Abstract
The commercialization of new point of care technologies holds great potential in facilitating and advancing precision medicine in heart, lung, blood, and sleep (HLBS) disorders. The delivery of individually tailored health care to a patient depends on how well that patient’s health condition can be interrogated and monitored. Point of care technologies may enable access to rapid and cost-effective interrogation of a patient’s health condition in near real time. Currently, physiological data are largely limited to single-time-point collection at the hospital or clinic, whereas critical information on some conditions must be collected in the home, when symptoms occur, or at regular intervals over time. A variety of HLBS disorders are highly dependent on transient variables, such as patient activity level, environment, time of day, and so on. Consequently, the National Heart Lung and Blood Institute sponsored a request for applications to support the development and commercialization of novel point-of-care technologies through small businesses (RFA-HL-14-011 and RFA-HL-14-017). Three of the supported research projects are described to highlight particular point-of-care needs for HLBS disorders and the breadth of emerging technologies. While significant obstacles remain to the commercialization of such technologies, these advancements will be required to achieve precision medicine.
Keywords: Biosensor, commercialization, point-of-care technologies, precision medicine
A discussion of the importance of point-of-care (POC) technologies in facilitating precision medicine for heart, lung, blood, and sleep disorders, including three such technologies currently undergoing commercialization.
I. Introduction
Precision medicine promises to deliver medical care that is tailored to individual patients based on personal pharmacodynamics and pharmacokinetics, genetic makeup, lifestyle, and environment. This movement away from traditional “one size fits all” therapies represents a dramatic paradigm shift in patient data collection, utilization of this information by healthcare providers, and application of this information to the discovery of new therapies in the context of individuals as opposed to an “average patient.” The Precision Medicine Initiative, announced by President Obama during the 2015 State of the Union address and supported by the National Institutes of Health, underscores the importance and exciting promise of this transition in healthcare.
While the Precision Medicine Initiative is initially focused on the collection of data from over one million patients to generate a dataset from which new insights may be gleaned, leveraging these discoveries for patient care will require the development of new technologies. Indeed, the ability to tailor medical care to an individual patient is intrinsically tied to capabilities for collecting information on that individual. The accrual of the Precision Medicine Initiative dataset may illuminate limitations of our current molecular and cellular analysis methods for monitoring the health status of individuals.
For example, the utility of a new blood-based biomarker for myocardial infarction may be highly dependent on how quickly and accurately blood can be analyzed upon onset of symptoms. Alternatively, new biomarkers may be discovered that can guide therapies on an hour-to-hour or on a day-to-day basis, similarly to the way self-monitoring of blood glucose has improved outcomes for diabetic patients through enhanced timing and dosage of insulin treatment [1]. At a more basic level, biomarkers discovered through the Precision Medicine Initiative may require the dissemination of new point-of-care tests to fully leverage the new discovery across various populations and clinical settings. The commercialization of new technologies will be required to deliver on the promise of precision medicine. While additional significant factors need to be addressed such as technology dissemination, patient adherence, incorporation of information into clinician workflow, and regulatory approval, this publication focuses on new underlying assay technologies that will make novel data collection possible.
II. Opportunities for Point-of-Care Technologies in Heart, Lung, Blood, and Sleep Disorders
Prevalent heart, lung, blood, and sleep (HLBS) disorders, such as hypertension, asthma, chronic obstructive pulmonary disease (COPD), and insomnia, have a complex and dynamic symptomatic nature that precludes the ability to gather a complete picture of a patient’s health during a clinic visit or through a single blood draw. The shortcomings of static patient information are most obvious in emergent cases, such as myocardial infarction (MI). Cardiac troponins, which can indicate an emerging MI, require periodic, frequent blood analysis to yield their full diagnostic capability [2], [3]. The lifestyle and day-to-day activity dependence of both chronic and emergent HLBS disorders creates many opportunities for significant patient benefit through POC testing. New technologies that encourage patient engagement and allow for improved self-monitoring may become critical tools for HLBS disease prevention, management, and recovery [4], [5]. Other new point-of-care technologies that improve the quality of available information through improved assay sensitivity, decreased test turn-around-time, or accurate determination of disease pathogenesis can be similarly utilized to deliver increasingly personalized healthcare. The new information made possible by such technologies will contribute to clinicians’ ability to deliver precision medicine.
HLBS diseases comprise a significant disease burden in the United States. Cardiovascular disease (CVD) remains the leading cause of death in the United States, accounting for 1 in 3 deaths [5]. Direct healthcare costs attributed to CVD and stroke amounted to $195.6 billion in 2011. By comparison, an estimated $86.6 billion was spent on cancer and benign neoplasms direct healthcare costs in 2009 [6]. Each year, approximately 965,000 people in the US suffer either a new or recurrent coronary attack that results in hospitalization [5]. Patients experiencing a diagnosed coronary attack only make up 6-16% of patients presenting in the ED with suspected coronary attack, indicating the magnitude of population that may benefit from point of care monitoring in potential emergent cardiac episodes [7], [8]. Asthma affects 22.2 million US patients, with 20% of asthmatics requiring emergency treatment for exacerbations [9]. Direct costs of COPD totaled $29.5B in 2014, affecting over 38 million US adults [10]. Acute COPD exacerbations are correlated to transient factors such as physical activity, respiratory infection, and air pollution, so POC testing may be instructive as to causes of exacerbations [11], [12]. While the prevalence of sleep disorders is difficult to ascertain given the lack of consensus defining disorders, an estimated one third of the population suffers from some degree of insomnia [13]. The recent popularization of sleep related consumer technologies (largely “apps”) highlights a broad interest in sleep characterization and monitoring [14]. Many patients stand to benefit from the advancement of HLBS diagnostic point-of-care technologies.
III. NHLBI Efforts
Recognizing the potential of POC technologies in advancing precision medicine, the National Heart, Lung, and Blood Institute (NHLBI) convened a point-of-care technologies working group on June 22, 2012. The goals of the working group were to discuss knowledge gaps and barriers and to recommend research areas where POC technologies could be best supported for the diagnosis and monitoring of heart, lung, blood, and sleep (HLBS) disorders. The recommendations included: convening collaborative groups to establish a roadmap; support for proof-of-concept studies and larger validation studies; development of POC technology for integrated measurements; development of standards; and support for access to biobanks and clinical datasets [15], [16]. Following the working group’s recommendations, NHLBI sponsored a request for applications using the SBIR/STTR grant mechanism for technology commercialization through small businesses (RFA-HL-14-011 and RFA-HL-14-017). The intention of this funding is to support research using advanced technologies to develop novel point-of-care devices, or to implement existing devices in clinical settings, towards the goal of improved patient outcomes for HLBS disorders. The SBIR/STTR mechanism was used to direct funding towards commercial efforts that will lead to technology dissemination. Nineteen projects have been funded to date, including the following three examples.
IV. Examples of NHLBI Funded Technologies
A. Semiconductor Electronic Label-Free Assay (SELFA)
Ischemic heart disease (IHD) is the leading cause of mortality worldwide. Patients presenting with acute coronary syndrome (ACS) to an emergency room (ER) are screened for myocardial infarction (MI) via electrocardiogram (ECG) and/or cardiac troponin (cTn) quantitation. ECGs are unfortunately non-diagnostic for 50% of MI patients. In contrast, tracking changes in concentration of cTn, which has isoforms that are unique to cardiac myocytes, plays a central role in MI diagnosis [17]. Quantitative troponin tests are readily available in hospital laboratories, but may not be available in relatively small clinics and doctor’s offices. POC cTn devices with a short turnaround time (TAT) can alternatively be used in clinics and doctor’s offices for prescreening purposes, although their use is limited by an inadequate limit of detection (LOD) [18]. To date, a quantitative POC cTn assay that can expedite ER triage with a TAT 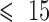 min remains lacking [19].
min remains lacking [19].
To address the said unmet need, the Semiconductor Electronic Label-Free Assay (SELFA) technology is being developed and commercialized as a sample-to-answer POC diagnostic device by Selfa, Inc. Originated from the University of California, Los Angeles (UCLA), SELFA is a holistic biomolecular quantitation platform which integrates hardware, software, reagents, and protocol. The core technology behind SELFA is a novel amplifying nanowire field-effect transistor biosensor that performs label-free biomolecular-to-electrical signal transduction as well as on-the-spot, pure-electrical signal amplification [20]–[22]. As previously determined, the amplifying nanowire transistor biosensor delivers an analytical lower limit of detection (LLOD) of <10 fg/ml of cTnI in spiked serum [22].
The label-free operation of SELFA eliminates labeling reagents (e.g., enzymatic, fluorescent, magnetic, etc.), their conjugations and tedious washing steps. Also, SELFA’s direct electrical outputs greatly simplify downstream signal processing, storage, and transmission. As a result, SELFA can significantly minimize onerous requirements for infrastructure (e.g., light source, microscope, optical reader, etc.), cost, personnel, and TAT.
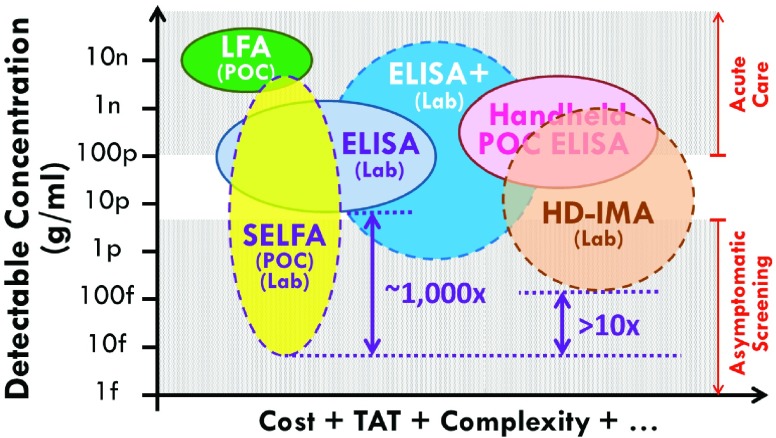
The aforementioned attributes of SELFA are benchmarked with other relevant POC and lab-based assays (Fig. 1). The detectable concentration ranges are based on reported values whereas requirements of the cost, TAT, etc. are subjectively assigned by the authors. Based on a clinical threshold of 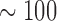 pg/ml of cTnI, only the handheld ELISA system like Abbott i-STAT® is capable of diagnosing acute MI among the currently available POC assays. Nonetheless, the high cost of this handheld system renders its POC adoption outside of major facilities unrealistic. It should be reiterated though such major facilities would already be equipped with the very cost effective lab-based standard ELISA or equivalent.
pg/ml of cTnI, only the handheld ELISA system like Abbott i-STAT® is capable of diagnosing acute MI among the currently available POC assays. Nonetheless, the high cost of this handheld system renders its POC adoption outside of major facilities unrealistic. It should be reiterated though such major facilities would already be equipped with the very cost effective lab-based standard ELISA or equivalent.
FIGURE 1.
Benchmarking SELFA with relevant POC and lab-based assays. POC assays include lateral flow assay (LFA) and handheld enzyme-linked immunosorbent assay (ELISA). Lab-based assays include standard ELISA, ultra-sensitive ELISA (ELISA+), and high-definition immunoassay (HD-IMA). Solid and dashed outlines represent current and emerging assays, respectively. The detectable concentration ranges measured by the vertical axis are based on reported values. The cost, turnaround time, complexity, etc. gauged by the horizontal axis are based on the authors’ subjective estimation.
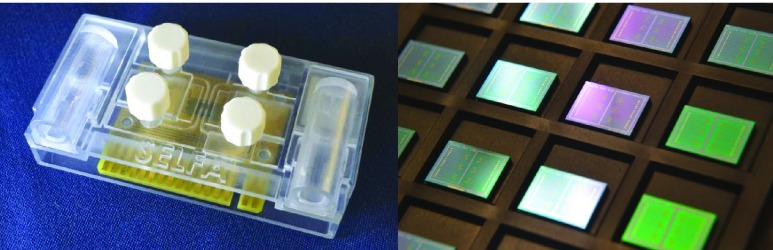
Since SELFA is capable of achieving about 3 orders of magnitude lower LLOD than standard ELISA, the former is poised to become a high sensitivity (quantitative) POC assay if its cost and other requirements can be kept very low. One such manifestation is a disposable cartridge which costs only a few US dollars in volume and a portable electronic reader (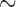 US$200-300) to interface with the cartridge. The cartridge comprises specimen and reagent handling microfluidics (Fig. 2, left) and a printed circuit board mounted with the foundry fabricated SELFA semiconductor chip (Fig. 2, right). The amplifying nanowire transistor biosensors integrated on the SELFA chip are functionalized and calibrated during cartridge production. During actual operation at POC, the SELFA TAT is expected to be within 15 min. Very recently, the clinical performance of SELFA in quantitating cTnI in patient specimens has also been validated, demonstrating an outstanding correlation with clinical laboratory measurements.
US$200-300) to interface with the cartridge. The cartridge comprises specimen and reagent handling microfluidics (Fig. 2, left) and a printed circuit board mounted with the foundry fabricated SELFA semiconductor chip (Fig. 2, right). The amplifying nanowire transistor biosensors integrated on the SELFA chip are functionalized and calibrated during cartridge production. During actual operation at POC, the SELFA TAT is expected to be within 15 min. Very recently, the clinical performance of SELFA in quantitating cTnI in patient specimens has also been validated, demonstrating an outstanding correlation with clinical laboratory measurements.
FIGURE 2.
SELFA POC product prototyping. The SELFA disposable cartridge prototype with integrated microfluidics for specimen preparation and assay operation (left). The foundry fabricated SELFA semiconductor chips with integrated amplifying nanowire transistor biosensor arrays (right).
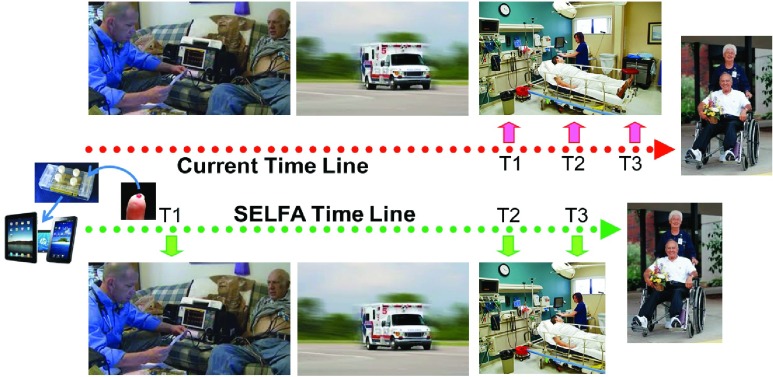
It has been established that serial measurements of highly sensitive cTn can improve the early diagnosis of acute MI (AMI) [23] although none of the current POC assay has a sufficient LLOD for very early detection (i.e., <10 pg/ml) (Fig. 1). As a result, the very first measurement of the series cannot currently be performed before arrival at the ER (Fig. 3, top row), leading to serious complications in many cases. Using a highly sensitive POC assay like SELFA, the very first cTn reading can be obtained and sent out at the patient’s location while ECG is being done (Fig. 3, bottom row). For patients with non-ST-elevated MI (NSTEMI), this timely piece of evidence would unequivocally enhance the overall emergency medical services (EMS) including triage and transport decisions and locations. This additional information would also minimize false activation of the catheterization lab and certainly shorten wait times in the ER. For the roughly 15 million patients per year from the US and Europe who present to the ER with signs of MI, the potential for enhanced health benefits and reduced costs associated with rapid, sensitive and accurate POC determinations of cTn levels are substantial [24].
FIGURE 3.
Serial blood sampling time lines for suspected AMI. Currently, the first blood biomarker test (T1) for the non-STEMI is mostly done in the ER setting (top row). With SELFA, the first test result can be obtained and transmitted out along with ECG data at the patient’s location (bottom row). This should clearly facilitate EMS triage and transport, increase ER throughput, permit alternative treatment earlier, and most importantly reduce patient restlessness.
Besides its utility in risk stratifying symptomatic and stable ACS patients for major adverse cardiac events, cTnI is also used to identify chronic injury in asymptomatic persons with unrecognized myocardial structural disease at high risk for adverse cardiovascular (CV) outcomes [25]. Most importantly, cTnI has also been established as a key biomarker associated with increased CV mortality 8 to 15 years in the future in healthy asymptomatic people according to a population-based study of CV disease risk factors [26]. These require a cTnI assay with substantially lower LLOD than any of the currently available ones. While the ultra-sensitive ELISA (ELISA+) may be marginally qualified, the high-definition immunoassay (HD-IMA) [26] is the only truly capable one as benchmarked in Fig. 1. Nevertheless, the HD-IMA requires special reagents, multiple tedious steps and expensive equipment, yet it only offers an LLOD of 100 fg/ml, which is 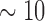 -fold higher than the SELFA’s LLOD.
-fold higher than the SELFA’s LLOD.
In summary, the successful development and commercialization of SELFA POC diagnostic device should bring immediate impacts to two major sectors: (i) emergency department and ambulance service for acute care, and (ii) outpatient clinics and physician offices for prognosis and stratification.
B. Continuous Bloodstream Monitoring (‘SmartIV™’)
A major advantage of POC diagnostics over sample processing in a central lab is the decreased logistical burden and time required to make a diagnostic measurement. This convenience offers the possibility of using more frequent measurements to guide therapies. For example, in the treatment of Ebola virus disease (EVD) during the recent crisis, many facilities in the US and Europe used the Abbott i-STAT® to guide electrolyte replacement therapy, and there is evidence that this improved patient outcomes [27]. Likewise, the Surviving Sepsis Campaign bundles have been shown to decrease mortality from sepsis by 40% and 36%, respectively, for the 3-hour and 6-hour bundles, when they are properly administered [28]. This rapid, measurement-driven intervention (which includes monitoring of glucose, lactate, electrolytes and several other parameters) is ideally suited for POC monitoring and perhaps cannot practically be implemented without it.
It is interesting to consider the logical culmination of this trend. Monitoring analytes in vivo– without withdrawing a sample– would provide continuous biochemical data in the manner of current vital stats (blood pressure, pulse, pulse oxygenation) and could be used to drive tightly-controlled therapeutic interventions. Perhaps early systems would utilize a human in-the-loop, making a decision to effect a therapy based on the stream of continuous data, with a movement towards human on-the-loop (monitoring for exceptions or warnings). Eventually a fully closed-loop system could automatically control interventions based on an algorithm and the measured parameters. This paradigm has long been pursued for the treatment of diabetes, and outpatient trials with diabetic management based on an insulin pump under the control of a subcutaneous glucose sensor have proved extremely promising, with widespread clinical application predicted in as early as four years [30].
In the case of analytes such as glucose and lactate, systemic concentrations can be deduced from continuous monitoring of interstitial fluid with a subcutaneous implant. For many parameters, however, the interstitial levels are either too low or are not in stable equilibrium with systemic circulation. In those cases (including most drugs, many biomarkers, blood electrolytes, and many circulating proteins) it is preferable to sample chemistry from a platform actually within the systemic circulation. There are inherent challenges to such an approach, including the need to prevent or mitigate bio-fouling and biologically-mediated degradation over the necessary measurement time scales, the need for flow-independent, repeatable and quantitative measurements, and the need for a method of deploying the sensor that is practical from the viewpoint of clinical practice and workflows.
With funding from the NHLBI, and building on work pioneered by Kevin Plaxco and Tom Soh and their collaborators at the University of California at Santa Barbara [31], Diagnostic Biochips has demonstrated the continuous monitoring of the chemotherapeutic agent doxorubicin in whole blood. The electrochemical sensors are embedded in a standard IV catheter, or the “SmartIV™” platform. Doxorubicin is an anthracycline antitumor antibiotic that has a narrow therapeutic range, bounded by cardiotoxic effects at high cumulative doses and poor efficacy at low doses. Estimates of cardiotoxicity among patients who receive doxorubicin treatments range from 3% to 26%, for patients who receive a cumulative dose of 300 mg/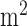 and 550 mg/
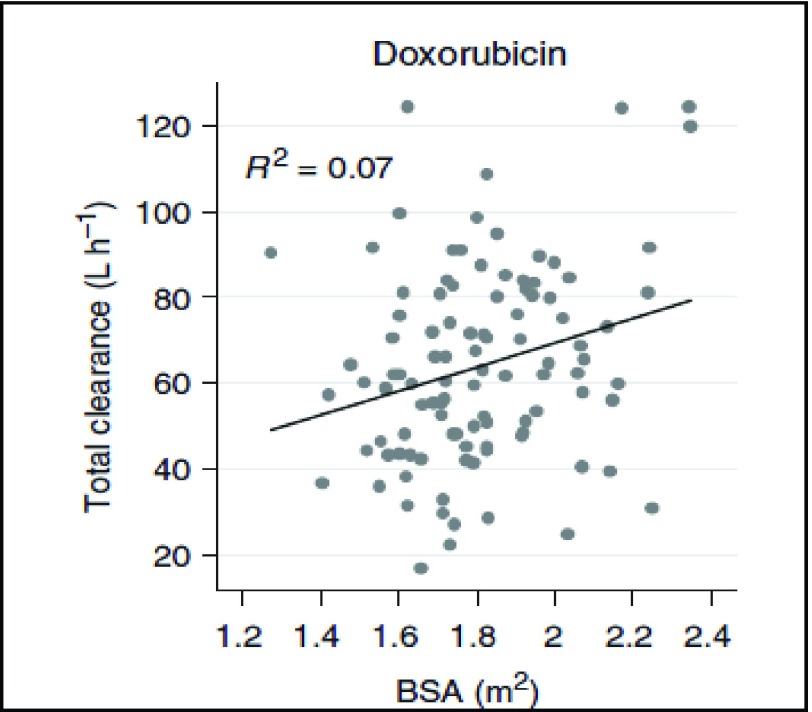
and 550 mg/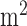 , respectively [32], [33]. Toxicity is often quantified by >30% reduction in left ventricle ejection fraction (LVEF), and is irreversible after ceasing chemotherapy [34]. These facts, combined with a remarkably high patient-to-patient variability in doxorubicin clearance rates (Fig. 4) and its extremely common usage for a wide variety of cancers, mean that a closed-loop system for controlling individual patient dosage could offer substantial potential treatment benefits in terms of more effective dosing with lower risks of severe side effects such as cardiotoxicity.
, respectively [32], [33]. Toxicity is often quantified by >30% reduction in left ventricle ejection fraction (LVEF), and is irreversible after ceasing chemotherapy [34]. These facts, combined with a remarkably high patient-to-patient variability in doxorubicin clearance rates (Fig. 4) and its extremely common usage for a wide variety of cancers, mean that a closed-loop system for controlling individual patient dosage could offer substantial potential treatment benefits in terms of more effective dosing with lower risks of severe side effects such as cardiotoxicity.
FIGURE 4.
Total clearance (liters per hour) of doxorubicin in human subjects, as a function of body surface area (BSA). Note poor correlation, 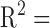 .07 (adapted from [29]).
.07 (adapted from [29]).
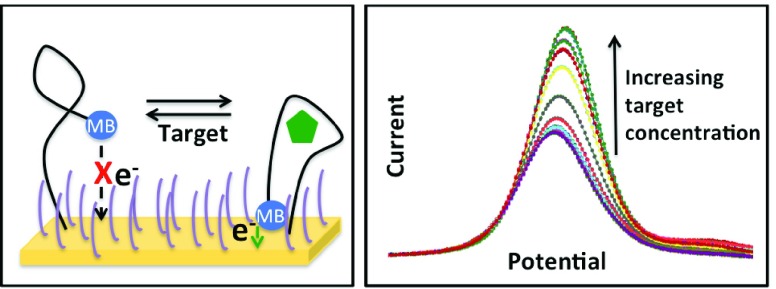
Diagnostic Biochips has developed a sensor using an aptamer biosensing element that has been modified by the addition of a methylene blue (MB) redox center at its 5’ terminus. An aptamer is a short (typically 20-60 base pair) RNA or DNA sequence that is selected from a starting library through a sequential process of selective binding and amplification called SELEX [35], to produce a molecule with a high affinity and specificity for a selected analyte. The aptamer is attached to the gold surface of a microelectrode through an alkane thiol group conjugated to the 3’ terminus of the aptamer, as has been described elsewhere [36]. A self-assembled monolayer of mercaptohexanol between the surface-bound aptamers reduces the surface energy of the gold surface and thus prevents non-specific binding. As the aptamer undergoes a conformational change during binding with the target analyte, the distance from the MB center to the electrode surface is modulated (Fig. 5, left). In order to transduce that change into a measurable signal, a time-varying voltage near the reduction/oxidation potential of MB is applied using a protocol known as square wave voltammetry [37]. The peak current resulting from that applied voltage is a measure of the number of MB centers in proximity to the electrode (Fig. 5, right). The total current flowing in the electrode circuit is the summed total from a very large number of contributing molecules, and is thus a measure of the proportion of bound to unbound states, which is linearly dependent upon the analyte concentration at equilibrium. This is true only for concentrations within a few orders of magnitude of the aptamer dissociation constant, 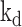 .) The voltage signal and resulting current are applied and measured, respectively, by an external potentiostat circuit which is connected to the electrode. A commercially-available potentiostat is used for device testing and characterization (Gamry Instruments, Warminster, PA). Diagnostic Biochips is also developing a highly miniaturized, low power potentiostat that can be carried by a laboratory animal (rat or primate) for pre-clinical studies.
.) The voltage signal and resulting current are applied and measured, respectively, by an external potentiostat circuit which is connected to the electrode. A commercially-available potentiostat is used for device testing and characterization (Gamry Instruments, Warminster, PA). Diagnostic Biochips is also developing a highly miniaturized, low power potentiostat that can be carried by a laboratory animal (rat or primate) for pre-clinical studies.
FIGURE 5.
The conformational change between the bound and unbound aptamer (left) modulates the distance of the methylene blue redox center from the electrode surface, resulting in a concentration-dependent current through the electrode.
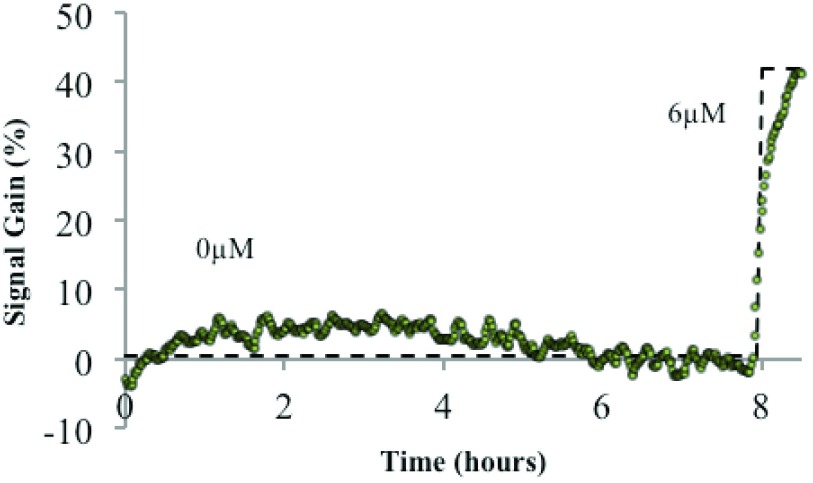
By mechanically integrating the functionalized microelectrode into the lumen of an IV catheter, this sensor can be inserted into a blood vessel to measure local chemical concentrations. A baseline flow of normal saline through the device provides a protective barrier to biofouling by large proteins and blood cells, while still allowing smaller molecules (molecular weight < 20,000 Da.) to diffuse to the sensor surface and be detected. In this manner these sensors have demonstrated stable baselines and linear sensor responses after 8 hours of deployment in whole blood (Fig. 6).
FIGURE 6.
Aptamer biosensor response measured in an experimental model of circulating whole blood from a “Smart IV™” platform during 8 hours in whole blood before and after the addition of 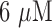 doxorubicin.
doxorubicin.
Future work on this sensor includes tuning of sensor dynamic range and patency based on optimizing buffer fluid flow rates, the inclusion of additional biofouling mitigation strategies, and improvements to sensor baseline drift (currently ± 10% over a time scale of 8 hours.) Diagnostic Biochips is also developing sensors that are sensitive to a variety of other analytes – including both drugs and naturally-occurring biomarkers – by functionalizing the electrochemical sensor platform with aptamers selected for sensitivity to various analytes of interest. Finally, by integrating a micro-fabricated array of lithographically-defined microelectrodes [38] into the same indwelling catheter or IV package, it should be possible to create a multi-analyte assay for continuous bloodstream monitoring.
Successful commercialization of this technology will allow for the tailored delivery of therapies based on individual pharmacokinetics and pharmacodynamics. The immediate feedback generated through continuous, real-time monitoring will permit the dosage of drugs to a given serum level, as opposed to an estimated serum level based on patient mass or surface area. This capability may both improve existing therapies, and make possible a new dosage strategy for novel therapeutics.
C. Acoustofluidics Sputum Analysis
Asthma continues to be a serious health problem for many people in the USA and worldwide. In 2011, 300 million people globally were diagnosed with asthma, and it caused 250,000 deaths [39]. In the US, the number of people living with asthma increased by 28% during 2001–2011, according to Centers for Disease Control and Prevention (CDC) [40]. In 2010 alone, asthma accounted for 3,404 deaths, 439,400 hospitalizations, 1.8 million emergency department visits, and 14.2 million physician office visits in the US. An estimated 39.5 million people (12.9% of the population), including 10.5 million children (14.0% of population), in the US have been diagnosed with asthma in their lifetimes.
The heterogeneous pathogenesis and clinical characteristics of asthma have been increasingly recognized, such that new therapeutic approaches may only be successful if they are targeted in a personalized fashion to individuals whose asthma is mediated by the targeted pathway [41]. By analyzing the different cell populations found in sputum, the mucus within the airways of the lungs, researchers have identified the distinct immunological phenotypes associated with the disease. Identifying these phenotypes has led to hopes of developing individually tailored therapeutic treatments that will more effectively target the mechanisms unique to each phenotype.
Despite its tremendous promise, sputum analysis has not been widely used among patients and healthcare providers. This is due to the following obstacles:
-
1)
The current methods for processing and analysis of induced sputum samples involve complex, labor-intensive procedures that are typically performed only at specialized centers, such as university hospitals.
-
2)
In conventional sputum processing/analysis assays, sputum samples need to be handled manually by the operator, often in open air, and run through several instruments. This presents significant biosafety concerns since all human specimens, even from healthy individuals, are potentially infectious.
-
3)
Since the processing and analysis of induced sputum samples is operator-dependent, the possibility of error or inter-operator variability can potentially confound the results.
-
4)
The conventional sputum processing and analysis assays generally require induced sputum volume of more than 1,
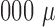 l; many asthmatic patients have difficulty producing such a significant amount of induced sputum sample.
l; many asthmatic patients have difficulty producing such a significant amount of induced sputum sample.
To address the above challenges of sputum processing/analysis approaches, Ascent Bio-Nano Technologies and The Pennsylvania State University are developing and commercializing an acoustofluidic-based (i.e., combination of acoustics and microfluidics technologies [42]–[47]), automated, point-of-care system that would allow on-chip processing of induced sputum samples and analysis of the percentage of inflammatory cells, such as eosinophils, neutrophils, lymphocytes, and macrophages, present in induced sputum samples from asthmatic patients.
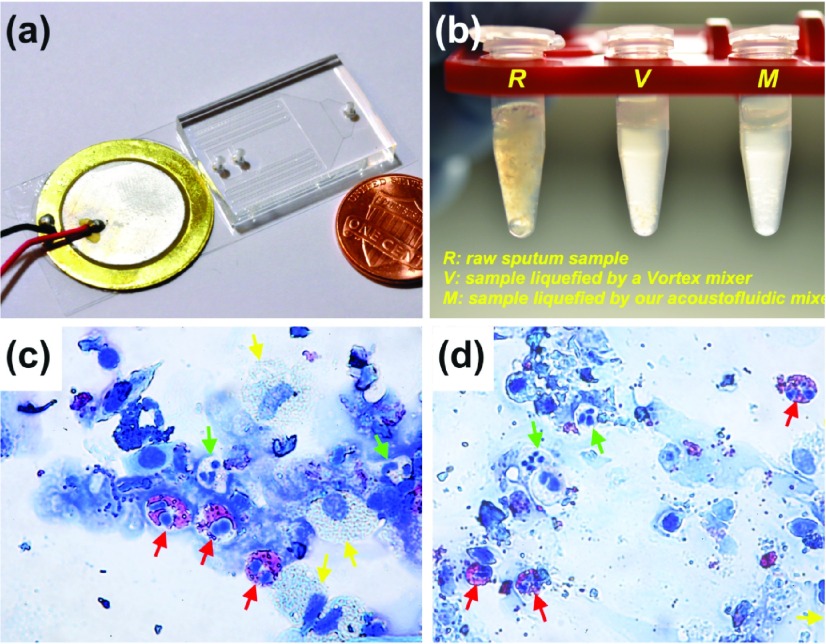
An acoustofluidic unit for on-chip sputum liquefaction is shown in Fig. 7 [44]. To liquefy sputum, the human sputum sample and the sputolysin (DTT) were co-injected into the microfluidic device (Fig. 7a) to be mixed in the presence of an acoustic field. Non-liquefied samples (i.e., the raw sputum sample) and liquefied sputum samples were compared visually. In Fig. 7b, R, V and M denote, respectively, the non-liquefied sputum sample, the sputum sample liquefied using a vortex mixer, and the sputum sample liquefied using the acoustofluidic device. As shown in the figure, the non-liquefied sputum sample was cloudy and contained visible clumps of mucus. The liquefied sputum samples, however, were translucent, presenting as a uniform mixture with a negligible amount of viscous mucus sputum. The sputum sample liquefied using the acoustofluidic device was similar to those liquefied using a vortex mixer, demonstrating that on-chip liquefaction may be achieved with the acoustofluidic approach. Post-liquefaction samples were inspected for cell morphologies. Using this acoustofluidic mixer, immune cells were retrieved and identified, such as eosinophils (red-arrow), neutrophils (green-arrow), and macrophages (yellow-arrow). The results are comparable to those obtained using a standard liquefaction procedure (Figs. 7c-d).
FIGURE 7.
(a) An optical image of the microfluidic device for liquefaction of human sputum samples. (b) Photograph of human sputum samples: an un-liquefied raw sputum sample (R), a liquefied sputum sample by Vortex mixer (V), and a liquefied sputum sample by the microfluidic mixer (M). Diff-Quik staining results of cell samples obtained from (c) on-chip liquefied sputum sample and (d) Vortex mixer-liquefied sputum sample.
Compared with the conventional approach for sputum processing and analysis that uses vortex mixing, centrifugation, and benchtop flow cytometry, this acoustofluidic system has the following advantages: 1) ability to perform accurate sputum analysis over a much wider range of sample size (volume: 50–3,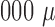 L) than the conventional approach (volume: 1,000–3,
L) than the conventional approach (volume: 1,000–3,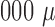 L); 2) automation and rapid turnaround time; 3) biohazard containment; and 4) low-cost, point-of-care devices. With these advantages, this acoustofluidic platform may both replace existing sputum processing/analysis tools, as well as fulfill unmet needs that have not been addressable with available techniques. For example, this technology may allow for analysis in applications where the amount of sputum induced from asthmatic patients is not enough to run the standard tests and/or the expertise and equipment to perform this analysis are not available, such as most practice locations outside of large hospitals. In this regard, this acoustofluidic platform could significantly increase the availability of personalized treatment strategies to asthmatic patients in both research and community settings.
L); 2) automation and rapid turnaround time; 3) biohazard containment; and 4) low-cost, point-of-care devices. With these advantages, this acoustofluidic platform may both replace existing sputum processing/analysis tools, as well as fulfill unmet needs that have not been addressable with available techniques. For example, this technology may allow for analysis in applications where the amount of sputum induced from asthmatic patients is not enough to run the standard tests and/or the expertise and equipment to perform this analysis are not available, such as most practice locations outside of large hospitals. In this regard, this acoustofluidic platform could significantly increase the availability of personalized treatment strategies to asthmatic patients in both research and community settings.
V. Barriers and Obstacles
New point-of-care technologies such as the above examples will help advance precision medicine, but significant challenges beyond technical feasibility remain to be addressed. Perhaps most significantly, the path to regulatory approval is relatively uncharted for highly novel assay technologies and at-home devices. Currently FDA-approved “home use tests” include assays that mostly achieve similar sensitivity and specificity to laboratory tests, including cholesterol, pregnancy, and fecal occult blood tests [48]. Generally, these tests result in follow-up clinical care, and so are relatively low-risk. Blood glucose monitoring devices are unique in their clearance to inform patient-driven dosing decisions due to the conferred improvement in diabetes management. In order for point-of-care devices to advance precision medicine, new technologies will have to demonstrate both an evident improvement in care as well as safe use by laypersons. An additional regulatory consideration is the secure transmission of confidential patient data. To fully utilize point-of-care devices, the collected data must integrate with patient health records. The design and implementation of user training will be critical, especially for consumer operated POC devices. Furthermore, data supporting significantly improved patient outcomes using new technologies will be required to justify physician use and payer support.
VI. Conclusion
While the obstacles are substantial, new POC technologies are required to achieve precision medicine. Continued financial support for technical advancement of novel assays, clear guidance on regulatory pathways, and backing from strategic partners with broad distribution networks will facilitate the commercialization of these devices. As better information on individual health becomes available, utility of currently available therapies may be expanded, patient adherence may be improved, and more targeted therapies may be developed. New point-of-care technologies will play a critical role in this promising transformation in healthcare.
VII. Acknowledgment
The authors thank the organizers of the NIH-IEEE 2015 Strategic Conference on Healthcare Innovations and Point-of-Care Technologies for Precision Medicine for the opportunity to assemble this group to discuss emerging point-of-care technologies for HLBS diseases.
Biographies

Mary Emma Gorham Bigelow received the B.S. degree in biological engineering from Cornell University, Ithaca, NY, in 2011. She previously worked at Scientific & Biomedical Microsystems before co-founding Diagnostic Biochips, Inc., as its Vice President, Research and Development. Her research interests include sensors for continuous physiological monitoring for use in research and clinical applications. She is a member of the Society for Neuroscience, and the Professional Organization Women in Bio.

Brian G. Jamieson was born in Livingston, NJ, in 1969. He received the B.S. degree in physics from Yale University in New Haven, CT, in 1991, and the M.S. and Ph.D. degrees in electrical and biomedical engineering from the University of Michigan, Ann Arbor, MI, in 2000 and 2003, respectively.
He held technical positions with the Naval Research Laboratory and the Savannah River Site from 1991 to 1996, before returning to graduate school in 1996. In 2002, he joined the NASA Goddard Space Flight Center as an Electronics Engineer, working on MEMS and the miniaturization of analytical instrumentation and sensors, including mass spectrometers, for space exploration and science. In 2006, he founded Scientific & Biomedical Microsystems, a company that develops custom sensor solutions for government and commercial clients, and in 2011, he founded a spin-out company, Diagnostic Biochips, Inc., to commercialize and market sensors for continuous in vivo monitoring.
Dr. Jamieson’s research and commercialization interests are in the development and deployment of fielded autonomous sensors for continuous monitoring of physical, biological, and chemical parameters of interest.

Chi On Chui (S’00–M’04–SM’08) received the B.Eng. degree in electronic engineering from the Hong Kong University of Science and Technology, Hong Kong, in 1999, and the M.S. and Ph.D. degrees in electrical engineering from Stanford University, Stanford, CA, USA, in 2001 and 2004, respectively.
He joined Intel Corporation, Santa Clara, CA, USA, in 2004, as a Senior Device Engineer. During his tenure with Intel, he also served as a Researcher-in-Residence with the University of California at Berkeley, Berkeley, CA, USA, and Stanford University. From 2005 to 2006, he was also appointed as a Consulting Assistant Professor of Electrical Engineering with Stanford University. Since 2007, he has been with the University of California at Los Angeles (UCLA), Los Angeles, CA, USA, where he is currently an Associate Professor of Electrical Engineering and Bioengineering, and a member of the California NanoSystems Institute, UCLA. He has authored and co-authored over 140 peer-reviewed and invited archival journal and conference papers, and six book chapters. He delivered two keynote speeches and numerous invited talks, and holds ten issued patents. His current research interests include bioelectronics and nanoelectronics.
Dr. Chui has served on the Technical Program Committees and International Advisory Committees of several conferences on electronic devices and circuits. He has received several recognitions for his work, including three best paper awards and three poster prizes. He received the Okawa Foundation Research Grant in 2007. He was a first recipient of the IEEE Electron Device Society Early Career Award in 2009, which is regarded as one of the society’s highest honors. In 2011, he received the Chinese-American Faculty Association Robert T. Poe Faculty Development Award, the UCLA Faculty Career Development Award, and the University of California at San Diego’s von Liebig Entrepreneurism Center Regional Health Care Innovation Challenge Award. He also received the UCLA Henry Samueli School of Engineering and Applied Science Northrop Grumman Excellence in Teaching Award in 2011.

Yufei Mao received the B.S. degree in electrical engineering from Beihang University, China, in 2010, and the M.S. degree from the Department of Electrical Engineering. He is currently pursuing the Ph.D. degree with the University of California, Los Angeles, under the mentorship of Prof. C. O. Chui. He joined the Nanostructure and Device Technology Laboratory in 2010. His research interests include field-effect transistor-based biosensing and biosensors and optoelectronic tweezers for bioelectronics applications.

Kyeong-Sik Shin received the Ph.D. degree from the Department of Electronics Engineering, Korea University, Seoul, South Korea, in 2007. He was a Post-Doctoral Researcher with the University of California, Los Angeles, from 2008 to 2013. In 2014, he joined the Korea Institute of Science and Technology, Seoul. His current research area includes MEMS, electrochemical sensor, and nano/microbiosensors.

Tony Jun Huang received the Ph.D. degree in mechanical and aerospace engineering from the University of California, Los Angeles, in 2005. He is currently a Professor and The Huck Distinguished Chair in Bioengineering Science and Mechanics with The Pennsylvania State University. He has authored or co-authored over 160 peer-reviewed journal publications in these fields. His research interests are in the fields of acoustofluidics, optofluidics, and micro/nanosystems for biomedical diagnostics and therapeutics.

Po-Hsun Huang received the B.Sc. degree in mechanical engineering from the National Taiwan University of Science and Technology, Taipei, Taiwan, in 2006, and the M.Sc. degree in mechanical engineering from the National Taiwan University, Taipei, in 2008. He is currently pursuing the Ph.D. degree in engineering science and mechanics from The Pennsylvania State University, University Park, USA. Since 2012, he has been working with Dr. T. J. Huang with The Acoustotfluidics Laboratory, The Pennsylvania State University.

Liqiang Ren received the B.S. degree in optics from the Harbin Institute of Technology, Harbin, China, in 2010, and the M.S. degree in optics from Fudan University, Shanghai, China, in 2013. He is currently pursuing the Ph.D. degree with the Engineering of Science and Mechanics Department, The Pennsylvania State University, PA. USA. His research focuses on the development and applications of acoustiofluidc and optofluidic systems.

Bishow Adhikari received the Ph.D. degree in molecular biophysics from Florida State University in 1997. He is currently a Program Director with the Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, National Institutes of Health. His responsibilities include the administration, planning, and oversight of a portfolio of basic and translational research programs in several forms of heart diseases/disorders, including heart failure, arrhythmias, cardiotoxicities, and various cardiomyopathies. He has organized and contributed to several initiatives, workshops, and working groups in these research areas. Prior to NIH, he conducted basic research work in skeletal and cardiac muscle function, applying biophysical, biochemical, and bioengineering approaches to study the structure, function, and regulation of key sarcomeric proteins involved in muscle contraction and force generation.

Jue Chen received the Bachelor of Medicine degree in preventive medicine and the master’s degree in toxicology from Fudan University, Shanghai, China, in 2001 and 2004, respectively, and the Ph.D. degree in pharmacology from Emory University in 2011. She joined the Laboratory of Biochemistry, Intramural Program, National Heart, Lung, and Blood Institute (NHLBI). In 2015, she then joined the NHLBI extramural program as a Program Officer with the Division of Cardiovascular Sciences (DCVS).
Dr. Chen’s past research experience includes the investigation of cardiotoxicity of environmental and occupational cadmium exposure, understanding the role of an antioxidative response enzyme in the pathogenesis of Parkinson’s disease, and determining the biological functions of redox signaling mediated by methionine oxidation and reduction. She currently manages basic and preclinical research grants studying atherosclerosis in the Atherothrombosis and Coronary Artery Disease Branch of the DCVS in the NHLBI.

Erin Iturriaga received the Bachelors of Science degree from the School of Medicine, George Washington University, and the Masters of Science degree in nursing from the School of Nursing, George Washington University. She was recently accepted into the Doctorate of Nursing Practice Program with the School of Nursing, Yale University.
She serves as a Program Officer and Clinical Trials Specialist with the National Heart, Lung, and Blood Institute. She led an RFA called Onsite Tools and Technologies for Heart, Lung, and Blood Clinical Research Point-of- Care and has an interest in technology for home use especially in the aging population. She was an Invited Speaker at the American Heart Association Scientific Session 2015, Beyond COI: Academic/Industry Partnership in the Future of Health Technology, Orlando, FL, in 2015, and served as a Session Moderator on Condition Management with Mobile and Connected Health at the mHealth Summit Annual Meeting held in Washington, DC, in 2014. She also served as a Session Chair and Speaker at the Drug Information Association’s Annual Meeting on What’s the Point? Can Point of Care Technology Really Enhance Clinical Trials? held in Boston in 2013. She has a strong background in clinical research, including clinical trials management, education, and regulatory responsibilities.
Funding Statement
This work was supported by the National Heart, Lung, and Blood Institute within the National Institutes of Health under Award R43HL126441, Award R44HL123909, and Award R43HL126473.
References
- [1].Martin S., et al. , “Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: An epidemiological cohort study,” Diabetologia, vol. 49, no. , pp. 271–278, 2006. [DOI] [PubMed] [Google Scholar]
- [2].Keller T., et al. , “Sensitive troponin I assay in early diagnosis of acute myocardial infarction,” New England J. Med., vol. 361, no. 9, pp. 868–877, 2009. [DOI] [PubMed] [Google Scholar]
- [3].Thygesen K., et al. , “How to use high-sensitivity cardiac troponins in acute cardiac care,” Eur. Heart J., vol. 33, no. 18, pp. 2252–2257, 2012. [DOI] [PubMed] [Google Scholar]
- [4].Mcdonald H. P., Garg A. X., and Haynes R. B., “Interventions to enhance patient adherence to medication prescriptions,” J. Amer. Med. Assoc., vol. 288, no. 22, pp. 2868–2879, 2002. [DOI] [PubMed] [Google Scholar]
- [5].Mozaffarian D., “Heart disease and stroke statistics–2015 update: A report from the American Heart Association,” Circulation, vol. 131, no. 4, p. e29-322, 2015. [DOI] [PubMed] [Google Scholar]
- [6].(2015). Global Status Report on Non-Communicable Diseases (WHO). [Online]. Available: http://apps.who.int/iris/bitstream/10665/44579/1/9789240686458_eng.pdf
- [7].Sun B. C., et al. , “Comparison of the HEART and TIMI risk scores for suspected acute coronary syndrome in the emergency department,” Critical Pathways Cardiol, J. Evidence-Based Med., vol. 15, no. 1, pp. 1–5, 2016. [DOI] [PubMed] [Google Scholar]
- [8].Shah A. S. V., et al. , “High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study,” Lancet, vol. 386, no. 10012, pp. 2481–2488, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dougherty R. H. and Fahy J. V., “Acute exacerbations of asthma: Epidemiology, biology and the exacerbation-prone phenotype,” Clin. Experim. Allergy, vol. 39, no. 2, pp. 193–202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Global Initiative for Chronic Obstructive Lung Disease, Global Initiative Chronic Obstructive Lung Disease, Inc, Florence, Italy, 2014. [Google Scholar]
- [11].Pitta F., et al. , “Physical activity and hospitalization for exacerbation of COPD,” Chest, vol. 129, no. 3, pp. 536–544, 2006. [DOI] [PubMed] [Google Scholar]
- [12].Esteban C., et al. , “Impact of changes in physical activity on health-related quality of life among patients with COPD,” Eur. Respiratory J., vol. 36, no. 2, pp. 292–300, 2010. [DOI] [PubMed] [Google Scholar]
- [13].Lee-Chiong T. L., Ed., Sleep: A Comprehensive Handbook. New York, NY, USA: Wiley, 2006, pp. 73–78. [Google Scholar]
- [14].Ko P. R., Kientz J. A., Choe E. K., Kay M., Landis C. A., and Watson N. F., “Consumer sleep technologies: A review of the landscape,” J. Clin. Sleep Med., vol. 11, no. 12, pp. 1455–1461, Dec. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].NHLBI. (2013). Point-of-Care Technology for Cardiovascular Care. [Online]. Available: http://www.nhlbi.nih.gov/research/reports/2013-onsite-tools
- [16].King K. R., et al. , “Point-of-care technologies for precision cardiovascular care and clinical research,” JACC Basic Transl. Sci., vol. 1, nos. 1–2, pp. 73–86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morrow D. A., et al. , “National academy of clinical biochemistry laboratory medicine practice guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes,” Circulation, vol. 53, no. 4, pp. 552–574, 2007. [DOI] [PubMed] [Google Scholar]
- [18].Friess U. and Stark M., “Cardiac markers: A clear cause for point-of-care testing,” Anal. Bioanal. Chem., vol. 393, no. 5, pp. 1453–1462, 2009. [DOI] [PubMed] [Google Scholar]
- [19].McCord J., et al. , “Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I,” Circulation, vol. 104, no. 13, pp. 1483–1488, 2001. [DOI] [PubMed] [Google Scholar]
- [20].Shin K.-S., Lee K., Kang J. Y., and Chui C. O., “Novel T-channel nanowire FET with built-in signal amplification for pH sensing,” in IEDM Tech. Dig., 2009, pp. 1–4.
- [21].Shin K.-S., Lee K., Park J.-H., Kang J. Y., and Chui C. O., “Schottky contacted nanowire field-effect sensing device with intrinsic amplification,” IEEE Electron Device Lett., vol. 31, no. 11, pp. 1317–1319, Nov. 2010. [Google Scholar]
- [22].Mao Y., Shin K-S., Wang X., Ji Z., Meng H., and Chui C. O., “Semiconductor electronic label-free assay for predictive toxicology,” Sci. Rep., vol. 6, 2016, Art. no. 24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reichlin T. and Mueller C., “Serial changes in highly sensitive cardiac troponin improve the early diagnosis of acute myocardial infarction,” Evidence Based Med., vol. 17, no. 6, p. e10, 2012. [DOI] [PubMed] [Google Scholar]
- [24].Reichlin T., et al. , “Early diagnosis of myocardial infarction with sensitive cardiac troponin assays,” New England J. Med., vol. 361, no. 9, pp. 858–867, 2009. [DOI] [PubMed] [Google Scholar]
- [25].Alcalai R., Planer D., Culhaoglu A., Osman A., Pollak A., and Lotan C., “Acute coronary syndrome vs nonspecific troponin elevation: Clinical predictors and survival analysis,” Arch. Internal Med., vol. 167, no. 3, pp. 276–281, 2007. [DOI] [PubMed] [Google Scholar]
- [26].Apple F. S., Steffen L. M., Pearce L. A., Murakami M. M., and Luepker R. V., “Increased cardiac troponin I as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: The minnesota heart survey,” Clin. Chem., vol. 58, no. 5, pp. 930–935, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wong K. K., et al. , “Supportive care of the first 2 Ebola virus disease patients at the monrovia medical unit,” Clin. Infectious Diseases, vol. 61, no. 7, pp. e47– e51, 2015. [DOI] [PubMed] [Google Scholar]
- [28].Levy M. M., et al. , “The surviving sepsis campaign: Results of an international guideline-based performance improvement program targeting severe sepsis,” Intensive Care Med., vol. 36, no. 2, pp. 222–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chatelut E., White-Koning M. L., Mathijssen R. H. J., Puisset F., Baker S. D., and Sparreboom A., “Dose banding as an alternative to body surface area-based dosing of chemotherapeutic agents,” Brit. J. Cancer, vol. 107, no. 7, pp. 1100–1106, Sep. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Russell S. J., “Progress of artificial pancreas devices toward clinical use: The first outpatient studies,” Current Opinion Endocrinol., Diabetes, Obesity, vol. 22, no. 2, pp. 106–111, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Plaxco K. W. and Soh H. T., “Switch-based biosensors: A new approach towards real-time, in vivo molecular detection,” Trends Biotechnol., vol. 29, no. 1, pp. 1–5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Swain S. M., Whaley F. S., and Ewer M. S., “Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials,” Cancer, vol. 97, no. 11, pp. 2869–2879, Jun. 2003. [DOI] [PubMed] [Google Scholar]
- [33].Legha S. S., et al. , “Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion,” Ann. Internal Med., vol. 96, no. 2, pp. 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- [34].O’Brien M. E. R., et al. , “Brien, “Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer,” Ann. Oncol., vol. 15, no. 3, pp. 440–449, Mar. 2004. [DOI] [PubMed] [Google Scholar]
- [35].Tuerk C. and Gold L., “Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase,” Science, vol. 249, no. 4968, pp. 505–510, Aug. 1990. [DOI] [PubMed] [Google Scholar]
- [36].Swensen J. S., et al. , “Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-based sensor,” J. Amer. Chem. Soc., vol. 131, no. 12, pp. 4262–4266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramaley L. and Krause M. S. Jr., “Theory of square wave voltammetry,” Anal. Chem., vol. 41, no. 11, pp. 1362–1365, 1969. [Google Scholar]
- [38].Csicsvari J., et al. , “Massively parallel recording of unit and local field potentials with silicon-based electrodes,” J. Neurophysiol., vol. 90, no. 2, pp. 1314–1323, Aug. 2003. [DOI] [PubMed] [Google Scholar]
- [39].Masoli M., Fabian D., Holt S., and Beasley R., “The global burden of asthma: Executive summary of the GINA Dissemination Committee report,” Allergy, Eur. J. Allergy Clin. Immunol., vol. 59, no. 5, pp. 469–478, 2004. [DOI] [PubMed] [Google Scholar]
- [40].2011 National Health Interview Survey (NHIS) Data: Lifetime Asthma, Current Asthma, Asthma Attacs Among Those With Current Asthma, accessed on Dec. 17, 2015. [Online]. Available: http://www.cdc.gov/asthma/nhis/2011/data.htm
- [41].Kraft M., “Asthma phenotypes and interleukin-13—Moving closer to personalized medicine,” New England J. Med., vol. 365, no. 12, pp. 1141–1144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ding X., et al. , “Standing surface acoustic wave (SSAW) based multichannel cell sorting,” Lab Chip, vol. 12, no. 21, pp. 4228–4231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shi J., Huang H., Stratton Z., Huang Y., and Huang T. J., “Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW),” Lab Chip, vol. 9, no. 23, pp. 3354–3359, 2009. [DOI] [PubMed] [Google Scholar]
- [44].Huang P. H., et al. , “An acoustofluidic sputum liquefier,” Lab Chip, vol. 15, no. 15, pp. 3125–3131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen Y., et al. , “Standing surface acoustic wave (SSAW)-based microfluidic cytometer,” Lab Chip, vol. 14, no. 5, pp. 916–923, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huang P.-H., et al. , “An acoustofluidic micromixer based on oscillating sidewall sharp-edges,” Lab Chip, vol. 13, no. 19, pp. 3847–3852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li S., et al. , “Standing surface acoustic wave (SSAW)-based cell washing,” Lab Chip, vol. 15, no. 1, pp. 331–338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].FDA. (2014). Home Use Tests. [Online]. Available: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/HomeUseTests/default.htm