Abstract
Asthma is characterized by airway inflammation, airway obstruction, and airway hyperresponsiveness (AHR), and it affects 300 million people worldwide. However, our current understanding of the molecular mechanisms that underlie asthma remains limited. Recent studies have suggested that transient receptor potential ankyrin 1 (TRPA1), one of the transient receptor potential cation channels, may be involved in airway inflammation in asthma. The present review discusses the relationship between TRPA1 and neurogenic inflammation in asthma, hoping to enhance our understanding of the mechanisms of airway inflammation in asthma.
MeSH Keywords: Asthma; Nerve Fibers, Unmyelinated; Neurogenic Inflammation; Transient Receptor Potential Channels
Background
According to the World Health Organization (WHO), asthma is the most common chronic disease among children, and the global prevalence is increasing, particularly in developing countries. Although current therapies, such as β-agonists and glucocorticoids, may be effective at attenuating symptoms, they do not limit disease progression [1]. Current evidence suggests that many of the TRP channels are associated with sensory perception and the pathogenesis of a range of diseases, including obstructive airway diseases such as asthma.
TRP channels were first observed in the photoreceptor cells of the fly eye in response to light, produced by a mechanism that consists of an initial activation of a transient inward current associated with receptor stimulation [2]. These channels are highly conserved among a wide variety of species, including fungi and animals from fruit flies to humans, but not in plants, bacteria, and protozoa [3–6]. They are cation-selective transmembrane proteins showing a preference for Ca2+ ions, which play a critical role in several cellular processes, including muscle contraction, cellular proliferation, cell death, gene transcription, and the release of transmitters, through changes in intracellular calcium concentration [7,8]. The family consists of 28 members in 6 subfamilies – ankyrin (A, 1 member), canonical (C, 7 members), Melastatin (M, 8 members), mucolipin (ML, 3 members), polycystic (P, 3 members), and vanilloid (V, 6 members) [8] – based on similarities in amino acid sequences expressed in all tissues. They play diverse signaling roles in mammalian physiology and are gated by a wide variety of endogenous ligands, physical stimuli, and environmental irritants, such as lipoxygenase products, low pH, heat, proinflammatory peptides, and/or reactive oxygen species (ROS) [9]. This helps respond to changes in the local environment, sharing a general role in sensory transduction because they contribute to vision, taste, olfaction, hearing, touch, and thermo- and osmo-sensation. TRP channels are formed by 4 subunits which assemble as homo- or hetero-tetramers and exhibit 6 transmembrane-spanning domains (S1–S6) with both the amino (NH2) and carboxylic acid (COOH) termini localized to the cytosol and the channel pore located between transmembrane domains 5 and 6, which permit a non-selective influx of cations. The C termini is highly conserved among TRP channels, whereas the N termini usually has varying degrees of ankyrin repeats [10].
Several TRP channels, like TRPA1, are expressed in the airway, and are increasingly being linked to key features in the development of asthma. Here, we summarize the emerging role of a member of the TRP family, TRPA1, in the mechanism of asthma and we hope to contribute to the development of drugs targeting TRPA1 to treat the asthmatic response.
TRPA1
Expression of TRPA1
As a member of the superfamily of TRP channels and the sole member of the TRP ankyrin subfamily, TRPA1 was first isolated from human fetal lung fibroblasts in 1999 [11], then in primary sensory neurons. Its gene is composed of 27 exons, spanning 55 701 base pairs of the human chromosome 8q13 [12], expressing a protein of about 1100 amino acids (120–130 kDa). This was originally named ANKTM1 due to the 13–18 ankyrin repeats likely to communicate with the pore [11,13–16] in its termini. This was thought to provide TRPA1 with mechanosensitivity and ability to interact with intracellular proteins and small molecules, compared with the only 0–8 ankyrin repeats in other TRPs [17]. In mammals, TRPA1 is co-expressed withTRPV1 in a subset of C-fibers which have cell bodies in nodose, dorsal root, and trigeminal ganglia (NG, DRG, and TG) [13,18,19] where it contributes to the perception of chemical pain and possibly to cold and mechanical stimuli [18,20,21], and innervate a variety of peripheral targets, including airway, skin, and gastrointestinal tract (GI). These channels are also found in many non-neuronal cell types and tissues, including hair cells, pancreas, heart, brain, keratinocytes, urinary bladder, prostate gland, endothelium, other vascular and perivascular cells, enterochromaffin cells, gastrointestinal tract, odontoblasts, dental pulp, synovial fibroblasts, and epithelial and smooth muscle cells of the airways and lung [11].
Stimuli of TRPA1 and covalent modification
Mammalian TRPA1 plays a key role in detecting, and is robustly activated by, a wide variety of harmful environmental and internal stimuli that cause pain and inflammation [13]. It is emerging as an essential sensor of noxious stimuli and tissue damage, and is also suggested to mediate the inflammatory actions of the irritants and pro-analgesic agents. Some pungent compounds that target TRPA1 are contained in various spicy foods, such as allyl isothiocyanate (mustard oil AITC) contained in horseradish, allicin, and diallyl disulfide contained in garlic, cinnamaldehyde contained in cinnamon, and capsiate. A large number of airborne irritants are present in toxic and volatile air pollution and cigarette smoke also activates TRPA1. These irritants include acetaldehyde, formaldehyde, hydrogen peroxide, hypochlorite, isocyanates, ozone, carbon dioxide, ultraviolet light, and acrolein, a highly reactive α,β-unsaturated aldehyde (present in tear gas), smoke from burning vegetation and vehicle exhaust, hydrogen sulfide (H2S), isocyanates, and heavy metals produced during the manufacturing of polymers, fertilizers, pesticides. Similarly, some drugs activate TRPA1, including nonsteroidal anti-inflammatory drugs (e.g., acetaminophen) metabolites, diclofenac, indomethacin, and mefenamic acid, as well as general anesthetics such as isoflurane or lidocaine. TRPA1 is also a target of endogenous inflammatory agents involved in inflammation and tissue injury. ROS from oxygen stress can eprosidate membrane lipids, resulting in the formation of reactive carbonyl species, such as 4-hydroxynonenal (4-HNE) and 4-oxononenal (4-ONE), which act directly on TRPA1. Nitrative stress is caused by reactive nitrogen species (RNS) that can produce nitrated fatty acids like nitro-oleic acid, which directly activates TRPA1 [13,21–23]. There is evidence that TRPA1 channels are activated by noxious cold (<17), although this proposal remains controversial [24–26]. There is also evidence for both sensitization and activation of TRPA1 channel by inflammatory mediators, such as bradykinin and prostaglandin E2 (PGE2) via G protein-coupled receptor (GPCR) activation [21]. With worsening air pollution, thousands of people experience accidental high-level irritant exposures each year, the consequence of which is persistent airway hyperresponsiveness and the clinical picture of asthma, referred to as reactive airways dysfunction syndrome (RADS). Repeated irritant exposures may lead to continual airway hyperresponsiveness and chronic inflammation.
TRPA1 is somewhat different in activation modality. Diverse stimuli activate TRPA1 to form covalent modification of just a few reactive cysteine residues located in the cytoplasmic N-terminal domain of the channel. Most TRPA1 agonists are strong electrophiles able to react with cysteines susceptible to electrophilic modification. The pre-S1 region is composed of pre-S1 helix and a preceding linker region. It connects the ankyrin repeat domain (ARD) to S1. Solvent-accessible cysteine and lysine residues, such as Cys 621, Cys 641, and Cys 665, which detect electrophile agonists, provide a driving force for conformational change and dedicated electrophile sensitivity [27]. In human TRPA1, 3 critical cysteine residues are clustered within the so-called linker region that connects the ankyrin-rich domain to the transmembrane core. Mutation of all 3 residues render the channel insensitive to activation by isothiocyanates, except at very high (>100 μM) concentrations, where modification of a nearby lysine elicits small and persistent responses [28–30]. TRPA1can also be activated through non-covalent protein modifications in which non-electrophilic compounds often behave via a bimodal mechanism. The covalent modification is well accepted, but not well understood, and how the channel activation is switched off is not clear. It may play a positive role in preventing progression of inflammation by switching off the channel quickly and effectively.
Neurogenic Inflammation induced by TRPA1 via C-fibers
Neurogenic inflammation
It was first demonstrated that electrical stimulation of dorsal roots induces cutaneous vasodilation, which led to the concept of a neurogenic inflammation [31], often provoked by the sensitization of small-diameter sensory neurons (or C-nociceptors) expressing TRPA1 [32]. Neuropeptides engaged in the process are produced by the peripheral terminals of nociceptive nerve fibers after the back-propagating action potentials induced by the noxious peripheral stimulation. Both the axon and the dorsal root reflex, including the neurokinin A (NKA), substance P (Sub P), and calcitonin gene-related peptide (CGRP) activating tachykinin (NK1, NK2, and NK3) and CGRP receptors on effector cells, particularly at the vascular level, play an important role in neurogenic inflammation, inflammatory pain, and chemo-nociception [13,33,34]. Sub P, a kind of neuropeptide, is an inflammatory mediator in peripheral tissues, inducing neurogenic inflammation and a ‘pain transmitter’ to transmit nociceptive signals from primary afferents to spinal dorsal horn neurons expressing the NK1 receptor (one of the tachykinin receptors). This occurs because of its synthesis and release from both central and peripheral terminals of primary afferent sensory neurons [35]. Sub P is also a pleiotropic peptide with specific neural activities, sharing common bio-physical and bio-chemical properties with antimicrobial peptides, suggested to take part in antimicrobial host defense at specialized locations such as the airway [20].
C-fibers in neurogenic inflammation
In recent research TRPA1 has emerged as a key regulator of neuropeptide release and neurogenic inflammation by C-fibers, which are polymodal and activated by noxious thermal, mechanical, and chemical stimuli. C-fiber presents a variety of receptors and channels in its termini localized from the nose to the lower airway beneath the epithelium. This forms a dense network that innervates the airway and acts as a sentinel for potential damaging agents to activate the system in emergence and initiate protective reflex responses [36,37]. They are divided into 2 major classes. Medium-diameter myelinated (Ad) afferents Ad are subdivided into 2 main classes, type I and Type II, mediating acute or fast pain. Small-diameter unmyelinated C fibers are responsible for chronic or slow pain, responding to heat and mechanical stimuli. Stimulation of these fibers in the airway leads to the release of inflammatory neuropeptides that induce neurogenic inflammation. Prolonged inflammation can ultimately lead to cough, asthma, and chronic obstructive pulmonary disease (COPD).
In mammals, TRPA1 channels are expressed in C-fiber nociceptors, each of which plays a central role in pain sensation and neurogenic inflammation, leading to the release of Sub P, NKA, and CGRP. This can promote vasodilation, extravasation of plasma proteins, the recruitment of immune cells, local inflammation, mechanical hyperalgesia, and hyperthermalgesia. It can also activate non-neuronal cells, such as keratinocytes, epithelial cells, and fibroblasts that reside within the damaged tissue, and immune cells that infiltrate the damaged area to release inflammatory mediators, including ATP, adenosine, bradykinin, prostaglandins (PGs), leukotrienes, histamine, tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), proteases, and glutamate, forming an “inflammatory soup” [38,39]. Evidence regarding TRP channels suggests that neurogenic inflammation induced by the TRPA1 mechanism may take part in the airway inflammation of respiratory diseases such as asthma [14].
The role of TRPA1 in asthma mechanism
Asthma, a chronic inflammatory condition of the airway, is one of the most prevalent diseases in the world, particularly in developing countries. Asthma is characterized by variable airflow obstruction, AHR, plasma extravasation, shortness of breath, wheezing, coughing, and chest tightness. Asthma is often associated with exposure to environmental allergens, with subsequent infiltration in the lungs of eosinophils and mast cells degranulation [40].
TRPA1 plays a central role in asthma. It is expressed on airway cells and C-fibers in the airway and keeps reactivity to a broad array of airborne chemicals inducing neurogenic inflammation. TRPA1 is an ideal sensor for irritants in the airway, in which there are complex interactions between airway cells and nerve fibers [23,41,42]. There is a growing list of airborne chemicals, such as industrial pollutants (e.g., isocyanates, heavy metals, and oxidizing agents) and general anesthetics that result in neurogenic inflammation in local airways by activating TRPA1 [33,43,44]. For example, crotonaldehyde, acrolein, acetaldehyde, and other reactive molecules and nicotine (all contained in cigarette smoke) [45–48] induce airway neurogenic inflammation through TRPA1 on airway sensory nerve terminals to release neuropeptides, other proinflammatory cytokines, and chemokines. These in turn mediate the early inflammatory response, such as plasma protein, leukocytes extravasation, mucus hypersecretion, and airway constriction in rodent airways, which can be inhibited by the nonselective TRP blocker, ruthenium red [13,45].
Schematic representation of the mechanisms of neurogenic inflammation (Figure 1). TRPA1 is expressed by the termini of C-fiber. The influx of Ca2+ is robustly activated by a wide variety of harmful environmental and internal stimuli that cause pain and inflammation through forming covalent modification with just a few reactive cysteine residues located in cytoplasmic N-terminal domain of the channel. This is required for the release of neuropeptides, such as substance P, calcitonin gene-related peptide (CGRP), and neurokinin A (NKA) to promote and modulate inflammatory responses. Neuropeptides engaged in the process activate tachykinin (NK1, NK2, and NK3). CGRP receptors on effector cells, particularly at the vascular level, play an important role in the release of some proinflammatory cytokines, chemokines, and inflammatory mediators. These in turn mediate the early inflammatory response, such as vasodilation, plasma protein, leukocytes extravasation, mucus hypersecretion, and airway constriction.
Figure 1.
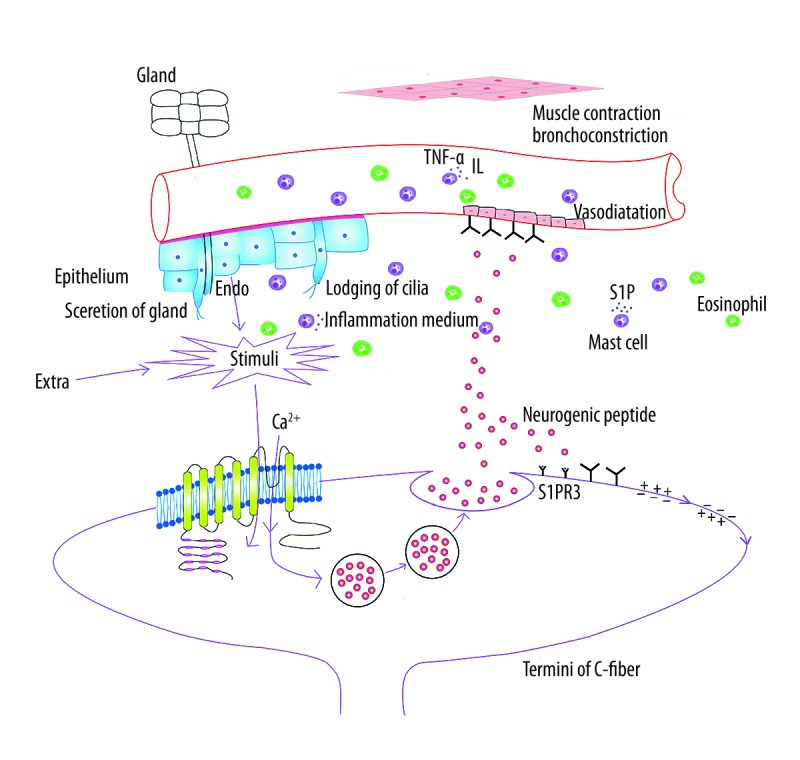
Schematic representation of the mechanisms of neurogenic inflammation.
There is also a functional TRPA1 channel in enterochromaffin cells of the GI, vascular endothelial cells, keratinocytes, and asthma-relevant cell types (e.g., CD4+ T cells, CD8+ T cells, B cells, and mast cells) and cell factors (e.g., IL17). This suggests that TRPA1 agonists may act on channels expressed by non-neuronal cells to promote key inflammatory responses [49–51]. Asthma patients can also suffer from disease exacerbations often associated with respiratory infections, leading to the acceleration of asthma progression. For instance, infection with Gram-negative bacteria shows that lipopolysaccharide (LPS), the most potent immunostimulatory cue produced by Gram-negative bacteria, binds to and activates the protein complex in immune cells via TRPA1. Moreover, LPS can cause the release of neuropeptide by the TRPA1 activation in nociceptive sensory neurons [20,52], in which the role of Sub P is anti-bacterial, or inducing neurogenic inflammation, or both. In addition, some inflammatory and immune cells are also proven to synthesize and release tachykinins, such as Sub P, under certain physiological conditions [53]. It is unclear whether a non-neurogenic inflammatory response or a neuro-immune interaction promoted by TRPA1 contributes to asthma.
Some experimental results also show that TRPA1 is linked to the development of asthma. Exposure to an allergen such as ovalbumin (OVA) induces asthma-like responses in wild-type mice, whereas TRPA-null mice are protected [54]. In animals sensitized with OVA, infiltration of immune cells, bronchoconstriction, and wheezing can be monitored after exposure to aerosolized OVA. As a bioactive sphingolipid metabolite mediating diverse biological responses, including smooth muscle contraction, Sphingosine-1-phosphate (S1P) is produced following the activation of sphingosine kinases (SPK). It exerts most of its effects by binding 5 distinct GPCRs, designated as Sphingosine-1-phosphate receptor1–5 (S1PR1–5), the levels of which are dramatically increased in mice sensitized by OVA [55,56]. The AHR caused by smooth muscle contraction can be abolished by the agonist of S1PR3, which may be present in the sensory neurons with the co-expression of TRPA1 and TRPV1 [57]. S1P is also a chemotactic agent for eosinophils persisting in asthma and is a trigger of T-cells, IgE, and mast cells, which are closely related to asthma-like symptoms in mice [56]. Further research is needed to determine if there is any contact or interaction between TRP channels (TRPA1) and S1P or its receptors. In addition, a significant increase in wild-type and reduction in TRPA1−/− mice of leukocyte number, an airway hyperactivity marker, shows the reduced influx of basophils in TRPA1−/− mice. This is often implicated in allergic reactions. Decreasing release of airway epithelium protecting mucins, inflammatory neuropeptides (e.g., CGRP, Sub P and NKA), and other proinflammatory cytokines (e.g., TNF-α), and IL6 in TRPA1-null mice or in pharmacologic inhibition of the TRPA1 were shown. This also suggests that the antagonists of the TRPA1 channels may be of value in the treatment of the allergic asthma [58]. Studies on tachykinin receptors have shown the potential of NK receptor antagonist to decrease airway responsiveness and to improve lung function in asthmatic patients, but it has poor effects on airway inflammation and asthma symptoms. However, selective tachykinin receptors antagonists tested on guinea pigs have been shown to inhibit the pathological process of airway hyperresponsiveness and reduce eosinophilic infiltration and vascular permeability, indicating a possible role in the genesis of asthma.
Conclusions
Asthma, associated with chronic neuropathic pain, inflammation, and tissue injury, continues to be a devastating clinical problem, with few effective therapies available to limit disease progression. This review suggests a significant role of the TRPA1 expressed in C-fiber nociceptors. It appears to play a central role in neurogenic inflammation to lead to the release of Sub P. This can promote vasodilation, extravasation of plasma proteins, and the recruitment of immune cells, as well as the promiscuous receptor activated by a wide range of known or novel stimuli. Although evidence suggests a potential as a therapeutic pharmacological target in neurogenic inflammation, inflammation pain, and asthma, more work is required. This characterized mechanism leaves many questions. It is unclear whether the neurogenic inflammation via TRPA1 is the initial factor of asthma attacks or just a contributing factor. The interaction between immune factors (e.g., IL17, S1P, and SubP), triggered via TRPA1, and immune cells (e.g., dendritic cells presenting antigens and playing key roles in the recognition of microbial products via Toll-like receptors) are involved in the development of asthma. Many studies indicate that interleukin (IL)-25 is involved in airway viral response by bonding with its receptor (IL-17RB) expressed by DCs. Exacerbation of asthma is closely related to microbial infections. If it can be established that the activation of TRPA1 alters IL-25 through affecting DCs with airway infection in asthma sufferers, it will be a great contribution to the exploration of the interaction between the immune system and TRPA1. Research and development of the specific antagonists would be greatly helped by development of a tracer technique to detect the specific binding sites of stimuli or an imaging technique combined with three-dimensional construction to define the channel structure and to determine how to switch it off. As research adds to our understanding of the pathogenesis of asthma, additional therapeutic options will be developed.
Footnotes
Source of support: Self financing
References
- 1.Baker KE, Bonvini SJ, Donovan C, et al. Novel drug targets for asthma and COPD: lessons learned from in vitro and in vivo models. Pulm Pharmacol Ther. 2014;29:181–98. doi: 10.1016/j.pupt.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–68. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 3.Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–96. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 5.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 6.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ, Lipp P, Bootman MD. Signal transduction. The calcium entry pas de deux. Science. 2000;287:1604–5. doi: 10.1126/science.287.5458.1604. [DOI] [PubMed] [Google Scholar]
- 8.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 9.Radresa O, Pare M, Albert JS. Multiple roles of transient receptor potential (TRP) channels in inflammatory conditions and current status of drug development. Curr Top Med Chem. 2013;13:367–85. doi: 10.2174/1568026611313030012. [DOI] [PubMed] [Google Scholar]
- 10.Sedgwick SG, Smerdon SJ. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–16. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- 11.Benemei S, Fusi C, Trevisan G, Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br J Pharmacol. 2014;171:2552–67. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflugers Arch. 2012;464:425–58. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 13.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Ann Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Hwang SW. Emerging roles of TRPA1 in sensation of oxidative stress and its implications in defense and danger. Arch Pharm Res. 2013;36:783–91. doi: 10.1007/s12272-013-0098-2. [DOI] [PubMed] [Google Scholar]
- 15.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–89. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 16.Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PloS One. 2012;7:e43597. doi: 10.1371/journal.pone.0043597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 18.Schulze A, Hartung P, Schaefer M, Hill K. Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine-type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium. 2014;55:200–7. doi: 10.1016/j.ceca.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Hackos DH. TRPA1 as a drug target-promise and challenges. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:451–63. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Bhatia M. Substance P at the neuro-immune crosstalk in the modulation of inflammation, asthma and antimicrobial host defense. Inflamm Allergy Drug Targets. 2014;13:112–20. doi: 10.2174/1871528113666140323202419. [DOI] [PubMed] [Google Scholar]
- 21.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–84. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 22.Grace MS, Baxter M, Dubuis E, et al. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br J Pharmacol. 2014;171:2593–607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geppetti P, Patacchini R, Nassini R, Materazzi S. Cough: The emerging role of the TRPA1 channel. Lung. 2010;188(Suppl 1):S63–68. doi: 10.1007/s00408-009-9201-3. [DOI] [PubMed] [Google Scholar]
- 24.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 25.Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–65. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 26.Latorre R. Perspectives on TRP channel structure and the TRPA1 puzzle. J Gen Physiol. 2009;133:227–29. doi: 10.1085/jgp.200910199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen CE, Armache JP, Gao Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–17. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macpherson LJ, Dubin AE, Evans MJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–45. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 29.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–68. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi N, Mizuno Y, Kozai D, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2:287–98. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 31.Koivisto A, Chapman H, Jalava N, et al. TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin Pharmacol Toxicol. 2014;114:50–55. doi: 10.1111/bcpt.12138. [DOI] [PubMed] [Google Scholar]
- 32.Nassenstein C, Kwong K, Taylor-Clark T, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Bautista DM, Movahed P, Hinman A, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–52. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura Y, Une Y, Miyano K, et al. Activation of transient receptor potential ankyrin 1 evokes nociception through substance P release from primary sensory neurons. J Neurochem. 2012;120:1036–47. doi: 10.1111/j.1471-4159.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 36.Plato M, Kummer W, Haberberger RV. Structural and neurochemical comparison of vagal and spinal afferent neurons projecting to the rat lung. Neurosci Lett. 2006;395:215–19. doi: 10.1016/j.neulet.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 37.Oh EJ, Mazzone SB, Canning BJ, Weinreich D. Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol. 2006;573:549–64. doi: 10.1113/jphysiol.2005.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basbaum AI, Woolf CJ. Pain. Current biology: CB. 1999;9:R429–31. doi: 10.1016/s0960-9822(99)80273-5. [DOI] [PubMed] [Google Scholar]
- 39.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 41.Belvisi MG, Dubuis E, Birrell MA. Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest. 2011;140:1040–47. doi: 10.1378/chest.10-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology. 2008;23:360–70. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nassini R, Pedretti P, Moretto N, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PloS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bessac BF, Sivula M, von Hehn CA, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre E, Campi B, Materazzi S, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–82. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bang S, Kim KY, Yoo S, et al. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci. 2007;26:2516–23. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- 47.Talavera K, Gees M, Karashima Y, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–99. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–48. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nozawa K, Kawabata-Shoda E, Doihara H, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–13. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104:987–94. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129:2312–15. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- 52.Meseguer V, Alpizar YA, Luis E, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groneberg DA, Harrison S, Dinh QT, et al. Tachykinins in the respiratory tract. Curr Drug Targets. 2006;7:1005–10. doi: 10.2174/138945006778019318. [DOI] [PubMed] [Google Scholar]
- 54.Caceres AI, Brackmann M, Elia MD, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA. 2009;106:9099–104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiba Y, Suzuki K, Uechi M, et al. Downregulation of sphingosine-1-phosphate receptors in bronchial smooth muscle of mouse experimental asthma. Pharmacol Res. 2010;62:357–63. doi: 10.1016/j.phrs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Roviezzo F, Sorrentino R, Bertolino A, et al. S1P-induced airway smooth muscle hyperresponsiveness and lung inflammation in vivo: Molecular and cellular mechanisms. Br J Pharmacol. 2015;172:1882–93. doi: 10.1111/bph.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trankner D, Hahne N, Sugino K, et al. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci USA. 2014;111:11515–20. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raemdonck K, de Alba J, Birrell MA, et al. A role for sensory nerves in the late asthmatic response. Thorax. 2012;67:19–25. doi: 10.1136/thoraxjnl-2011-200365. [DOI] [PubMed] [Google Scholar]


