Abstract
Based on research carried out over the last decade, it has become increasingly evident that bile acids act not only as detergents, but also as important signaling molecules that exert various biological effects via activation of specific nuclear receptors and cell signaling pathways. Bile acids also regulate the expression of numerous genes encoding enzymes and proteins involved in the synthesis and metabolism of bile acids, glucose, fatty acids, and lipoproteins, as well as energy metabolism. Receptors activated by bile acids include, farnesoid X receptor α, pregnane X receptor, vitamin D receptor, and G protein-coupled receptors, TGR5, muscarinic receptor 2, and sphingosine-1-phosphate receptor (S1PR)2. The ligand of S1PR2, sphingosine-1-phosphate (S1P), is a bioactive lipid mediator that regulates various physiological and pathophysiological cellular processes. We have recently reported that conjugated bile acids, via S1PR2, activate and upregulate nuclear sphingosine kinase 2, increase nuclear S1P, and induce genes encoding enzymes and transporters involved in lipid and sterol metabolism in the liver. Here, we discuss the role of bile acids and S1P signaling in the regulation of hepatic lipid metabolism and in hepatobiliary diseases.
Keywords: liver metabolism, fatty acid, lysosphingolipid, sphingosine kinase, bile duct cancer
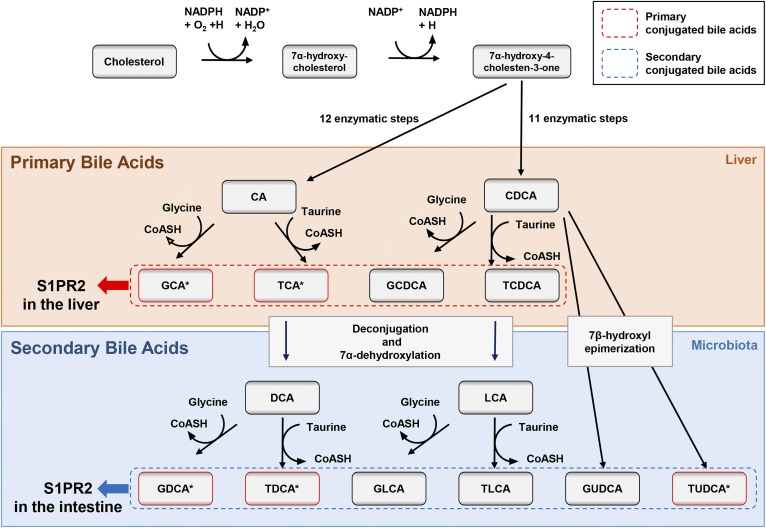
Bile acids are steroid acids that constitute one of the major components of bile. They are known to play multiple crucial roles in lipid and glucose homeostasis in the liver (1). Primary bile acids are synthesized from cholesterol in hepatocytes, and are actively secreted from the liver following conjugation to either glycine or taurine (Fig. 1). Following excretion of primary bile acids into the gastrointestinal tract, colonic bacteria form secondary bile acids by removal of the 7α-hydroxy group (Fig. 1). In the gallbladder, conjugated bile acids form mixed micelles of cholesterol and phospholipids. Gallbladder micelles solubilize cholesterol and inhibit cholesterol crystallization, preventing cholesterol gallstone formation. In the small intestine, micelles containing conjugated bile acids function to solubilize, digest, and promote the absorption of dietary lipids, cholesterol, and fat-soluble vitamins (A, D, E, and K) (2). In addition to fat and cholesterol solubilization, bile acids have bacteriostatic properties that inhibit bacterial growth in the biliary tree. Disruption of normal bile acid synthesis and metabolism is associated with cholestasis, cholesterol gallstone formation, lipid and fat malabsorption, fat-soluble vitamin deficiency, and intestinal bacterial dysbiosis (2).
Fig. 1.
Metabolism of cholesterol and bile acid synthesis. The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are formed from cholesterol in the liver and stored in the gallbladder. The secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA), are formed by microbiota. Primary conjugated bile acids stimulate S1PR2 in the liver. On the other hand, secondary conjugated bile acids stimulate S1PR2 in the intestine. *These bile acids were shown to stimulate S1PR2 previously (8). GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; GDCA, glycodeoxycholic acid; TDCA, taurodeoxycholic acid; GLCA, glycolithocholic acid; TLCA, taurolithocholic acid; GUDCA, glycoursodeoxycholic acid, TUDCA, tauroursodeoxycholic acid.
Bile acids regulate the expression of numerous genes encoding enzymes and proteins involved in the synthesis and metabolism of bile acids, glucose, fatty acids, and lipoproteins. In addition, bile acids regulate energy metabolism by activating specific nuclear receptors and G protein-coupled receptors (GPCRs) in cells of the liver and gastrointestinal tract. Those receptors include the farnesoid X receptor (FXR)α (3–5), as well as other nuclear receptors (pregnane X receptor, vitamin D receptor), and GPCRs, such as TGR5 (also known as GPBAR1), muscarinic receptors 2 and 3, and sphingosine-1-phosphate receptor (S1PR)2 (6–8). Bile acids also activate cellular signaling pathways, such as c-Jun N-terminal kinase 1/2 (JNK1/2) (9). Dent and colleagues have previously reported that conjugated bile acids activate protein kinase B (AKT) and extracellular regulated protein kinases 1 and 2 (ERK1/2) via Gαi protein-coupled receptors (10). Bile acids have also been implicated in the inflammatory response and various liver diseases, as well as the promotion of cancers of the colon, liver, and bile duct (9). Increasingly, bile acids have been proposed to also function as hormones and nutrient signaling molecules that contribute to glucose and lipid metabolism. In this regard, we have recently reported that conjugated bile acids activate S1PR2, upregulating the expression and activity of sphingosine kinase (SphK)2, thereby increasing nuclear sphingosine-1-phosphate (S1P), upregulating gene expression, and regulating lipid and sterol metabolism in the liver (11). These results indicate that the S1P signaling via S1PR2 and SphK2 play pivotal roles in lipid metabolism. Here, we will discuss the role of bile acid and S1P signaling in the regulation of hepatic lipid metabolism and in hepatobiliary diseases.
S1P, A LIPID MEDIATOR
The lysosphingolipid, S1P, is a bioactive lipid mediator that regulates various physiological and pathophysiological cellular processes that are important in cell proliferation, angiogenesis/lymphangiogenesis, immunity, immune cell trafficking, endothelial barrier integrity, inflammation, and malignant transformation (12–15). S1P can act intracellularly, or through the activation of five specific cell surface GPCRs (S1PR1–5), regulating different biological functions (16).
The S1P biosynthetic pathway is conserved across various cell types. S1P is produced from sphingosine by SphK1 and SphK2. Ceramide is produced from sphingomyelin by sphingomyelinases, and sphingosine is produced from ceramide by ceramidases. S1P can be converted to sphingosine by cytosolic S1P phosphatases or degraded by S1P lyase to ethanolamine phosphate and hexadecanal (palmitaldehyde) (17). SphK1 and SphK2 are located in different subcellular compartments. Various external stimuli activate SphK1, stimulating its translocation to the plasma membrane where it converts sphingosine to S1P. Plasma membrane transporters of S1P have been identified and they include ABC transporter family members (ABCC1, ABCG2) (18) and the major facilitator superfamily member, Spinster 2 (Spns2) (19–22). The “inside-out-signaling” process refers to the intracellular synthesis of S1P and transport out of the cell to activate S1PRs differentially expressed on mammalian cells activating autocrine and paracrine signaling (21).
S1P levels are relatively high (1–2 μM) in the blood and finely regulated. It was reported that the half-life of S1P in plasma is about 15 min in mice, suggesting rapid clearance by degenerative enzymes, such as S1P phosphatases and S1P lyase, and/or uptake of S1P into the cells. The rapid turnover of plasma S1P also implies the presence of a high-capacity cellular source involved in the maintenance of high plasma S1P levels (23). It has been hypothesized that various cells are responsible for synthesizing and secreting S1P into the blood, including red blood cells, endothelial cells, thrombocytes, macrophages, and mast cells (24). S1P is found at lower levels (<0.2 μM) in lymph and lymphoid tissues compared with blood. It has been reported that a S1P gradient may play a crucial role in controlling immune cell trafficking between the circulation and lymphoid tissues (25–27).
The SphK/S1P/S1PR axis is important in many physiological processes, and is an emerging therapeutic target for treating several pathobiologic and inflammatory diseases (12, 28, 29). Recently, it was reported that S1P can act through intracellular targets for cell signaling. In this regard, TNF-α and interleukin-1 activate SphK1, thus increasing intracellular S1P that binds directly to the TNF-α receptor-associated factor 2 (TRAF2). TRAF2 is an important component in nuclear factor-κB (NF-κB) signaling and cellular inhibition of apoptosis 2 (cIAP2). In addition, it enhances E3 ubiquitin ligase activities via lysine-63-linked poly-ubiquitylation (30).
Little is known about the biological function of SphK2 and its possible role in cancer and other diseases. In many cell types, SphK2 is localized in several organelles, including the nucleus, mitochondria, and intracellular membranes (31). It has been reported that pERK1/2 phosphorylates and activates SphK2, thereby increasing the synthesis of S1P (31). It has been shown that nuclear S1P produced by either SphK2 or through inhibition of S1P lyase, specifically binds and inhibits the histone deacetylases (HDACs), HDAC1 and HDAC2, linking sphingolipid metabolism to epigenetic gene expression that is relevant to cancer and inflammatory diseases (31–33). In this regard, SphK2 downregulation or inhibition decreases cancer cell growth as well as xenograft growth of tumor cells in mice (34, 35). Studies with FTY720, a S1P mimetic prodrug, have also served to demonstrate the role of S1P. FTY720 is phosphorylated in the nucleus by SphK2 and FTY720-phosphate, a potent class I HDAC inhibitor that facilitates fear extinction memory in mice (36). In addition, FTY720 also activates estrogen receptor (ER)-α expression to enhance hormonal therapy for breast cancer (37).
It has been demonstrated that mitochondrial S1P, produced by SphK2, interacts with prohibitin 2 (PHB2) that is important for mitochondrial assembly and function (38). Unlike SphK1, high expression of SphK2 was observed mainly in adult kidney, liver, and brain, compared with other tissues (39, 40). Recently, it was demonstrated that conjugated bile acids signal through the S1PR2 and activate SphK2 (11). S1PR2 is highly expressed in various tissues, including the liver (Table 1). In fact, S1PR2−/− and SphK2−/− mice (11) rapidly develop fatty livers on a high-fat diet, indicating the importance of the conjugated bile acids, S1PR2 and SphK2, in regulating hepatic lipid metabolism (Fig. 2).
TABLE 1.
List the various S1P receptors, tissue expression, and physiological functions
| Receptors | G protein Coupling | Tissue Expression | Signaling Pathways | Physiological Functions | Reference |
| S1PR1 | Gαi/o | Ubiquitously expressed, high in CD19+ B cells and cerebellum. | Protein kinase AKT and the small GTPase Rac, MAPK, activates NF-κB, STAT3. | Trafficking of lymphocytes and hematopoietic cells including T and B lymphocytes, NKT cells, dendritic cells, macrophages, neutrophils, hematopoietic progenitors, mast cells, and osteoclasts in both homeostatic and disease settings. Vascular development and integrity, promoting metastasis. | (26, 55–60) |
| S1PR2 | Gi, Gq, Gα12/13 | Ubiquitously expressed, immune cells (dendritic cells, macrophages, eosinophils, mast cells, NKT) Smooth muscle cells, cardiomyocytes, hepatocytes, cholangiocytes, gut epithelial cells. | GTPase Rho activation, Rho-dependent Rac inhibition and 3′-specific phosphoinositide phosphatase (PTEN) stimulation, NF-κB activation. | Inhibits cell motility through inhibition of Rac, involved in cell proliferation, motility and transcriptional activation, heart development, vascular development, role in atherosclerosis, bone maintenance, muscle regeneration, B cell function, promoting metastasis, hepatic lipid metabolism and gene expression. | (9, 11, 55, 56, 59, 61) |
| S1PR3 | Gαi/o, Gαq, and Gα12/13 | Highest expression in heart, lung, spleen, kidney, intestine, diaphragm; immune cells (dendritic cells, eosinophils, lymphocytes B) endothelial cells smooth muscle cells, cardiomyocytes neuronal cells (astrocytes, oligodendrocytes). | Leading to the generation of inositol trisphosphate and diacylglycerol with subsequent calcium mobilization and activation of PKC pathways respectively. | Cell motility, ER+ breast cancer progression, regulates endothelial barrier function, involved in sepsis, regulation of heart rate, regulation of vascular tone (relaxation) survival in ischemia-reperfusion cardiomyocytes. | (55, 56, 58, 59) |
| S1PR4 | GαI, Gα12/13 | Primarily expressed in lymphoid tissues and blood cells, especially CD19+ B cells, dendritic cells, T cells, NKT cells and lung | Preferentially activates phospholipase C/IP3/Ca2+, MAPK, and Rho. | Migration of dendritic and neutrophil cells, ER− breast cancer poor prognosis. | (55, 56, 59) |
| S1PR5 | Gi, Gα12/13 | Mostly expressed in brain, skin and natural killer cells. | Preferentially activates phospholipase C channels/IP3/Ca2+, PI3K/AKT Rho, and inhibition of adenylate cyclase. | Similar to the role S1PR1, involved in T and B cell trafficking, promotes the egress of NK cells from bone marrow and lymph nodes into blood and other tissues. | (55, 56, 59) |
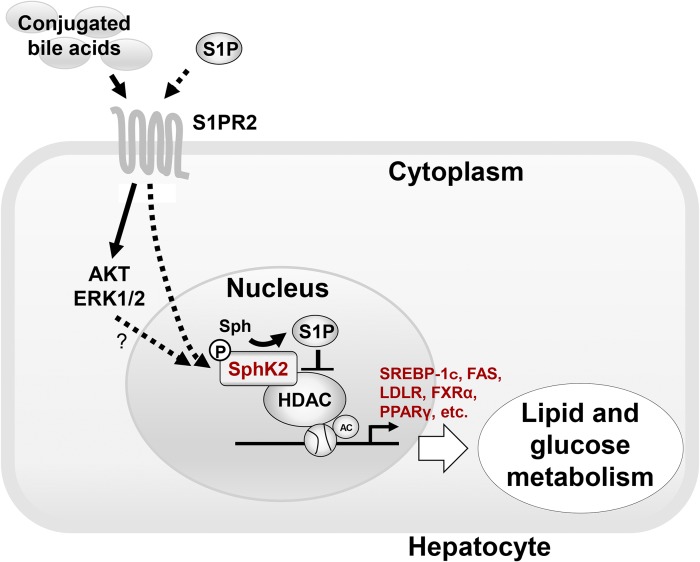
Fig. 2.
Model of regulation of hepatic genes encoding enzymes involved in nutrient metabolism by conjugated bile acids and S1P. Conjugated bile acids and S1P activate S1PR2 and then activate nuclear SphK2 via cell signaling pathways such as AKT or ERK1/2 (8, 11, 54), increasing the levels of S1P in the nucleus. Nuclear S1P inhibits specific HDACs, causing an increase in acetylation of histones and upregulation of genes encoding nuclear receptors and enzymes involved in lipid and glucose metabolism. CBA, conjugated bile acid; Sph, sphingosine.
CONJUGATED BILE ACIDS ACTIVATE S1PR2
Conjugated bile acids have been demonstrated to activate ERK1/2 and AKT in a manner sensitive to pertussis toxin and dominant-negative Gαi, thereby implicating GPCRs in this signaling pathway (8, 41). Activation of the AKT pathway by conjugated bile acids was shown to activate glycogen synthase activity in vitro and in vivo in a Gαi-dependent manner (10). Further, conjugated bile acids were shown to repress the gluconeogenic genes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), both in vitro and in vivo (42). Importantly, repression of PEPCK and G6Pase mRNA by conjugated bile acids was shown to be pertussis toxin sensitive in primary rat hepatocytes. Finally, it was reported that activation of the AKT pathway was required for optimal induction of small heterodimer partner (SHP) mRNA, an FXR target gene, by conjugated bile acids in vivo (42). It has also been reported that activation of the ERK1/2 pathway plays an important role in regulating the rate of turnover of SHP protein (43). Taken together, these data suggest that conjugated bile acids may be important regulators of hepatic glucose and lipid metabolism through activation of a specific Gi protein-coupled receptor and FXR in a coordinated manner, although the specific GPCR activated by S1P remains unknown.
By screening various GPCRs in the lipid-activated phylogenetic family, our group discovered that S1PR2 is activated by taurocholate (TCA) and other conjugated bile acids, but not unconjugated bile acids (8) (Fig. 1). S1PR2 is highly expressed in liver hepatocytes (9). The S1PR2 antagonist, JTE-013, has been shown to inhibit activation of ERK1/2 and AKT by S1P, TCA, taurodeoxycholic acid, tauroursodeoxycholic acid, glycocholic acid, and glycodeoxycholic acid (8) (Fig. 1). Further, shRNA knockdown of S1PR2 (S1PR2−/−) in mice markedly inhibited the activation of ERK1/2 and AKT by TCA and S1P (8).
Finally, structural modeling of the S1PRs demonstrated that only S1PR2, and not other S1P receptors, can accommodate TCA binding (8). In that study, we reported modeling of SIPR2, which predicted that S1P, a high-affinity ligand, generates hydrogen bonds to three amino acid residues (Ser6, Leu173, and Glu177) on S1PR2. In contrast, TCA, a low-affinity agonist, is predicted to generate hydrogen bonds only to Leu173. Both S1P and TCA activate the S1PR2 in rodent hepatocytes, leading to activation of both the ERK1/2 and AKT pathways in primary hepatocytes. TCA also activated the same signaling pathways in the chronic bile fistula rat model. Furthermore, its activity was inhibited by a specific S1PR2 antagonist, JTE-013, demonstrating the association between TCA and S1PR2. Activation of the AKT pathway appears to be essential for optimal activation of the nuclear receptor, FXR, by conjugated bile acids. Taken together the current data suggest that TCA specifically activates S1PR2 in hepatocytes.
CONJUGATED BILE ACIDS, S1PR2 AND SPHK2, REGULATE HEPATIC LIPID METABOLISM
S1PR2 is involved in the regulation of hepatic lipid metabolism as evidenced by studies in S1PR2−/− mice, where S1PR2−/− mice rapidly develop overt fatty livers when placed on high-fat diet as compared with wild-type mice (11). Furthermore, infusion of TCA into the chronic bile fistula rat model, or overexpression of S1PR2, resulted in significant upregulation of hepatic SphK2, but not SphK1 (11). These data suggest that a bile acid induced an increase in SphK2 through S1PR2 activation. In fact, mice deficient in SphK2 also rapidly developed fatty livers on a high-fat diet, suggesting the importance of S1PR2 and SphK2 in regulating liver lipid metabolism (9, 11). In mice fed a high-fat diet, overexpression of SphK2 led to elevated S1P and reduced ceramide, sphingomyelin, and glucosylceramide in plasma and in the liver (44). In response to accumulation of lipids in the liver, SphK2 facilitates upregulation of genes encoding enzymes in fatty acid transport and oxidation (44).
The liver is also intricately involved in nutrient metabolism. Notably, in mouse livers deficient in S1PR2 and SphK2, key genes encoding nuclear receptors and enzymes involved in nutrient metabolism, such as sterol regulatory element-binding protein (SREBP)-1c, FAS, LDLR, FXRα, and PPARγ, were significantly downregulated (11) (Fig. 2). This illustrates the importance of S1PR2 and SphK2 in regulating genes encoding enzymes and transporters involved in nutrient metabolism
BILE ACIDS AND S1PR2 SIGNALING IN REGULATING HEPATIC GLUCOSE METABOLISM
Bile acid-mediated activation of the ERK1/2 and AKT signaling pathways through S1PR2 was shown to play an important role in hepatic lipid metabolism and glucose regulation (9, 11). In fact, in primary rat hepatocytes, bile acids activated glycogen synthesis to a similar level as insulin due to the effect of AKT and ERK1/2 signaling (10, 11). In addition, TCA induced a rapid downregulation of the gluconeogenesis genes, PEPCK and G6Pase, and a marked upregulation of SHP mRNA in the livers (42). This illustrates that bile acid activation of S1PR2 has insulin-like activity in hepatic glucose regulation (9). Further, it has been reported that hepatic overexpression of SphK2 in mice led to elevated S1P and reduced ceramide, sphingomyelin, and glucosylceramide in plasma and liver, and ameliorated glucose intolerance and insulin resistance by improving hepatic insulin signaling (44). Considering that SphK2 can be activated by conjugated bile acids via S1PR2, which results in elevation of S1P and reduction of ceramide, sphingomyelin, and glucosylceramide, both S1PR2 and SphK2 appear to play important roles in hepatic glucose metabolism (Fig. 2).
S1PR2 AND BILE DUCT CANCER
It has been suggested that bile acids promote bile duct cancer, also known as cholangiocarcinoma, although the underlying mechanisms have not been fully elucidated. The earliest findings regarding bile acids and bile duct cancer were observed two decades ago, where it was demonstrated that bile acids stimulate proliferation of biliary cells (45). Later, it was reported that bile acids activate the epidermal growth factor receptor (EGFR) via a transforming growth factor-a (TGF-a)-dependent mechanism in human cholangiocarcinoma cells (46). The activation of EGFR by bile acids resulted in increased expression of cyclooxygenase-2 (COX-2). Moreover, conjugated bile acids have been shown to decrease FXR expression in vitro and to promote cholangiocellular carcinoma growth in vivo (47). However, the potential interaction between bile acids and sphingolipids has been overlooked until recently.
For the last few years, bile acids and S1PR2 have been identified as contributors to bile duct cancer (48). Unlike unconjugated bile acids, conjugated bile acids increase the activity of NF-κB, leading to higher levels of interleukin-6 and COX-2 in mouse cholangiocarcinoma models (48). COX-2-derived prostaglandin E2 is among the most abundant prostaglandins found in cancer. High COX-2 levels are associated with a variety of cancers due to their activation of EGFR (49). In cholangiocarcinoma, activation of EGFR has been implicated in enhanced growth and apoptosis resistance in cholangiocarcinoma cells (49). COX-2 expression has been negatively associated with survival in cholangiocarcinoma (48).
In addition to COX-2-based mechanisms, interaction of conjugated bile acids with S1PR2 has been found to promote invasive growth of cholangiocarcinoma in a human HuCCT1 cholangiocarcinoma cell line (48). In that study, invasive growth of cholangiocarcinoma correlated with S1PR2-mediated upregulation of COX-2 expression and PGE2 synthesis. Additionally, inhibition of S1PR2 with JTE-013 resulted in decreased COX-2 expression and also in decreased TCA-induced activation of EGFR. Similar results were seen when S1PR2 was silenced with shRNA (48). Taken together, these data suggest that S1PR2 plays a critical role in TCA-induced COX-2 expression and progression of cholangiocarcinoma, and can be a promising novel therapeutic target for cholangiocarcinoma.
S1P IN BILE
Because S1P signaling through S1PR2 and SphK2 is important in bile acid signaling in the liver, we cannot help but speculate that S1P itself plays important roles in the liver and intestines (Fig. 2). However, the role of S1P in bile acid signaling has yet to be rigorously investigated. In fact, we still do not know the normal range of bile S1P concentration in healthy or pathological conditions. Because liver and cholangiocytes highly express S1PR2, S1P should exist with certain levels in bile. Determining the levels of S1P in bile and its targeting organs, such as the liver, bile duct, and intestines, will be crucial to unveil the pathophysiology of S1P in hepatobiliary diseases.
It has been reported that S1P affects the mucosal integrity of the intestine in an animal model (50). We have previously shown that expression of S1P phosphatase (Sgpp1 and Sgpp2) was readily detectable in intestinal epithelial cells isolated from wild-type mice. Degradation of S1P to sphingosine was greatly reduced in intestinal extracts from Sgpp1 and Sgpp2 knockouts compared with wild-type mice. Thus, it appears that some of the S1P delivered with bile to the intestinal lumen can be taken into the intestinal epithelial cells and degraded by the S1P phosphatases. Because bile acids are important for intestinal homeostasis, bile acids and S1P may cooperate to maintain the epithelium of the intestine.
It has been reported that intravenously administered S1P is actively accumulated in the liver (23). As a consequence, the clearance of S1P from the portal vein in the liver occurs rapidly. Interestingly, this rapid clearance of S1P appears to be similar to the clearance of bile acids from the portal vein in the liver. Moreover, it has been demonstrated that hepatocyte-specific apoM overexpression facilitates formation of large apoM/S1P-enriched HDL by promoting formation of large nascent HDL and stimulating sphingolipid synthesis and S1P secretion. These results suggest that there is coordination between sphingolipid and cholesterol metabolism (51, 52). Taken together, it is possible that the liver regulates not only plasma bile acid levels, but also plasma S1P levels by regulating its uptake and secretion. Further, it was reported that induction of cellular sphingolipid storage stimulated cholesterol synthesis by activating SREBP1 (53). Considering that synthesis of bile acids is the major route of cholesterol secretion, metabolism of sphingolipids and bile acids should be tightly coordinated. Indeed, we found that S1P levels in bile are altered in the animals with high-fat diet (unpublished observations). It suggests that disorders of lipid metabolism in the liver affect S1P metabolism and its levels in bile. Further studies will be needed to investigate the role of S1P in bile and organs under pathological conditions.
CONCLUSION
There is growing evidence that bile acids play a much larger role than merely cholesterol and lipid homeostasis. Emerging studies point to bile acid function spanning glucose regulation, nutrient metabolism, and malignant transformation of cholangiocytes. These effects seem to be mediated through the S1P axis with close involvement of bile acids with S1PR2. It is more than likely that bile acids are also involved in regulating inflammation. These all point to future therapeutic avenues for targeting bile acids and/or the S1P axis for the treatment of a range of hepatobiliary conditions, including cholangiocarcinoma, glucose, and lipid management. Linking bile acids to the regulation of S1PR2 and SphK2 shows the interaction between these two important signaling molecules in the gastrointestinal tract.
Footnotes
Abbreviations:
- AKT
- protein kinase B
- COX-2
- cyclooxygenase-2
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- ERK1/2
- extracellular regulated protein kinases 1 and 2
- FXR
- farnesoid X receptor
- G6Pase
- glucose-6-phosphatase
- GPCR
- G protein-coupled receptor
- HDAC
- histone deacetylase
- NF-κB
- nuclear factor-κB
- PEPCK
- phosphoenolpyruvate carboxykinase
- S1P
- sphingosine-1-phosphate
- S1PR
- sphingosine-1-phosphate receptor
- SHP
- small heterodimer partner
- SphK
- sphingosine kinase
- SREBP
- sterol regulatory element-binding protein
- TCA
- taurocholate
This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 15H05676 and 15K15471 (M.N.), and 15H04927 and 16K15610 (T.W.). Support was also received from the Uehara Memorial Foundation (M.N.), the Nakayama Cancer Research Institute (M.N.), the Takeda Science Foundation (M.N.), the Tsukada Medical Foundation (M.N.), the Intramural Research Program of the Roswell Park Cancer Institute (N.C.H.), National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK057543 and R01DK104893 (P.B.H., H.Z., K.T.), National Cancer Institute Grant R01CA160688 (K.T.), and the Susan G. Komen Investigator Initiated Research Grant IIR12222224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Chiang J. Y. 2009. Bile acids: regulation of synthesis. J. Lipid Res. 50: 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Aguiar Vallim T. Q., Tarling E. J., and Edwards P. A.. 2013. Pleiotropic roles of bile acids in metabolism. Cell Metab. 17: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., and Shan B.. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Chen J., Hollister K., Sowers L. C., and Forman B. M.. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553. [DOI] [PubMed] [Google Scholar]
- 6.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khurana S., Yamada M., Wess J., Kennedy R. H., and Raufman J. P.. 2005. Deoxycholyltaurine-induced vasodilation of rodent aorta is nitric oxide- and muscarinic M(3) receptor-dependent. Eur. J. Pharmacol. 517: 103–110. [DOI] [PubMed] [Google Scholar]
- 8.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M., Pandak W. M., Dent P., Spiegel S., Shi R., et al. 2012. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 55: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong E., Li Y., Hylemon P. B., and Zhou H.. 2015. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharm. Sin. B. 5: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y., Studer E., Mitchell C., Grant S., Pandak W. M., Hylemon P. B., and Dent P.. 2007. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol. Pharmacol. 71: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 11.Nagahashi M., Takabe K., Liu R., Peng K., Wang X., Wang Y., Hait N. C., Wang X., Allegood J. C., Yamada A., et al. 2015. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 61: 1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maceyka M., Harikumar K. B., Milstien S., and Spiegel S.. 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagahashi M., Ramachandran S., Kim E. Y., Allegood J. C., Rashid O. M., Yamada A., Zhao R., Milstien S., Zhou H., Spiegel S., et al. 2012. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 72: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyagi T., Nagahashi M., Yamada A., and Takabe K.. 2012. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat. Res. Biol. 10: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W. C., Nagahashi M., Terracina K. P., and Takabe K.. 2013. Emerging role of sphingosine-1-phosphate in inflammation, cancer, and lymphangiogenesis. Biomolecules. 3: 408–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takabe K., Paugh S. W., Milstien S., and Spiegel S.. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maceyka M., and Spiegel S.. 2014. Sphingolipid metabolites in inflammatory disease. Nature. 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takabe K., Kim R. H., Allegood J. C., Mitra P., Ramachandran S., Nagahashi M., Harikumar K. B., Hait N. C., Milstien S., and Spiegel S.. 2010. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 285: 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishi T., Kobayashi N., Hisano Y., Kawahara A., and Yamaguchi A.. 2014. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta. 1841: 759–765. [DOI] [PubMed] [Google Scholar]
- 20.Nagahashi M., Kim E. Y., Yamada A., Ramachandran S., Allegood J. C., Hait N. C., Maceyka M., Milstien S., Takabe K., and Spiegel S.. 2013. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takabe K., and Spiegel S.. 2014. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 55: 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahashi M., Takabe K., Terracina K. P., Soma D., Hirose Y., Kobayashi T., Matsuda Y., and Wakai T.. 2014. Sphingosine-1-phosphate transporters as targets for cancer therapy. BioMed Res. Int. 2014: 651727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salous A. K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G. A., Smyth S. S., and Morris A. J.. 2013. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 54: 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thuy A. V., Reimann C. M., Hemdan N. Y., and Graler M. H.. 2014. Sphingosine 1-phosphate in blood: function, metabolism, and fate. Cell. Physiol. Biochem. 34: 158–171. [DOI] [PubMed] [Google Scholar]
- 25.Pham T. H., Baluk P., Xu Y., Grigorova I., Bankovich A. J., Pappu R., Coughlin S. R., McDonald D. M., Schwab S. R., and Cyster J. G.. 2010. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyster J. G., and Schwab S. R.. 2012. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30: 69–94. [DOI] [PubMed] [Google Scholar]
- 27.Donoviel M. S., Hait N. C., Ramachandran S., Maceyka M., Takabe K., Milstien S., Oravecz T., and Spiegel S.. 2015. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 29: 5018–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W. C., Hait N. C., Allegood J. C., Price M. M., Avni D., et al. 2013. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 23: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagahashi M., Matsuda Y., Moro K., Tsuchida J., Soma D., Hirose Y., Kobayashi T., Kosugi S. I., Takabe K., Komatsu M., et al. 2016. DNA damage response and sphingolipid signaling in liver diseases. Surg. Today. 46: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., et al. 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 465: 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihlefeld K., Claas R. F., Koch A., Pfeilschifter J. M., and Meyer Zu Heringdorf D.. 2012. Evidence for a link between histone deacetylation and Ca(2)+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem. J. 447: 457–464. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen-Tran D. H., Hait N. C., Sperber H., Qi J., Fischer K., Ieronimakis N., Pantoja M., Hays A., Allegood J., Reyes M., et al. 2014. Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy. Dis. Model. Mech. 7: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu K., Guo T. L., Hait N. C., Allegood J., Parikh H. I., Xu W., Kellogg G. E., Grant S., Spiegel S., and Zhang S.. 2013. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS One. 8: e56471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beljanski V., Lewis C. S., and Smith C. D.. 2011. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol. Ther. 11: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hait N. C., Wise L. E., Allegood J. C., O’Brien M., Avni D., Reeves T. M., Knapp P. E., Lu J., Luo C., Miles M. F., et al. 2014. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat. Neurosci. 17: 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hait N. C., Avni D., Yamada A., Nagahashi M., Aoyagi T., Aoki H., Dumur C. I., Zelenko Z., Gallagher E. J., Leroith D., et al. 2015. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis. 4: e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strub G. M., Paillard M., Liang J., Gomez L., Allegood J. C., Hait N. C., Maceyka M., Price M. M., Chen Q., Simpson D. C., et al. 2011. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 25: 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., and Spiegel S.. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275: 19513–19520. [DOI] [PubMed] [Google Scholar]
- 40.Blondeau N., Lai Y., Tyndall S., Popolo M., Topalkara K., Pru J. K., Zhang L., Kim H., Liao J. K., Ding K., et al. 2007. Distribution of sphingosine kinase activity and mRNA in rodent brain. J. Neurochem. 103: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dent P., Fang Y., Gupta S., Studer E., Mitchell C., Spiegel S., and Hylemon P. B.. 2005. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 42: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 42.Cao R., Cronk Z. X., Zha W., Sun L., Wang X., Fang Y., Studer E., Zhou H., Pandak W. M., Dent P., et al. 2010. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J. Lipid Res. 51: 2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao J., Xiao Z., Kanamaluru D., Min G., Yau P. M., Veenstra T. D., Ellis E., Strom S., Suino-Powell K., Xu H. E., et al. 2009. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S. Y., Hong I. K., Kim B. R., Shim S. M., Sung Lee J., Lee H. Y., Soo Choi C., Kim B. K., and Park T. S.. 2015. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 62: 135–146. [DOI] [PubMed] [Google Scholar]
- 45.Alpini G., Glaser S., Robertson W., Phinizy J. L., Rodgers R. E., Caligiuri A., and LeSage G.. 1997. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am. J. Physiol. 273: G518–G529. [DOI] [PubMed] [Google Scholar]
- 46.Werneburg N. W., Yoon J. H., Higuchi H., and Gores G. J.. 2003. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G31–G36. [DOI] [PubMed] [Google Scholar]
- 47.Dai J., Wang H., Shi Y., Dong Y., Zhang Y., and Wang J.. 2011. Impact of bile acids on the growth of human cholangiocarcinoma via FXR. J. Hematol. Oncol. 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu R., Li X., Luo L., Qiang X., Hylemon P. B., Jiang Z., Zhang L., and Zhou H.. 2015. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J. Biol. Chem. 290: 30988–31002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones H., Alpini G., and Francis H.. 2015. Bile acid signaling and biliary functions. Acta Pharm. Sin. B. 5: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W. C., Liang J., Nagahashi M., Avni D., Yamada A., Maceyka M., Wolen A. R., Kordula T., Milstien S., Takabe K., et al. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB J. Epub ahead of print. April 29, 2016; doi:10.1096/fj.201600394R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M., Seo J., Allegood J., Bi X., Zhu X., Boudyguina E., Gebre A. K., Avni D., Shah D., Sorci-Thomas M. G., et al. 2014. Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J. Biol. Chem. 289: 2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu M., Allegood J., Zhu X., Seo J., Gebre A. K., Boudyguina E., Cheng D., Chuang C. C., Shelness G. S., Spiegel S., et al. 2015. Uncleaved ApoM signal peptide is required for formation of large ApoM/sphingosine 1-phosphate (S1P)-enriched HDL particles. J. Biol. Chem. 290: 7861–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puri V., Jefferson J. R., Singh R. D., Wheatley C. L., Marks D. L., and Pagano R. E.. 2003. Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J. Biol. Chem. 278: 20961–20970. [DOI] [PubMed] [Google Scholar]
- 54.Hait N. C., Bellamy A., Milstien S., Kordula T., and Spiegel S.. 2007. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 282: 12058–12065. [DOI] [PubMed] [Google Scholar]
- 55.Kihara Y., Maceyka M., Spiegel S., and Chun J.. 2014. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 171: 3575–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adada M., Canals D., Hannun Y. A., and Obeid L. M.. 2013. Sphingosine-1-phosphate receptor 2. FEBS J. 280: 6354–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendelson K., Evans T., and Hla T.. 2014. Sphingosine 1-phosphate signalling. Development. 141: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spiegel S., and Milstien S.. 2011. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blaho V. A., and Hla T.. 2014. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 55: 1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagahashi M., Hait N. C., Maceyka M., Avni D., Takabe K., Milstien S., and Spiegel S.. 2014. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv. Biol. Regul. 54: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez T., Skoura A., Wu M. T., Casserly B., Harrington E. O., and Hla T.. 2007. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 27: 1312–1318. [DOI] [PubMed] [Google Scholar]