1. Introduction
The rapid, effective and safe delivery of a broad range of therapeutics via the gastrointestinal (GI) tract remains a significant challenge in the field of drug delivery. The mucus layer coating the tissue, along with the low pH and wealth of degradative species, largely limit the kinetics and types of therapeutics that may be administered. As a result, technologies beyond formulation-based approaches are needed to enable the delivery of a wide-range of therapeutics in a rapid manner.
Physical enhancers, such as ultrasound (US), may afford the capacity to achieve ultra-rapid delivery via the GI tract of a wide-range of therapeutics currently limited to injection. Ultrasound (US) is a longitudinal pressure wave with a frequency above the audible range (>20 kHz) [1]. US has been investigated for decades for transdermal drug delivery [2]. US, when propagating through a fluid, can spontaneously nucleate voids (bubbles) in the solution, a phenomenon known as cavitation [1]. Research interest first focused on high-frequency (≥1 MHz) US to enhance the permeability of the skin [3]. More recently, research has focused on the use of low-frequency US (≤100 kHz) because of the observation of transient cavitation occurring at these frequencies. At low frequencies, the cavitation bubbles grow over cycles of the US pressure wave through a phenomenon known as rectified diffusion [4]. Eventually, the bubbles become unstable in size and implode, causing the surrounding fluid to rush into that void space, generating a microjet [1]. In transdermal applications, it is these microjets that have been shown to be the dominant mechanism of enhancement in drug delivery [2]. This phenomenon has been shown to enable the delivery of macromolecules, including biologics, such as insulin, via the skin [5].
Since those reports, low-frequency US has been explored extensively with respect to transdermal drug delivery and has been previously reviewed [1], [6]. The general findings from the transdermal field include the capacity to deliver nucleic acids [7], peptides [8], proteins [5], [6], as well as significantly enhance the delivery of small molecules [1], [9]. It was this capacity for delivery of a broad range of molecules, which motivated further exploration of this technology for drug delivery to parts of the body other than the skin. The application of low-frequency US in the GI tract was initially proposed by Kost and Langer in the mid 1980s through their patent application focusing on buccal delivery [10]. However, until recently, there were few reports in the literature investigating this method. Our group recently reported on the use of US in the GI tract (Figure 1). This work was motivated by the significant delivery potential of low-frequency US observed in the vast transdermal literature, the lack of a keratinized barrier in the GI tract, and the potential for modulation of beam profiles, enabling circumferential delivery through a single brief application. As a result, we hypothesized that application of US to the GI tract could provide rapid delivery of small molecules and also facilitate the delivery of macromolecules via the GI tract.
Figure 1. Ultrasound-mediated Gastrointestinal Drug Delivery.
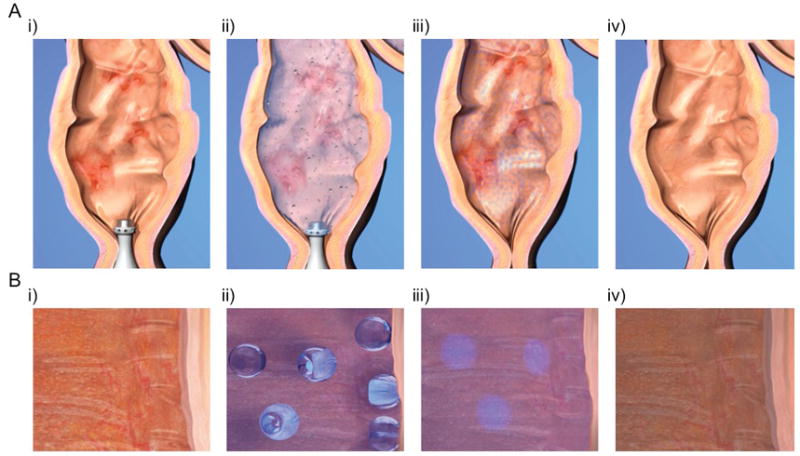
Macroscopic (A) and microscopic (B) view of the use of ultrasound in the colon for the treatment of inflamed mucosa in the setting of ulcerative colitis. i) Treatment is started by inserting the enema syringe into the colon. ii) When treatment is started, the enema is instilled in the colon and low-frequency ultrasound nucleates cavitation bubbles, which implode and drive microjets of drug (light blue) into the inflamed tissue. iii) After treatment, the device is removed and the drug begins to reduce inflammation. iv) After a course of treatment, inflammation is resolved.
2. Ultrasound-Mediated Gastrointestinal Drug Delivery
As part of this effort we initially conducted a drug delivery survey of the pig GI tract with a small molecule (glucose) co-administered with US [11]. This demonstrated up to 10-fold enhancement in the delivery of glucose utilizing an US treatment time of only one minute. This unique capacity was further expanded on by the evaluation of delivery of inulin (5 kDa), mesalamine, and hydrocortisone. Furthermore 3 kDa and 70 kDa dextrans serving as model macromolecules were successfully delivered with US ex vivo and shown to penetrate the entire thickness of the tissue with only one-minute of US treatment. This is in stark contrast to the typical treatment times required for transdermal drug delivery, which can exceed 10 minutes and requires co-administration with surfactants [1], [9]. The efficacy observed with short treatment times in the GI tract to achieve significant enhancement in delivery is thought to be a result of the architecture of the GI epithelium. The GI tract, in contrast to the skin, is designed to absorb nutrients and lacks a barrier analogous to the stratum corneum of the skin. Further, when used in the GI tract, the drug of interest may be co-administered with the US because of the short treatment time. As a result, the enhancement may also be due to US physically driving the drug into the tissue, as opposed to the US acting exclusively on the tissue. Longer treatment times used in transdermal delivery limit the co-administration of US and drug due to concerns of prolonged US treatments denaturing the therapeutic to be delivered.
The mechanism of enhancement in the GI tract was also investigated. Indeed, the onset of cavitation was confirmed through the detection of sub-harmonic frequencies when using 20 kHz US in the GI tract [11], [12]. In addition to cavitation, the contribution of thermal effects and acoustic streaming (bulk fluid motion) were investigated. While both heating and agitation of the permeant solution were found to enhance delivery over passive diffusion, the enhancement was significantly less than that achieved using 20 kHz US [11]. Interestingly, co-administration of US along with the drug was noted to provide greater delivery of the drug as compared to initial pre-treatment with US followed by exposure of the tissue to the drug. This observation supports the hypothesis that US also acts on the therapeutic, driving it into the tissue. This is an important and surprising feature of this technology.
We hypothesized that given the potential for radial emission and ease and acceptance of rectal administration, a potential initial indication for this technology could be for the treatment of ulcerative colitis. This subtype of inflammatory bowel disease (IBD) is characterized by inflammation of the colon in a distal to proximal distribution [13]. The standard recommendation for the treatment of mild to moderate disease includes enema treatments with mesalamine. The challenge with enema administration is the requirement for extended retention of the medicated enema to maximize the opportunity for diffusion of mesalamine into the tissue. Overnight retention of the liquid formulation is generally recommended, which, given the recognized symptoms of diarrhea and urgency, can be particularly challenging [14]. We therefore reasoned that treatment of ulcerative colitis might be significantly aided by US technology to enable ultra-rapid delivery of mesalamine.
We first set out to examine the safety and tolerability of this treatment in a large-animal model. We performed histological examination of biopsies taken from animals having received a single treatment, as well as daily US treatment for an extended period of time. Colon biopsies showed minor epithelial disruption in less than 5% of the area treated with US. Minor observations included patchy saponification of the adipose tissue and minimal congestion of intramucosal capillary vessels located in the superficial submucosa. Mucosal integrity was preserved and there was no evidence of epithelial damage. The lack of any significant tissue disruption is attributed to the short treatment duration. Additionally, the mucus layer over GI tissue may help prevent significant injury to the tissue as a result of US treatment. We then evaluated the capacity of US to deliver mesalamine in a large animal model and observed an order of magnitude enhancement in delivery as compared to standard diffusion, which would be observed with standard enema administration [11]. We also investigated the potential for US to deliver biologics that cannot be given via the GI tract currently. We chose insulin as a model biologic to allow for real-time quantification of the animal’s blood-glucose level to assess delivery. Indeed, insulin, when administered with US, resulted in a robust hypoglycemic effect. Insulin administration alone had no effect [11]. This highlights the potential platform nature of this technology and its capacity to deliver a wide-range of therapeutics without the need for significant reformulation.
To characterize the potential treatment benefits of enhanced mesalamine delivery, we then evaluated US treatment in a rodent model of colitis. Specifically, the dextran sodium sulfate model was treated with mesalamine with and without simultaneous US. Significant resolution in inflammation was observed when daily enemas were co-administered with US. This was supported by significant reduction in histological inflammation scores and significant improvement in a composite clinical score of stool consistency and occult bleeding [11]. The administration of US every other day demonstrated a trend towards improvement as well, supporting the likely benefit when used on an infrequent basis.
3. Conclusions
US has been investigated extensively for transdermal drug delivery. Only recently has it been investigated for its ability to enable GI-based drug delivery. US-mediated GI delivery was found to enable significant enhancement in the delivery of small molecule therapeutics and proteins in an ultra-rapid manner. Furthermore, these short ultrasound treatments reassuringly did not induce significant histological disruption or upregulation of pro-inflammatory cytokines, supporting the likely safety of this treatment. The ability to deliver both small and large molecules without significant reformulation is surprising and promising, underscoring the potential of this technology.
4. Expert Opinion
Physical enhancers, such as US, are a broadly applicable technology with significant clinical utility for the delivery of a wide-range of therapeutics without the need for reformulation. The ability of US to propagate through fluid media and act on a field stands to revolutionize our capacity to treat the GI tract, particularly in cases of IBD where the disease can affect a broad segment of mucosa. The capacity of US to significantly enhance drug uptake could have a significant positive impact on the inflamed mucosa as disease outcomes have been shown to correlate with local drug levels in inflamed tissue [14]. Therefore, a lead-indication for this technology could be for the treatment of IBD. This could free patients from having to retain the enema for extended periods as well as enable superior outcomes by maximizing delivery to the inflamed tissue. The capacity to deliver biologics should also prove useful in those patients who do not respond to mesalamine therapy and must be escalated to monoclonal antibodies targeting tumor necrosis factor alpha. Current systemic delivery of these antibodies has significant adverse effects and local delivery in the rectum may afford decreased side effect profiles.
Beyond IBD, US-mediated GI delivery (UMGID) could have significant utility in other GI diseases, including cancer, infections, and other inflammatory conditions. Translation of the technology to the clinic is aided by the already broad use of US clinically for other applications and by the fact that previous US-based systems have been FDA approved for transdermal drug delivery. Indeed, our study having demonstrated the safety and tolerability of treatments in large-animals is an important step towards translating these technologies.
Future developments in UMGID should include exploration of a wider repertoire of drugs including nucleic acids and other macromolecules for both topical and systemic delivery. Additionally, we see significant potential in novel probe design to facilitate use throughout the GI tract. Indeed, the potential utility of this technology will be aided by the ability to optimize device configurations for use in different parts of the body. In addition to potential at-home use by patients, devices might be created for use in clinical settings. Ultimately, we believe technological developments will enable fully ingestible devices capable of emitting US for systemic oral delivery. In the near-term, work should focus on developing these systems further for translation into the clinic.
Acronyms
- GI
Gastrointestinal
- IBD
Inflammatory Bowel Disease
- UMGID
Ultrasound-Mediated GI Delivery
- US
Ultrasound
Footnotes
Declaration of Interest
The authors were supported by NIH grants EB-00351 and DK-007191. CM Schoellhammer is co-inventor of: Provisional patent application 62/144,842 filed on 4/8/15. G Traverso is co-inventor of: Provisional patent application 62/144,842 filed on 4/8/15, described in [11]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1••.Polat BE, Hart D, Langer R, et al. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. Journal of Controlled Release. 2011;152:330–48. doi: 10.1016/j.jconrel.2011.01.006. Excellent review of the field of ultrasound-mediated transdermal drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. doi: 10.1038/nbt.1504. Excellent review of the field of transdermal drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman MK, Kill M, Frampton G. Effects of ultrasound alone and combined with hydrocortisone injections by needle or hydrospray. American Journal of Physical Medicine. 1958;37:206–9. [PubMed] [Google Scholar]
- 4.Eller A, Flynn HG. Rectified Diffusion during Nonlinear Pulsations of Cavitation Bubbles. J Acoust Soc Am. 1965;37:493–503. [Google Scholar]
- 5•.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–3. doi: 10.1126/science.7638603. The first demonstration of transdermal protein delivery using ultrasound. [DOI] [PubMed] [Google Scholar]
- 6.Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv. 2014;11:393–407. doi: 10.1517/17425247.2014.875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezel A, Dokka S, Kelly S, et al. Topical Delivery of Anti-sense Oligonucleotides Using Low-Frequency Sonophoresis. Pharm Res. 2004;21:2219–25. doi: 10.1007/s11095-004-7674-6. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Kalluri H, Herwadkar A, et al. Transcending the skin barrier to deliver peptides and proteins using active technologies. Crit Rev Ther Drug Carrier Syst. 2012;29:265–98. doi: 10.1615/critrevtherdrugcarriersyst.v29.i4.10. [DOI] [PubMed] [Google Scholar]
- 9.Schoellhammer CM, Srinivasan S, Barman R, et al. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. Journal of Controlled Release. 2015;202:93–100. doi: 10.1016/j.jconrel.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kost J, Langer RS. Ultrasound enhancement of transbuccal drug delivery. 1990 [Google Scholar]
- 11••.Schoellhammer CM, Schroeder A, Maa R, et al. Ultrasound-mediated gastrointestinal drug delivery. Science Translational Medicine. 2015;7:310ra168–310ra168. doi: 10.1126/scitranslmed.aaa5937. Demonstrates the utility of ultrasound-mediated gastrointestinal drug delivery in both small and large animal models for the first time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston K, Tapia-Siles C, Gerold B, et al. Periodic shock-emission from acoustically driven cavitation clouds: a source of the subharmonic signal. Ultrasonics. 2014;54:2151–8. doi: 10.1016/j.ultras.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Danese S, Fiocchi C. Ulcerative Colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 14.Frieri G, Giacomelli R, Pimpo M, et al. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000;47:410–4. doi: 10.1136/gut.47.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]


