Significance
Human brain evolution is often considered synonymous with cortical expansion, in particular of the prefrontal cortex, a cortical region required for our remarkable cognitive abilities such as personality expression, planning, and decision making. In this study, we show that the expansion of numbers of cortical neurons in human and nonhuman primate evolution occurred in a similar manner across the cortex, without an increase in the relative number of neurons in the prefrontal region, and without a relative increase in the number of cells in the prefrontal white matter. One thing that distinguishes the human brain from other primate brains is thus not the relative size of its prefrontal cortex but its absolute number of neurons.
Keywords: cortical expansion, evolution, number of neurons, primate, prefrontal cortex
Abstract
Human evolution is widely thought to have involved a particular expansion of prefrontal cortex. This popular notion has recently been challenged, although controversies remain. Here we show that the prefrontal region of both human and nonhuman primates holds about 8% of cortical neurons, with no clear difference across humans and other primates in the distribution of cortical neurons or white matter cells along the anteroposterior axis. Further, we find that the volumes of human prefrontal gray and white matter match the expected volumes for the number of neurons in the gray matter and for the number of other cells in the white matter compared with other primate species. These results indicate that prefrontal cortical expansion in human evolution happened along the same allometric trajectory as for other primate species, without modification of the distribution of neurons across its surface or of the volume of the underlying white matter. We thus propose that the most distinctive feature of the human prefrontal cortex is its absolute number of neurons, not its relative volume.
Human evolution was long thought to have involved a selective enlargement of the prefrontal cortex, the cortical region related to some of our remarkable cognitive abilities such as personality expression, planning, and decision making (1, 2). However, recent publications showed that the gray matter of the human prefrontal cortex has the expected volume for a primate with the same brain size (3–5) and therefore does not diverge from the allometric rule of great apes—although Passingham and Smaers (6) still claim that the volume of the human prefrontal gray matter is larger than expected based on the volumes of the striate and motor areas in the brain. The amount of white matter is also still a subject of debate. The prefrontal white matter was observed to be significantly expanded in humans compared with other primates (4), especially in the left hemisphere (5), compared with the gray matter of the same region. However, analyzing the same data but accounting for the statistical effects of phylogenetic nonindependence among species, Barton and Venditti (7) observed instead that the volume of the prefrontal white matter in humans is not larger than expected for the volume of prefrontal gray matter, nonprefrontal white matter, or nonprefrontal gray matter. It seems from the controversy that any possible exceptionality of the white matter volume of the human prefrontal cortex must be small, because an exceptionality depends on the statistical analysis applied. Still, those studies were limited to the use of the only parameter available then, cortical volume, which we now know not to be a simple, direct reflection of numbers of neurons across cortical areas (8, 9). If the human prefrontal cortex was preferentially expanded in evolution, then its gray matter should hold a relatively larger fraction of all cortical neurons. If, instead, the human prefrontal white matter was preferentially expanded in evolution, then it should be composed of more cells than expected for its volume and for its number of prefrontal neurons. Alternatively, if the human prefrontal gray matter does have a relatively enlarged fraction of all cortical neurons, then the underlying white matter would also be expected to contain a relatively enlarged population of cells myelinating a larger number of connecting fibers.
Here we test these hypotheses by performing a systematic analysis of neuron distribution along the anteroposterior (A-P) axis of the cerebral cortex across eight primate species, including humans (9). We use the isotropic fractionator (10), a nonstereological method that yields results similar to those obtained with stereology (11), to analyze numbers of neurons and other cells in a systematic series of 2-mm coronal sections along the A-P axis of one cortical hemisphere of each species. For the purposes of this study, we define the prefrontal region of the cerebral cortex as all gray and white matter anterior to the corpus callosum in the coronal plane, according to Schoenemann et al. (4). In nonhuman primates, this region includes granular frontal cortex, orbital frontal cortex, ventromedial frontal cortex, and some rostral cingulate cortex, but not motor cortex and little of premotor cortex (12, 13). Although defining prefrontal region as anterior to the callosum is necessary due to the lack of reliable anatomical criteria to define the prefrontal cortex across all species (14), and because the thin histological slices required for cytoarchitectural analysis preclude using the isotropic fractionator to count neurons, it is an easily applicable criterion to all primate species and, most importantly, it makes our findings directly comparable to those of Schoenemann et al. (4).
Results
Prefrontal Cortex: Smaller Fiber Caliber.
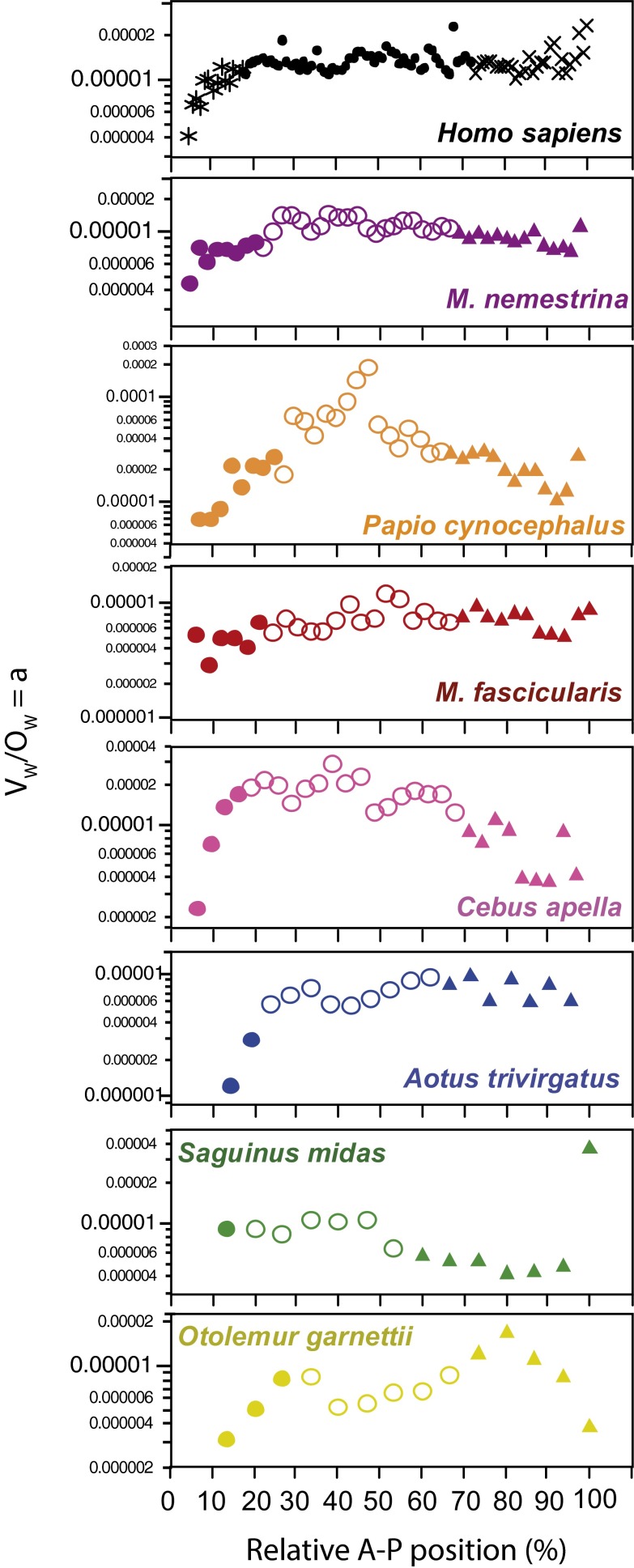
Along the A-P axis, we find that the ratio of the volume of the white matter to the number of other cells in the white matter (VW/OW) is smaller in those sections anterior to the corpus callosum (Fig. S1). The VW/OW ratio is not an accurate estimate of absolute white matter fiber caliber across species, because the fraction of nonmyelinated fibers and the proportion of myelin to fiber caliber are unknown. Still, the VW/OW ratio is a useful parameter for comparing variations in average fiber caliber within an individual cortex, because the ratio is proportional to the average cross-sectional area of myelinated axons (15). Thus, the smaller VW/OW ratio in sections anterior to the callosum (and therefore defined as prefrontal) suggests that these sections contain fibers of smaller caliber, in line with the previous finding that callosal fibers are thinnest at the genu (9, 16, 17). This consonance supports the conclusion that cortex anterior to the callosum indeed defines comparable prefrontal cortical regions across primate species, including humans (4, 18–21), even if these zones do not correspond to the entire associative areas in the frontal lobe.
Fig. S1.
Smaller average fiber caliber in cortex anterior to corpus callosum, defined here as prefrontal cortex. The ratio between VWM and OWM, which approximates the average fiber caliber, is shown for each coronal section along the A-P axis. Filled circles, prefrontal cortex (coronal sections anterior to corpus callosum); triangles, occipital cortex (posterior third of VGM along the A-P axis); and unfilled circles, intermediate regions. Species are shown in order of decreasing gray matter volume.
Cumulative Distribution of Neurons and Other Cells.
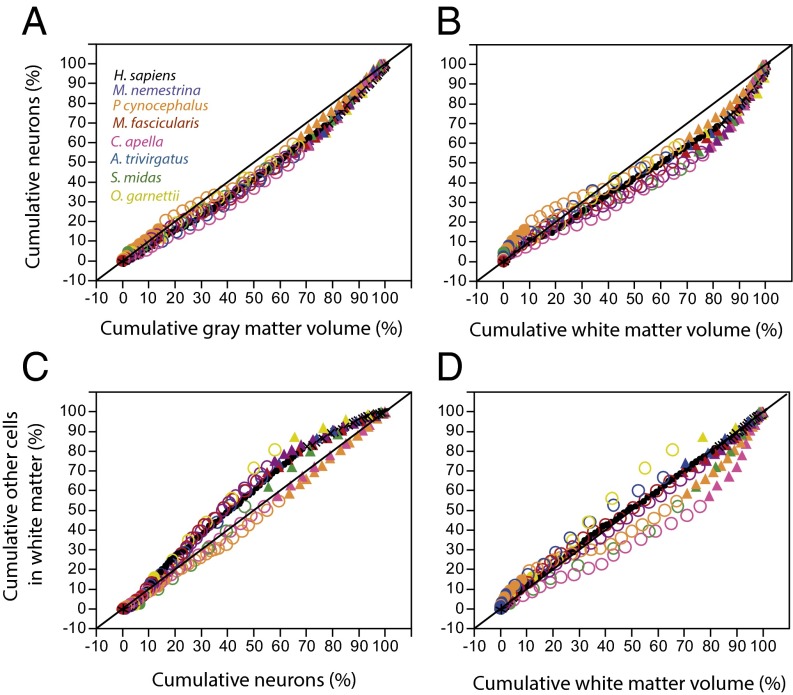
The distribution of cumulative gray matter volume (VGM) along the A-P axis does not correspond linearly to the cumulative distribution of cortical neurons in the same axis. In most species, the prefrontal region accumulates fewer neurons than expected for its cumulative volume (Fig. 1A and Table S1), which can be explained by the heterogeneous neuron density distribution across the A-P axis, with a large extent of cortex displaying higher neuronal densities in the posterior pole (Fig. 2). The prefrontal region corresponds to the anteriormost 10% of VGM or less in all species but Papio and accumulates 6–8% of all cortical neurons in six of the eight species, including humans (Table S1), whereas the posteriormost 10% of the VGM accumulates 20% of the cortical neurons.
Fig. 1.
Similar distribution of neurons along the A-P cortex across all species. Cumulative distributions (in percent) begin in the anterior (frontal) pole, at 0%, and reach 100% at the occipital pole. Cumulative distribution of neurons varies similarly with the cumulative distribution of VGM (A) and with the cumulative distribution of VWM (B) across all species, whereas there are two patterns of variation in the cumulative distribution of other cells in the white matter with the cumulative number of neurons (C) and VWM (D). However, the human cortex fits the distribution that applies to macaque species.
Table S1.
Percent volume and number of cortical neurons in prefrontal region
| Species | % VGM | % VWM | % OWM | % neurons |
| O. garnettii | 9.2 | 3.7 | 7.6 | 8.3 |
| S. midas | 7.4 | 3.6 | 2.8 | 6.4 |
| A. trivirgatus | 9.2 | 1.9 | 7.4 | 7.8 |
| C. apella | 6.6 | 3.0 | 3.6 | 4.2 |
| M. fascicularis | 7.7 | 4.4 | 6.5 | 6.9 |
| P. cynocephalus | 13.5 | 8.0 | 14.4 | 16.2 |
| M. nemestrina | 7.5 | 4.6 | 6.8 | 7.8 |
| Homo sapiens | 10.0 | 5.5 | 7.2 | 7.8 |
% VGM, percentage of gray matter volume contained in prefrontal region; % VWM, percentage of white matter volume contained in prefrontal region; % OWM, percentage of all other (nonneuronal) cells in the white matter contained in prefrontal region; and % neurons, percentage of all cortical neurons contained in prefrontal region.
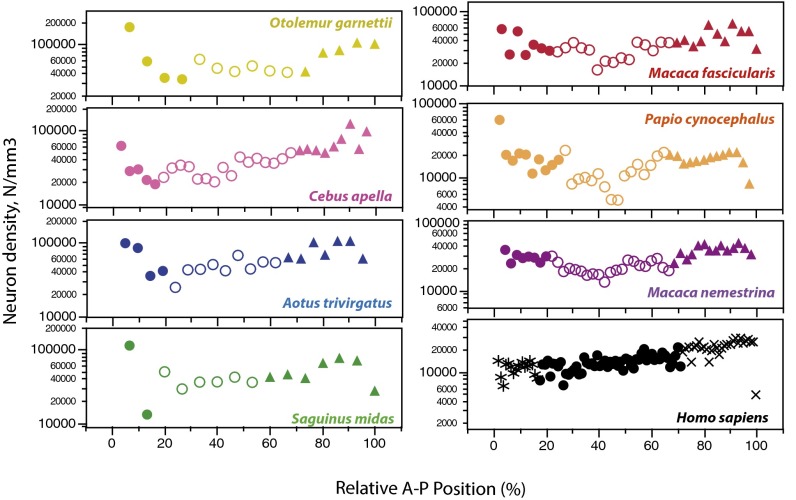
Fig. 2.
Neuronal density varies as a double gradient along the cortex. Each data point represents one cortical location in one species (colors) along the A-P axis. Neuronal density is highest in the frontal and occipital poles in all nonhuman species.
Importantly, across all species examined the cumulative distribution of neurons along the A-P axis varies as a similar function of the cumulative percentage of VGM (Fig. 1A). The overlap suggests that the eight species, including humans, share a similar neuronal distribution along the cortical surface, notwithstanding the 40-fold variation in volume across species. These findings are compatible with a scenario in which cortical expansion in primate evolution happened without a change in the pattern of neuron distribution across the surface.
The cumulative percentage of neurons along the A-P axis also varies as a similar function of the cumulative percentage of the volume of white matter (VWM) across all eight species (Fig. 1B). Again, the two cumulative distributions are not linearly related: Whereas the prefrontal region holds 8% of all cortical neurons and less than 10% of VGM in most species, it contains typically less than 4% of VWM (Fig. 1B and Table S1).
Interestingly, the cumulative distribution of other cells in the white matter (OWM) along the A-P axis varies with the cumulative number of cortical neurons in a similar fashion across five species, including humans, but differently from Papio, Cebus, and Saguinus, for which OWM accumulates linearly with the number of neurons along the axis (Fig. 1C). Likewise, the cumulative distribution of VWM along the A-P axis varies similarly across human and macaque cortices, although it differs in Cebus and Papio (Fig. 1D).
Neuronal Density Along the Cerebral Cortex.
The distribution of neurons along the A-P axis of the cerebral cortex exhibits a double gradient of neuronal density in all nonhuman primates, with the highest densities found in the frontal and occipital poles (Fig. 2). Within each nonhuman species, neuronal densities are higher in the prefrontal pole than in the intermediate region and increase posteriorly within the occipital region. In the human cortex, in contrast, the lowest neuronal densities occur in the prefrontal region and part of intermediate cortex (possibly motor cortex), and a gradient of neuronal densities is only seen within the occipital region (9) (Fig. 2, black).
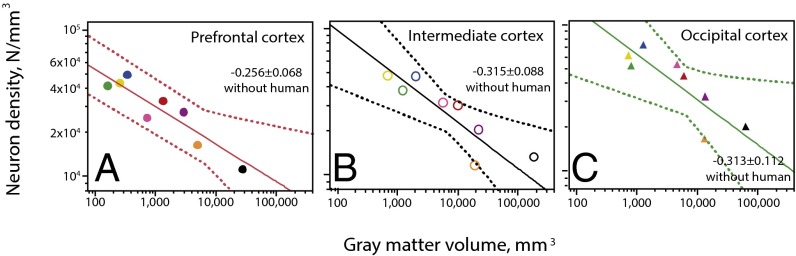
At first sight, the lowest neuronal densities in the human prefrontal region suggest a particularity of this species. However, quantitative analysis across nonhuman species shows that average neuronal density in the prefrontal region scales across species, decreasing as a power function of increasing prefrontal VGM (Fig. 3A), which suggests that average neuronal cell size increases with increasing gray matter volume in the prefrontal region. Most importantly, the low neuronal density in the human prefrontal region conforms to this scaling. A similar scaling of average neuronal density, decreasing with increasing local VGM across nonhuman species, applies to the intermediate (Fig. 3B) and occipital (Fig. 3C) regions of the cortex, and there again the human cortex fits the rules. Functions calculated using phylogenetically independent contrasts are similar to the functions calculated for raw data for these and all other analyses (Table S2), and therefore all figures show only the plotted raw data.
Fig. 3.
Neuronal density varies as a double gradient along the cortex. (A) Average neuronal density in the prefrontal cortex scales with VGM across nonhuman primate species with an exponent of −0.256 ± 0.068 (95% confidence interval, −0.392 to −0.120; r2 = 0.738, P = 0.0132). (B) Average neuronal density in the intermediate cortex scales with VGM across nonhuman primate species with an exponent of −0.315 ± 0.088 (95% confidence interval −0.491 to −0.139, r2 = 0.720, P = 0.0157). (C) The occipital cortex also shows a similar behavior with an exponent of −0.313 ± 0.112 (95% confidence interval, −0.537 to −0.089, r2 = 0.610, P = 0.0381). In all three regions, the neuronal density in the human cortex (black data points) conforms to the relationship that applies to other primates.
Table S2.
Slopes of allometric relationships for raw data and phylogenetically independent contrasts
| Raw data | Phylogenetically independent contrasts | |||||||
| Dependent variable | Independent variable | Region | Slope ± SE | r2 | P value | Slope ± SE | r2 | P value |
| Neuron density | VGM | PF | −0.256 ± 0.068 | 0.738 | 0.0132 | −0.328 ± 0.073 | 0.801 | 0.0065 |
| Int | −0.315 ± 0.088 | 0.720 | 0.0157 | −0.324 ± 0.113 | 0.620 | 0.0355 | ||
| Occ | −0.313 ± 0.112 | 0.610 | 0.0381 | −0.289 ± 0.147 | 0.435 | 0.1070 | ||
| VGM | Neurons | PF | 1.290 ± 0.118 | 0.960 | 0.0001 | 1.405 ± 0.153 | 0.944 | 0.0002 |
| Int | 1.349 ± 0.173 | 0.924 | 0.0006 | 1.297 ± 0.218 | 0.876 | 0.0019 | ||
| Occ | 1.285 ± 0.210 | 0.872 | 0.0017 | 1.158 ± 0.240 | 0.824 | 0.0047 | ||
| VWM | Other cells, WM | PF | 1.176 ± 0.222 | 0.848 | 0.0032 | 1.206 ± 0.303 | 0.760 | 0.0105 |
| Int | 1.241 ± 0.254 | 0.827 | 0.0045 | 1.310 ± 0.347 | 0.740 | 0.0130 | ||
| Occ | 1.137 ± 0.211 | 0.853 | 0.0030 | 1.158 ± 0.198 | 0.837 | 0.0021 | ||
| VWM | Neurons | PF | 1.656 ± 0.298 | 0.860 | 0.0026 | 1.765 ± 0.415 | 0.783 | 0.0081 |
| Int | 1.644 ± 0.208 | 0.926 | 0.0005 | 1.695 ± 0.258 | 0.896 | 0.0012 | ||
| Occ | 1.557 ± 0.260 | 0.878 | 0.0019 | 1.553 ± 0.271 | 0.868 | 0.0023 | ||
| Neurons, PF | Neurons, NPF | 0.917 ± 0.220 | 0.776 | 0.0088 | 0.779 ± 0.210 | 0.733 | 0.0139 | |
| Other cells, PF WM | Other cells, NPF WM | 0.966 ± 0.256 | 0.739 | 0.0131 | 0.914 ± 0.237 | 0.749 | 0.0119 | |
All functions calculated without human data points (n = 7). Neuron density in neurons per cubic millimeter. VGM, volume of cortical gray matter (cubic millimeters). VWM, volume of subcortical white matter (cubic millimeters). Int, intermediate region; NPF, nonprefrontal region; Occ, occipital region; and PF, prefrontal region.
Scaling of the Cortical Gray Matter.
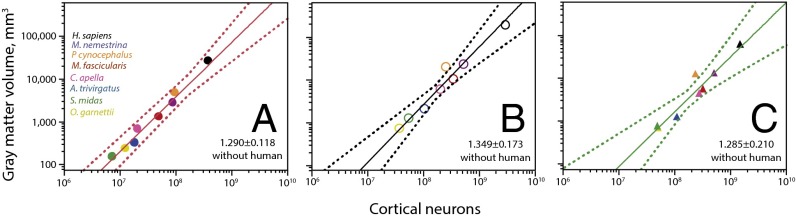
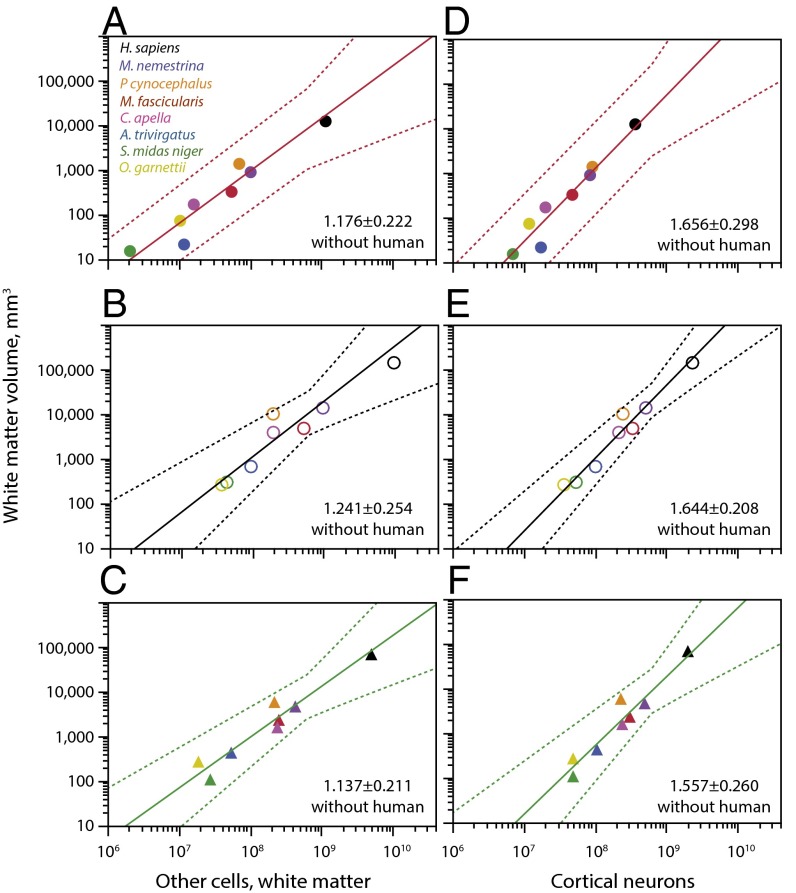
Across the seven nonhuman primate species, VGM scales as similar power functions of the number of neurons in each of the three cortical regions (prefrontal, intermediate, and occipital; Fig. 4). In particular, and in agreement with the decreasing neuronal density with increasing VGM in each region (which indicates increasing average neuronal cell size), the gray matter volume of the prefrontal region varies across nonhuman species as a power function of the number of cortical neurons in the region with an exponent of 1.290 ± 0.118 (r2 = 0.960, P = 0.0001; Fig. 4A). Strikingly, the value for the human cerebral cortex fits the relationship for nonhumans (Fig. 4A, black). The same pattern is found for the intermediate (Fig. 4B) and occipital (Fig. 4C) regions, in which the human cortical gray matter has the volume expected for its number of neurons. The similar exponents across all cortical regions suggest that the prefrontal region does not become preferentially expanded over other cortical regions as it gains neurons. Most importantly, the conformity of the human cortex to the nonhuman scaling relationships indicates that the human prefrontal gray matter does not hold an extraordinarily larger volume of gray matter for its number of neurons.
Fig. 4.
Human prefrontal gray matter scales in volume as expected for its number of neurons. Each graph shows how the total VGM in the prefrontal (A), intermediate (B), and occipital regions (C) in each species scales with the number of neurons in the cortical regions. Plotted functions exclude the human data points (in black). Exponents are (A, prefrontal) 1.290 ± 0.118 (r2 = 0.960, P = 0.0001), (B, intermediate) 1.349 ± 0.173 (r2 = 0.924, P = 0.0006), and (C, occipital) 1.285 ± 0.210 (r2 = 0.872, P = 0.0017). Notice that the human cortex has the expected VGM for the local number of neuron in each of the three cortical regions, including the prefrontal cortex.
Scaling of the Cortical White Matter.
Across the seven nonhuman species, VWM in each cortical region scales with OWM as similar power functions across cortical regions (Fig. 5 A–C). Again, the human VWM in each cortical region remains well inside the calculated confidence intervals (Fig. 5 A–C, black), indicating that the white matter of each region of the human cortex has the expected volume for its cellular composition.
Fig. 5.
Human prefrontal white matter volume scales as expected for its number of other cells and neurons in the adjacent gray matter. Each graph shows how the total VWM in the prefrontal (A and D), intermediate (B and E), and occipital regions (C and F) in each species scales with the number of other cells in the white matter (A–C) or gray matter (D–F) of that cortical region. Plotted functions exclude the human data points (in black). Exponents are (A, prefrontal) 1.176 ± 0.222 (r2 = 0.848, P = 0.0032), (B, intermediate) 1.241 ± 0.254 (r2 = 0.827, P = 0.0045), (C, occipital) 1.137 ± 0.211 (r2 = 0.853, P = 0.0030), (D, prefrontal) 1.656 ± 0.298 (r2 = 0.860, P = 0.0026), (E, intermediate) 1.644 ± 0.208 (r2 = 0.926, P = 0.0005), and (F, occipital) 1.557 ± 0.260 (r2 = 0.878, P = 0.0019). Notice that the human subcortical white matter has the expected volume both for the local number of other cells composing the white matter and for the number of neurons in the gray matter in each of the three cortical regions, including the prefrontal cortex.
Most importantly, we find that the human subcortical white matter in each cortical region also has the volume expected for the number of cortical neurons in the region as predicted from the relationships that apply to nonhuman primate species (Fig. 5 D–F). The conformity of human white matter volumes in cortical regions to the values predicted for their respective numbers of neurons suggests that human evolution has involved neither a significant expansion of prefrontal white matter volume beyond the expected for the local number of neurons nor a significant expansion of the white matter volume beneath other cortical regions.
Human Prefrontal Cortex Is Not Relatively Expanded.
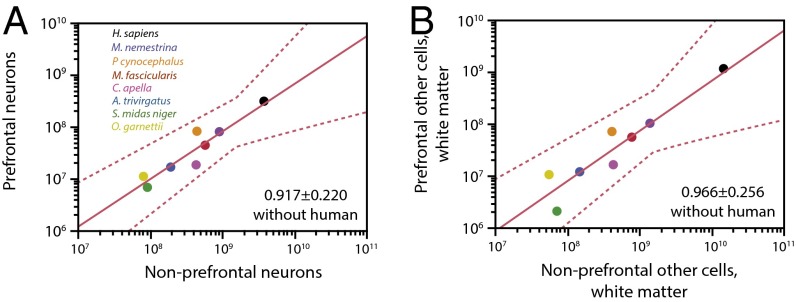
To test the hypothesis that the human prefrontal cortex is expanded in its number of neurons or white matter cells compared with the remainder cortical regions relative to other primates, regardless of the volume of the structures, we performed the analyses shown in Fig. 6. We find that, across nonhuman primates, the number of neurons in the prefrontal region varies with the total number of neurons in the other, nonprefrontal regions as a power function of exponent of 0.917 ± 0.220 (r2 = 0.776, P = 0.0088), not significantly different from linearity (Fig. 6A). This linearity indicates that, across nonhuman primates, the prefrontal region gains neurons proportionately with the rest of the cortex, without positive allometry, and therefore without significant expansion. Crucially, we find that the number of neurons in the human prefrontal region matches the expected for its number of neurons in nonprefrontal regions, given the relationship found across nonhuman primates (Fig. 6A, black). The human prefrontal region, therefore, is not expanded in its number of neurons compared with other cortical regions.
Fig. 6.
The human prefrontal region is not expanded in number of neurons or total length of myelinated fibers in the white matter relative to other cortical regions. (A) Total number of neurons in the prefrontal region of each nonhuman species varies as a power function of the total number of neurons in the remainder (nonprefrontal) regions with exponent 0.917 ± 0.220 (r2 = 0.776, P = 0.0088), not significantly different from linearity. The human prefrontal region (black data point) matches the nonhuman relationship. (B) Total number of other cells in the prefrontal region of the white matter (supposedly oligodendrocytes in their majority, and therefore proportional to the total length of myelinated fibers in the prefrontal white matter) varies across nonhuman species as a power function of the total number of other cells in the nonprefrontal white matter regions with exponent 0.966 ± 0.256 (r2 = 0.739, P = 0.0131), also not significantly different from linearity. The human prefrontal white matter (black data point) matches the nonhuman relationship.
Finally, we find that, across nonhuman primates, the number of other cells in the prefrontal white matter varies with the total number of other cells in the remainder (nonprefrontal) white matter as a power function of exponent 0.966 ± 0.256 (r2 = 0.739, P = 0.0131), also not significantly different from linearity (Fig. 6B). Because other cells in the white matter are presumably myelinating oligodendrocytes in their majority, this linearity indicates an isometric expansion of the total length of myelinated fibers in the primate prefrontal white matter compared with the other cortical regions. Again, the number of other cells in the human prefrontal white matter matches the expected for its number of other cells in the white matter of other, nonprefrontal cortical regions (Fig. 6B, black), conforming to the scaling rules that apply to other primates. The human prefrontal white matter is therefore not expanded over other cortical regions, compared with other primate species.
Discussion
Here we show that the human prefrontal region, in comparison with that of other primates, has (i) the expected number of neurons for its gray matter volume, (ii) the expected number of neurons for the total number of neurons in the remainder of the cortex, (iii) the white matter volume expected for the number of neurons in the region, and (iv) the expected volume and number of other cells in the white matter for the volume and number of other cells in the remainder of the nonprefrontal subcortical white matter. In particular, we find that the prefrontal region of the cortex (anterior to the corpus callosum) holds a similar 8% of all neurons in human and nonhuman primates alike. All these results point to the same two conclusions: that primate evolution did not involve a shift in the distribution of cortical neurons toward prefrontal regions and that human evolution, in particular, did not involve an expansion of the relative number of neurons or relative total length of myelinated fibers in the prefrontal region over other primates. Our findings thus concur with the conclusion of Barton and Venditti (7) that human frontal cortex is not proportionately larger than expected for a nonhuman primate of our cortical size, with no evidence of a relative enlargement of the human prefrontal white matter.
The present analysis of a single hemisphere of each species does not allow the analysis of variables such as interindividual variability, gender, and age on the distribution of neurons along the cerebral cortex. However, the fact that we found overlapping cumulative distributions of numbers of neuronal and nonneuronal cells and gray and white matter volumes even when single hemispheres were analyzed for each species (Fig. 2), when interindividual variability is known to exist (22, 23), only makes it more likely that the distribution of neurons and white matter is indeed similar in human and nonhuman primate species. We find this similarity all the more remarkable given that our sample does not include nonhuman ape species.
Our analysis of the distribution of neurons along the A-P axis of the cerebral cortex demonstrates a previously unknown U-shaped distribution of neuronal densities, with higher densities in the prefrontal pole, lower densities in the intermediate area, and densities increasing again toward the occipital pole. Because our analysis was restricted to the A-P axis, we cannot at this point address the possibility of yet another orthogonal gradient superposed onto the double A-P gradients. Still, the U-shaped pattern found here is in contrast to the single gradient proposed by Finlay et al. (24) and, in particular, by Charvet et al. (25), both based partially on the data of Collins et al. (8). The inconsistency across those studies and our results cannot be assigned to the method used to count cells, because Collins et al. (8) also used the isotropic fractionator, and this method has proven to have high internal consistency and to be at least as reliable as stereology (11). We believe that those authors failed to detect this U-shaped pattern because the original data they used were not based on a systematic, front-to-back analysis of neuronal densities in the primate cerebral cortex, but rather a bidimensional distribution of data points obtained from flattened cortices that make the dorsal cortex of the frontal pole no longer appear as the most anterior region of the cortex. Indeed, the flattened baboon cortex shown originally in Collins et al. (8) and recently in one flattened chimpanzee cortex (26) had a clear cluster of high neuronal densities in prefrontal patches that are located in the frontal pole of the folded cortex (although not in the anteriormost part of the unfolded, flattened cortex). In contrast, our systematic analysis of coronal sections along the A-P pole, starting with the frontal pole and ending with the occipital pole, indicates clearly the existence of two inverted gradients of neuronal density, peaking at both cortical poles.
That such a U-shaped pattern is not found in the human cortex (9) is explained simply by the scaling of the prefrontal cortical gray matter with decreasing densities across species as the number of cortical neurons increases (that is, with increasing average neuronal size in the gray matter as the cortex gains neurons), which leads to an overall flattening of the distribution of neuronal densities across the A-P axis. In line with this possibility, there is also no distinguishable U-shape pattern in Macaca nemestrina, the second largest cortex analyzed. The low neuron densities in the human prefrontal cortex are consistent with the increasing distance between pyramidal neurons in area 10 compared with other regions (27), in addition to a higher arborization of pyramidal neurons in layer III in comparison with the rest of the cortex (28, 29). These findings suggest that the dendritic length in human area 10, the frontal pole, is the highest of the cerebral cortex, which in turn suggests larger neurons with more synapses in this region, consistent with their associative function (30).
Importantly, the U-shaped pattern of neuronal densities across the primate cortex departs from the correlation between order of neurogenesis across the cortical surface and neuronal density proposed by Finlay et al. (24). Order of neurogenesis may indeed explain the generation of a larger number of neurons in the developing occipital region—although this order does not provide a mechanism to account for how these neurons are smaller and so occur at a greater neuronal density—but neurogenesis order does not explain the large neuronal densities also found in the frontal pole. It seems that other factors also determine the average size of cortical neurons and the fraction connected through the white matter in each cortical zone, as proposed previously (9, 31). By recognizing that the A-P gradient of neuron generation time does not truly account for the variation in their sizes and densities, further study will be necessary to identify the different mechanisms by which the quantitative differences between the three regions observed here are formed, including the generation of the two neuronal density gradients found peaking in the prefrontal and occipital poles.
Expansion of Cortical Areas Without a Change in the Relative Distribution of Cortical Neurons.
We have previously found that the primary visual cortex, the primary auditory cortex, and the primary motor cortex occupy a similar percentage of the cortical surface and hold a similar percentage of all cortical neurons across nonhuman primates (32–34). Those findings are consistent with the present report of a similar pattern of distribution of neurons along the A-P axis of the cerebral cortex across primate species. In contrast, Chaplin et al. (35) have shown that some associative cortical areas are relatively larger in larger primate species (capuchin and macaque) than in a smaller species (marmoset), which suggests that they have undergone relative expansion in primate evolution, and therefore might be expected to have relatively larger numbers of neurons. Similarly, Hill et al. (36) found that, compared with the macaque, the human cerebral cortex has relatively expanded lateral temporal, frontal, and parietal regions, which suggests an expansion of these areas in human evolution.
How can the relative expansion of some cortical areas over others be reconciled with our findings of no significant change in the relative distribution of neurons over the A-P axis of the cortex? We suggest that the shared cumulative distribution of neurons along the A-P axis is the result of a basic layout of neurons in the cortical sheet, according to mechanisms that control numbers of neurons and cell size during development (9, 31), that is conserved across species. These neurons are then allocated to particular functions depending on the pattern of thalamic and cortical connectivity that they establish. Thus, the functional identity of a particular anatomical region of cortex may change across species, becoming relatively expanded or contracted and thus containing more or fewer neurons, without a necessary change in the distribution of neurons along the A-P axis of the cortical sheet.
What Distinguishes Humans from Other Primates?
Using the same criterion to define prefrontal regions as Schoenemann et al. (4), our data on the distribution of neurons support the conclusions of Barton and Venditti (7) regarding the nonextraordinary nature of the human prefrontal cortex. In addition, we show that the A-P distribution of cortical neurons is similar in human and nonhuman species, and just as there are not relatively more neurons in the human prefrontal region, there are not relatively fewer neurons in other cortical regions. Instead, it is only absolute numbers of neurons that are increased in human cortex compared with other species. The increased number of nonprefrontal neurons presumably contributes to improving nonassociative functions in human brains relative to other species. Additionally, an overall increase in the number of cortical areas proposed for primates with increasing cortical size (37), and therefore increasing number of neurons, should also contribute to adding complexity to sensory-motor processing in larger cortices.
We also extend the conclusions of Barton and Venditti (7) to the number of neurons in the human prefrontal cortex and the total length of myelinated fibers in the prefrontal white matter, inferred from the number of other cells (presumably mostly oligodendrocytes) in the white matter, showing that the human prefrontal cortex does not deviate from the primate pattern in these criteria. Our findings indicate that the number of neurons and white matter connectivity of the human prefrontal region did not expand disproportionately to other cortical areas in human evolution. Incidentally, the prefrontal region that is considered to be associative in the mouse cortex also accumulates 8% of all cortical neurons (31), which raises the possibility that the relative allocation of neurons to associative functions is shared at least between primates and rodents, which are closely related in mammalian evolution.
Not surprisingly, the same 8% of all cerebral cortical neurons found in the prefrontal region correspond to a much larger absolute number of neurons in human (1.3 billion) than in other primate brains (e.g., macaque, 137 million), regardless of the relative size of the prefrontal cortex. Although a number of genetic changes specific to the human brain have been found (38–43) and certainly play some role in human cognition, we propose that this larger absolute number of neurons in human prefrontal, associative cortical regions is the main factor underlying the complexity of our cognitive abilities in comparison with other primates, and possibly all other mammals. As for nonassociative regions (37), the ensuing increase in number of cortical prefrontal areas expected with an increased absolute number of neurons and absolute cortical size would be an additional factor to contribute to increased complexity of associative processing in humans compared with other primate species.
Materials and Methods
To ensure that the distribution of cells along the entire cerebral cortex could be compared directly across species, we chose to examine the numbers of neuronal and other cells found in the gray and white matter systematically across a complete series of 2 mm coronal sections along the A-P axis of one hemisphere of each species. Each section had the cortical gray and white matter dissected and counted separately, using the isotropic fractionator (10). Each section was assigned as prefrontal (that is, located anterior to the corpus callosum), occipital (that is, situated in the posterior third of the cortical gray matter volume), or intermediate (located between prefrontal and occipital). Occipital region is defined after the finding by Ribeiro et al. (9) that the posterior third of the human cortical gray matter scales differently from the rest of the cerebral cortex. That a similar criterion could be applied to other, nonhuman primate species is supported by the findings of Collins et al. (34) that V1 occupies a similar fraction of the cortical surface and is comprised of a similar percentage of all cortical neurons in a range of nonhuman primate species comparable to those analyzed here. Within each section in the intermediate region of the A-P axis, temporal and dorsal areas were processed separately, and their numbers later combined for each section. This approach allowed the comparison of the absolute, relative and cumulative distributions of numbers of neurons and other cells along the A-P axis across nonhuman primate species and their comparison with similar published data for the human cerebral cortex (9), despite their different cortical volumes. Further, this approach also allowed the determination of the scaling rules that apply to each cortical region.
Animals.
We analyzed one hemisphere of each of the following seven primate species: Macaca fascicularis, Papio cynocephalus, Saguinus midas niger, Otolemur garnettii, Aotus trivirgatus, Cebus apella, and M. nemestrina. All animals were adults at the time of the experiments; more specifically, Papio was a 6-y-old female, Saguinus was a 4-y-old male, and Cebus was a 6-y-old female. The sex and precise age of the other animals was not recorded. The hemispheres of M. fascicularis, O. garnettii, P. cynocephalus, A. trivirgatus, and M. nemestrina were obtained from colonies in the Department of Psychology at Vanderbilt University. All veterinary care and procedures reported herein were performed according to the ethical standards of the Vanderbilt Institutional Animal Care and Use Committee. The S. midas niger and C. apella hemispheres were obtained from the colony at the Centro de Primatologia at Belém, Pará, Brazil, in accordance with the local ethics committee and with authorization from the Brazilian Institute of the Environment and Renewable Natural Resources.
The human cortex studied here was obtained from the death certification service at Universidade de São Paulo, Brazil. That cortex was previously analyzed by Ribeiro et al. (9), whose data were analyzed here.
Surface and Volume Reconstruction.
Nonhuman primate brains were removed from the skull after transecting the spinal cord at the level of the foramen mag num, weighed, and postfixed for 2 wk to 12 mo by immersion in 4% (vol/vol) phosphate-buffered paraformaldehyde. The cerebral cortex in all animals was defined as all cortical regions lateral to the olfactory tract, including hippocampus and piriform cortex. Each hemisphere was embedded in 3% (vol/vol) agar and sectioned in a coronal series of 2-mm sections along the A-P axis with a large-capacity industrial deli cutter (Filizola). Sections were scanned on a flatbed scanner at a resolution of 1,200 dots per inch.
For each section, we defined surface area (AG) as the total gray matter surface including gyri and sulci and the external surface area (AE) as the smallest surface of gray matter that did not enter sulci. Other measures include surface area of the white matter (AW), volume of the gray matter subcortical (VW), and the volume of the gray matter (VG). To determine surface and cortical volume we applied formulas that are sensitive to cortical inclination (9). Total volume and surface of gray and white matter for each cortical region were calculated using perimeter (P) and area values for each section as
Isotropic Fractionator.
The total number of neurons and other cells in the gray and white matter were estimated applying the isotropic fractionator (10), which consists of transforming the tissue of interest in a homogeneous solution of free nuclei that when labeled with DAPI (diluted 20–50× from a stock solution of 20 mg/L; Invitrogen) allows the quantification of the total number of cells in a structure. To determine the total number of neurons in the solution, we performed immunocytochemistry for NeuN (Cy3-conjugated rabbit polyclonal antibody; Millipore) by calculating the fraction of at least 500 DAPI-positive nuclei in each sample that expressed NeuN. The fraction of NeuN-positive nuclei in each region of interest multiplied by the total number of cells in the structure yields the total number of neurons in the sample. The total number of nonneuronal cells (that is, total number of cells minus number of NeuN-positive nuclei) in the white matter is referred to as the number of “other cells,” which are presumably mostly myelinating oligodendrocytes. Due to overfixation that precluded immunocytochemistry, the fraction of neuronal nuclei in Papio samples was performed according to morphological criteria, which increases the risk of confusing small interneuronal nuclei with oligodendrocyte nuclei (44), and thus might reduce estimates of numbers of neurons and neuronal density.
Statistical Analysis.
Power functions were calculated using least-squares regression of log-transformed raw data in JMP 9.0 (SAS). Phylogenetically independent contrasts for each variable were calculated using the PDAP module in Mesquite (mesquiteproject.wikispaces.com) applying the evolutionary tree in Purvis (1995). Least-squares linear regressions through the origin were then calculated for the contrasts using JMP 9.0. The power functions for raw data and phylogenetically independent contrasts, which are indistinguishable, are given in Table S2.
Acknowledgments
This work was supported by grants from National Council for Scientific and Technological Development (CNPq), Research Support Foundation of the State of Rio de Janeiro (FAPERJ), Ministry of Science and Technology/National Institutes of Science and Technology (MCT/INCT), the James McDonnell Foundation (to S.H.-H.), and the Mathers Foundation (to J.H.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610178113/-/DCSupplemental.
References
- 1.Passingham R. What Is Special About the Human Brain? Oxford Univ Press; Oxford: 2008. [Google Scholar]
- 2.Passingham R, Wise S. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 3.Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5(3):272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 4.Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8(2):242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- 5.Smaers JB, et al. Primate prefrontal cortex evolution: Human brains are the extreme of a lateralized ape trend. Brain Behav Evol. 2011;77(2):67–78. doi: 10.1159/000323671. [DOI] [PubMed] [Google Scholar]
- 6.Passingham RE, Smaers JB. Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain Behav Evol. 2014;84(2):156–166. doi: 10.1159/000365183. [DOI] [PubMed] [Google Scholar]
- 7.Barton RA, Venditti C. Human frontal lobes are not relatively large. Proc Natl Acad Sci USA. 2013;110(22):9001–9006. doi: 10.1073/pnas.1215723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci USA. 2010;107(36):15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro PF, et al. The human cerebral cortex is neither one nor many: Neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat. 2013;7:28. doi: 10.3389/fnana.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25(10):2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herculano-Houzel S, von Bartheld CS, Miller DJ, Kaas JH. How to count cells: The advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res. 2015;360(1):29–42. doi: 10.1007/s00441-015-2127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 13.Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423(1):140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Johann Ambrosius Barth; Leipzig, Germany: 1909. [Google Scholar]
- 15.Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity-driven white matter scaling and folding in primate cerebral cortex. Proc Natl Acad Sci USA. 2010;107(44):19008–19013. doi: 10.1073/pnas.1012590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caminiti R, Ghaziri H, Galuske R, Hof PR, Innocenti GM. Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proc Natl Acad Sci USA. 2009;106(46):19551–19556. doi: 10.1073/pnas.0907655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598(1–2):143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 18.Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behav Brain Res. 2002;136(1):51–59. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 19.Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49(3):195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
- 20.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 21.McBride T, Arnold SE, Gur RC. A comparative volumetric analysis of the prefrontal cortex in human and baboon MRI. Brain Behav Evol. 1999;54(3):159–166. doi: 10.1159/000006620. [DOI] [PubMed] [Google Scholar]
- 22.Andrews TJ, Halpern SD, Purves D. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J Neurosci. 1997;17(8):2859–2868. doi: 10.1523/JNEUROSCI.17-08-02859.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song C, Schwarzkopf DS, Kanai R, Rees G. Reciprocal anatomical relationship between primary sensory and prefrontal cortices in the human brain. J Neurosci. 2011;31(26):9472–9480. doi: 10.1523/JNEUROSCI.0308-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finlay BL, Hersman MN, Darlington RB. Patterns of vertebrate neurogenesis and the paths of vertebrate evolution. Brain Behav Evol. 1998;52(4-5):232–242. doi: 10.1159/000006566. [DOI] [PubMed] [Google Scholar]
- 25.Charvet CJ, Cahalane DJ, Finlay BL. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex. 2015;25(1):147–160. doi: 10.1093/cercor/bht214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins CE, et al. Cortical cell and neuron density estimates in one chimpanzee hemisphere. Proc Natl Acad Sci USA. 2016;113(3):740–745. doi: 10.1073/pnas.1524208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semendeferi K, et al. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex. 2011;21(7):1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative golgi study. Cereb Cortex. 2001;11(6):558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi S, et al. Dendritic morphology of pyramidal neurons in the chimpanzee neocortex: Regional specializations and comparison to humans. Cereb Cortex. 2013;23(10):2429–2436. doi: 10.1093/cercor/bhs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herculano-Houzel S, Watson C, Paxinos G. Distribution of neurons in functional areas of the mouse cerebral cortex reveals quantitatively different cortical zones. Front Neuroanat. 2013;7:35. doi: 10.3389/fnana.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong P, et al. Faster scaling of auditory neurons in cortical areas relative to subcortical structures in primate brains. Brain Behav Evol. 2013;81(4):209–218. doi: 10.1159/000350709. [DOI] [PubMed] [Google Scholar]
- 33.Young NA, Collins CE, Kaas JH. Cell and neuron densities in the primary motor cortex of primates. Front Neural Circuits. 2013;7:30. doi: 10.3389/fncir.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins CE, Leitch DB, Wong P, Kaas JH, Herculano-Houzel S. Faster scaling of visual neurons in cortical areas relative to subcortical structures in non-human primate brains. Brain Struct Funct. 2013;218(3):805–816. doi: 10.1007/s00429-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaplin TA, Yu H-HH, Soares JG, Gattass R, Rosa MG. A conserved pattern of differential expansion of cortical areas in simian primates. J Neurosci. 2013;33(38):15120–15125. doi: 10.1523/JNEUROSCI.2909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill J, et al. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Essen DC, Glasser MF, Dierker DL, Harwell J. Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cereb Cortex. 2012;22(10):2227–2240. doi: 10.1093/cercor/bhr290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans PD, Anderson JR, Vallender EJ, Choi SS, Lahn BT. Reconstructing the evolutionary history of microcephalin, a gene controlling human brain size. Hum Mol Genet. 2004;13(11):1139–1145. doi: 10.1093/hmg/ddh126. [DOI] [PubMed] [Google Scholar]
- 39.Dumas LJ, et al. DUF1220-domain copy number implicated in human brain-size pathology and evolution. Am J Hum Genet. 2012;91(3):444–454. doi: 10.1016/j.ajhg.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enard W, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137(5):961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Dennis MY, et al. Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell. 2012;149(4):912–922. doi: 10.1016/j.cell.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charrier C, et al. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell. 2012;149(4):923–935. doi: 10.1016/j.cell.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somel M, Liu X, Khaitovich P. Human brain evolution: Transcripts, metabolites and their regulators. Nat Rev Neurosci. 2013;14(2):112–127. doi: 10.1038/nrn3372. [DOI] [PubMed] [Google Scholar]
- 44.Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133(1–2):328–332. doi: 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]