Abstract
Background
Helicobacter pylori is the primary cause of gastric cancer, but about 9% of cases harbor Epstein-Barr virus (EBV) in the tumor cells. There is limited evidence on the possible interaction or antagonism between these infectious agents in gastric carcinogenesis.
Methods
We compared H. pylori serological profiles of EBV-positive (n=58) and -negative (n=111) noncardia gastric cancer patients from the United States National Cancer Institute's International EBV-Gastric Cancer Consortium. EBV positivity of tumors was assessed by in situ hybridization. Serum levels of 15 antibodies to immunogenic proteins of H. pylori (Cad, CagA, Cagδ, CagM, Catalase, GroEL, HcpC, HP0231, HP0305, HpaA, HyuA, NapA, Omp, UreA, VacA) were assessed using bead-based multiplex serology. Logistic regression models were used to adjust odds ratios (OR) for country, age, sex, and year of diagnosis.
Results
Seropositivity to individual proteins ranged up to 90% overall. Antibodies to Catalase were borderline associated with tumor EBV positivity (adjusted OR=3.15, p=0.0024, Bonferroni corrected p=0.036). Distributions of other antibodies did not vary by tumor EBV status.
Conclusion
Similarity of host response indicates the essential etiological role of H. pylori in EBV-positive gastric cancer.
Keywords: EBV, gastric cancer, H. pylori, interaction, serology
Introduction
Gastric cancer represents the third leading cause of cancer death worldwide [1]. Chronic Helicobacter pylori infection is the primary cause of these tumors in the noncardia stomach. Antibody reactivity is commonly used in epidemiologic studies to identify exposure to H. pylori infection. The sensitivity and specificity of serologic assays depend on the antigen(s), population characteristics, and the presumed gold standard. Commercially available enzyme-linked immunosorbent assays generally use whole bacterial cell preparations as antigens. Serological reactivity to individual H. pylori proteins provides a more detailed characterization of host immune response, and has been recently applied in case-control studies of preneoplastic [2, 3] and neoplastic gastric lesions [4, 5].
Epstein-Barr virus (EBV) is also implicated in gastric carcinogenesis, as about 9% of gastric tumors harbor monoclonal viral episomes [6]. Presence of EBV in tumors can be reliably determined by in situ hybridization for EBV-encoded RNA [7]. EBV-positive gastric tumors have demographic and clinicopathologic differences from EBV-negative tumors. Tumor EBV positivity is increased with male sex, smoking, non-antral gastric subsites and post-gastrectomy [6, 8]. In addition, patients with EBV-positive gastric tumors have better overall survival as compared to those with EBV-negative tumors [9]. A comprehensive evaluation of 295 primary gastric tumors by The Cancer Genome Atlas project [10] identified EBV-positive gastric cancer as one of the four molecular subtypes. In particular, EBV-positive tumors are characterized by recurrent PIK3CA mutation, almost complete absence of TP53 mutation, JAK2 amplification and extreme DNA hypermethylation. Taken together, these findings suggest that EBV-positive gastric cancer is a distinct disease entity.
There is limited evidence on the possible interaction or antagonism between H. pylori and EBV in gastric carcinogenesis. In an in vitro study, Minoura-Etoh et al. [11] found that reactive products from H. pylori infection (e.g., monochloramine) trigger EBV reactivation in latently infected gastric epithelial cells. In a nested case-control study, Levine et al. [12] reported significantly elevated total immunoglobulin (Ig) G anti-H. pylori antibody levels in participants who later developed EBV-negative gastric tumors, but not among those developing EBV-positive tumors, as compared to cohort controls. However, in a gastric cancer case series, Wu et al. [13] found similar prevalence of H. pylori seropositivity in patients with EBV-positive and -negative tumors.
To further address this question, and test the hypothesis that EBV-positive gastric cancer is an H. pylori-driven malignancy, we examined the association of H. pylori antibody levels with tumor EBV status using samples from the United States National Cancer Institute's International EBV-Gastric Cancer Consortium [9].
Materials and Methods
Study population
Five case series of noncardia gastric cancer (ICD-10 codes C16.1 - C16.9) from Korea, Japan, Poland, Mexico and Honduras were included in this analysis. For each series, serum samples from all available EBV-positive cases and a subset of EBV-negative cases were selected, frequency matched for sex, age at diagnosis (± 5 years), and year of diagnosis (± 2 years). This study comprises a total of 58 EBV-positive and 111 EBV-negative tumors (Table 1). Informed consent was obtained from all patients.
Table 1. Patient characteristics by tumor EBV status.
| Characteristics | EBV-positive tumors (n=58) | EBV-negative tumors (n=111) | ||
|---|---|---|---|---|
| Period of diagnosis, n(%) | 1994-2003 | 21 (30) | 50 (70) | |
| 2004-2010 | 19 (40) | 28 (60) | ||
| 2011-2012 | 18 (35) | 33 (65) | ||
| Country, n(%) | Korea | 15 (32) | 32 (68) | |
| Japan | 14 (50) | 14 (50) | ||
| Poland | 11 (50) | 11 (50) | ||
| Mexico | 6 (23) | 20 (77) | ||
| Honduras | 12 (26) | 34 (74) | ||
| Age in years, mean (SD) | 59 (11) | 60 (12) | ||
| Sex, n(%) | Male | 46 (38) | 75 (62) | |
| Female | 12 (25) | 36 (75) | ||
| Histological classification, n(%) | Diffuse | 29 (34) | 57 (66) | |
| Intestinal | 22 (37) | 38 (63) | ||
| Mixed | 1 (17) | 5 (83) | ||
| Unspecified | 6 (35) | 11 (65) | ||
Abbreviations: SD, standard deviation.
Tumor EBV detection
For all cases, the presence of EBV in cancer cells had been previously assessed by in situ hybridization for EBV-encoded RNA (EBER), using either an automated system or a manual staining method as previously described [8, 14, 15].
Helicobacter pylori multiplex serology assay
Serum samples were analyzed with multiplex serology based on a glutathione S-transferase capture immunosorbent assay combined with fluorescent-bead technology, as described elsewhere [16]. Seroprevalence of antibodies to 15 specific H. pylori proteins (Cad, CagA, Cagδ, CagM, Catalase, GroEL, HcpC, HP0231, HP0305, HpaA, HyuA, NapA, Omp, UreA, and VacA) was analyzed with a multiplex H. pylori serology assay [17]. Briefly, bead sorts each carrying a different antigen were mixed and incubated with human sera at 1:1000 dilutions. Antibodies bound to the beads via the bacterial antigens were stained with biotinylated goat anti-human IgG, IgA, IgM (Dianova, Hamburg, Germany) and streptavidin-R-phycoerythrin. Beads were examined in a Luminex 200 analyzer (Luminex, Austin, TX, USA) that identifies the pseudocolor of each bead sort and quantifies the antibody bound to viral antigen via the median R-phycoerythrin fluorescence intensity (MFI) of at least 100 beads of the same internal color. Seroprevalence cut-off values used for each antigen were calculated (mean MFI + 3 standard deviations, excluding positive outliers) in 30 H. pylori-negative sera previously classified for H. pylori status run within the same experiment [17]. Overall prevalence of infection was defined as seropositivity to at least 4 of the 15 proteins. Serum samples were tested in two batches, using the same lot of custom reagents. In addition to the in-house controls, we inserted six blinded replicates across plates. There was perfect agreement between replicates for all markers, with the exception of one discordant result for Cagδ.
Statistical analysis
Seroprevalence of antibodies for each antigen was compared in patients with EBV-positive and -negative gastric tumors using the Pearson χ2 test. A t-test was used to determine whether there was a difference by tumor group in the mean number of seroreactive proteins. Begg and Zhang [18] have demonstrated that the most efficient way to identify etiologic heterogeneity among subgroups is by directly comparing their risk profiles in a case-only study design. Following this analytical approach, we compared H. pylori serological patterns between patients with EBV-positive and -negative gastric tumors. Logistic regression models were used to estimate odds ratios (OR) with 95% confidence intervals (CI) of tumor EBV-positivity for antibodies to each H. pylori protein. ORs were adjusted for country, age (ordinal), sex (male vs. female), and year of diagnosis (tertiles based on the distribution in EBV-negative cases). Log-transformed MFI levels were alternatively analyzed as linear predictors, with exclusion of the Honduran cases (12 EBV-positive and 34 EBV-negative) for which the quantitative values were unavailable. Since some cases of overlapping and unspecified subsites (ICD-10 codes C16.8 - C16.9) may have arisen in the cardia (presumably unrelated to chronic H. pylori infection), we performed sensitivity analyses restricted to 139 cases with definite noncardia subsites (ICD-10 codes C16.1-C16.6). All p-values were two-sided, and Bonferroni correction was used to account for multiple comparisons. Statistical analyses were performed in Stata (version 10; Stata Corp, College Station, Texas, USA).
Results
Seropositivity to H. pylori proteins ranged from 14% (anti-CagM) to 90% (anti-CagA) overall. Patients with EBV-positive and -negative tumors were seroreactive to similar numbers of H. pylori proteins (means 8.6 vs. 8.1, respectively; p=0.32). Based on a pre-defined criterion of seropositivity to 4 or more bacterial proteins [17] , the overall seroprevalence of H. pylori infection was also comparable between patient groups: 95% for EBV-positive tumors vs. 92% for EBV-negative tumors (p=0.48).
Sixty-two percent of patients with EBV-positive tumors vs. 36% of patients with EBV-negative tumors had anti-Catalase antibodies (unadjusted OR=2.9, p=0.001, Bonferroni corrected p=0.022). In a multivariable analysis, seropositivity to Catalase was associated with tumor EBV status (OR=3.15, p=0.0024, Bonferroni corrected p=0.036). With the exception of anti-Catalase, the other bacterial antibodies were not associated with tumor EBV positivity (Table 2). Sensitivity analyses restricted to patients with definite noncardia tumors showed similar results (data not shown).
Table 2. Specific anti-Helicobacter pylori antibody prevalences (%) and associations with EBV-positive gastric cancer.
| Helicobacter pylori proteins | EBV-positive tumors (n=58) | EBV-negative tumors (n=111) | Adjusted odds ratio* (95% confidence interval) | |
|---|---|---|---|---|
| Short name (gene symbol) | Full name | |||
| Cad (HP1104) | Cinnamyl-alcohol dehydrogenase ELI3-2 | 22 | 23 | 0.68 (0.29-1.60) |
| CagA (HP0547) | Cytotoxin-associated gene A | 95 | 87 | 2.63 (0.66-10.5) |
| Cagδ (HP0522) | CAG pathogenicity island protein 3 | 9 | 19 | 0.43 (0.14-1.31) |
| CagM (HP0537) | CAG pathogenicity island protein 16 | 19 | 11 | 1.45 (0.55-3.82) |
| Catalase (HP0875) | Catalase | 62 | 36 | 3.15 (1.50-6.61) |
| GroEL (HP0010) | GroEL (also known as HSP60) | 83 | 81 | 0.83 (0.32-2.18) |
| HcpC (HP1098) | Cysteine–rich protein C | 66 | 59 | 0.96 (0.45-2.05) |
| (HP0231) | 55 | 51 | 0.90 (0.43-1.84) | |
| (HP0305) | 69 | 60 | 1.32 (0.63-2.77) | |
| HpaA (HP0410) | Putative neuraminyllactose-binding hemagglutinin homologue | 31 | 42 | 0.50 (0.23-1.06) |
| HyuA (HP0695) | Hydantoin utilization protein A | 66 | 65 | 0.85 (0.40-1.83) |
| NapA (HP0243) | Neutrophil activating protein A | 72 | 73 | 0.78 (0.35-1.74) |
| Omp (HP1564) | Outer membrane protein | 91 | 85 | 1.40 (0.43-4.51) |
| UreA (HP0073) | Urease alpha subunit | 45 | 45 | 0.97 (0.47-1.98) |
| VacA (HP0887) | Vacuolating cytotoxin A | 78 | 73 | 1.24 (0.53-2.89) |
Adjusted for country, age, sex and year of diagnosis
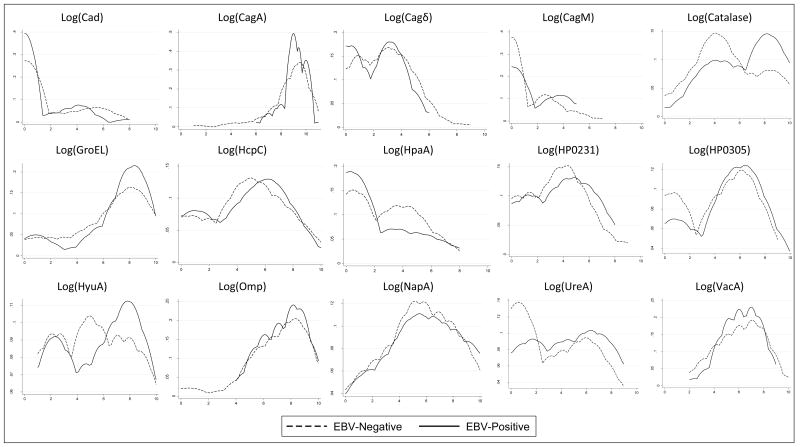
Distributions of log-transformed MFI for each antibody by tumor EBV status are presented in the Figure. Except for anti-Catalase, levels of other individual antibodies were generally similar between the two patient groups. Multivariable quantitative analyses of log-transformed antibody levels were generally null. In particular, the OR per unit change of anti-Catalase was 1.16 (p-value=0.049, Bonferroni corrected p=0.74).
Figure. Distribution of log-transformed antibody levels (MFI) to specific-H. pylori antigens by tumor EBV status.
Discussion
This case-case comparison found no interaction of EBV status with H. pylori serology, based on either overall or protein-specific humoral responses. Associations were consistent in analyses of qualitative and quantitative antibody levels. The small proportion of patients with overall seronegativity was similar between EBV-positive and negative cases, likely representing false negative serology since anti-H. pylori antibodies tend to diminish with progression of gastric atrophy [19]. On balance, the largely similar host-response to bacterial-specific proteins in both EBV-positive and -negative tumors reflects the essential role of H. pylori infection in gastric carcinogenesis. Our multicenter case series of gastric cancer represents the largest and most comprehensive evaluation of this association to date.
EBV is acquired early in life and establishes chronic latency in B-lymphocytes following primary infection. [20] EBV entry into either B lymphocytes or epithelial cells involves fusion of the virus envelope with a cell membrane. Fusion with an epithelial cell requires three envelope glycoproteins, gB and a binary complex of gHgL [21]. The exact stage during carcinogenesis when EBV enters gastric epithelial cells is unknown. Shibata and Weiss found EBER expression in dysplastic epithelium adjacent to cancer [22]. However, zur Hausen et al. [23] were unable to demonstrate EBER transcripts in lesions of intestinal metaplasia or dysplasia from patients who subsequently developed EBV-positive tumors either of the intact stomach or gastric stump.
Oxidative stress may play a critical role mediating EBV reactivation from latency [24], a postulated mechanism for the development of EBV-associated malignancies [25]. H. pylori Catalase counters host generation of reactive oxygen species by converting hydrogen peroxide into molecular oxygen and water. Higher seroreactivity to Catalase in patients with EBV-positive tumors may reflect immune-mediated oxidative stress and a concomitant increase in H. pylori Catalase production. Given the marginal statistical significance of our finding, whether or not Catalase is particularly involved in the development of EBV-positive tumors needs to be addressed in independent studies.
Although we did not assess the main effect of the bacterial infection with a non-cancer group, it is well-known from other evidence that chronic H. pylori infection is the primary cause of gastric cancer overall. We can therefore infer that H. pylori infection is also important in EBV-positive gastric cancer.
Acknowledgments
This study was funded by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, United States of America.
Footnotes
Conflicts of interest: none reported
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1 0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11. Lyon, France: International Agency for Research on Cancer; 2013. [Internet] [Google Scholar]
- 2.Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between Chronic Atrophic Gastritis and Serum Antibodies to 15 Helicobacter pylori Proteins Measured by Multiplex Serology. Cancer Research. 2009;69:2973–2980. doi: 10.1158/0008-5472.CAN-08-3477. [DOI] [PubMed] [Google Scholar]
- 3.Pan KF, Formichella L, Zhang L, Zhang Y, Ma JL, Li ZX, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer. 2014;134:2118–2125. doi: 10.1002/ijc.28560. [DOI] [PubMed] [Google Scholar]
- 4.Epplein M, Zheng W, Xiang YB, Peek RM, Li HL, Correa P, et al. Prospective Study of Helicobacter pylori Biomarkers for Gastric Cancer Risk among Chinese Men. Cancer Epidemiology Biomarkers & Prevention. 2012;21:2185–2192. doi: 10.1158/1055-9965.EPI-12-0792-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H, Michel A, Nyren O, Ekstrom AM, Pawlita M, Ye W. A CagA-independent cluster of antigens related to the risk of noncardia gastric cancer: associations between Helicobacter pylori antibodies and gastric adenocarcinoma explored by multiplex serology. Int J Cancer. 2014;134:2942–2950. doi: 10.1002/ijc.28621. [DOI] [PubMed] [Google Scholar]
- 6.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. Journal of Molecular Diagnostics. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo MC, Koriyama C, Matsuo K, Kim WH, Herrera-Goepfert R, Liao LM, et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. Int J Cancer. 2014;134:948–953. doi: 10.1002/ijc.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236–243. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minoura-Etoh J, Gotoh K, Sato R, Ogata M, Kaku N, Fujioka T, et al. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. Journal of Medical Microbiology. 2006;55:905–911. doi: 10.1099/jmm.0.46580-0. [DOI] [PubMed] [Google Scholar]
- 12.Levine PH, Stemmermann G, Lennette ET, Hildesheim A, Shibata D, Nomura A. Elevated antibody titers to Epstein-Barr virus prior to the diagnosis of Epstein-Barr-virus-associated gastric adenocarcinoma. Int J Cancer. 1995;60:642–644. doi: 10.1002/ijc.2910600513. [DOI] [PubMed] [Google Scholar]
- 13.Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H.pylori infection and genetic alterations. Gastroenterology. 2000;118:1031–1038. doi: 10.1016/s0016-5085(00)70355-6. [DOI] [PubMed] [Google Scholar]
- 14.Ryan JL, Morgan DR, Dominguez RL, Thorne LB, Elmore SH, Mino-Kenudson M, et al. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Lab Invest. 2009;89:80–90. doi: 10.1038/labinvest.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Kang WK, et al. Host Inflammatory Response Predicts Survival of Patients With Epstein-Barr Virus-Associated Gastric Carcinoma. Gastroenterology. 2010;139:84–U123. doi: 10.1053/j.gastro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 17.Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14:525–535. doi: 10.1111/j.1523-5378.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Zhang ZF. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev. 1994;3:173–175. [PubMed] [Google Scholar]
- 19.Kokkola A, Kosunen TU, Puolakkainen P, Sipponen P, Harkonen M, Laxen F, et al. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–624. doi: 10.1034/j.1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]
- 20.Shah KM, Young LS. Epstein-Barr virus and carcinogenesis: beyond Burkitt's lymphoma. Clin Microbiol Infect. 2009;15:982–988. doi: 10.1111/j.1469-0691.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 21.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alpha v beta 6 or alpha v beta 8. Proc Natl Acad Sci U S A. 2009;106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 23.Zur Hausen A, van Rees BP, van Beek J, Craanen ME, Bloemena E, Offerhaus GJ, et al. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: a late event in gastric carcinogenesis. J Clin Pathol. 2004;57:487–491. doi: 10.1136/jcp.2003.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang SY, Fang CY, Wu CC, Tsai CH, Lin SF, Chen JY. Reactive oxygen species mediate Epstein-Barr virus reactivation by N-methyl-N′-nitro-N-nitrosoguanidine. PLoS One. 2013;8:e84919. doi: 10.1371/journal.pone.0084919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol Immunol. 2014;58:307–317. doi: 10.1111/1348-0421.12155. [DOI] [PubMed] [Google Scholar]