Abstract
Rationale: Previous work found the lung microbiome in healthy subjects infected with HIV was similar to that in uninfected subjects. We hypothesized the lung microbiome from subjects infected with HIV with more advanced disease would differ from that of an uninfected control population.
Objectives: To measure the lung microbiome in an HIV-infected population with advanced disease.
Methods: 16s RNA gene sequencing was performed on acellular bronchoalveolar lavage (BAL) fluid from 30 subjects infected with HIV with advanced disease (baseline mean CD4 count, 262 cells/mm3) before and up to 3 years after starting highly active antiretroviral therapy (HAART) and compared with 22 uninfected control subjects.
Measurements and Main Results: The lung microbiome in subjects infected with HIV with advanced disease demonstrated decreased alpha diversity (richness and diversity) and greater beta diversity compared with uninfected BAL. Differences improved with HAART, but still persisted up to 3 years after starting therapy. Population dispersion in the group infected with HIV was significantly greater than in the uninfected cohort and declined after treatment. There were differences in the relative abundance of some bacteria between the two groups at baseline and after 1 year of therapy. After 1 year on HAART, HIV BAL contained an increased abundance of Prevotella and Veillonella, bacteria previously associated with lung inflammation.
Conclusions: The lung microbiome in subjects infected with HIV with advanced disease is altered compared with an uninfected population both in diversity and bacterial composition. Differences remain up to 3 years after starting HAART. We speculate an altered lung microbiome in HIV infection may contribute to chronic inflammation and lung complications seen in the HAART era.
Keywords: lung microbiome, HIV infection, advanced disease, microbial diversity
At a Glance Commentary
Scientific Knowledge on the Subject
A recently published article demonstrated that the lung microbiome in healthy subjects infected with HIV with preserved CD4 counts is similar to that of an uninfected population.
What This Study Adds to the Field
In this work we found that treatment-naive subjects infected with HIV with more advanced disease have an altered lung microbiome compared with uninfected control subjects. Furthermore, longitudinal data indicated that the microbiome was affected by antiretroviral therapy but still did not return to normal after up to 3 years of treatment.
Recognition of the potential impact of the human microbiome on health and disease led the National Institutes of Health to add the Human Microbiome Project to the National Institutes of Health Roadmap in 2007. Microbiomes of the gastrointestinal tract, oral and nasal cavities, skin, and urogenital tract have now been extensively characterized in healthy individuals and in various disease states. Analysis of the respiratory microbiome was initially hindered by the belief that the healthy lung is sterile. However, use of culture-independent microbial detection methods, such as 16S ribosomal RNA (rRNA) gene sequencing, has strongly suggested that a lung microbiome is present, both in healthy (1–3) and diseased (1, 4, 5) populations.
In 2009 the Lung HIV Microbiome Project (LHMP) consortium was formed by the NHLBI to study the lung microbiome. Because pulmonary complications remain a major cause of morbidity in subjects infected with HIV even in the highly active antiretroviral therapy (HAART) era (6), the fundamental question raised by the LHMP was whether HIV infection altered the respiratory microbiome. In a recently published consortium paper, the primary finding was that in a large healthy HIV cohort (median CD4 count in treatment-naive subjects, 668 cells/mm3; in subjects on HAART, 618 cells/mm3), the respiratory microbiome measured in whole bronchoalveolar lavage (BAL) was indistinguishable between an HIV-infected and uninfected population (7). To further address whether subjects infected with HIV with more advanced disease had a respiratory microbiome that differed from uninfected subjects and to determine the effect of HAART on the respiratory microbiome, we took advantage of a cohort of subjects infected with HIV who were studied with BAL before and up to 3 years after starting HAART. We found that treatment-naive subjects infected with HIV with lower CD4 counts have a respiratory microbiome that differs significantly from uninfected subjects. Furthermore, we show that HAART induces significant changes in the respiratory microbiome, although it remains different from uninfected control subjects even up to 3 years after starting therapy.
Methods
Participants
Twenty-two participants uninfected with HIV were recruited in Indianapolis, Houston, and Atlanta as part of the LHMP. The HIV cohort consisted of 30 subjects who underwent bronchoscopy before and at 1 month, 1 year, and 3 years after starting HAART as part of a prospective trial to assess risk factors for the immune reconstitution syndrome (IRIS) in subjects starting HAART. All subjects with HIV had to have a CD4 count less than 500 cells/mm3 at baseline. This research was approved by the Institutional Review Board at Indiana University, the University of Arizona, University of Houston, and Emory University. For more details on subject characteristics see the Methods section in the online supplement.
BAL Collection and Processing
Bronchoscopy with BAL was performed as previously described (8). Lavage fluid was passed through a 100-μm mesh to remove debris and then centrifuged at 400 × g for 10 minutes to pellet BAL cells. The acellular supernatant was harvested and stored at −70°C for subsequent batch DNA extraction and 16S rRNA gene sequencing. In this study we have analyzed acellular BAL fluid (not whole BAL as in the consortium paper) because of our belief that acellular BAL yields a more distinct respiratory microbiome (compared with oral washes) than whole BAL (9).
16S Gene Sequencing
Acellular BAL obtained at other sites was shipped to Indiana for DNA isolation and sequencing. Samples from all sites were processed at the same time. Thus, other than BAL acquisition variables, all samples were processed identically. DNA was extracted from 5 ml of acellular BAL as previously described (7) and shipped to The Genome Institute at Washington University for sequencing using Roche 454 FLX Titanium platform (Branford, CT) as previously described (2, 4). Primers for variable regions 1 through 3 (V1–3) were used. The 16S sequences were processed with the Mothur package (version 1.29) (10). High-quality 16S sequences from each sample were classified using the RDP Classifier (version 2.5) (11) with the default cutoff value of 0.8 from phylum to genus level. Species-level operational taxonomic units (OTUs) were produced by Mothur and BLASTed (12) against the RDP 162 database release 11.4 (13). Top BLAST hits were used as species-level annotations if the BLAST alignment achieved greater than or equal to 95% coverage and greater than or equal to 98% sequence identity. The Methods section in the online supplement provides more details on sequencing.
Data Analysis and Statistics
Baseline subject characteristics were compared using chi-square tests and two-sample Student’s t tests after logarithmic transformation if data were skewed. To account for the uneven sequencing depth between samples, a normalized procedure was used as previously described (14, 15). Differences in the abundance of specific genera between groups (i.e., uninfected vs. untreated, uninfected vs. 1-yr treated, and uninfected vs. 3-yr treated) were analyzed using negative binomial regression models. Alpha diversity richness was measured using Chao 1 and abundance-based coverage estimators (ACE) indices, and diversity evenness was assessed using the Shannon diversity index and Simpson’s index of diversity (1-D) (16). Alpha diversity between uninfected and infected groups was compared using linear regression models. Alpha diversity between untreated and treated groups was compared using mixed linear regression models (i.e., subject as random effect) by taking the correlations among the longitudinal data from the same subject into consideration. Beta diversity was visualized using nonmetric multidimensional scaling (17) and analyzed by measuring Bray-Curtis dissimilarity (18, 19) and UniFrac distances (20, 21). The PERMANOVA test was used to compare UniFrac distances or Bray-Curtis dissimilarities (22, 23) between various populations. Multivariate dispersion of groups was compared using the betadisper command in R vegan package (24, 25). Principal coordinates analysis (26) was performed at http://unifrac.colorado.edu. Benjamini-Hochberg corrections were used to adjust for multiple testing. The Methods section in the online supplement provides statistical details.
Results
Subject Demographics
There were BAL samples from 22 uninfected control subjects and 30 subjects infected with HIV (Table 1). Subjects infected with HIV were significantly younger, more likely to be white, and had a higher incidence of smoking than the uninfected control population. Table 2 shows baseline CD4 counts and viral loads in the HIV-infected cohort and the changes that occurred on HAART. All 30 subjects had a minimum follow-up of 1 year on HAART, and 12 subjects had 3 years of follow-up. HIV infection was advanced at baseline as evident by a median CD4 count of 280 cells/mm3 and a median viral load of 76,556 copies/ml. Antiretroviral treatment was effective as evidenced by a rapid and sustained drop in viral load and improved CD4 counts over the course of the study, although CD4 counts still remained well below those in the LHMP consortium paper (7). Importantly, although the HIV cohort was from a prospective cohort to identify IRIS in subjects infected with HIV starting antiretroviral therapy, none of the subjects had clinically significant IRIS events at the time of study. Furthermore, despite the increased incidence of smoking in the HIV population, spirometry was normal in this group (see Table E1 in the online supplement). As a result, no subject was on inhaled steroids and only 3 of the 30 subjects were on inhaled albuterol.
Table 1.
Baseline Characteristics
| HIV (n = 30) | Uninfected (n = 22) | P Value | ||
|---|---|---|---|---|
| Baseline demographics |
||||
| Age |
37.3 (9.2) | 47.9 (10.9) | 0.0004 | |
| Race, n (%) |
||||
| African American |
10 (33.3) | 14 (63.6) | 0.03 | |
| White |
20 (66.7) | 8 (36.4) | ||
| Sex, n (%) |
||||
| Female |
6 (20.0) | 5 (22.7) | 1.0 | |
| Male |
24 (80.0) | 17 (77.3) | ||
| Smoker, n (%) |
||||
| No |
9 (30.0) | 14 (63.6) | 0.016 | |
| Yes |
21 (70.0) | 8 (36.4) | ||
| Duration of HIV, yr |
||||
| Mean |
3.99 | N/A | ||
| Median |
1.75 | N/A | ||
| Obstructive lung disease, n |
||||
| COPD |
0 | N/A | ||
| Asthma |
2 | N/A | ||
| Pulmonary HTN, n |
0 | N/A | ||
| Prior PCP infection, n |
2 | N/A | ||
| Prior Kaposi sarcoma, n |
2 | N/A | ||
| | ||||
| BAL yields |
||||
| BAL fluid, ml |
123.1 ± 29.4 | 118.3 ± 33.0 | 0.589 | |
| Total cells, 106 |
59.5 ± 33.4 | 14.0 ± 16.3 | 0.006 | |
| Macrophages, % |
84.7 ± 12.3 | 88.2 ± 11.5 | 0.31 | |
| Lymphocytes, % |
13.5 ± 12.2 | 8.9 ± 9.8 | 0.16 | |
| Neutrophils, % |
1.5 ± 3.0 | 0.8 ± 1.0 | 0.25 | |
| Eosinophils, % | 0.3 ± 0.8 | 0.1 ± 0.3 | 0.31 | |
Definition of abbreviations: BAL = bronchoalveolar lavage; COPD = chronic obstructive pulmonary disease; HTN = hypertension; N/A = not applicable; PCP = Pneumocystis carinii pneumonia.
Table 2.
Response to HAART in the Population Infected with HIV (Median and Interquartile Range)
| Baseline | 4 Weeks | Baseline versus 4 Weeks | 1 Year | Baseline versus 1 Year | 3 Years | Baseline versus 3 Years | |
|---|---|---|---|---|---|---|---|
| CD4 count | 280 (92–385) | 311 (189–459) | 0.222 | 400 (236–469) | 0.012 | 375 (318–529) | 0.029 |
| Viral load | 76,554 (24,100–125,000) | 299 (156–1,090) | <0.0001 | 25 (25–79) | <0.0001 | 25 (25–407) | <0.0001 |
Definition of abbreviation: HAART = highly active antiretroviral therapy.
Sequencing Yields
Genomic DNA was isolated from 5 ml of stored acellular BAL. The amount of genomic DNA isolated from acellular BAL was similar in the two groups. Genomic DNA was amplified with V1–V3 primers and 16S rRNA gene sequencing was performed. The average numbers of high-quality sequences were similar in the different groups, as was the number of bacteria identified at the genus level using the default confidence cutoff of 80% (see Table E2). After removing samples with less than 500 reads, there were 20 uninfected subjects (10 from Indiana, five from Emory, five from the University of Houston), 26 subjects infected with HIV at baseline, 22 subjects infected with HIV at 1 month of treatment, 25 subjects infected with HIV at Year 1 of treatment, and 9 subjects infected with HIV at Year 3 of treatment.
Baseline Comparison of HIV with Uninfected BAL Microbiome
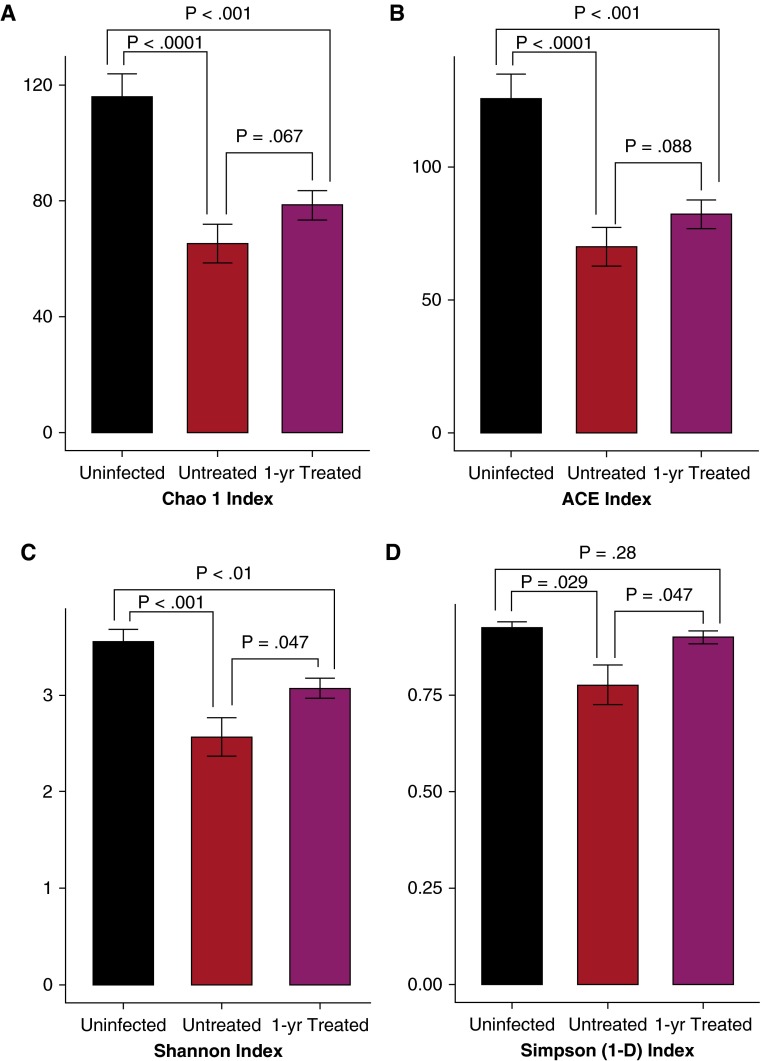
16S rRNA gene sequences from acellular BALs of 20 uninfected and 26 treatment-naive subjects infected with HIV were compared. At the OTU level Chao1 and ACE analysis demonstrated BAL from uninfected subjects was significantly richer compared with a treatment-naive HIV population (Figures 1A and 1B). Furthermore, Shannon and Simpson alpha diversity analysis showed that BAL from the uninfected population was significantly more diverse compared with BAL from the subjects with HIV (Figures 1C and 1D). Differences in alpha diversity indices remained even after adjusting for age, race, and smoking status (results not shown). Nearly identical results were seen when the same analysis was performed at the genera level instead of OTUs (see Figure E1).
Figure 1.
Comparison of alpha diversity in acellular bronchoalveolar lavage fluid at the operational taxonomic unit level between uninfected subjects (black), untreated subjects infected with HIV (red), and subjects infected with HIV treated with highly active antiretroviral therapy for 1 year (purple). Richness (A and B) and diversity (C and D) are significantly greater in the uninfected population compared with a treatment-naive population infected with HIV. However, differences between the population infected with HIV and uninfected control subjects are less after 1 year of treatment. ACE = abundance-based coverage estimators.
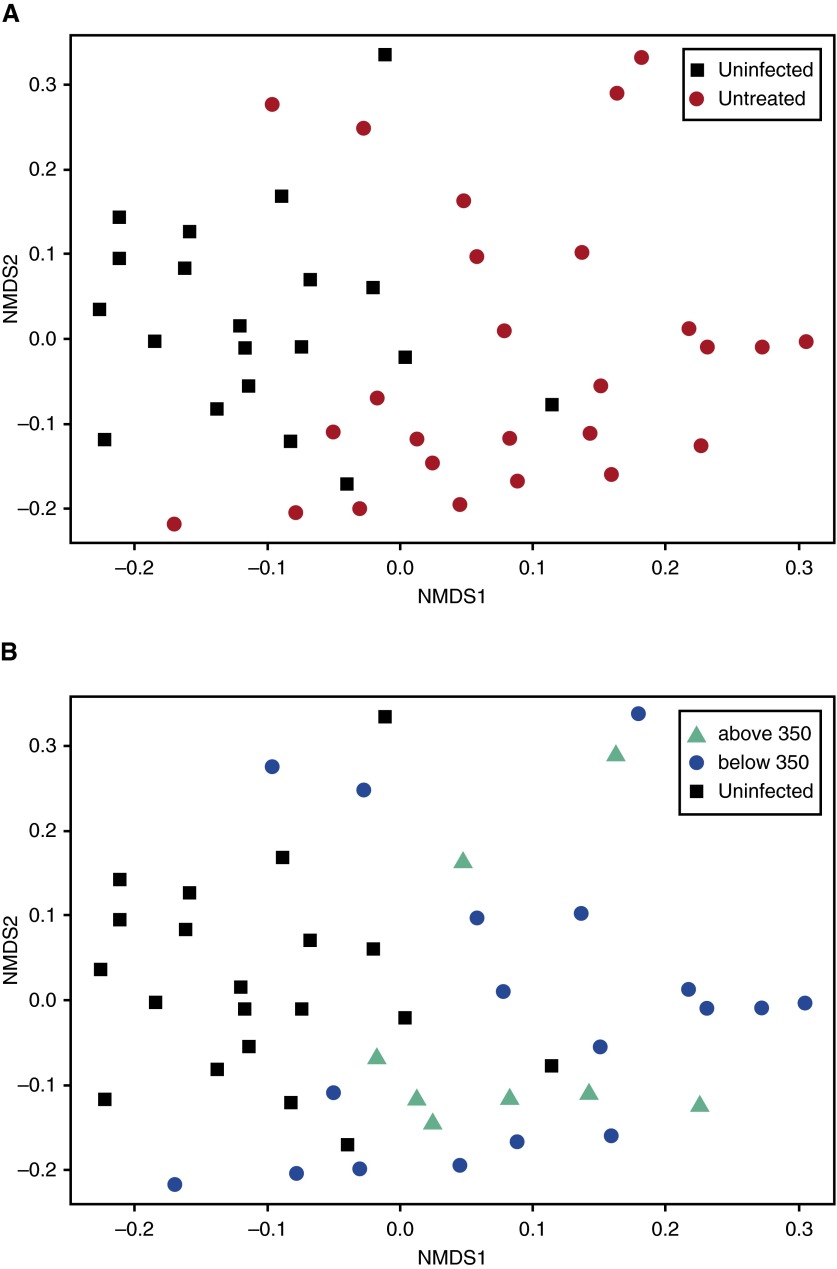
Next, population beta diversity at the OTU level was examined using weighted UniFrac analysis. BAL from subjects infected with HIV was significantly different compared with BAL from uninfected subjects (Figure 2A). These findings held even when considering the different sites from which uninfected control subjects came (see Figure E2). Subdividing the HIV cohort into subjects with CD4 counts above and below 350 cells/mm3 (Figure 2B) did not alter the results, although it should be emphasized that all of our subjects had CD4 counts less than 500 cells/mm3 at baseline. Similar results were obtained when analyzing population beta diversity at the genera level using Bray-Curtis Dissimilarity (see Figure E3). Interestingly, when dispersion of the two communities was analyzed, the untreated population infected with HIV was significantly more spread out compared with the normal population (average weighted UniFrac distance to centroid at the OTU level: 0.167 for the uninfected group, 0.204 for the untreated HIV-infected group; P = 0.041). Thus, although BAL microbiome diversity is low in an individual untreated subject infected with HIV compared with an uninfected volunteer, as a group the BAL microbiome from untreated subjects infected with HIV is much more varied than in uninfected subjects, with large differences between members of the HIV cohort.
Figure 2.
Comparison of beta diversity in bronchoalveolar lavage at the operational taxonomic unit level between uninfected subjects and untreated subjects infected with HIV using UniFrac Principal Coordinate Analysis. (A) The population infected with HIV (red) was significantly different compared with bronchoalveolar lavage from uninfected subjects (black). P = 0.001 using the PERMANOVA test. (B) Subdividing subjects infected with HIV into those with CD4 counts higher than (green) or lower than (blue) 350 cells/mm3 did not affect the results. NMDS = nonmetric multidimensional scaling.
We next investigated whether any bacterial taxa were different between uninfected subjects and subjects infected with HIV using a negative binomial model shown to be suitable for microbiome data (27). We limited this analysis to bacteria that were present in greater than 0.5% abundance in at least one population. The top 15 most abundant genera in uninfected subjects and subjects infected with HIV are shown in Table E3. After correction for multiple test comparisons, seven bacteria genera were found in different abundance between uninfected subjects and untreated subjects with HIV (Table 3). The most notable differences were an increase in the abundance of Streptococcus in the HIV population and an increase in Flavobacterium in the control population.
Table 3.
Most Significant Differences between Uninfected Subjects and Subjects Infected with HIV at Baseline
| Organism | Uninfected (%) |
HIV (%) |
P Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Streptococcus | 11.9 | 10.44 | 27.9 | 24.64 | 0.017 |
| Flavobacterium | 13.2 | 10.86 | 0.008 | 0.04 | <0.00001 |
| Curvibacter | 0.6 | 0.54 | 0 | 0 | 0.0004 |
| Methylobacterium | 0.7 | 1.24 | 0.1 | 0.44 | 0.04 |
| Corynebacterium | 1.9 | 1.86 | 0.4 | 1.12 | 0.04 |
| Rickettsia | 0.6 | 0.72 | 0 | 0 | 0.002 |
| Borrelia | 0.6 | 1.02 | 0 | 0 | 0.004 |
Effect of HAART on the HIV BAL Microbiome
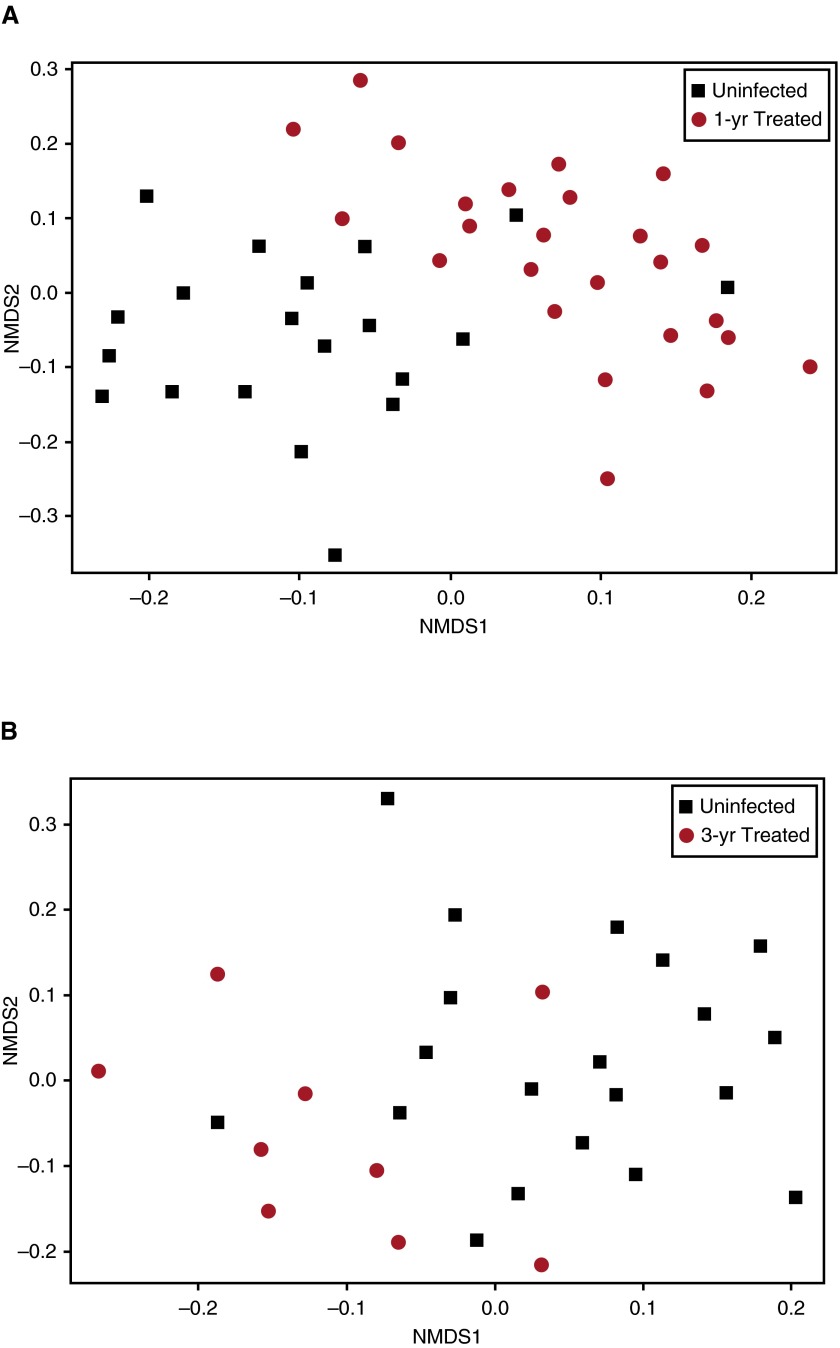
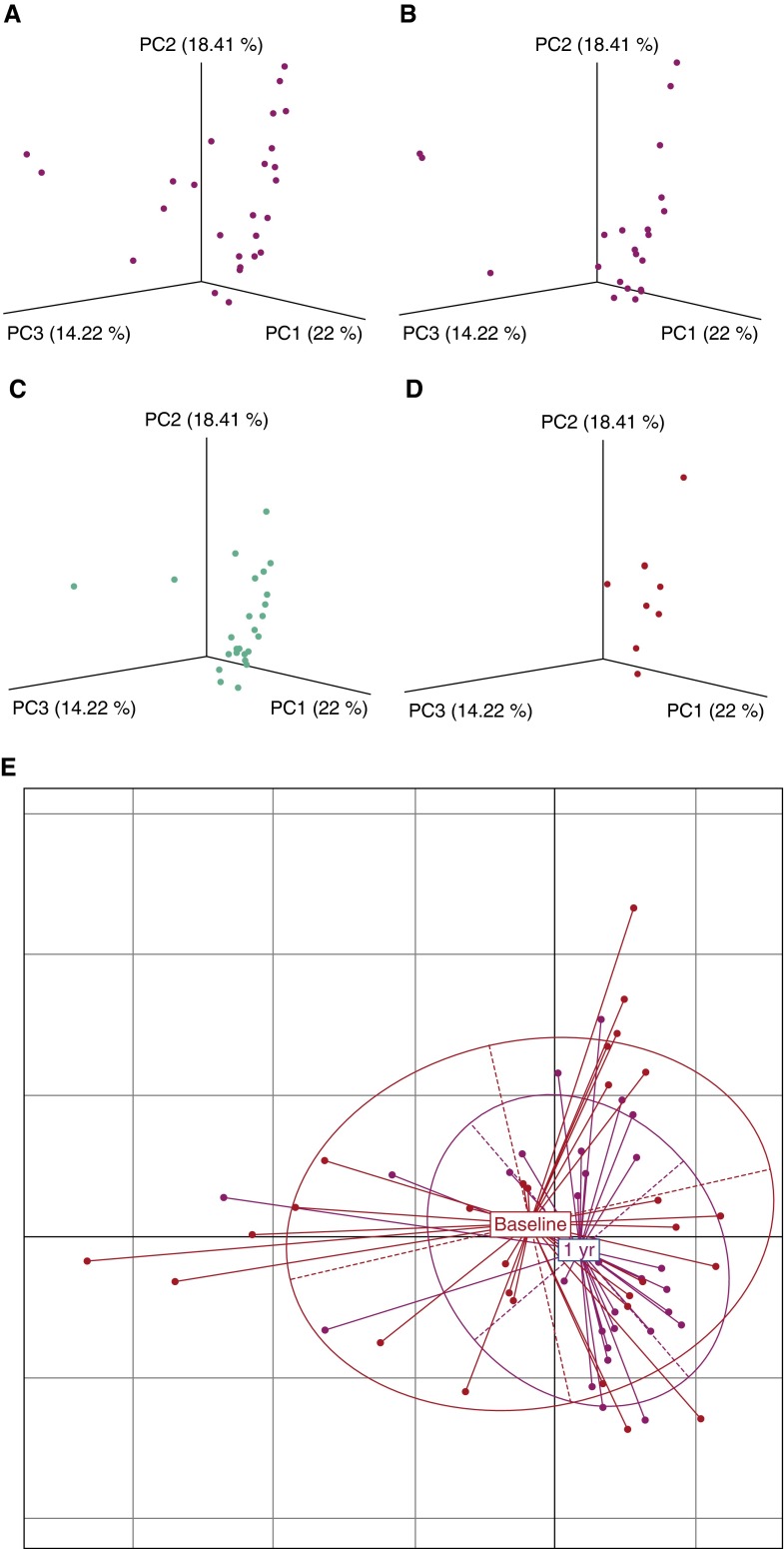
After 1 year of therapy differences in alpha diversity between uninfected and HIV BAL persisted, although in most instances the difference declined (Figure 1). Differences in beta diversity between uninfected BAL and BAL from treated subjects infected with HIV also persisted after 1 year on therapy (Figure 3 shows OTU level; Figure E4 shows genera level). However, UniFrac distances between uninfected subjects and subjects infected with HIV treated for 1 year were significantly less than distances between uninfected subjects and untreated subjects infected with HIV (P = 0.012) (see Figure E5), suggesting after 1 year of treatment HIV BAL contained more taxa similar to uninfected control subjects. Furthermore, principal coordinates analysis using weighted and normalized UniFrac distance showed beta diversity within the population infected with HIV decreased with time on HAART (Figures 4A–4D), suggesting the population was becoming more uniform over time. This was confirmed by dispersion analysis using weighted UniFrac distance (Figure 4E), showing that the population spread in subjects infected with HIV after 1 year of HAART was significantly less than in the untreated population (average distance to centroid: 0.157 for the 1-yr treated group, 0.204 for the untreated group; P = 0.005) and was no longer significantly different from uninfected control subjects (average distance to centroid: 0.167 for the uninfected group, 0.157 for the 1-yr treated group; P = 0.48). Results were nearly identical if analysis was confined to only those subjects who had both baseline and 1-year data available for comparison (P = 0.01). These data suggest BAL differences in alpha and beta diversity between uninfected subjects and subjects infected with HIV decrease with antiretroviral treatment, but have not returned to normal after 1 year of therapy.
Figure 3.
Comparison of beta diversity in bronchoalveolar lavage at the operational taxonomic unit level between uninfected subjects and treated subjects infected with HIV using weighted UniFrac Distance visualized by NMDS. Subjects infected with HIV who have been treated for 1 year (A), and for 3 years (B). The population infected with HIV (red) remains significantly different compared with bronchoalveolar lavage from uninfected subjects (black) even after 1 year on therapy. This difference is slightly less after 3 years of highly active antiretroviral therapy. NMDS = nonmetric multidimensional scaling.
Figure 4.
(A–D) UniFrac principal coordinates analysis of bronchoalveolar lavage samples from subjects infected with HIV before treatment and at 4 weeks, 1 year, and 3 years after therapy. With time on therapy, the subjects infected with HIV demonstrate more clustering, indicating a decrease in beta diversity within the population. (E) Principal coordinates analysis scatter plot of baseline and 1 year after treatment groups using weighted UniFrac distances. Multivariate homogeneity of group dispersions analysis showed dispersion of the population infected with HIV after 1 year of therapy was significantly less than in the untreated population (P = 0.005). PC = principal coordinate.
Next, we investigated which specific bacterial taxa might be different between uninfected subjects and subjects with HIV after 1 year of treatment (Table 4). The top 15 most abundant genera after 1 year of treatment are shown in Table E3. Streptococcus remained more abundant in the HIV population and Flavobacterium remained more abundant in the control population. Interestingly, after 1 year of HAART BAL from the HIV group now contained a greater amount of Veillonella and Prevotella compared with uninfected BAL. By 3 years after starting HAART the differences in the abundance of Streptococcus and Flavobacterium were much less compared with previous time points (P = 0.03 for Streptococcus and P = 0.04 for Flavobacterium). Furthermore, at this time point differences in abundance of Veillonella and Prevotella were no longer statistically different between the two groups. However, 3-year data should be viewed cautiously because they are based on only nine subjects infected with HIV from the original cohort.
Table 4.
Most Significant Differences between Uninfected Subjects and Subjects Infected with HIV after 1 Year of Treatment
| Organism | Uninfected (%) |
HIV (%) |
P Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Streptococcus | 11.9 | 10.50 | 21.6 | 14.66 | 0.02 |
| Flavobacterium | 13.2 | 10.86 | 0 | 0 | <0.00001 |
| Prevotella | 9.8 | 8.40 | 21.9 | 9.38 | 0.001 |
| Veillonella | 3.9 | 3.88 | 8.1 | 5.84 | 0.02 |
| Corynebacterium | 1.9 | 1.86 | 0.2 | 0.80 | 0.001 |
| Curvibacter | 0.6 | 0.54 | 0 | 0 | 0.0001 |
| Methylobacterium | 0.7 | 1.24 | 0 | 0 | 0.0001 |
| Staphylococcus | 2.0 | 4.40 | 0.04 | 0.16 | 0.0008 |
| Rickettsia | 0.6 | 0.71 | 0 | 0 | 0.002 |
| Borrelia | 0.6 | 1.03 | 0 | 0 | 0.001 |
| Actinomyces | 2.0 | 2.24 | 7.9 | 6.08 | <0.00001 |
| Burkholderia | 0.5 | 0.64 | 0.02 | 0.08 | 0.0001 |
| Propionibacterium | 1.0 | 1.18 | 0.2 | 0.42 | 0.001 |
| Sneathia | 0.11 | 1.65 | 1.1 | 1.68 | 0.003 |
| Atopobium | 0.4 | 0.54 | 0.9 | 0.68 | 0.01 |
| Parvimonas | 0.5 | 0.64 | 1.1 | 1.0 | 0.02 |
Finally, given the potential importance of Veillonella and Prevotella as components of a proinflammatory lung microbiome (28), we specifically assessed changes in these organisms over time in the population infected with HIV. Table 5 shows that the relative abundance of these genera increased between baseline and 1-year treated subjects. By 3 years of treatment the abundance of Prevotella had significantly decreased, whereas the abundance of Veillonella remained stable.
Table 5.
Changes in Relative Abundance of Prevotella and Veillonella in BAL over Time after Starting HAART
| Baseline | 1-Year Treated | Baseline vs. 1-Year Treated | 3-Years Treated | 1-Year Treated vs. 3-Years Treated | |
|---|---|---|---|---|---|
| Prevotella | 13.9 ± 11.2 | 21.9 ± 9.4 | 0.044 | 15.8 ± 11.3 | 0.003 |
| Veillonella | 5.5 ± 5.6 | 8.1 ± 5.8 | 0.12 | 9.5 ± 8.2 | 0.73 |
Definition of abbreviations: BAL = bronchoalveolar lavage; HAART = highly active antiretroviral therapy.
Data shown as percentage ± SD.
Discussion
We have demonstrated that untreated subjects infected with HIV with advanced disease have an altered respiratory microbiome measured in acellular BAL characterized by decreased alpha diversity (less richness and diversity) and greater beta diversity compared with normal BAL. These differences improve with time on HAART, but still have not returned to normal up to 3 years after starting therapy, at a time when the peripheral blood CD4 count has improved but is still significantly below normal. Population dispersion in the untreated group infected with HIV is significantly greater than in the uninfected cohort, but declines with treatment. We further identify differences in relative bacterial abundances in HIV-infected BAL compared with uninfected BAL both in untreated subjects and those who have been on HAART for 1 year. Finally, after 1 year on HAART, HIV BAL contains an increased abundance of Prevotella and Veillonella, bacteria previously associated with lung inflammation (28).
These results differ from a recent LHMP consortium paper demonstrating that the lung microbiome measured in whole BAL was indistinguishable between a healthy population infected with HIV and uninfected control subjects (7). This finding was unexpected given the high incidence of pulmonary complications in this population. Our results demonstrate that subjects infected with HIV with more advanced disease have a significantly different respiratory microbiome when compared with uninfected subjects and that differences decrease with time on HAART. Taken together, these two reports suggest that in healthy subjects infected with HIV, even those who have low CD4 counts before starting antiretroviral therapy, it is possible to attain a more normal-appearing lung microbiome as assessed by various diversity indices and the presence of signature bacteria. Our findings and those of the consortium further support the evolving practice of starting patients infected with HIV on antiretroviral therapy at earlier time points to try and prevent more severe immunologic and inflammatory perturbations and limit HIV-related complications (29, 30).
Our cohort also differs significantly from the work of Iwai and colleagues (31), who examined the lung microbioime in a Ugandan cohort of subjects infected with HIV with pneumonia. Despite differences in the cohorts, some interesting similar results were seen. First, Prevotella was one of the shared taxa identified in the entire Ugandan population, and Prevotella was the most significant contributor to the bacteria found in our patients infected with HIV after 1 year of HAART. Second, both the Ugandan and U.S. cohort demonstrated a large amount of beta diversity within the population suggesting it was not unusual for different specific bacteria to dominate the lung microbiome in individual subjects infected with HIV. In fact, we speculate that the large beta diversity in an untreated population infected with HIV makes comparisons of specific bacterial abundances between this group and an uninfected population problematic. This could explain why after 1 year of treatment, at a time when beta diversity has declined and the population infected with HIV has become more uniform, greater number of different bacterial abundances are now found between the HIV-infected and uninfected population comparison.
The presence of Prevotella and Veillonella as dominant organisms in subjects infected with HIV after 1 year of HAART is intriguing in light of recent work by Segal and colleagues (28) demonstrating that the presence of these organisms defines a microbiome pneumotypeSCT believed to be most reflective of a true lung microbiome from chronic microaspiration. Furthermore, these investigators demonstrate this pneumotype is associated with markers of lung inflammation. Numerous studies have demonstrated persistence of chronic lung inflammation even in subjects infected with HIV on HAART (32–34). It is tempting to speculate that this chronic inflammation may contribute to lung complications seen in the HAART era, which have moved away from classic opportunistic infections toward more chronic lung diseases, such as chronic obstructive pulmonary disease, pulmonary hypertension, and lung cancer (35). It is important to determine if similar correlations between the lung microbiome and markers of inflammation exist in these cohorts.
The other major difference between the two groups was an increased abundance of Streptococcus and a lower abundance of Flavobacterium in the HIV-infected group compared with uninfected subjects both at baseline and after 1 year of HAART. Interestingly, by 3 years after starting HAART all these differences (including the abundance of Prevotella and Veillonella) were less pronounced or had resolved. Although these results should be viewed with caution because of smaller sample size, they do indicate the possibility that antiretroviral therapy can return the lung microbiome to a more normal-appearing phenotype.
A key feature of our analysis is that it was performed on acellular BAL. The literature is mixed with some reports using acellular BAL (28), whereas others use whole BAL (2). Dickson and colleagues (36) have shown that analysis of whole and acellular BAL microbiomes yields different results. We also found that analysis of acellular and whole BAL yields different results (9). Furthermore, we found that acellular BAL is more distinct from oral wash (the presumed major source of upper airway contamination when obtaining BAL using bronchoscopic techniqes) compared with whole BAL, which we speculate is because of the pulling down of larger upper airway biofilms and bacterial clumps during the cell separation process (9). Because analysis of acellular BAL contains fewer bacteria shared with the upper airway, it may more closely represent true unique lung organisms. However, the loss of potential cell-associated bacteria during this process is an important consideration when viewing our results in the context of other published data.
Our study has several strengths and weaknesses. The greatest strengths of this work are the longitudinal nature of the data and that we studied subjects infected with HIV with more advanced disease but who were otherwise healthy at the time of bronchoscopy. Our cohort was also large enough to examine confounders between the uninfected and HIV-infected population, including age, race, and smoking differences. Finally, all samples were processed at the same time in the same place, minimizing the potential of confounding batch effects despite getting samples from different sites.
Despite this, a weakness in our study is the lack of upper airway and environmental controls in the longitudinal HIV-infected cohort. The importance of upper airway and environmental controls when trying to define a unique lower respiratory microbiome has been emphasized (37). Because the HIV-infected cohort came from a prior prospective study, environmental and upper airway controls were not available. Nevertheless, the longitudinal nature of this cohort strongly suggests that the changes observed over time are significant. Furthermore, our uninfected volunteer population did have oral wash and environmental controls. As previously described (4), we saw clear separation between BAL and oral wash samples in the uninfected population (see Figure E6). Furthermore, environmental controls demonstrated that preprocedure bronchoscopic washes, an appropriate control for environmental contamination (37), were completely different from lung and oral specimens (see Figure E6). Finally, we had fewer subjects who participated in research BAL at 3 years after initiation of HAART, limiting conclusions that can be drawn at this time point.
In summary, we have compared the microbiome in acellular BAL fluid from a population infected with HIV with advanced disease before HAART and an uninfected control population, and differences over time with initiation of HAART. In contrast to subjects infected with HIV with preserved CD4 counts, those with lower CD4 cell counts have an altered alveolar microbiome characterized by a loss of richness and diversity within individuals, but an increase in differences between subjects. These differences decrease with time on HAART, but have not returned to normal up to 3 years after starting treatment, when CD4 counts have improved but still remain lower than normal. Finally, the alveolar microbiome in subjects infected with HIV contain increased amounts of some signature bacteria even after 1 year of HAART, some of which have been previously associated with chronic lung inflammation. Future studies need to link lung microbiome alterations in HIV infection with pulmonary immunologic and inflammatory perturbations as potential mechanisms to explain the changing spectrum of pulmonary complications in patients living with HIV.
Supplementary Material
Footnotes
Supported by the National Institutes of Health/NHLBI grant U01 HL098960. In addition, this project was supported by the Indiana Clinical and Translational Sciences Institute funded in part by National Institutes of Health grant UL1 TR001108.
Author Contributions: Subject recruitment: H.L.T., K.S.K., K.A.C., and R.B.D. Experimental work (sequencing): D.E.N., E.T., E.S., and G.M.W. Data analysis: Q.D., J.Z., X.G., and H.L. Statistics: D.M. and B.P.K. Writing: H.L.T. (primary), K.S.K., K.A.C., Q.D., B.P.K., and G.M.W.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201509-1875OC on February 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, et al. Lung HIV Microbiome Project. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twigg HL, III, Morris A, Ghedin E, Curtis JL, Huffnagle GB, Crothers K, Campbell TB, Flores SC, Fontenot AP, Beck JM, et al. Lung HIV Microbiome Project. Use of bronchoalveolar lavage to assess the respiratory microbiome: signal in the noise. Lancet Respir Med. 2013;1:354–356. doi: 10.1016/S2213-2600(13)70117-6. [DOI] [PubMed] [Google Scholar]
- 4.Lozupone C, Cota-Gomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E, Mitreva M, Abubucker S, Martin J, Yao G, et al. Lung HIV Microbiome Project. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187:1110–1117. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr. 2011;23:319–324. doi: 10.1097/MOP.0b013e32834604f2. [DOI] [PubMed] [Google Scholar]
- 6.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS. 2006;20:1095–1107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 7.Beck JMSP, Schloss PD, Venkataraman A, Twigg H, III, Jablonski KA, Bushman FD, Campbell TB, Charlson ES, Collman RG, Crothers K, et al. Lung HIV Microbiome Project. Multi-center comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected individuals. Am J Respir Crit Care Med. 2015;192:1335–1344. doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twigg HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, Schnizlein-Bick CT. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159:1439–1444. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 9.Twigg HL , Day RB, Gregory RL, Dong Q, Rong R, Knox KS, Crothers K, Sodergren E, Weinstock G. Comparison of whole and acellular bronchoalveolar lavage to oral wash microbiomes. Should acellular bronchoalveolar lavage be the standard? Ann Am Thorac Soc. 2014;11:S72–S73. [Google Scholar]
- 10.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maidak BL, Olsen GJ, Larsen N, Overbeek R, McCaughey MJ, Woese CR. The ribosomal database project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawlena H, Rynkiewicz E, Toh E, Alfred A, Durden LA, Hastriter MW, Nelson DE, Rong R, Munro D, Dong Q, et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 2013;7:221–223. doi: 10.1038/ismej.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou M, Rong R, Munro D, Zhu C, Gao X, Zhang Q, Dong Q. Investigation of the effect of type 2 diabetes mellitus on subgingival plaque microbiota by high-throughput 16S rDNA pyrosequencing. PLoS One. 2013;8:e61516. doi: 10.1371/journal.pone.0061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faith DP. Phylogenetic pattern and the quantification of organismal biodiversity. Philos Trans R Soc Lond B Biol Sci. 1994;345:45–58. doi: 10.1098/rstb.1994.0085. [DOI] [PubMed] [Google Scholar]
- 17.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H.Vegan: community ecology package. R package version 2.3-0. 2015[accessed 2015 Sept 1]. Available from: https://cran.r-project.org/web/packages/vegan/index.html
- 18.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw. 2007;22:1–19. [Google Scholar]
- 20.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 23.McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- 24.Anderson MJ, Walsh DCI. Permanova, anosim, and the mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr. 2013;83:557–574. [Google Scholar]
- 25.Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006;62:245–253. doi: 10.1111/j.1541-0420.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamady M, Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res. 2009;19:1141–1152. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang R, Wagner BD, Harris JK, Fillon SA.Application of zero-inflated negative binomial mixed model to human microbiota sequence data. PeerJ PrePrints 2014[accessed 2015 Sept 1]. Available from: http://dx.doi.org/10.7287/peerj.preprints.215v1 [Google Scholar]
- 28.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z, Chen H, Berger KI, Goldring RM, Rom WN, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1:19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, et al. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, Ouattara E, Anzian A, Ntakpé JB, Minga A, et al. TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 31.Iwai S, Huang D, Fong S, Jarlsberg LG, Worodria W, Yoo S, Cattamanchi A, Davis JL, Kaswabuli S, Segal M, et al. The lung microbiome of Ugandan HIV-infected pneumonia patients is compositionally and functionally distinct from that of San Franciscan patients. PLoS One. 2014;9:e95726. doi: 10.1371/journal.pone.0095726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, Hage CA, Brahmi Z, Khoruts A, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris A, Crothers K, Beck JM, Huang L American Thoracic Society Committee on HIV Pulmonary Disease. An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc. 2011;8:17–26. doi: 10.1513/pats.2009-047WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twigg HL, III, Knox KS. HIV-related lung disorders. Drug Discov Today Dis Mech. 2007;4:95–101. doi: 10.1016/j.ddmec.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Cell-associated bacteria in the human lung microbiome. Microbiome. 2014;2:28. doi: 10.1186/2049-2618-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.