenoids in serum revealed that both β-carotene and the zeaxanthin metabolite existed in approximately 12 μM concentrations (Fig. 1C). Liver carotenoid levels differed greatly between the two dietary groups and were 3-fold higher in the β-carotene group when compared with the zeaxanthin-supplemented group (Fig. 1D). Control DKO mice fed a diet without supplemented carotenoids had no detectable levels of carotenoids in either the serum or liver tissue.
Carotenoids induce changes in the hepatic transcriptome
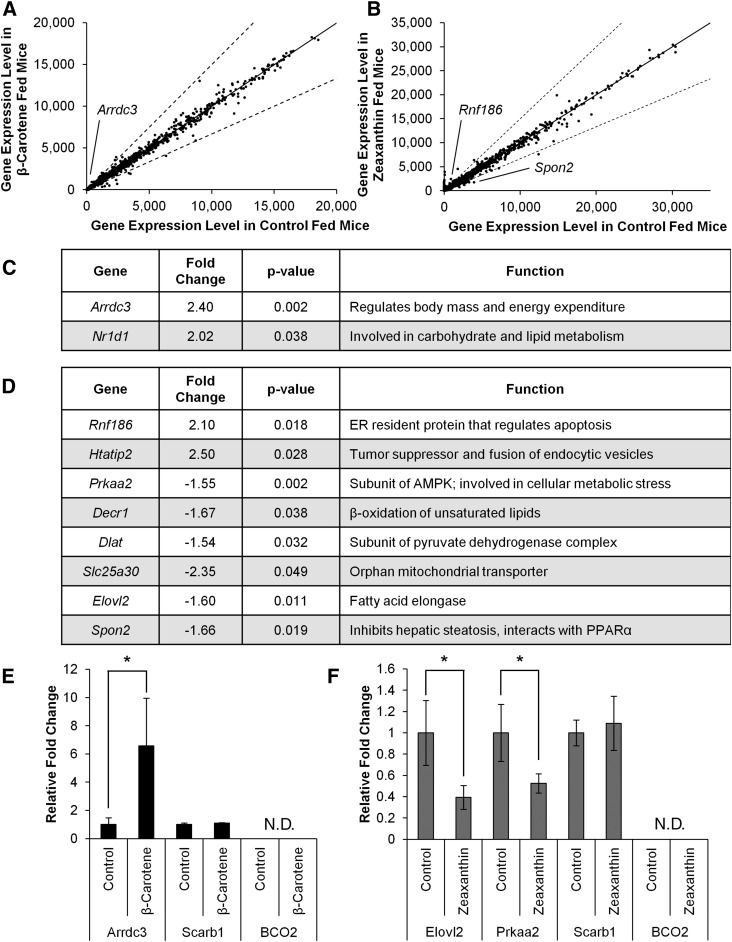
To assess which physiological pathways are affected by carotenoid accumulation, we performed a transcriptome analysis of liver tissues by Illumina gene expression array. The overall profile, 30,855 distinct genes measured, of differential expression did not demonstrate a large change in the overall hepatic expression signature as a result of either carotenoid supplementation (Fig. 2A, B). However, within the β-carotene group, changes in expression of 1,207 genes were statistically significant between the treated and control group; compared with 2,133 genes in the zeaxanthin group to its control.
Fig. 2.
Carotenoid accumulation has a modest effect on the liver expressome. Five-week-old Bco1−/− Bco2−/− (DKO) female mice were supplemented on either a zeaxanthin (n = 5, control = 4) or β-carotene (n = 5, control = 6) diet for 10 weeks. Effects of carotenoid accumulation on the liver expressome was assessed by Illumina beadchip. Overall expression profile of individual expression levels for genes for β-carotene-fed (A) and zeaxanthin-fed (B) mice as compared with their littermate controls. Solid line depicts no change in expression between carotenoid-supplemented and control-diet mice. Dashed line represents the 1.5-fold change. Several genes were identified to have a significant change in expression in response to β-carotene (C) or zeaxanthin (D) supplementation. Results from both data sets, β-carotene (E) and zeaxanthin (F), were validated by real-time PCR of select genes. Values are means, error bars represent standard deviation. *P < 0.05 (by two-tailed t-test). N.D., not detected.
To narrow down candidate genes that may be altered in their expression levels by carotenoid accumulation; we filtered out genes that had signal intensities less than three times the background. Additionally, we excluded genes that had less than a 1.5-fold change in their expression levels in either direction. Under such stringent conditions, six genes were identified in the liver genome that were differentially regulated as a result of β-carotene accumulation, and 22 genes as a result of zeaxanthin accumulation (Fig. 2C, D; supplemental Fig. S2A, B). There was no overlap between the two different carotenoid treatment groups. Of note, several genes involved in lipid metabolism, energy, and mitochondrial homeostasis were identified to have had their mRNA expression levels modified by carotenoid accumulation when these stringent criteria were applied. In the β-carotene group, Arrdc3 and Nr1d1 genes, respectively encoding a regulator of EE (29) and a nuclear receptor involved in the control of sterol metabolism (30) were significantly increased in their expression levels (Fig. 2C). In the zeaxanthin group, candidate genes revealed by our stringent criteria were generally downregulated. These involved genes in lipid metabolism, such as Prkaa2, which encodes a subunit of the AMPK complex and is associated with change in lipoprotein levels (31), Elovl2 encoding a fatty-acid elongase (32), Decr1 encoding a protein involved in β-oxidation of fatty acids (33), and Slc25a30 encoding an orphan mitochondrial transporter (34).
To validate the results of the gene expression array, we performed quantitative real-time PCR of a few select genes. For this purpose, we selected genes which were differentially regulated, had no change in expression, and as negative control we used Bco2 expression. We observed comparable results between real-time PCR and beadchip analyses in both supplementation groups, thus validating our results (Fig. 2E, F). The Arddc3 gene was upregulated in hepatic RNA preparations of β-carotene-supplemented animals, while Elovl2 and Prkaa2 genes were downregulated in RNA preparations of zeaxanthin-supplemented animals when compared with the respective nonsupplemented controls (Fig. 2C–F). Accordingly, Scrab1 mRNA expression remained unchanged between experimental groups and Bco2 mRNA was undetectable (CT >35) in all groups.
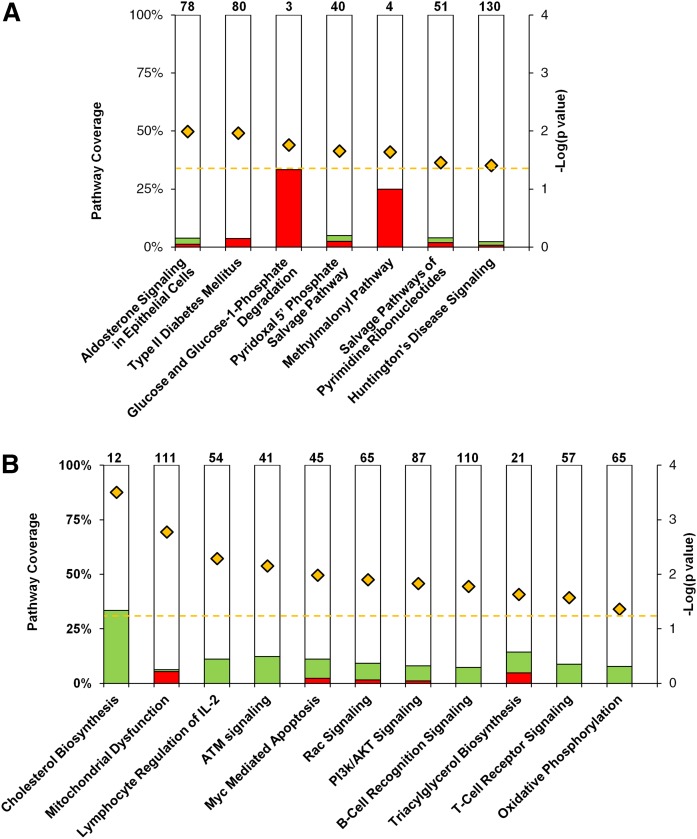
A pathway analysis using Ingenuity software was performed to assess whether multiple changes of genes within a singular pathway could amount to a physiological change. To more completely capture the changes in cellular metabolism, we included all genes that had a signal value 3-fold above background and showed differences that were statistically significant (P < 0.05) in expression between the two groups. This included all genes shown in Fig. 2 and those that had a lower fold change; a total of 102 genes for the β-carotene supplemented group and 423 for the zeaxanthin supplemented group were input for this analysis. The computational pathway analysis revealed that mice on the β-carotene diet had changes in the expression levels of genes in pathways related to energy metabolism, among them glucose degradation and those involved in type 2 diabetes (Fig. 3A, supplemental Fig. S3). On the zeaxanthin diet, mice had changes in the expression levels of genes in cholesterol metabolism, mitochondrial dysfunction, and reduction in mitochondrial oxidative phosphorylation (Fig. 3B, supplemental Fig. S4). As with the individual gene changes, there was no overlap in pathways that were differentially regulated between the β-carotene and zeaxanthin groups. The outcome of the computational and expressome analysis was then used to investigate potential biochemical changes at the cellular level.
Fig. 3.
β-Carotene and zeaxanthin supplementation affects pathways involved in lipid metabolism and mitochondrial function. Results from the differential liver expressome were analyzed computationally to assess which cellular pathways may have changed as a result of carotenoid accumulation. Selected results from β-carotene (A) and zeaxanthin (B) are shown here. Names of individual pathways are given below each bar. Numbers at the top of each bar depict the total number of genes identified in that pathway. Coloration of each bar represents the percentage (left axis) of genes differentially regulated. Red coloration indicates an upregulation of genes in that pathway, while green depicts a downregulation. P values for each pathway are reported as −Log (P value) (yellow diamonds, right axis), the dashed line represents the cutoff of P = 0.05 (by two-tailed t-test).
Carotenoid accumulation alters lipid composition in liver and blood
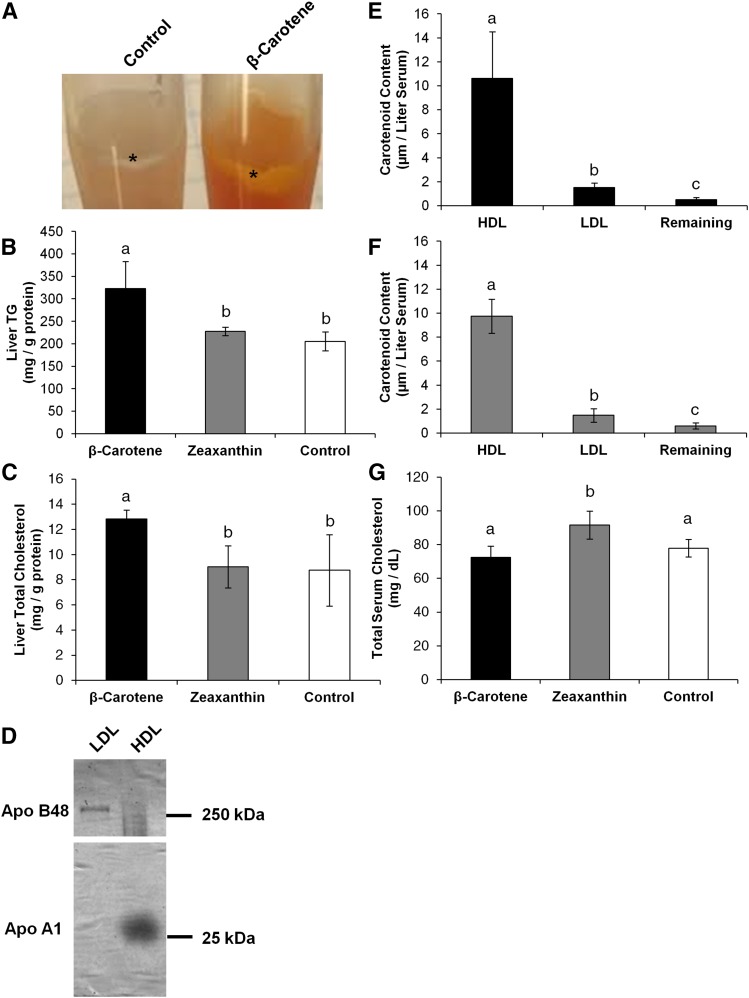
The liver is central to whole-body energy metabolism as well as the distribution of lipids to the periphery. We observed that carotenoids accumulated in the liver and altered expression levels of genes associated with critical hepatic function(s). To assess the biochemical consequences of carotenoid accumulation on lipid metabolism, we determined hepatic cholesterol and triglyceride levels. During tissue extraction, we observed that β-carotene accumulated in the floating lipid droplet fraction of the hepatic homogenate (Fig. 4A). Quantification of hepatic lipids revealed an overall increase of triglycerides within the β-carotene-supplemented group when compared with zeaxanthin and control groups (Fig. 4B). Similarly, total liver cholesterol levels within the β-carotene group were elevated in comparison to both the zeaxanthin and control groups (Fig. 4C). For both lipid classes, the increases in the β-carotene group reached statistical significance when compared with the other dietary groups.
Fig. 4.
Cholesterol and triglycerides of the liver and serum, and the presence of carotenoids in lipoproteins. Five-week-old Bco1−/− Bco2−/− (DKO) female mice were supplemented on either β-carotene (n = 4), zeaxanthin (n = 4), or a control carotenoid-free diet (n = 4) for 10 weeks. A: During initial tissue homogenization, we observed increased lipid separation, as compared with controls, and carotenoid incorporation into this layer. The * denotes the carotenoid-rich lipid layer. Measurements of triglycerides (B) and cholesterol (C) within liver tissue of all groups. Lipoproteins were precipitated by dextran sulfate. D: Purity was assessed by SDS-PAGE and observing bands corresponding to ApoB48 (250 kDa) within the LDL fraction and ApoA1 (27 kDa) within the HDL fraction. Molar quantification of carotenoids in lipoprotein fractions of β-carotene-supplemented (E) and zeaxanthin-supplemented (F) animals. Values are means, error bars represent standard deviation; values which do not share the same letter are statistically significant (P < 0.05, by two-tailed t-test).
In addition to metabolizing lipids, the liver is paramount to lipoprotein metabolism and lipid homeostasis of the body. To better understand how carotenoid accumulation may impact this process, we measured its occurrence in lipoprotein classes. This analysis also aimed to clarify whether the related but structurally distinct carotenoids, β-carotene and zeaxanthin, were transported in the same lipoprotein class in the blood. Serum from zeaxanthin- and β-carotene-fed mice was separated into HDL and LDL fractions by dextran sulfate precipitation. Purity of the HDL and LDL fractions was determined by separating proteins by SDS-PAGE. After staining with Coomassie blue dye, we observed bands corresponding to the ApoB48 protein (∼250 kDa) present within LDL and ApoA1 (∼27 kDa) found in HDL (Fig. 4D). Subsequently, lipids were extracted from these fractions and subjected to HPLC analysis (Fig. 4E, F). The majority of serum carotenoids were found within the HDL lipoprotein fraction for both β-carotene-supplemented animals (10.63 ± 3.86 μM/l) and zeaxanthin-supplemented animals (9.75 ± 1.42 μM/l). The carotenoids transported within the LDL fraction constituted only one-sixth of the total serum levels (β-carotene, 1.51 ± 0.38 μM/l; zeaxanthin, 1.48 ± 33.21 μM/l).
To assess whether the incorporation of large quantities of carotenoids within lipoprotein complexes altered lipid composition of the serum, we determined cholesterol levels in β-carotene- and zeaxanthin-fed mice. We observed that mice supplemented with a β-carotene-rich diet did not have a change in total serum cholesterol (72.44 ± 6.75 mg/dl) compared with control mice on the carotenoid-free diet (77.95 ± 5.13 mg/dl) (Fig. 4G). In contrast to this, zeaxanthin-fed mice (91.68 ± 8.30 mg/dl) had a significant increase in total serum cholesterol compared with either β-carotene- or control-fed mice.
Carotenoids affect EE and respiration
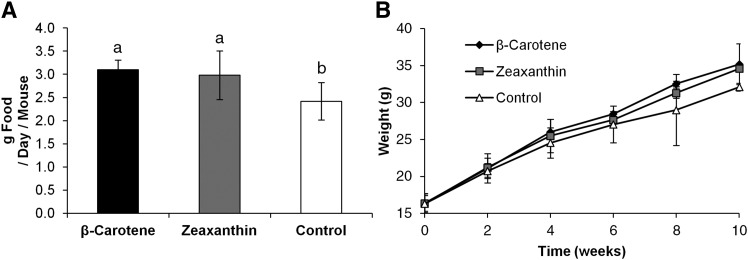
Our initial investigation provided evidence that carotenoid accumulation affected expression of genes involved in energy metabolism and changed lipid profiles in liver and blood. Therefore, we sought to discern whether carotenoids exerted a global effect on oxygen consumption and EE of mice. DKO mice were initially randomized between the different dietary groups. All dietary groups gained significant weight during the experimental period (Fig. 5A). The β-carotene- and zeaxanthin-supplemented mice displayed a slight increase in the rate of weight gain at the end of the feeding period when compared with nonsupplemented DKO control mice. However, this difference did not reach statistical significance. Median food intake was greater in carotenoid-supplemented animals when compared with controls (Fig. 5B).
Fig. 5.
Carotenoid diet affects food intake, but not weight. Five-week-old Bco1−/− Bco2−/− (DKO) female mice were supplemented on either β-carotene (n = 4), zeaxanthin (n = 4), or a control carotenoid-free diet (n = 4) for 10 weeks. Food (A) and mice (B) were weighed twice, weekly. Values are means, error bars represent standard deviation; values which do not share the same letter are statistically significant (P < 0.05, by two-tailed t-test).
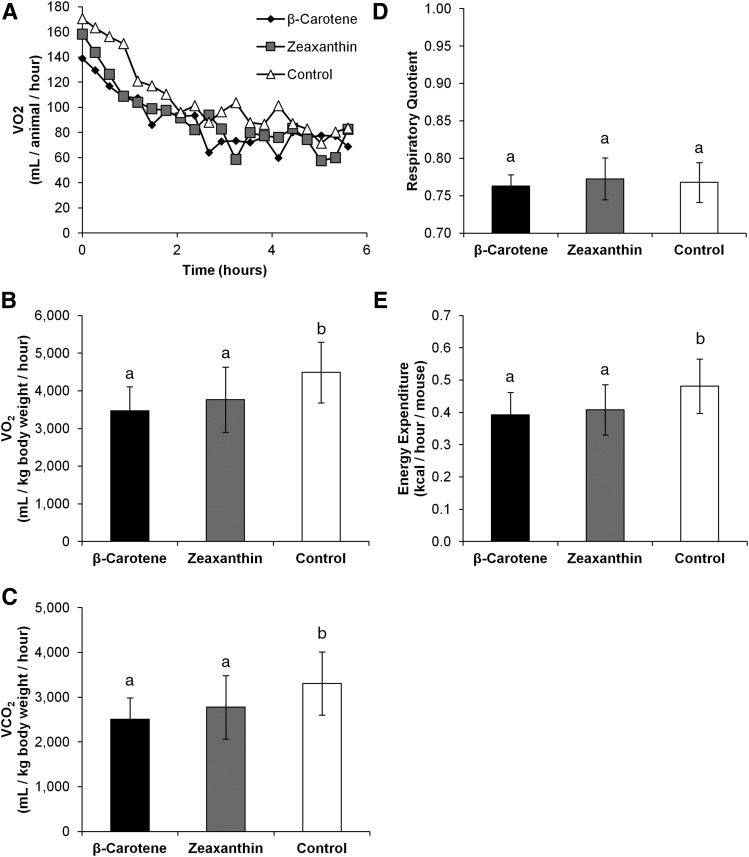
To establish whether carotenoid supplementation affected energy metabolism, we subjected DKO mice (n = 3 for each dietary group) to whole-body respiration analysis to assess oxygen consumption (VO2) and carbon dioxide production (VCO2). We calculated total EE and respiration quotient (RER), to determine whether there was a change in preferred substrate utilization. A decrease in average oxygen consumption and carbon dioxide production was measured in the β-carotene- and zeaxanthin-fed mice when compared with control diet-fed siblings (Fig. 6A–C). The calculated average RER values (VCO2/VO2) did not differ between groups (Fig. 6D). Average EE was calculated as described in the Materials and Methods (based on VCO2 and VO2 values). We observed that both β-carotene and zeaxanthin supplementation resulted in a slight reduction in EE as compared with control animals (Fig. 6E). Comparisons of respiration values as area under the curve (AUC), displayed similar trends, though numerous values only approached statistical significance (supplemental Fig. S5). Of note, the AUC-RER value for the β-carotene was statistically significant when compared with the value of controls (P < 0.05), while zeaxanthin did not reach a statistical difference (P = 0.053).
Fig. 6.
Carotenoid accumulation affects whole-body respiration rates. Five-week-old Bco1−/− Bco2−/− (DKO) female mice were supplemented on either β-carotene (n = 3), zeaxanthin (n = 3), or a control carotenoid-free diet (n = 3) for 10 weeks. Mice were then subjected to whole-body respiration analysis. A: Oxygen consumption and carbon dioxide production were measured every 15 min for 6 h. Average values of oxygen consumption (B) and carbon dioxide production (C) were lower in the carotenoid-fed group. D: RER was calculated as the ratio of oxygen consumption to carbon dioxide production. E: Total EE was then calculated from these values. Values are means, error bars represent standard deviation; values which do not share the same letter are statistically significant (P < 0.05, by two-tailed t-test).
DISCUSSION
Low carotenoid status, especially blood and tissue levels of xanthophylls, has been associated with a number of degenerative diseases, such as cardiovascular disease, cognitive impairments, visual impairments and certain forms of cancer (5, 6, 9, 10). However, the effects of carotenoids on mammalian physiology are not well defined because of the dual role of these compounds as parent compounds and precursors for apocarotenoid production. Here, we genetically dissected the effects of carotenoids from the effects of their metabolites. We accomplished this by subjecting DKO mice deficient for both carotenoid-oxygenases to feeding with either β-carotene or zeaxanthin. The principal findings of our study are that: i) carotenoids are chemically modified in the absence of carotenoid-oxygenases; ii) carotenoids have a modest but statistically significant impact on the liver expressome; iii) carotenoids impact lipid profiles of liver and blood; and iv) carotenoids affect whole-body respiratory rates and EE. The characteristics of these effects differed between the two structurally related carotenoids, suggesting that distinct carotenoids display a specific mode of action.
Carotenoids are metabolized in mammals by two carotenoid-oxygenases. These enzymes play an important role in carotenoid homeostasis of blood and tissues, as seen by genetic variability in the corresponding genes in humans and animals (35–39). However, little is known as to whether an additional mechanism exists in cells to chemically modify these lipids. Here, we observed that carotenoids in the absence of carotenoid-oxygenases underwent metabolic transformation in mice. In β-carotene-supplemented animals, a putative β-carotene oxidation product was formed (Fig. 1). The change in molecular mass, spectral characteristics, and hydrophobicity indicated the formation of a β-carotene epoxide. To date, the formation of β-carotene epoxides has been observed mainly in cell culture and in vitro systems (40). Similarly, zeaxanthin underwent oxidative modification in mice (Fig. 1). Zeaxanthin (3,3′-di-ol-β-carotene)-supplemented mice accumulated 3,3′-di-keto-β-carotene, whereas the parent compound was almost entirely absent. We can exclude that this metabolite was already present within food given to the animals, as this putative source was investigated. The keto-form of zeaxanthin also occurs as a low level metabolite in mice sufficient for carotenoid-oxygenases (Bco1+/+ Bco2+/+) and humans (41–43). In contrast to β-carotene, zeaxanthin was almost completely converted in the absence of carotenoid oxygenases. The very low levels of this metabolite in wild-type mice and humans might be best explained by a rapid metabolic turnover of keto-derivative compounds. Future research should clarify whether the observed carotenoid metabolites derive from specific (enzymatic) or nonenzymatic oxidation reactions.
Similar to other dietary lipids, carotenoids are not soluble in water and must be sequestered in lipophilic compartments. We previously demonstrated that zeaxanthin preferentially accumulates in liver mitochondria (44). We now observed that β-carotene accumulated in the floating lipid droplet fraction of homogenized hepatocytes. Thus, in an effort to sequester β-carotene, BCO1-deficient hepatocytes may generate lipid droplets to accommodate this hydrocarbon. Such a storage model has been described for the chemically and structurally related retinyl esters (45). The formation of lipid droplets for β-carotene storage may explain, in part, the increase of the triglyceride and cholesterol content of the liver in supplemented animals. An impact of BCO1 genotype on hepatic lipid profiles has previously been reported in Bco1 single knockout mice by others, though it was attributed to different mechanisms (26, 46).
We also provide evidence that carotenoids are transported in circulating lipoproteins in mice. When serum was separated into LDL and HDL fractions, both carotenoids were found predominantly in the HDL fraction. This contrasts with findings in humans, where β-carotene is primarily transported in LDL and zeaxanthin within HDL (47). This difference may be explained by the observation that mice predominately transport cholesterol in HDL particles as compared with LDL in humans. Notably, zeaxanthin supplementation was associated with an increase in total serum cholesterol. This increase may be related to the specific chemical and physical properties of the different carotenoids. The hydrocarbon β-carotene may be transported within the lipophilic lipoprotein core, whereas the hydroxylated zeaxanthin may exist at the membrane surface (48, 49). The latter component is much smaller in volume and, thus, additional lipoproteins may be required to accommodate relatively large amounts of zeaxanthin in the serum.
A previous study with mice showed that β-carotene supplementation had a significant impact on the transcriptome of mouse tissues expressing carotenoid-oxygenases. This effect was largely mediated by retinoic acid signaling, clearly demonstrating that this effect depends on BCO1 (50). In fact, when we removed the ability of tissues to synthesize retinoids, carotenoids had only a modest effect on the transcriptome. This is consistent with the notion that the parent carotenoids do not directly interact with transcription factors as opposed to their apocarotenoid metabolites (13). These findings strongly suggest that retinoic acid, or any other retinoic acid receptor ligand, cannot be generated in the absence of BCO1 and BCO2.
Our transcriptome analyses revealed that the accumulation of carotenoids affected the expression of numerous genes. The most significantly changed transcripts included genes associated with EE (Arrdc3 and Prkaa2), lipid metabolism (Nr1d1 and Elovl2), and cellular stress (Spon2 and Rnf186). Notably, we compared isogenic littermates, thus removing background effects of genetic-knockout on the expressome in this study. Others have reported that Arrdc3 is required for lipid droplet formation, a gene we observed with a positive 2.4-fold change. This upregulation may drive the changes in liver lipids observed here and in other reports (26, 29).
The transcriptome examination was further expanded on by pathway analyses, which revealed significant changes in expression of genes in pathways related to lipid metabolism and mitochondrial stress. In agreement with the predicted changes at the transcriptional level, we observed biochemically detectable changes in lipid metabolism, as discussed above. In addition, we provide evidence for reduced whole-body respiration rates and EE in mice supplemented with both carotenoids. These effects may be related to an accumulation of carotenoid metabolites in mitochondria and a direct interaction of carotenoids with the mitochondrial respiratory chain (25, 44). Such an interaction of carotenoids has been previously demonstrated for lutein in BCO2-deficient mice (25). This interaction was also reflected by an induction of genes of pathways related to mitochondrial stress in zeaxanthin-supplemented mice in the present study. Because β-carotene had a comparable impact and does not concentrate in mitochondria (44), additional mechanisms may contribute to the observed reduced rates in respiration. These may include direct interactions between carotenoids and other lipids that alter the metabolic flux of these compounds in cells and tissues. A different mode of action of the two carotenoids was further supported by our pathway analyses, which revealed distinct patterns of changes for zeaxanthin- and β-carotene-supplemented animals. Though a direct cause-effect relationship for either carotenoid was not definitively determined by this study, our data indicate that carotenoid accumulation interfered with lipid metabolism at several levels leading to modest alterations in lipid composition of liver and blood, whole-body respiration, and EE.
Taken together, this study scrutinized the effects of carotenoids on hepatic physiology in mice. For this, we established a novel mouse model that can accumulate large amounts of carotenoids, in a tunable fashion, based on the dosage supplemented. Our findings with this mouse model indicate that parent carotenoids interact with cells and tissues both on the genomic and nongenomic level. Hereby, our study provides novel insights into the metabolism, transport, and cellular sequestration of carotenoids. It is of interest that the hydrocarbon, β-carotene, and its hydroxylated derivative, zeaxanthin, displayed a different metabolic fate and biological effects. It will be fascinating to employ this novel mouse model to study the effects and function of other carotenoids. The tunable accumulation of carotenoids in combination with modern analytic methods will help to further elucidate the roles of these compounds in various tissues and physiological conditions. This knowledge is critical to understand the biochemical foundation of the putative beneficial effects of carotenoids in the prevention of chronic disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Colleen Croniger (Case Western Reserve University) for help with mouse respiration studies and acknowledge the Genomics Core of Cleveland Clinic’s Lerner Research Institute for their help with the Illumina beadchip.
Footnotes
Abbreviations:
- AUC
- area under the curve
- BCO1
- β,β-carotene-15,15′-oxygenase
- BCO2
- β,β-carotene-9′,10′-oxygenase
- DKO
- Bco1−/− Bco2−/−
- EE
- energy expenditure
- RER
- respiration quotient
- VCO2
- volume carbon dioxide
- VO2
- volume oxygen
This research was supported by Foundation for the National Institutes of Health Grants EY020551, EY023948, and EY07157 and Case Western Reserve University [Mouse Metabolic Phenotyping Center (MMPC)] Grant U24 DK76174. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.DellaPenna D., and Pogson B. J.. 2006. Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 57: 711–738. [DOI] [PubMed] [Google Scholar]
- 2.Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 57: 761–780. [DOI] [PubMed] [Google Scholar]
- 3.Moise A. R., Al-Babili S., and Wurtzel E. T.. 2014. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 114: 164–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Álvarez R., Vaz B., Gronemeyer H., and de Lera Á. R.. 2014. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 114: 1–125. [DOI] [PubMed] [Google Scholar]
- 5.Krinsky N. I., and Johnson E. J.. 2005. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26: 459–516. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein P. S., Li B., Vachali P. P., Gorusupudi A., Shyam R., Henriksen B. S., and Nolan J. M.. 2016. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 50: 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X. D. 2012. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 96: 1214S–1222S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaulmann A., and Bohn T.. 2014. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 34: 907–929. [DOI] [PubMed] [Google Scholar]
- 9.Moran N. E., Erdman J. W. Jr., and Clinton S. K.. 2013. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 539: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinton S. K. 1998. Lycopene: chemistry, biology, and implications for human health and disease. Nutr. Rev. 56: 35–51. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E., Ascherio A., Rimm E. B., Stampfer M. J., Colditz G. A., and Willett W. C.. 1995. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 87: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal J. M., Kim J., de Monasterio F., Thompson D. J., Bone R. A., Landrum J. T., de Moura F. F., Khachik F., Chen H., Schleicher R. L., et al. 2006. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest. Ophthalmol. Vis. Sci. 47: 5227–5233. [Erratum. 2007. Invest. Ophthalmol. Vis. Sci 48: 17.] [DOI] [PubMed] [Google Scholar]
- 13.Eroglu A., and Harrison E. H.. 2013. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 54: 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Lintig J. 2010. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30: 35–56. [DOI] [PubMed] [Google Scholar]
- 15.Kiser P. D., Golczak M., and Palczewski K.. 2014. Chemistry of the retinoid (visual) cycle. Chem. Rev. 114: 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhinn M., and Dollé P.. 2012. Retinoic acid signalling during development. Development. 139: 843–858. [DOI] [PubMed] [Google Scholar]
- 17.D’Ambrosio D. N., Clugston R. D., and Blaner W. S.. 2011. Vitamin A metabolism: an update. Nutrients. 3: 63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo G. P., Amengual J., Palczewski G., Babino D., and von Lintig J.. 2012. Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim. Biophys. Acta. 1821: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyss A., Wirtz G., Woggon W., Brugger R., Wyss M., Friedlein A., Bachmann H., and Hunziker W.. 2000. Cloning and expression of beta,beta-carotene 15,15′-dioxygenase. Biochem. Biophys. Res. Commun. 271: 334–336. [DOI] [PubMed] [Google Scholar]
- 20.Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., and von Lintig J.. 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276: 14110–14116. [DOI] [PubMed] [Google Scholar]
- 21.Lindqvist A., and Andersson S.. 2002. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J. Biol. Chem. 277: 23942–23948. [DOI] [PubMed] [Google Scholar]
- 22.Amengual J., Widjaja-Adhi M. A., Rodriguez-Santiago S., Hessel S., Golczak M., Palczewski K., and von Lintig J.. 2013. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 288: 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.dela Seña C., Narayanasamy S., Riedl K. M., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2013. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 288: 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babino D., Palczewski G., Widjaja-Adhi M. A., Kiser P. D., Golczak M., and von Lintig J.. 2015. Characterization of the role of beta-carotene 9,10-dioxygenase in macular pigment metabolism. J. Biol. Chem. 290: 24844–24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., and von Lintig J.. 2011. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25: 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., et al. 2007. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282: 33553–33561. [DOI] [PubMed] [Google Scholar]
- 27.Lobo G. P., Amengual J., Baus D., Shivdasani R. A., Taylor D., and von Lintig J.. 2013. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J. Biol. Chem. 288: 9017–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo G. P., Hessel S., Eichinger A., Noy N., Moise A. R., Wyss A., Palczewski K., and von Lintig J.. 2010. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 24: 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patwari P., Emilsson V., Schadt E. E., Chutkow W. A., Lee S., Marsili A., Zhang Y., Dobrin R., Cohen D. E., Larsen P. R., et al. 2011. The arrestin domain-containing 3 protein regulates body mass and energy expenditure. Cell Metab. 14: 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solt L. A., Wang Y., Banerjee S., Hughes T., Kojetin D. J., Lundasen T., Shin Y., Liu J., Cameron M. D., Noel R., et al. 2012. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 485: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer-Jones N. J., Ge D., Snieder H., Perks U., Swaminathan R., Spector T. D., Carter N. D., and O’Dell S. D.. 2006. AMP-kinase alpha2 subunit gene PRKAA2 variants are associated with total cholesterol, low-density lipoprotein-cholesterol and high-density lipoprotein-cholesterol in normal women. J. Med. Genet. 43: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Torres-Gonzalez M., Tripathy S., Botolin D., Christian B., and Jump D. B.. 2008. Elevated hepatic fatty acid elongase-5 activity affects multiple pathways controlling hepatic lipid and carbohydrate composition. J. Lipid Res. 49: 1538–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanders R. J., Ruiter J. P., IJLst L., Waterham H. R., and Houten S. M.. 2010. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J. Inherit. Metab. Dis. 33: 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmieri F. 2013. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol. Aspects Med. 34: 465–484. [DOI] [PubMed] [Google Scholar]
- 35.Widjaja-Adhi M. A., Lobo G. P., Golczak M., and Von Lintig J.. 2015. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 24: 3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry S. D., Davis S. R., Beattie E. M., Thomas N. L., Burrett A. K., Ward H. E., Stanfield A. M., Biswas M., Ankersmit-Udy A. E., Oxley P. E., et al. 2009. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics. 182: 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian R., Pitchford W. S., Morris C. A., Cullen N. G., and Bottema C. D.. 2010. Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 41: 253–259. [DOI] [PubMed] [Google Scholar]
- 38.Våge D. I., and Boman I. A.. 2010. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindqvist A., Sharvill J., Sharvill D. E., and Andersson S.. 2007. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 137: 2346–2350. [DOI] [PubMed] [Google Scholar]
- 40.Gurak P. D., Mercadante A. Z., Gonzalez-Miret M. L., Heredia F. J., and Melendez-Martinez A. J.. 2014. Changes in antioxidant capacity and colour associated with the formation of beta-carotene epoxides and oxidative cleavage derivatives. Food Chem. 147: 160–169. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann D., Thurmann P. A., Spitzer V., Schalch W., Manner B., and Cohn W.. 2004. Plasma kinetics of zeaxanthin and 3′-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am. J. Clin. Nutr. 79: 410–417. [DOI] [PubMed] [Google Scholar]
- 42.Nagao A., Maoka T., Ono H., Kotake-Nara E., Kobayashi M., and Tomita M.. 2015. A 3-hydroxy beta-end group in xanthophylls is preferentially oxidized to a 3-oxo epsilon-end group in mammals. J. Lipid Res. 56: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yonekura L., Kobayashi M., Terasaki M., and Nagao A.. 2010. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 140: 1824–1831. [DOI] [PubMed] [Google Scholar]
- 44.Palczewski G., Amengual J., Hoppel C. L., and von Lintig J.. 2014. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 28: 4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orban T., Palczewska G., and Palczewski K.. 2011. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J. Biol. Chem. 286: 17248–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y. K., Zuccaro M. V., Costabile B. K., Rodas R., and Quadro L.. 2015. Tissue- and sex-specific effects of beta-carotene 15,15′ oxygenase (BCO1) on retinoid and lipid metabolism in adult and developing mice. Arch. Biochem. Biophys. 572: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker R. S. 1989. Carotenoids in human blood and tissues. J. Nutr. 119: 101–104. [DOI] [PubMed] [Google Scholar]
- 48.Gruszecki W. I., and Strzalka K.. 2005. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta. 1740: 108–115. [DOI] [PubMed] [Google Scholar]
- 49.Augustynska D., Jemiola-Rzeminska M., Burda K., and Strzalka K.. 2015. Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chem. Biol. Interact. 239: 19–25. [DOI] [PubMed] [Google Scholar]
- 50.Amengual J., Gouranton E., van Helden Y. G., Hessel S., Ribot J., Kramer E., Kiec-Wilk B., Razny U., Lietz G., Wyss A., et al. 2011. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS One. 6: e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.