Abstract
Lipoprotein (a) [Lp(a)] is a human plasma lipoprotein with unique structural and functional characteristics. Lp(a) is an assembly of two components: a central core with apoB and an additional glycoprotein, called apo(a). Ever since the strong association between elevated levels of Lp(a) and an increased risk for CVD was recognized, interest in the therapeutic modulation of Lp(a) levels has increased. Here, the past and present therapies aiming to lower Lp(a) levels will be reviewed, demonstrating that these agents have had varying degrees of success. The next challenge will be to prove that Lp(a) lowering also leads to cardiovascular benefit in patients with elevated Lp(a) levels. Therefore, highly specific and potent Lp(a)-lowering strategies are awaited urgently.
Keywords: apolipoproteins, drug therapy, drug therapy/hypolipidemic drugs, dyslipidemias, lipoproteins, lipoprotein(a)
Lipoprotein (a) [Lp(a)] is a unique plasma lipoprotein first described half a century ago (1). Lp(a) consists of two critical elements: a central LDL-like core containing a single molecule of apoB linked by a disulfide bridge to a signature protein called apo(a). Initial case-control studies showed a strong association between Lp(a) and the risk of CVD (2, 3), which was corroborated by recent genetic studies describing Lp(a) as a causal risk factor for CVD (4–8). However, for Lp(a) to reach an established modifiable CVD risk factor status, it should also be demonstrated that lowering Lp(a) levels leads to a reduction in CVD. In the present review, we will describe the therapeutic approaches evaluated for their ability to lower Lp(a) levels. Prior to discussing the available therapeutic strategies, we will describe how to identify the patients that are eligible for Lp(a)-lowering therapy.
WHAT PATIENT IS ELIGIBLE FOR Lp(a)-LOWERING THERAPY?
Despite the strong genetic component underlying Lp(a) levels in plasma, Lp(a) has not been fully acknowledged as a CVD risk factor in clinical practice. To improve the awareness of Lp(a), expert panels of the National Cholesterol Education Program Adult Treatment Panel, the European Atherosclerosis Society, and the National Lipid Association made an effort to advise clinicians on screening for and modulation of elevated Lp(a) (9–11). Whereas screening for elevated Lp(a) in the general population is not recommended at present, Lp(a) should be measured at least once in subjects at intermediate to high risk of CVD who present with: i) premature CVD; ii) familial hypercholesterolemia (FH); iii) a positive family history of elevated Lp(a) or premature CVD; iv) recurrent CVD despite statin therapy; or v) high risk scores [according to European guidelines ≥3% 10 year risk of fatal CVD; according to the US guidelines ≥10% 10 year risk of (non)fatal CHD]. In particular, if these subjects also lack signs of the more established risk factors for CVD, screening for Lp(a) level is warranted (9–11). Once identified, it has been recommended to strive for a desirable level of Lp(a) below the 80th percentile (less than 50 mg/dl for Caucasians) (11). Target levels for therapy are based on level Ia evidence obtained from meta-analysis of randomized controlled trials, as has also been done in the case of LDL cholesterol (LDL-C) (12, 13). At present, however, evidence from Lp(a)-lowering trials is still very limited. Hence, larger studies of longer duration of potent Lp(a)-lowering therapeutics in high-risk individuals are warranted to substantiate this advice. Below, we will discuss past and present therapeutics that have achieved varying levels of Lp(a) lowering (Table 1) and highlight the concomitant effects of these compounds on apoB and Lp(a) levels. (Fig. 1).
TABLE 1.
Lp(a)-lowering strategies rated for the capacity to lower Lp(a)
| Strategy | Mechanism | Reduction in Lp(a) (%) | Reduction in LDL-C (%) | Reference |
| Statins | Increased LDLR expression | 0 to +7 | 50 | (14–20) |
| Niacin | Reduced apo(a) transcription, or reduced apoB-100 secretion via inhibition of TG synthesis | 20 | 13 | (24–26) |
| CETP inhibitor | Attenuation of apoB-100 lipidation due to inhibited transfer of TG and cholesterol esters between apoB-100 lipoproteins and HDL | 24–36 | 36–42 | (35–36) |
| apoB antisense | Decrease hepatic apoB-100 synthesis through blockage of mRNA translation of apoB-100 | 26–27 | 38–48 | (39–42) |
| MTP inhibitor | Interfering apoB-100 lipidation due to inhibition of lipoprotein assembly in the liver | 17 | 30% | (28) |
| PCSK9 inhibitor | Inhibition of LDLR degradation, decreased apoB-100 formation | 25 | 40–59 | (46–48, 54) |
| Anti-IL6R | IL6 responsive element on APOA promotor region | 30–37 | 10 | (61) |
| Apheresis | Removal of circulating apoB-100 lipoproteins | 70 ± 10 | Up to 75 | (56–57) |
| Apo(a) antisense | Decrease hepatic apo(a) synthesis through blockage of mRNA translation of apo(a) | Up to 78 | No effect | (70) |
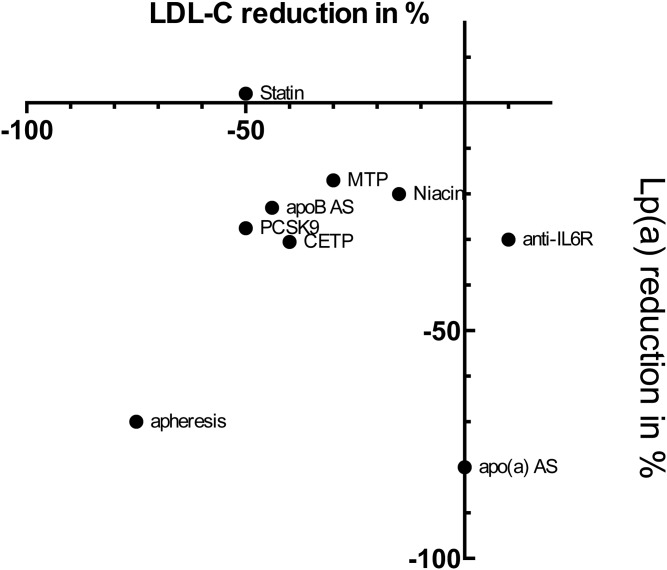
Fig. 1.
Association of LDL-C and Lp(a)-lowering effect per drug class. CETP is CETP inhibitors, PCSK9 is PCSK9 inhibition, apheresis is LA, MTP is microsomal TG transfer protein inhibitor, AS is antisense treatment.
Statins
Therapeutic options for Lp(a) arose alongside the developmental track for LDL-C-lowering drugs (Table 1). Statins have been around for more than 20 years and exert the majority of their LDL-lowering capacity by upregulating LDL receptor (LDLR) expression, subsequently leading to increased LDL-C clearance. Due to the structural similarities between Lp(a) and LDL, a hitchhiking-like process was proposed whereby Lp(a) attached to LDL could be removed by the LDLR pathway. Although statins represent one of the best-studied compounds in clinical research, there is no final answer to their effect on Lp(a) levels. Most recent studies report that statins do not affect Lp(a) levels [i.e., post hoc analysis of the Treating to New Targets (TNT) (14) and Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trials (15)] or perhaps even increase Lp(a) levels [Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACLE) (16), Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) (17), and Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) (18) trials]. In contrast, several smaller studies report decreases in Lp(a) levels (19, 20). Overall, there is no clear evidence that statin treatment lowers Lp(a) levels. Hence, the LDLR does not seem to be a major contributor to Lp(a) clearance in humans (21). A detailed discussion of this topic will be presented elsewhere in this review. The role of LDLR in Lp(a) clearance will be co-discussed again in the section on PCSK9 inhibition.
Niacin
Beyond the well-known capacity of niacin (nicotinic acid) to favorably influence the levels of HDL cholesterol (HDL-C), LDL-C, and TGs, niacin also decreases plasma levels of Lp(a). Potential mechanisms by which niacin lowers Lp(a) comprise a reduced apo(a) transcription (22), or a reduced apoB secretion via the inhibition of TG synthesis (23). The inhibitory effect of niacin on Lp(a) production was recently substantiated in vivo in an apo(a) kinetic study investigating the effect of extended-release niacin in eight obese male subjects with hypertriglyceridemia. Niacin resulted in a 50% reduction of newly synthesized apo(a), which was partly compensated by decreased catabolic clearance. The net effect was a 20% lowering of Lp(a) levels by niacin in this study (24).
In recent years, two large randomized controlled trials have studied the clinical effects of nicotinic acid derivatives. In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial, the effect of extended release niacin on a background of statin treatment was tested in 3,414 patients with stable atherosclerotic disease, low baseline HDL-C, and elevated TG levels, and niacin treatment resulted in 21% Lp(a) reduction compared with placebo (25). The larger Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial had a similar approach and enrolled 25,773 high-risk patients with prior CVD who were randomized to receive extended-release niacin in combination with laropiprant or placebo, on top of statin with or without ezetimibe background therapy (26). The prostaglandin D2 antagonist, laropiprant, was added to improve study adherence by reducing skin flushing, a common side-effect of niacin treatment. In HPS2-THRIVE, Lp(a) levels were only measured at 1 year in a randomly selected subset of 1,999 subjects and baseline levels are lacking. However results (50.7 vs. 60.3 nmol/l at year 1, 17.8% difference) were comparable to AIM-HIGH. Both studies did not show a reduction in cardiovascular event rate, despite a potential beneficial effect on lipoprotein levels, including an average 20% reduction in Lp(a) levels. Of greater concern was the increased rate of serious adverse events in patients receiving niacin/laropiprant in HPS2-THRIVE, including increased occurrence of diabetic complications and incidence, serious infections, serious bleeding, gastrointestinal complaints, and myopathy (26). In AIM-HIGH, without the addition of laropiprant, a similar profile of serious adverse event rates was observed, although not statistically significant, which is likely a reflection of the smaller study size (27).
Patients enrolled in HPS2-THRIVE and AIM-HIGH had very well-controlled baseline lipid profiles and the main criticism on these studies is centered on the question of whether these patients should have been treated at all, as they already met the most stringent criteria for lipid control at baseline (28). The same goes for Lp(a) levels in these studies, no current guidelines advise treatment of baseline levels of 36 nmol/l (AIM-HIGH) or 60 nmol/l (HPS2-THRIVE) (roughly equivalent to 15 mg/dl and 25 mg/dl) (29), and it must be stressed that these studies were not specifically designed for patients with elevated Lp(a).
Apart from the average Lp(a) reduction of 20% by niacin, the inter-individual response is variable and more potent Lp(a) lowering by niacin has been reported in some cases (30). Factors that determine Lp(a) response to niacin treatment have not been fully elucidated; for instance, apo(a) genotype status did not predict Lp(a) reduction and high Lp(a) levels at baseline were associated with increased response to niacin (31, 32).
One of the key questions is whether a selection of patients with high baseline Lp(a) levels benefitted from niacin treatment. A post hoc analysis of AIM-HIGH (32) showed that 30% of the study population had baseline Lp(a) levels >100 nmol/l. In addition, response to niacin was increased in the subgroups with higher Lp(a) levels: 20, 39, and 64% Lp(a) decrease in the 50th, 75th, and 90th percentile, respectively. There was no significant difference in event rate, however, between the niacin and placebo group for any quartile of baseline Lp(a), indicating the highest quartile of Lp(a) (median 125 nmol/l) did not benefit from the addition of niacin on top of statin background therapy. As Lp(a) levels in HPS2-THRIVE were only measured in a relatively small subset at year 1 and not at baseline (26), it is unlikely that post hoc analysis on high Lp(a) patients in HPS2-THRIVE will become available soon. Apart from the lessons learned from the impact of niacin on Lp(a), it should be realized that, while it continues to be available in the US, niacin is no longer available in the European market, in view of the unfavorable harm versus health balance of this compound.
CETP inhibitors
Originally developed as HDL-C raising agents, cholesteryl ester transfer protein (CETP) inhibitors have also been shown to reduce LDL-C and Lp(a) levels. CETP is a plasma glycoprotein that shuttles cholesterol esters between apoB-containing lipoproteins and HDL in exchange for TGs. To date, five CETP inhibitors have been developed, of which two developmental programs have been halted due to either off-target toxicity (torcetrapib) (33) or futility (dalcetrapib) (34). Data on Lp(a) levels have been reported for only two CETP inhibitors: anacetrapib treatment resulted in 24% Lp(a) reduction from baseline (35), and treatment with TA-8995 was associated with up to 36.9% Lp(a) reduction from baseline (36). Unfortunately, no data on Lp(a) levels have been released for dalcetrapib; this would be of great interest, as dalcetrapib is the only CETP inhibitor without an LDL-C-lowering effect. Given the similarities in lipoprotein changes induced by evacetrapib treatment compared with anacetrapib and TA-8995 (LDL-C reduced by 35.9–45.3% and HDL-C increased up to 128.8–157.1%) (35–37), it is likely that evacetrapib also lowers Lp(a) levels by 20–40%. Recently, the phase 3 outcome trial, Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes (ACCELERATE), was terminated early for futility by the data safety monitoring board (38), despite potent LDL-C lowering in phase 2 trials and, thus, presumably also a Lp(a)-lowering effect (37). Detailed study data from ACCELERATE will have to be awaited for a final verdict.
apoB antisense and MTP inhibitors: targeting apoB production
Targeting apoB in the process of gene translation can block apoB-100 mRNA translation through the use of a single-strand antisense oligonucleotide (ASO) that is complementary to the mRNA. Mipomersen, an ASO-targeting apoB, was shown to reduce apoB lipoproteins and Lp(a) in a dose-dependent manner by 38–48% and 26–27%, respectively (39, 40); whereas the results were slightly attenuated at two years in the ongoing open label extension program (41). A recent meta-analysis of four phase III trials found a mean reduction of 26.4% (42). In line with other ASO therapies, injection-site reactions and flu-like symptoms were the most common adverse effects, whereas liver fat accumulation was also observed. Awaiting CVD results from the FOCUS FH study evaluating mipomersen in a larger number of patients, the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use has issued a negative opinion for this drug; whereas, the U.S. Food and Drug Administration (FDA) has approved the use of this compound in patients with homozygous FH. Last August, Isis Pharmaceuticals announced that the FOCUS FH study had reached its primary efficacy endpoint, with similar LDL-C reduction to that observed in earlier phase 3 studies (43). Detailed study data and Lp(a) levels are expected to be presented in the near future.
apoB production and lipidation can also be attenuated via inhibition of microsomal TG transfer protein (MTP), which is an intracellular endoplasmic reticulum transfer protein responsible for the assembly of lipoproteins in the liver and the intestines. Treatment with the MTP inhibitor, lomitapide, resulted in a reduction in Lp(a) levels of 17% on top of a reduction in LDL-C of approximately 30% as monotherapy (44). However, adverse effects, including elevated liver enzymes and hepatic fat accumulation, as well as gastro-intestinal complaints, occur in the vast majority of patients, which excludes the widespread use of this drug for Lp(a) patients.
Currently, both apoB antisense and MTP inhibition therapy are only approved for lowering LDL-C levels in homozygous FH patients and, as noted, both of these agents have side effects. Therefore, these agents are unlikely to be approved for Lp(a) lowering in the near future.
PCSK9
Based on initial genetic studies, circulating proprotein convertase subtilisin kexin type 9 (PSCK9) was identified as a fundamental regulator of the LDLR and LDL-C levels, which resulted in the swift development of anti-PCSK9 therapies. Indeed, PCSK9-specific monoclonal antibodies have recently been shown to profoundly reduce LDL-C on top of statin background therapy and in exploratory post hoc analysis after 1 year of therapy, where shown to cause a reduction in cardiovascular event rates (45, 46). In addition to LDL-C lowering, PCSK9 inhibition was shown to reduce Lp(a) levels, which might also contribute to the anti-atherogenic potential of these drugs.
Currently, there are three monoclonal antibody-based compounds in development: evolocumab (Amgen), alirocumab (Sanofi and Regeneron Pharmaceuticals), and bococizumab (Pfizer). A meta-analysis of phase II trials investigating the effects of evolocumab showed that treatment resulted in an average 29.5% Lp(a) reduction compared with placebo (47). This is in line with the open label extension data from phase 2 and 3 studies with evolocumab, which showed a 25.5% Lp(a) lowering (46). In the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) study, Lp(a) levels were reduced by 25.6% compared with placebo (45). Finally, the third compound in clinical development, bococizumab, lowered Lp(a) by 27%, whereas Lp(a) was decreased by 12% in the placebo arm (48). Overall, PCSK9 inhibition results in a Lp(a) reduction of approximately 25%.
The obvious mechanistic link would be that increased hepatic LDLR expression, induced by PCSK9 inhibition, results in increased clearance of Lp(a) via this receptor, and this view is supported by in vitro data (49). However, the in vitro data contrasts with the clinical observation that statin treatment does not affect Lp(a) levels [and might even increase Lp(a)], whereas both treatment modalities exert their effect primarily by upregulation of the LDLR. In homozygous FH patients, characterized by a severe disruption of LDLR functionality, PCSK9 inhibition still reduced Lp(a) levels, albeit to a lesser extent (11–20%) (50, 51). Interestingly, these studies included three homozygous FH patients with complete absence of LDLR expression, in whom Lp(a) levels clearly went down in two patients, in the absence of a significant LDL-C lowering effect. In accordance, one of the rare Lp(a) kinetics studies performed in humans showed that the Lp(a) clearance rate was not affected in five homozygous FH patients when compared with wild-type family controls (21). Altogether, the clinical data support a minor role, at best, for Lp(a) clearance via the LDLR.
An alternative explanation is the direct impact of PCSK9 on apoB synthesis rate and MTP activity, as has been reported recently (52). This is in line with the aforementioned Lp(a)-lowering effect of apoB antisense therapy and MTP inhibition, both key processes in apoB synthesis.
The intriguing observation that PCSK9 and LDLR are regulated in a reciprocal fashion and that statin therapy results in PCSK9 upregulation, might explain part of the discrepancy between statin and PCSK9 inhibition on Lp(a) levels (53). If one is to assume that a (minor) part of Lp(a) is cleared via LDLR, any increased Lp(a) clearance might be counteracted by increased Lp(a) production via increased PCSK9 expression. Finally, it has been suggested that the relevance of LDLR-mediated Lp(a) clearance is increased in the situation of supraphysiological concentrations of LDLR, as is the case in the combination of statin treatment with PCSK9 inhibition (49). This would imply more effective Lp(a) lowering by PCSK9 inhibition in combination therapy with statins as compared with PCSK9 inhibitor monotherapy. However, this has not been substantiated. PCSK9 inhibition in statin-intolerant patients is equally effective in lowering Lp(a) (up to 27%) (54). In summary, clinical observations do not support a dominant role for the LDLR in Lp(a) clearance. To unravel the exact effects of anti-PCSK9 (and statin) therapy, the results from ongoing Lp(a) kinetic studies are needed.
Lipoprotein apheresis
Currently available interventions targeting the apoB component of Lp(a) only effectuate a modest (10–40%) reduction in Lp(a) levels, which is expected to be insufficient to attenuate the pro-atherogenic potential of strongly elevated Lp(a) levels. A more effective Lp(a)-lowering strategy is lipoprotein apheresis (LA). LA removes apoB-containing lipoproteins, as well as the levels of associated oxidized phospholipids (55). In a cohort study of 120 patients with elevated Lp(a) plasma levels (117.9 ± 42.0 mg/dl), but otherwise well-controlled risk factors, the mean annual major adverse coronary events rate per patient was reduced significantly from 1.06 to 0.14 after LA (56). In a recent prospective study performed in 170 patients (104.9 ± 45.7 mg/dl), the major adverse coronary events rate also declined from 0.41 for 2 years before LA to 0.09 for 2 years during LA (57). Whereas these small-scaled studies hint toward clinical benefit from lowering Lp(a) levels, the interpretation is hampered by the concomitant lowering of other atherogenic apoB-containing particles, including LDL-C, up to 75%. Obviously, the invasive nature and high costs also preclude wider use of this technique in large numbers of patients. Finally, one important caveat is the intermittent nature of the therapy. Lp(a) levels tend to rebound to 80% of baseline levels before the next apheresis session (57). The resulting saw-tooth pattern in lipid levels is likely to decrease any beneficial impact of Lp(a) reduction. Thus, in vitro studies suggest a rapid detrimental effect, within minutes, of elevated Lp(a) (58), indicating that the short rebound spikes in Lp(a) levels before apheresis might pose a repetitive pro-atherogenic challenge, supporting the need for therapies that result in stable and potent Lp(a) reduction.
Non-apoB-directed therapies
It is of interest that chronic inflammatory conditions, such as rheumatoid arthritis and Crohn’s disease, are associated with elevated Lp(a) levels, indicating a key regulatory role for the innate immune system (59, 60). Clinical studies in rheumatoid arthritis patients with tocilizumab, a specific monoclonal antibody against the interleukin 6 (IL6) receptor, resulted in a 30–37% reduction in Lp(a) levels, while total cholesterol and LDL-C levels were slightly increased (61). This is one of the few interventions that lower Lp(a) without concomitant LDL-C lowering. Recently, it was shown that this effect is likely mediated via an IL6 responsive element in the promoter region of the LPA gene (62). Other treatment modalities for rheumatoid arthritis, like TNF-a inhibitors, did not affect Lp(a) metabolism.
Given the emerging role of the innate immune system in relation to CVD risk, especially in the postmyocardial infarction window, it is interesting to note that IL6 is upregulated in acute myocardial infarction and levels remain elevated up to 12 weeks postmyocardial infarction (63). Additional studies are needed to support a role of IL6 inhibition in Lp(a) metabolism in patients without rheumatoid arthritis.
Other agents reported to decrease Lp(a) include: i) hormonal therapy with thyroxine replacement via eprotirome (20–40%), estrogen (24%) in combination with progestagens (up to 34%), the estrogen replacer tibolone (39%), and the anti-estrogen tamoxifen (23%); ii) supplements including L-carnitine (−8%) and ascorbic acid combined with L-lysine (−8 to 20%); and finally iii) drugs with anti-inflammatory effects, such as angiotensin-converting enzyme inhibitors (18%) and aspirin (20%). Others have reviewed these compounds previously (64–67).
FUTURE PERSPECTIVE: GOING FROM apoB TO apo(a)
Given the crucial role of apo(a) genotype in determining the plasma concentration of Lp(a), targeting apo(a) may provide a more selective and attractive therapeutic target. ASO therapies directed at apo(a) synthesis hold great promise as a future therapeutic strategy (68, 69). Recently, data from a phase 1 study was published indicating that Lp(a) lowering up to 78% could be reached (70). Such agents with specific Lp(a)-lowering efficacy will pave the way to establish the role of Lp(a) lowering in CVD prevention. These options will be discussed in another review article in this Thematic Review series.
CONCLUDING REMARKS
Whereas the Mendelian randomization approaches have firmly established the causality of Lp(a) in atherosclerosis and CVD risk, the next challenge will be to prove that Lp(a) lowering also leads to cardiovascular benefit in patients with elevated Lp(a) levels. Clearly, detailed studies of the metabolism of Lp(a) are required to aid in the design and development of selective and potent therapies to lower Lp(a). Given the critical role of apo(a) synthesis in determining the plasma concentration of Lp(a), targeting the synthesis of apo(a) would be most suitable. Such highly specific and potent Lp(a)-lowering strategies would provide us with the unique opportunity to resolve this missing criterion of Koch’s postulates.
Footnotes
Abbreviations:
- ASO
- antisense oligonucleotide
- CETP
- cholesteryl ester transfer protein
- FH
- familial hypercholesterolemia
- HDL-C
- HDL cholesterol
- IL6
- interleukin 6
- LA
- lipoprotein apheresis
- LDL-C
- LDL cholesterol
- LDLR
- LDL receptor
- Lp(a)
- lipoprotein (a)
- MTP
- microsomal TG transfer protein
- PSCK9
- proprotein convertase subtilisin kexin type 9
REFERENCES
- 1.Berg K. 1963. A new serum type system in man–the LP system. Acta Pathol. Microbiol. Scand. 59: 369–382. [DOI] [PubMed] [Google Scholar]
- 2.Seed M., Hoppichler F., Reaveley D., McCarthy S., Thompson G. R., Boerwinkle E., and Utermann G.. 1990. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N. Engl. J. Med. 322: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 3.Jansen A. C. M., van Aalst-Cohen E. S., Tanck M. W., Trip M. D., Lansberg P. J., Liem A. H., van Lennep H. W. O. R., Sijbrands E. J. G., and Kastelein J. J. P.. 2004. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2,400 patients. J. Intern. Med. 256: 482–490. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J., Collins R., and Peto R.. 2000. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 102: 1082–1085. [DOI] [PubMed] [Google Scholar]
- 5.Bennet A., Di Angelantonio E., Erqou S., Eiriksdottir G., Sigurdsson G., Woodward M., Rumley A., Lowe G. D. O., Danesh J., and Gudnason V.. 2008. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch. Intern. Med. 168: 598–608. [DOI] [PubMed] [Google Scholar]
- 6.Clayton D., and McKeigue P. M.. 2001. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet. 358: 1356–1360. [DOI] [PubMed] [Google Scholar]
- 7.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., and Nordestgaard B. G.. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. ; PROCARDIS Consortium. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 9.Grundy S. M.; National Cholesterol Education Program (NCEP)-The National Cholesterol Guidelines in 2001, Adult Treatment Panel (ATP) III. 2002. Approach to lipoprotein management in 2001 National Cholesterol Guidelines. Am. J. Cardiol. 90: 11i–21i. [DOI] [PubMed] [Google Scholar]
- 10.Davidson M. H., Ballantyne C. M., Jacobson T. A., Bittner V. A., Braun L. T., Brown A. S., Brown W. V., Cromwell W. C., Goldberg R. B., McKenney J. M., et al. 2011. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J. Clin. Lipidol. 5: 338–367. [DOI] [PubMed] [Google Scholar]
- 11.Nordestgaard B. G., Chapman M. J., Ray K., Borén J., Andreotti F., Watts G. F., Ginsberg H., Amarenco P., Catapano A., Descamps O. S., et al. ; European Atherosclerosis Society Consensus Panel. 2010. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 31: 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham I., Atar D., Borch-Johnsen K., Boysen G., Burell G., Cifkova R., Dallongeville J., De Backer G., Ebrahim S., et al. ; European Society of Cardiology (ESC) Committee for Practice Guidelines (CPG). 2007. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur. Heart J. 28: 2375–2414. [DOI] [PubMed] [Google Scholar]
- 13.Grundy S. M., Cleeman J. I., Merz C. N. B., Brewer H. B., Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., and Stone N. J.; Coordinating Committee of the National Cholesterol Education Program. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler. Thromb. Vasc. Biol. 24: e149–e161. [DOI] [PubMed] [Google Scholar]
- 14.Arsenault B. J., Barter P., DeMicco D. A., Bao W., Preston G. M., LaRosa J. C., Grundy S. M., Deedwania P., Greten H., Wenger N. K., et al. ; Treating to New Targets (TNT) Investigators. 2014. Prediction of cardiovascular events in statin-treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non-lipid biomarkers. PLoS One. 9: e114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera A. V., Everett B. M., Caulfield M. P., Hantash F. M., Wohlgemuth J., Ridker P. M., and Mora S.. 2014. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 129: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraley A. E., Schwartz G. G., Olsson A. G., Kinlay S., Szarek M., Rifai N., Libby P., Ganz P., Witztum J. L., and Tsimikas S.; MIRACL Study Investigators. 2009. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: Results from the MIRACL (Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering). J. Am. Coll. Cardiol. 53: 2186–2196. [DOI] [PubMed] [Google Scholar]
- 17.Choi S. H., Chae A., Miller E., Messig M., Ntanios F., DeMaria A. N., Nissen S. E., Witztum J. L., and Tsimikas S.. 2008. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J. Am. Coll. Cardiol. 52: 24–32. [DOI] [PubMed] [Google Scholar]
- 18.Capoulade R., Chan K. L., Yeang C., Mathieu P., Bossé Y., Dumesnil J. G., Tam J. W., Teo K. K., Mahmut A., Yang X., et al. 2015. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66: 1236–1246. [DOI] [PubMed] [Google Scholar]
- 19.Takagi H., and Umemoto T.. 2012. Atorvastatin decreases lipoprotein(a): a meta-analysis of randomized trials. Int. J. Cardiol. 154: 183–186. [DOI] [PubMed] [Google Scholar]
- 20.van Wissen S., Smilde T. J., Trip M. D., de Boo T., Kastelein J. J. P., and Stalenhoef A. F. H.. 2003. Long term statin treatment reduces lipoprotein(a) concentrations in heterozygous familial hypercholesterolaemia. Heart. 89: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rader D. J., Mann W. A., Cain W., Kraft H. G., Usher D., Zech L. A., Hoeg J. M., Davignon J., Lupien P., and Grossman M.. 1995. The low density lipoprotein receptor is not required for normal catabolism of Lp(a) in humans. J. Clin. Invest. 95: 1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chennamsetty I., Kostner K. M., Claudel T., Vinod M., Frank S., Weiss T. S., Trauner M., and Kostner G. M.. 2012. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J. Lipid Res. 53: 2405–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamanna V. S., and Kashyap M. L.. 2008. Mechanism of action of niacin. Am. J. Cardiol. 101: 20B–26B. [DOI] [PubMed] [Google Scholar]
- 24.Croyal M., Ouguerram K., Passard M., Ferchaud-Roucher V., Chétiveaux M., Billon-Crossouard S., de Gouville A-C., Lambert G., Krempf M., and Nobécourt E.. 2015. Effects of extended-release nicotinic acid on apolipoprotein (a) kinetics in hypertriglyceridemic patients. Arterioscler. Thromb. Vasc. Biol. 35: 2042–2047. [DOI] [PubMed] [Google Scholar]
- 25.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., and Weintraub W.. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 26.Landray M. J., Haynes R., Hopewell J. C., Parish S., Aung T., Tomson J., Wallendszus K., Craig M., and Jiang L.; HPS2-THRIVE Collaborative Group. 2014. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 27.Anderson T. J., Boden W. E., Desvigne-Nickens P., Fleg J. L., Kashyap M. L., McBride R., and Probstfield J. L.; AIM-HIGH Investigators. 2014. Safety profile of extended-release niacin in the AIM-HIGH trial. N. Engl. J. Med. 371: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuteja S., and Rader D. J.. 2014. Dyslipidaemia: cardiovascular prevention–end of the road for niacin? Nat. Rev. Endocrinol. 10: 646–647. [DOI] [PubMed] [Google Scholar]
- 29.Brown W. V., Ballantyne C. M., Jones P. H., and Marcovina S.. 2010. Management of Lp(a). J. Clin. Lipidol. 4: 240–247. [DOI] [PubMed] [Google Scholar]
- 30.Li M., Saeedi R., Rabkin S. W., and Frohlich J.. 2014. Dramatic lowering of very high Lp(a) in response to niacin. J. Clin. Lipidol. 8: 448–450. [DOI] [PubMed] [Google Scholar]
- 31.Cenarro A., Puzo J., Ferrando J., Mateo-Gallego R., Bea A. M., Calmarza P., Jarauta E., and Civeira F.. 2014. Effect of nicotinic acid/laropiprant in the lipoprotein(a) concentration with regard to baseline lipoprotein(a) concentration and LPA genotype. Metabolism. 63: 365–371. [DOI] [PubMed] [Google Scholar]
- 32.Albers J. J., Slee A., O’Brien K. D., Robinson J. G., Kashyap M. L., Kwiterovich P. O., Xu P., and Marcovina S. M.. 2013. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 62: 1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J-C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. ; dal-OUTCOMES Investigators. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 35.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. ; Determining the Efficacy and Tolerability Investigators. 2010. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 36.Hovingh G. K., Kastelein J. J. P., van Deventer S. J. H., Round P., Ford J., Saleheen D., Rader D. J., Brewer H. B., and Barter P. J.. 2015. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 386: 452–460. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls S. J., Brewer H. B., Kastelein J. J. P., Krueger K. A., Wang M-D., Shao M., Hu B., McErlean E., and Nissen S. E.. 2011. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 306: 2099–2109. [DOI] [PubMed] [Google Scholar]
- 38.Eli-Lilly. 2015. Lilly to discontinue development of evacetrapib for high risk atherosclerotic cardiovascular disease. Accessed December 16, 2015, at https://investor.lilly.com/releasedetail.cfm?ReleaseID=936130.
- 39.Visser M. E., Wagener G., Baker B. F., Geary R. S., Donovan J. M., Beuers U. H. W., Nederveen A. J., Verheij J., Trip M. D., Basart D. C. G., et al. 2012. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 33: 1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas G. S., Cromwell W. C., Ali S., Chin W., Flaim J. D., and Davidson M.. 2013. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 62: 2178–2184. [DOI] [PubMed] [Google Scholar]
- 41.Santos R. D., Duell P. B., East C., Guyton J. R., Moriarty P. M., Chin W., and Mittleman R. S.. 2015. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur. Heart J. 36: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos R. D., Raal F. J., Catapano A. L., Witztum J. L., Steinhagen-Thiessen E., and Tsimikas S.. 2015. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler. Thromb. Vasc. Biol. 35: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ISIS Pharmaceuticals. 2015. Isis Reports Positive Data from KYNAMRO® (mipomersen sodium) FOCUS FH Phase 3 Study in Patients with Severe Heterozygous Familial Hypercholesterolemia. Accessed December 16, 2015, at http://ir.isispharm.com/phoenix.zhtml?c=222170&p=irol-newsArticle&ID=2075147.
- 44.Samaha F. F., McKenney J., Bloedon L. T., Sasiela W. J., and Rader D. J.. 2008. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 5: 497–505. [DOI] [PubMed] [Google Scholar]
- 45.Robinson J. G., Farnier M., Krempf M., Bergeron J., Luc G., Averna M., Stroes E. S., Langslet G., Raal F. J., El Shahawy M., et al. ; ODYSSEY LONG TERM Investigators. 2015. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 46.Sabatine M. S., Giugliano R. P., Wiviott S. D., Raal F. J., Blom D. J., Robinson J., Ballantyne C. M., Somaratne R., Legg J., Wasserman S. M., et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. 2015. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 47.Raal F. J., Giugliano R. P., Sabatine M. S., Koren M. J., Langslet G., Bays H., Blom D., Eriksson M., Dent R., Wasserman S. M., et al. 2014. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 48.Ballantyne C. M., Neutel J., Cropp A., Duggan W., Wang E. Q., Plowchalk D., Sweeney K., Kaila N., Vincent J., and Bays H.. 2015. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am. J. Cardiol. 115: 1212–1221. [DOI] [PubMed] [Google Scholar]
- 49.Romagnuolo R., Scipione C. A., Boffa M. B., Marcovina S. M., Seidah N. G., and Koschinsky M. L.. 2015. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 290: 11649–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein E. A., Honarpour N., Wasserman S. M., Xu F., Scott R., and Raal F. J.. 2013. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 128: 2113–2120. [DOI] [PubMed] [Google Scholar]
- 51.Raal F. J., Honarpour N., Blom D. J., Hovingh G. K., Xu F., Scott R., Wasserman S. M., and Stein E. A.; TESLA Investigators. 2015. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 385: 341–350. [DOI] [PubMed] [Google Scholar]
- 52.Rashid S., Tavori H., Brown P. E., Linton M. F., He J., Giunzioni I., and Fazio S.. 2014. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation. 130: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavori H., Fan D., Blakemore J. L., Yancey P. G., Ding L., Linton M. F., and Fazio S.. 2013. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 127: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroes E., Colquhoun D., Sullivan D., Civeira F., Rosenson R. S., Watts G. F., Bruckert E., Cho L., Dent R., Knusel B., et al. ; GAUSS-2 Investigators. 2014. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 63: 2541–2548. [DOI] [PubMed] [Google Scholar]
- 55.Arai K., Orsoni A., Mallat Z., Tedgui A., Witztum J. L., Bruckert E., Tselepis A. D., Chapman M. J., and Tsimikas S.. 2012. Acute impact of apheresis on oxidized phospholipids in patients with familial hypercholesterolemia. J. Lipid Res. 53: 1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaeger B. R., Richter Y., Nagel D., Heigl F., Vogt A., Roeseler E., Parhofer K., Ramlow W., Koch M., Utermann G., et al. , Group of Clinical Investigators. 2009. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat. Clin. Pract. Cardiovasc. Med. 6: 229–239. [DOI] [PubMed] [Google Scholar]
- 57.Leebmann J., Roeseler E., Julius U., Heigl F., Spitthoever R., Heutling D., Breitenberger P., Maerz W., Lehmacher W., Heibges A., et al. ; Pro(a)LiFe Study Group. 2013. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 128: 2567–2576. [DOI] [PubMed] [Google Scholar]
- 58.Pellegrino M., Furmaniak-Kazmierczak E., LeBlanc J. C., Cho T., Cao K., Marcovina S. M., Boffa M. B., Côté G. P., and Koschinsky M. L.. 2004. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J. Biol. Chem. 279: 6526–6533. [DOI] [PubMed] [Google Scholar]
- 59.Koutroubakis I. E., Malliaraki N., Vardas E., Ganotakis E., Margioris A. N., Manousos O. N., and Kouroumalis E. A.. 2001. Increased levels of lipoprotein (a) in Crohn’s disease: a relation to thrombosis? Eur. J. Gastroenterol. Hepatol. 13: 1415–1419. [DOI] [PubMed] [Google Scholar]
- 60.Missala I., Kassner U., and Steinhagen-Thiessen E.. 2012. A systematic literature review of the association of lipoprotein(a) and autoimmune diseases and atherosclerosis. Int. J. Rheumatol. 2012: 480784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McInnes I. B., Thompson L., Giles J. T., Bathon J. M., Salmon J. E., Beaulieu A. D., Codding C. E., Carlson T. H., Delles C., Lee J. S., et al. 2015. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 74: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Müller N., Schulte D. M., Türk K., Freitag-Wolf S., Hampe J., Zeuner R., Schröder J. O., Gouni-Berthold I., Berthold H. K., Krone W., et al. 2015. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 56: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabriel A. S., Martinsson A., Wretlind B., and Ahnve S.. 2004. IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. Eur. J. Intern. Med. 15: 523–528. [DOI] [PubMed] [Google Scholar]
- 64.Norata G. D., Ballantyne C. M., and Catapano A. L.. 2013. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur. Heart J. 34: 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boffa M. B., and Koschinsky M. L.. 2013. Screening for and management of elevated Lp(a). Curr. Cardiol. Rep. 15: 417. [DOI] [PubMed] [Google Scholar]
- 66.Kolski B., and Tsimikas S.. 2012. Emerging therapeutic agents to lower lipoprotein (a) levels. Curr. Opin. Lipidol. 23: 560–568. [DOI] [PubMed] [Google Scholar]
- 67.Kronenberg F., and Utermann G.. 2013. Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273: 6–30. [DOI] [PubMed] [Google Scholar]
- 68.Merki E., Graham M., Taleb A., Leibundgut G., Yang X., Miller E. R., Fu W., Mullick A. E., Lee R., Willeit P., et al. 2011. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J. Am. Coll. Cardiol. 57: 1611–1621. [DOI] [PubMed] [Google Scholar]
- 69.Viney N. J., Graham M., Crooke R., Hughes S., and Singleton W.. 2013. Evaluation of ISIS apo(a) Rx, an antisense inhibitor to apolipoprotein (a), in healthy volunteers. Circulation. 128: A14196. [Google Scholar]
- 70.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]