Significance
The aggregation of α-synuclein (aSyn) is a pathological hallmark of Parkinson’s disease. Here we show that the enzymatic component of the innate inflammation system, known as caspase-1, hydrolyzes aSyn, rendering it aggregation-prone.
Keywords: synuclein, Parkinson's disease, caspase, inflammasome, aggregation
Abstract
The aggregation of α-synuclein (aSyn) leading to the formation of Lewy bodies is the defining pathological hallmark of Parkinson’s disease (PD). Rare familial PD-associated mutations in aSyn render it aggregation-prone; however, PD patients carrying wild type (WT) aSyn also have aggregated aSyn in Lewy bodies. The mechanisms by which WT aSyn aggregates are unclear. Here, we report that inflammation can play a role in causing the aggregation of WT aSyn. We show that activation of the inflammasome with known stimuli results in the aggregation of aSyn in a neuronal cell model of PD. The insoluble aggregates are enriched with truncated aSyn as found in Lewy bodies of the PD brain. Inhibition of the inflammasome enzyme caspase-1 by chemical inhibition or genetic knockdown with shRNA abated aSyn truncation. In vitro characterization confirmed that caspase-1 directly cleaves aSyn, generating a highly aggregation-prone species. The truncation-induced aggregation of aSyn is toxic to neuronal culture, and inhibition of caspase-1 by shRNA or a specific chemical inhibitor improved the survival of a neuronal PD cell model. This study provides a molecular link for the role of inflammation in aSyn aggregation, and perhaps in the pathogenesis of sporadic PD as well.
Over the past two decades, studies stimulated by the discovery of genetic mutations occurring in familial Parkinson’s disease (PD) have generated a number of hypotheses concerning potential mechanisms of PD pathogenesis. Nonetheless, the causes and mechanism of sporadic PD, which constitutes the majority of cases, remain unknown.
Before the genetic discoveries, epidemiologic evidence suggested environmental toxins, traumatic brain injury, and viral infection as potential causes of idiopathic PD (1–7). All of these insults could cause neural inflammation, a common feature of PD brains; however, whether neural inflammation contributes to the etiology of the disease or is part of its effect remained unclear. Suggestive evidence indicating the involvement of inflammation in the pathogenesis of PD emerged after an outbreak of encephalitis lethargica following the 1918 influenza pandemic, which killed approximately 1 million people and left many survivors with postencephalitic Parkinsonism (PEP). Affected persons presented with cardinal symptoms of typical Parkinson’s disease, including stooped posture, masklike faces, muscular rigidity, and tremorous extremities. Contemporary cases of viral infection-associated Parkinsonism are rare, but both epidemiology and patient case studies indicate that infection-associated PD still occurs today (8–10).
The role of viral infection in the pathogenesis of PD has been a controversial subject for more than 50 years. Proponents have pointed out the known close temporal association between infection and PEP, whereas opponents have cited studies that failed to find any viral remnants in the brains of affected patients. Moreover, there were no known routes for peripheral viral migration into the central nervous system (CNS). Recently, R. Smeyne and coworkers (11) found that in mice, the influenza virus (H5N1 strain) can travel from the peripheral nervous system into the CNS to higher levels of the neuroaxis. Moreover, in regions infected by the H5N1 virus, they observed aSyn aggregation that persisted long after resolution of the infection. This observation provides evidence for the involvement of viral infection and also possibly the immune response in generating aSyn aggregation and, potentially, the pathogenesis of PD (8, 10, 12). The mechanism by which infections cause aSyn aggregation remains unknown, however.
Several different posttranslational modifications of aSyn have been proposed to increase its aggregation propensity, including phosphorylation, ubiquitination, and truncation (13). Among these modifications, truncation of aSyn is irreversible and most strongly associated with PD pathology; that is, truncated aSyn is always observed in Lewy bodies and generally is not found in soluble fractions. Various engineered recombinant truncated forms of aSyn aggregate readily in vitro and form toxic inclusions when overexpressed in animal models (14–17). Furthermore, C-terminally truncated aSyn is ubiquitously present in Lewy bodies of PD brains, and the amount of truncated forms is correlated with the number and size of Lewy bodies (18, 19), suggesting that truncation of aSyn might be an early event that renders it more prone to aggregate into disease-associated conformations. A number of in vitro studies have investigated this possibility and have implicated different proteases that theoretically could truncate aSyn, including neurosin (20), 20S proteasome (21, 22), calpain-1 (23), matrix metallo-proteases (MMPs) (24), and cathepsin D (25). However, these are general proteases that cleave proteins, including aSyn, at multiple locations, and some are extracellular proteases (i.e., neurosin and MMPs); therefore, their roles in truncating aSyn intracellularly in vivo remain unclear. Moreover, none has a mechanism of activation that correlates with the known risk factors for sporadic PD (i.e., head trauma and other causes of brain inflammation, cholesterol deposits, lysosomal or mitochondrial dysfunction, and aging). A protease that specifically cleaves a C-terminal segment of aSyn in vitro and in vivo, producing the same fragment observed in Lewy bodies, has not yet been identified.
Here, we report that activation of an important component of the innate immune response system, the inflammasome, leads to the aggregation of aSyn in neuronal cell culture. This aggregation is caused by truncation of aSyn by the enzymatic component of the inflammasome, the cysteine protease caspase-1 (also known as IL-converting enzyme-1, or ICE). The level of toxicity to neuronal culture is correlated with the level of aSyn truncation and is rescued by chemical inhibition of caspase-1 or its genetic knockdown with shRNA. Taken together, our data demonstrate that activation of the inflammasome by environmental toxins, oxidative stress, and infection [i.e., bacterial lipopolysaccharide (LPS)] causes aSyn truncation and aggregation, which are believed to be associated with the pathogenesis of PD.
Results and Discussion
Activation of the Inflammasome Causes aSyn Aggregation in Neuronal Cells.
Inflammasomes are key parts of the innate immunity system that respond to pathogen-associated molecular patterns as well as to host-derived damage-associated molecular patterns, such as tissue damage after trauma and metabolic stress, leading to proteolytic activation of the proinflammatory cytokines IL-1β and IL-18 by the cysteine protease caspase-1 (26).
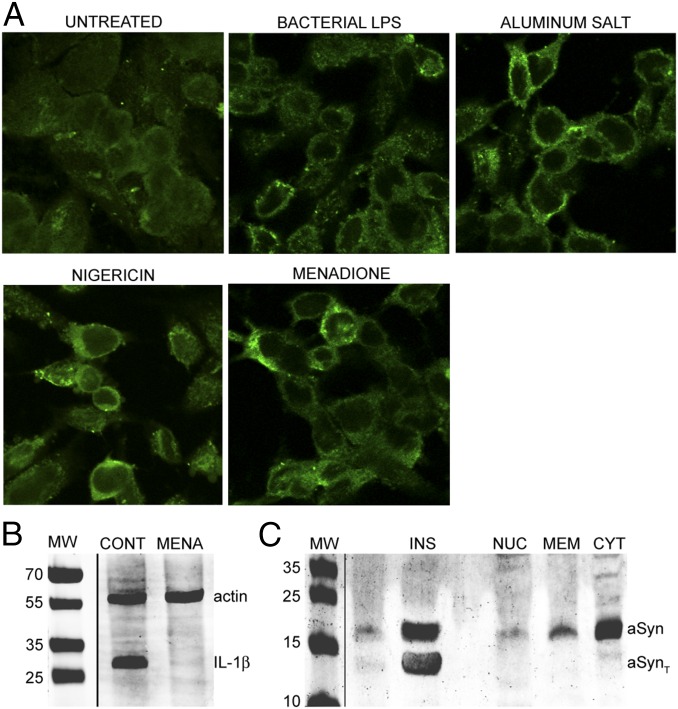
When we treated neuronal BE(2)-M17 cells overexpressing aSyn (M17-aSyn) with known inflammasome activators, including nigericin, aluminum potassium sulfate crystals (APSC), bacterial LPS, and vitamin K3 (menadione), we found that aSyn is more localized to the plasma membrane in the treated cells, which also developed aSyn-positive punctate inclusions that resemble the Lewy bodies found in PD (Fig. 1A). The redistribution of aSyn to the plasma membrane and formation of punctate structures are strikingly similar to events that occurred in a yeast model of PD (27).
Fig. 1.
Inflammasome activation leads to aSyn aggregation. (A) Confocal microscopy images (at 60× magnification) of M17-aSyn cells stained with anti-aSyn antibodies, showing concentration and punctate aSyn structures at the plasma membrane in cells treated with various inflammasome activators. (B) Western blot of pro-IL-1β from M17-aSyn showing that menadione treatment leads to pro-IL-1β hydrolysis, and thus caspase-1 activation. β-Actin (actin) was blotted as a loading standard. (C) Western blot of aSyn extracted from various cellular fractions, showing that the insoluble aggregates (INS) consist of truncated aSyn (aSynT) and are undetectable in soluble nuclear (NUC), membrane (MEM), and soluble cytoplasmic (CYT) fractions.
Nigericin, APSC, and LPS are canonical inflammasome activators. Whether menadione also activates the inflammasome-associated protease caspase-1 has been less clear. To determine this, we measured the levels of pro-IL-1β (the natural substrate of caspase-1) in cells treated with menadione and found significantly reduced levels in treated cells compared with untreated control cells (Fig. 1B), as would be expected if the inflammasome is activated.
Although the appearance of aSyn-positive punctate structures in the induced cells resembles the neuronal protein aggregates in PD, they also could simply represent clustering of aSyn onto membranes of microsomes or lipidic droplets. To determine whether these inclusions are genuine protein aggregates, as found in Lewy bodies, or are membrane-associated 2D clusters, we extracted the membrane fractions and nonmembranous inclusions from the menadione-treated cells. We found aSyn mainly in the detergent-insoluble fraction and barely detectable in the solubilized membrane fraction, indicating that the aSyn inclusions are likely genuine protein aggregates and not simply membrane-bound molecules (Fig. 1C).
Activation of the Inflammasome Results in aSyn Truncation.
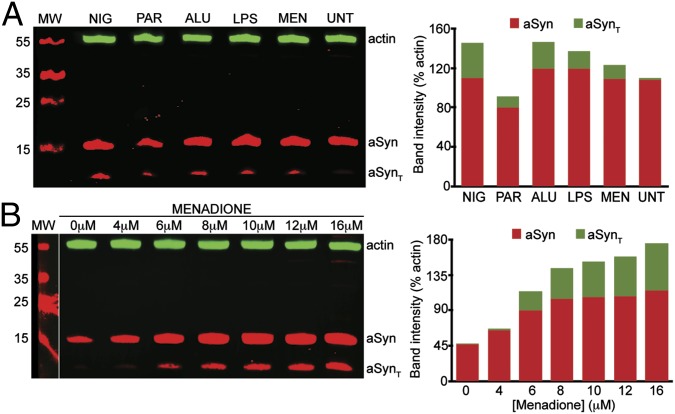
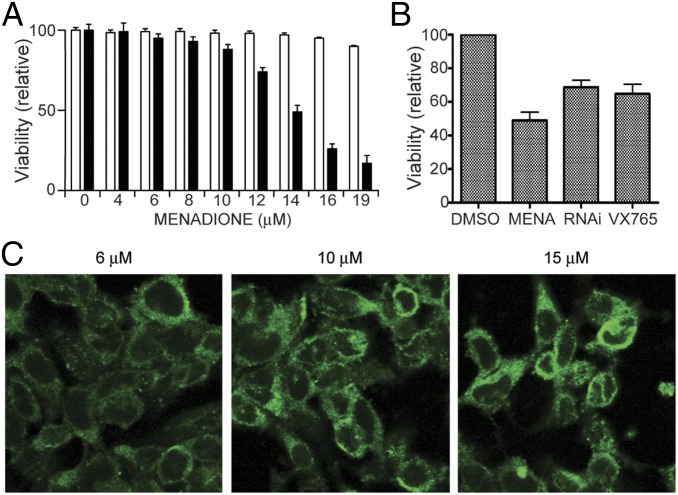
During our investigation of inflammasome activation and aSyn aggregation, we noticed that the presence of inflammasome activators produced a truncated fragment of aSyn. To investigate this in more detail, we treated M17-aSyn cells with various inflammasome activators and monitored their effects on aSyn truncation. We found that all of the activators tested do indeed cause truncation of aSyn in M17-aSyn cells (Fig. 2A). Activation of the inflammasome typically results in the conversion of procaspase-1 (a cysteine protease that is an integral component of the inflammasome) into active caspase-1; thus, our results suggest the possibility of activated caspase-1 directly cleaving aSyn into smaller fragments. Among the foregoing activators, menadione is the least toxic, the most titratable, and shows a dose-dependent response (Fig. 2B); therefore, we chose it as a representative activator for subsequent experiments. We selected a range of menadione concentrations of 0–16 μM for experimentation, because at higher concentrations we observed the presence of SDS-insoluble oligomers or high molecular weight species.
Fig. 2.
Inflammasome activation leads to aSyn truncation. (A) Western blot of aSyn from M17-aSyn cells showing that inflammasome activators, including nigericin (NIG), paraquat (PAR), aluminum crystals (ALU), bacterial lipopolysaccharide (LPS), and menadione (MEN), lead to truncated aSyn (aSynT). β-Actin (actin) was blotted as a loading standard. The bar graph shows the intensity of bands corresponding to aSyn and aSynT relative to β-actin. (B) Western blot of aSyn from M17-aSyn cells showing that the amount of aSynT increases with an increasing concentration of menadione. β-Actin (actin) was blotted as a loading standard.
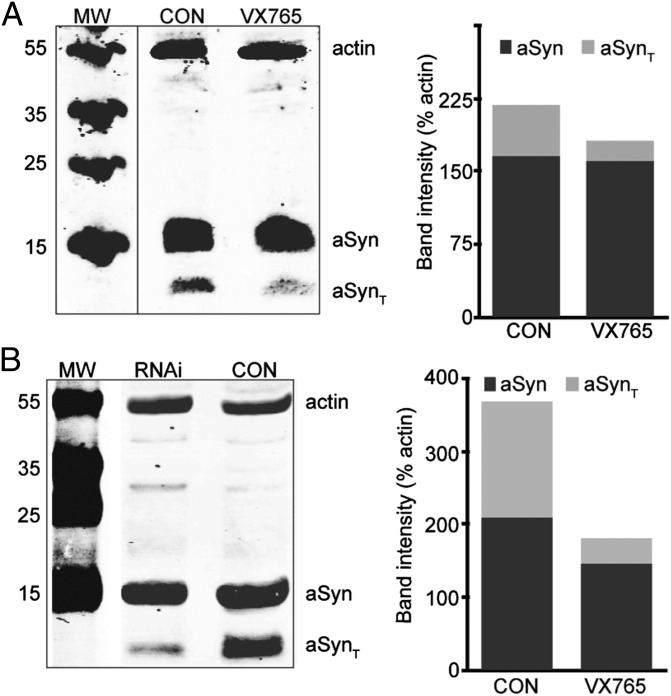
To determine whether caspase-1 is directly involved in the cleavage of aSyn, we treated M17-aSyn cells with the caspase-1–specific inhibitor VX765 and monitored its effects on aSyn truncation. We found that inhibition of caspase-1 with VX765 significantly reduced the amount of truncated aSyn in the treated M17-aSyn cells (Fig. 3A). Although VX765 is a potent and specific caspase-1 inhibitor, to verify that its rescue of aSyn truncation is not due to off-target effects, we also tested whether a similar rescue of aSyn truncation would occur when the caspase-1 gene is silenced with shRNA (Fig. S1). Indeed, we found that knockdown of the caspase-1 gene dramatically reduced aSyn truncation compared with control (Fig. 3B). These results suggest that caspase-1 directly cleaves aSyn in M17-aSyn cells.
Fig. 3.
Inflammasome inhibition leads to reduction of aSyn truncation. (A) Western blot of aSyn from M17-aSyn showing that the specific caspase-1 inhibitor VX765 inhibits aSyn truncation compared with control (CON). The bar graph shows the intensity of bands corresponding to aSyn and aSynT relative to β-actin. (B) Western blot of aSyn from M17-aSyn cells transfected with anti–caspase-1 shRNA and treated with 10 μM menadione (RNAi), showing that knockdown of caspase-1 expression diminishes aSyn truncation compared with control (CON).
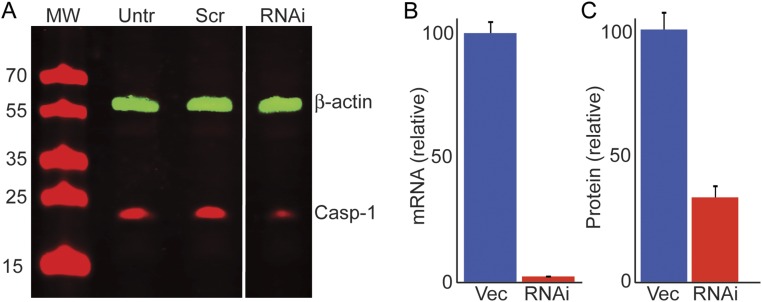
Fig. S1.
shRNA-mediated knockdown of caspase-1 (ICE). (A) Western blot of untreated M17 cells (Untr), transfected with control vector carrying a scrambled GFP shRNA (Scr) and an anti–caspase-1 shRNA (RNAi). (B) Levels of caspase-1 mRNA transfected with empty vector (Vec) and anti–caspase-1 shRNAs (RNAi) as determined by quantitative PCR. (C) Levels of caspase-1 protein in M17-aSyn cells transfected with empty vector before (Vec) and shRNAs against caspase-1 (RNAi).
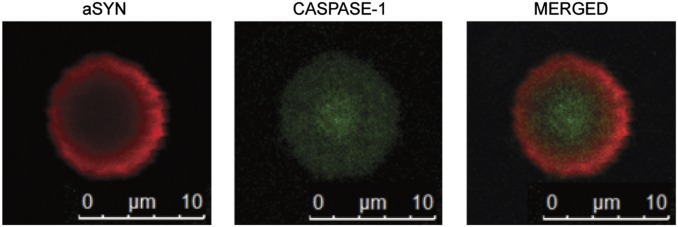
Truncation of aSyn has been shown to correlate with the rate of formation and the size of Lewy bodies found in brains of patients with PD (28). To determine whether caspase-1 is present in Lewy bodies of PD, we isolated Lewy bodies from postmortem PD brains and stained them with specific antibodies for caspase-1 and aSyn. We found that the Lewy bodies stained positive for both caspase-1 and aSyn (Fig. 4), confirming their coexistence in Lewy bodies and supporting the notion that caspase-1 also might be involved in generating the truncated aSyn found in Lewy bodies.
Fig. 4.
Caspase-1 and aSyn in Lewy bodies. Lewy bodies from PD brain stained with specific antibodies for aSyn (red) and caspase-1 (green), showing the presence of caspase-1 in Lewy bodies.
Caspase-1 Truncates aSyn at Asp121.
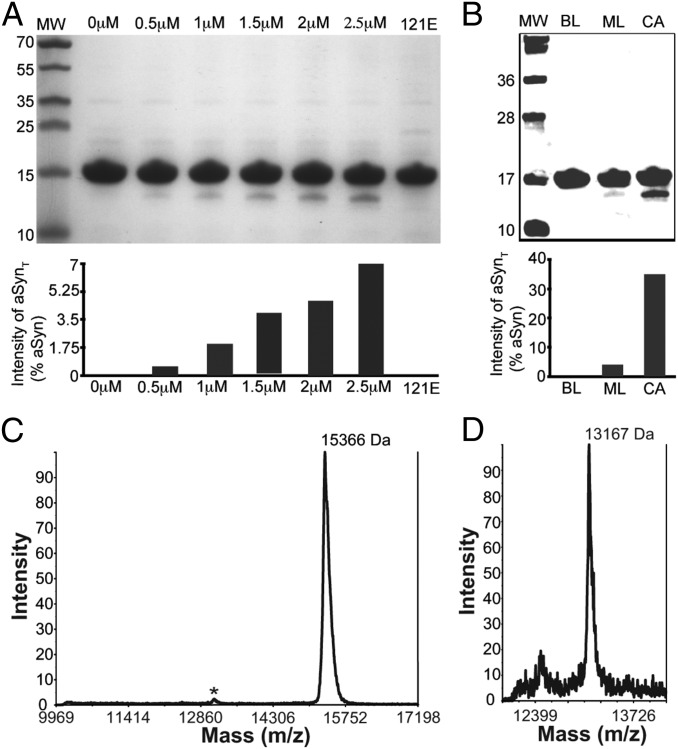
To further study the activity of caspase-1 on aSyn in more detail, we investigated the activity of human recombinant caspase-1, expressed and purified as described previously (Fig. S2) (29), with purified recombinant aSyn as its substrate in an isolated binary in vitro system. We incubated 10 μg of aSyn with increasing concentrations of caspase-1 (0.5, 1, 1.5, 2, and 2.5 μM) and analyzed the proteolyzed product using both SDS/PAGE and Western blot analysis. We found that the amount of truncated aSyn increased proportionately with increasing caspase-1 concentration (Fig. 5A), demonstrating that caspase-1 directly truncates aSyn in vitro. We observed that the cleavage of aSyn occurred slowly and approached nearly complete digestion after overnight incubation. Whereas the P1 position of natural caspase-1 substrates have 100% stringently conserved amino acid Asp (which we have identified as Asp121 in aSyn), the cleavage efficiency is determined by the surrounding residues (P10–P10′). The optimal sequence for caspase-1 is WEHDS/G at positions P4-P3-P2-P1-P1′, respectively, but the cleavage sequence in aSyn is VDPDN; thus, it is not optimal for efficient cleavage.
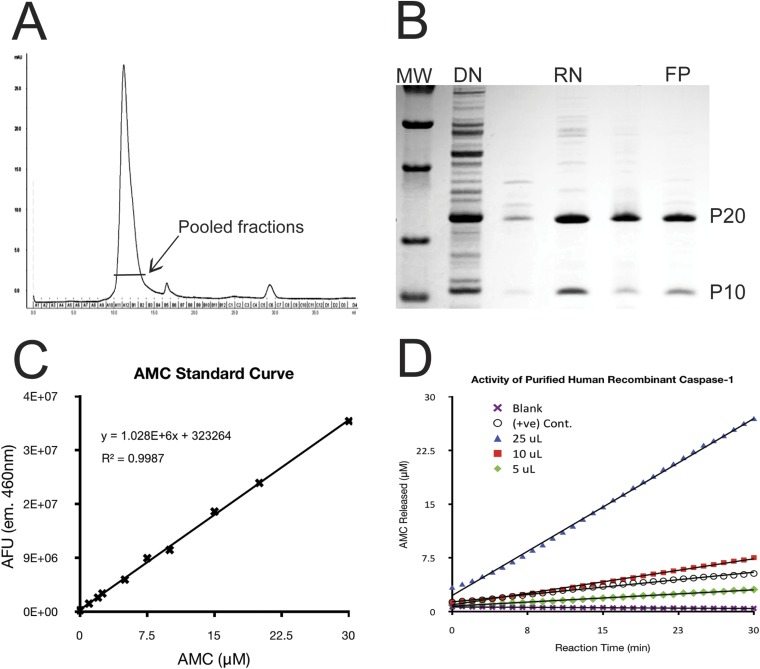
Fig. S2.
Purification and in vitro activity of recombinant caspase-1. (A) Elution profile of renatured caspase-1 from a Superdex 75 size-exclusion column. (B) SDS/PAGE of recombinant caspase-1 along the purification procedure, including resolubilization of inclusion bodies with denaturant (DN), refolded rapid dilution (RN), and purification through a Superdex 75 column (FP). The purified fraction consisted predominantly of caspase-1, which is composed of P20 and P10 domains. (C) Standard of fluorescence vs. AMC concentration used for determining the activity of recombinant caspase-1. (D) Activity of assay of our purified recombinant caspase-1 (green diamond, red square, and blue triangle), showing it to be at least as active as commercial positive control (open circle).
Fig. 5.
Caspase-1 directly cleaves aSyn. (A) SDS/PAGE of purified recombinant aSyn incubated with various concentrations of purified recombinant caspase-1, showing that a concentration-dependent truncation of aSyn and D121E mutation (121E) abolishes this activity. The bar graph shows the intensity of bands corresponding to truncated form relative to full-length aSyn. (B) Western blot of an in vitro aSyn truncation assay showing that the caspase-1–cleaved aSyn (CA) compared with control lacking caspase-1 (BL) and inhibitor of caspase-1 ML132 (ML) inhibit aSyn truncation. (C and D) MALDI-TOF mass spectrum of an in vitro aSyn truncation reaction with caspase-1 (C), showing full-length aSyn (15,366) and a small peak at ∼13 kDa (*), which, when focused (D), reveals a molecular weight corresponding to a fragment of aSyn consisting of residues 1–121 (13,167).
To ensure that the truncated fragment of aSyn is generated specifically by caspase-1 and not by a copurifying impurity, we treated the proteolysis reaction mixture with a caspase-1–specific inhibitor NCG00183434 (ML132) (30), and found that ML132 treatment resulted in significant reduction in truncated aSyn (Fig. 5B), confirming that caspase-1 directly truncates aSyn. (Note that VX765 was not used for the in vitro experiments because it is a prodrug that requires cellular esterase activity for conversion into the active inhibitor.)
To determine precisely where caspase-1 cuts aSyn, we used matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry to determine the size of the major caspase-1–cleaved fragment (Fig. 5 C and D). The truncated fragment of aSyn was found to have a molecular mass of 13,167 Da, which corresponds to the molecular mass of an aSyn fragment ending at residue aspartic acid 121 (theoretical molecular mass, 13,166 Da). This is consistent with the fact that caspases are aspartic acid-specific enzymes that cleave immediately after an aspartic acid.
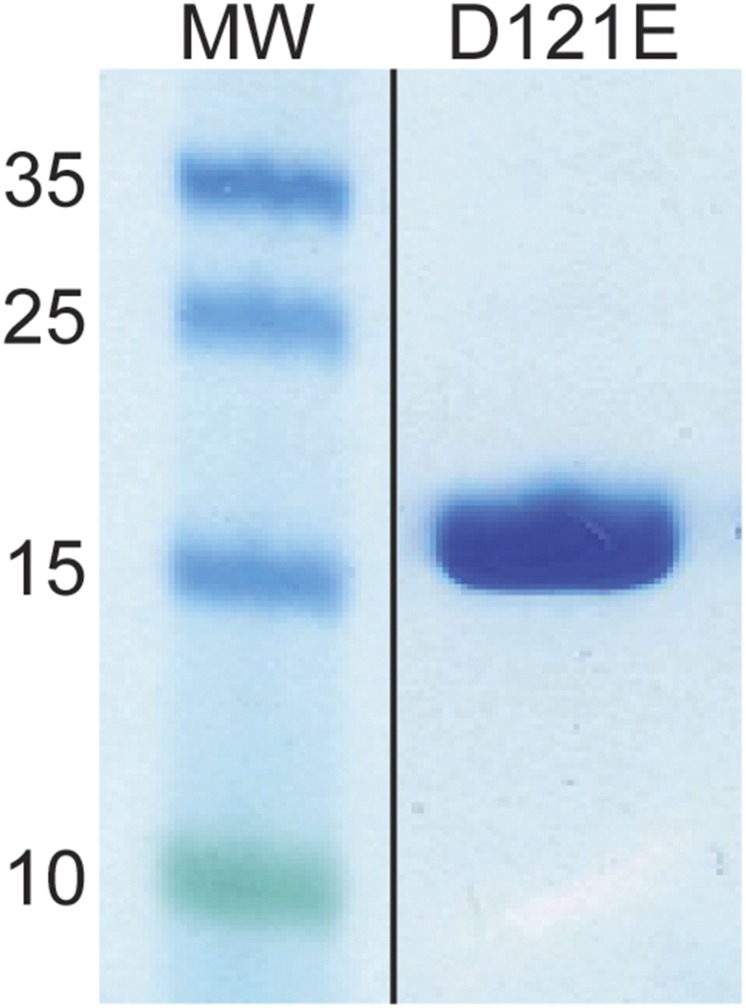
To confirm that we identified the correct cleavage site, we introduced a point mutation substituting Asp121 for glutamic acid, which we expressed and purified as described previously (31, 32) (Fig. S3). Introducing the D121E mutation completely abolished truncation of aSyn by caspase-1 in our in vitro experiments (Fig. 5A, lane 121E), confirming that caspase-1 does indeed cleave aSyn after residue D121. We created a construct for the expression of the D121E mutant in neuronal cells to determine whether this mutation would ameliorate inflammasome-induced toxicity; however, cells transfected with the D121E aSyn construct were synthetically lethal.
Fig. S3.
Purification of recombinant D121E mutant aSyn. SDS/PAGE of purified Asp to Glu substituted aSyn at residue 121.
aSyn Truncation Produces an Aggregation-Prone Species in Vitro.
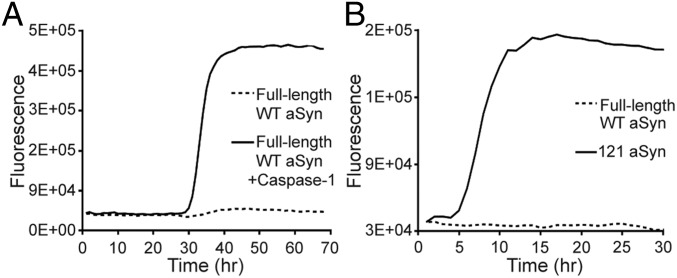
Lewy bodies consist of various truncated forms of aSyn, and it has been speculated that the more aggregation-prone truncated aSyn might first aggregate, forming seeds that then nucleate the aggregation of full-length aSyn (33, 34). Therefore, we set out to characterize the aggregation propensity of caspase-1–truncated aSyn. Full-length aSyn is stable against aggregation for more than 100 h, but we found that aSyn samples predigested with caspase-1 began to aggregate at approximately 30 h, as determined by a thioflavin-T (ThT) fluorescence aggregation assay, and rapidly increased to maximum intensity by 40 h (Fig. 6A). This suggests that caspase-1–truncated aSyn is more aggregation-prone than the full-length protein. The caspase-1–digested sample presented in Fig. 5B consisted of only approximately 10% of the truncated form, based on SDS/PAGE, which would have been insufficient to generate the large amount of aggregates observed, thus supporting the notion that truncated aSyn aggregates first and then seeds the aggregation of full-length aSyn.
Fig. 6.
Truncated aSyn is aggregation-prone. (A) ThT aggregation assay showing that aSyn sample preincubated with caspase-1 (solid line) is more aggregation-prone than control (dashed line). (B) ThT aggregation assay showing that C-terminal–truncated aSyn consisting of residues 1–121 (solid line) aggregates more readily than full-length aSyn (dashed line).
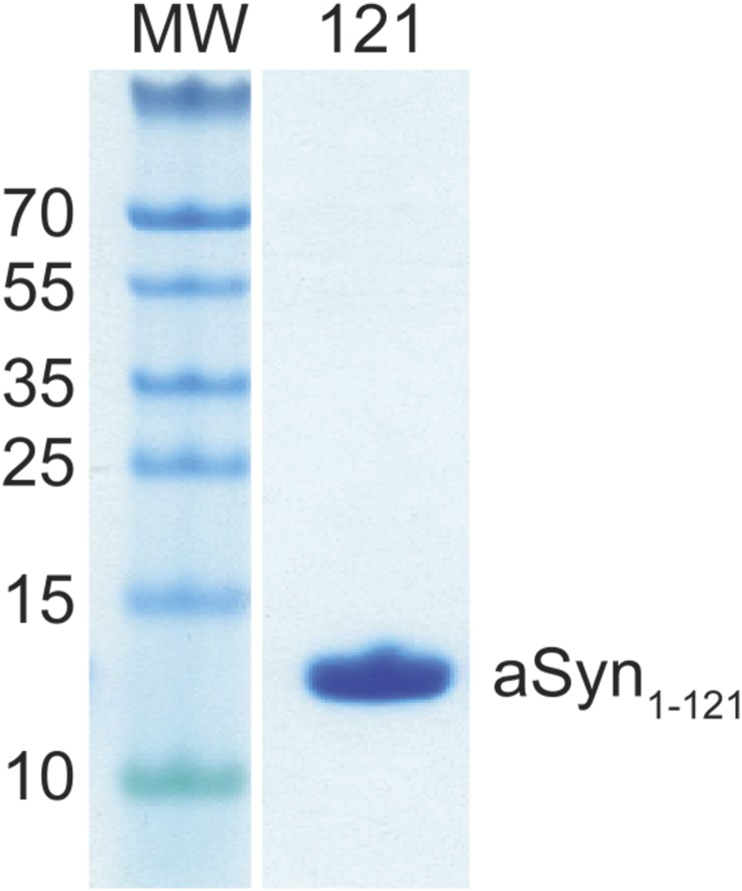
To investigate the aggregation propensity of the caspase-1–truncated aSyn fragment (residues 1–121) by itself, we subcloned, expressed, and purified the corresponding C-terminal–truncated construct (aSyn 1–121) using a procedure previously developed for full-length protein (Fig. S4). In a ThT fluorescence aggregation assay, the purified aSyn1-121 began to aggregate at approximately 5 h and reached its maximum by 10 h (Fig. 6B), significantly faster than any of the other forms of aSyn that we tested, including all of the disease-associated mutants and heat-denatured samples. This result confirms that caspase-1–truncated aSyn is aggregation-prone, and implies that caspase-1 activity might directly initiate aSyn aggregation, leading to Lewy body formation in vivo.
Fig. S4.
Purification of recombinant 1–121 truncated aSyn. SDS/PAGE of purified 1–121 C-terminally truncated aSyn (1–121).
Inhibition of Caspase-1 Rescues Neuronal Cells from aSyn-Induced Toxicity.
To investigate whether the inflammasome-associated truncation and aggregation of aSyn described above are associated with the aSyn-induced cytotoxicity believed to be the cause of neuronal death in PD, we first measured the viability of M17-aSyn cells in various concentrations of menadione (0–19 µM) and compared the values with the values of M17 cells transfected with empty pcDNA vectors. We found that M17-aSyn cells were more sensitive to menadione toxicity than the control cells (Fig. 7A). At 14 μM, the viability of M17-aSyn was approximately one-half that of control cultures. The level of toxicity was roughly correlated with the severity of aSyn aggregation (Fig. 7C).
Fig. 7.
Inhibition of caspase-1 rescues cell viability. (A) Live/dead cell viability assay of M17-aSyn cells in various concentrations of menadione, showing that cells overexpressing aSyn (solid bars) are more prone to menadione-induced toxicity than are cells carrying an empty vector (unfilled bars). (B) Live/dead cell viability assay of M17-aSyn cells treated with the caspase-1 inhibitor VX765 and genetic knockdown with anti–caspase-1 shRNA (RNAi), showing that a reduction in caspase-1 activity partially rescues menadione-induced toxicity in neuronal cells. (C) Confocal microscopy images of M17-aSyn cells stained with anti-aSyn antibodies, showing increasingly larger and denser aSyn aggregates with increasing menadione concentration.
We next investigated whether inhibiting caspase-1 activity could rescue these toxic effects of aSyn in 15 µM menadione by comparing the viability of M17-aSyn cells with and without the caspase-1 inhibitor VX-765. We found that VX-765 treatment partially rescued cell viability, from ∼50% viability to ∼65% (Fig. 7B), suggesting that caspase-1 activity might contribute to cellular toxicity. Although VX-765 is known to be specific for caspase-1, to rule out unanticipated nonspecific effects, we tested whether a similar rescuing effect would occur when the expression of caspase-1 is knocked down with shRNA. We found that the silencing of caspase-1 expression to 30% of WT protein levels with shRNA (Fig. S1) also partially rescued M17-aSyn cells, from ∼50% viability to ∼70% viability (Fig. 7B).
Conclusions
The PD brain is known to be inflamed (35–37), and IL-1β is known to be activated in synucleinopathies (38–41); however, whether neuroinflammation, and thus caspase-1 activation, is the cause or the consequence of the disease process has remained unclear. A number of studies have provided evidence supporting the role of inflammation in the pathogenesis of neurodegenerative diseases (42, 43). Specifically, known risk factors for PD, including aging (44), pesticide exposure (45), traumatic brain injury (46), the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (47), lysosomal storage diseases (48), and brain infection (49), can result in the activation of caspase-1. Chemical inhibition of caspase-1 or its gene deletion was found to rescue mouse models of PD from MPTP-induced toxicity (50), although the basis for that rescue was not completely understood. Activation of the inflammasome with bacterial endotoxin LPS selectively causes dopaminergic neuron cell death in mice (51). These different lines of evidence point to caspase-1 activation as a common intersection to disease.
Our results provide a direct association of caspase-1 truncation of aSyn with aggregation and neuronal cell toxicity, and hence suggest that inflammation may play an important role in the pathogenesis of PD, as numerous reports have suggested (8, 9, 52, 53), as well as other neurologic diseases (53–56). Our data suggest that modulation of caspase-1 activity (or inflammasome activity) might be an effective strategy for preventing or treating PD. In an accompanying paper (57), Bezard, Meissner, and colleagues show that chemical inhibition of caspase-1 mitigates alpha-synuclein pathology and mediates neuroprotection in a transgenic mouse model of multiple system atrophy.
Methods
Tissue Culture.
The production of stable cell lines overexpressing aSyn from parental BE(2)-M17 human dopaminergic neuroblastoma cells has been described elsewhere. Cells were cultured in Opti-MEM (Life Technologies) supplemented with 10% (vol/vol) FBS and 500 μg/mL G418.
Inflammasome Activation.
M17 cells were grown to 90% confluence in medium (Opti-MEM; Invitrogen) containing 10% (vol/vol) FBS (Atlanta Biologicals) and 500 μg/mL G418. The following inflammasome activators (all from InvivoGen) were added to the culture medium: monosodium urate crystals (100 μg/mL), LPS from E. coli K12 (LPS-EK) (1 μg/mL), and LPS-EK ultrapure (1 μg/mL). After incubation at 37 °C in a humidified 5% (vol/vol) CO2 incubator overnight, the cells were harvested for Western blot analysis.
Caspase-1 Activity Assay.
Recombined caspase-1 (rCASP) protein activity was measured using the Caspase-1 Drug Discovery Kit (BML-AK701; Enzo Life Sciences), with an rCASP stock solution concentration of 0.3 mg/mL. Protein samples (0, 50 U of caspase-1 standard, 5 μL of rCASP, 15 μL of rCASP, 25 μL of rCASP, 150 μL of rCASP) were added to assay buffer [50 mM Hepes (pH 7.4), 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, 10% (vol/vol) glycerol], to a total volume of 50 μL in a 96-well plate. The reaction was initiated by the addition of 50 μL of 100 μM Ac-YVAD-7-amino-4-methylcoumarin (AMC) as a substrate, and fluorescence was read (excitation wavelength, 360 nm; emission wavelength, 460 nm) continuously at 30 °C in 1-min intervals for a total of 30 min using a FlexStation2 fluorescence plate reader (Molecular Devices). The standard curve for fluorescence vs. AMC concentration was constructed by measuring the fluorescence emitted from various concentrations of AMC at 30 °C.
Caspase-1 activity on aSyn was conducted similarly; however, 10 μg of aSyn and 50 nM caspase-1 were incubated at 30 °C for 30 min and then analyzed by Western blot analysis (as described above) and MALDI-TOF mass spectrometry. For mass spectrometry analysis of caspase-1 truncated aSyn, 1 μL of sample was spotted on a MALDI target containing 1 μL of matrix and 10 mg/mL sinapic acid, and analyzed on an AB SCIEX 5800 TOF/TOF mass spectrometer (Applied Biosystems).
ThT Aggregation Assay.
Protein samples (full-length WT, full-length WT with 2 μM caspase 1, 90% full-length and 10% truncated form, truncated form) were added to 100 µL of 100 mM Hepes (pH 7.4), 150 mM NaCl, 10% (vol/vol) glycerol, 0.1% BOG, and 5 µM ThT to reach a final concentration of 0.2 mM, followed by incubation at 37 °C with frequent agitation. The fluorescence of the ThT (excitation wavelength, 440 nm; emission wavelength, 490 nm; cutoff wavelength, 475 nm) was measured with a FlexStationII scanner (Molecular Devices) at 37 °C in 1-h intervals over a 1-wk period.
Acknowledgments
We thank S. Lindquist for the yeast expression vectors of aSyn, and Z. Y .Z. Zhang and L. Chen for their generous support and access to the Chemical Genomics Core Facility at Indiana University. Q.Q.H., D.R., and G.A.P. were supported through a Community Fast Track grant from the Michael J. Fox Foundation. Q.Q.H. was also supported by National Institutes of Health Grants 1R21NS079881-01 and 5R01GM111639, an Indiana University School of Medicine Biomedical Research Grant, and an Indiana University–Purdue University Indianapolis Research Support Funds Grant. D.R. and G.A.P. also acknowledge support from the Fidelity Biosciences Research Initiative (with much useful discussion from Dr. S. Weninger) and early support from the Ellison Medical Foundation and the McKnight Endowment for Neuroscience. M.B.B. and C.J.T. acknowledge support from the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610099113/-/DCSupplemental.
References
- 1.Ben-Shlomo Y. The epidemiology of Parkinson’s disease. Baillieres Clin Neurol. 1997;6(1):55–68. [PubMed] [Google Scholar]
- 2.Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang AE, Lozano AM. Parkinson’s disease. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 4.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC. Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2(3):18. doi: 10.1186/alzrt42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee AC, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Furuya T, Mizuno Y, Mochizuki H. Inflammation and infection in Parkinson’s disease. Histol Histopathol. 2006;21(6):673–678. doi: 10.14670/HH-21.673. [DOI] [PubMed] [Google Scholar]
- 8.Harris MA, Tsui JK, Marion SA, Shen H, Teschke K. Association of Parkinson’s disease with infections and occupational exposure to possible vectors. Mov Disord. 2012;27(9):1111–1117. doi: 10.1002/mds.25077. [DOI] [PubMed] [Google Scholar]
- 9.Rail D, Scholtz C, Swash M. Post-encephalitic Parkinsonism: Current experience. J Neurol Neurosurg Psychiatr. 1981;44(8):670–676. doi: 10.1136/jnnp.44.8.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16(9):566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang H, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci USA. 2009;106(33):14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurizi CP. Why was the 1918 influenza pandemic so lethal? The possible role of a neurovirulent neuraminidase. Med Hypotheses. 1985;16(1):1–5. doi: 10.1016/0306-9877(85)90034-9. [DOI] [PubMed] [Google Scholar]
- 13.Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 14.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27(12):3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michell AW, et al. The effect of truncated human alpha-synuclein (1-120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transplant. 2007;16(5):461–474. doi: 10.3727/000000007783464911. [DOI] [PubMed] [Google Scholar]
- 16.Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M. Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur J Neurosci. 2010;32(3):409–422. doi: 10.1111/j.1460-9568.2010.07284.x. [DOI] [PubMed] [Google Scholar]
- 17.Wakamatsu M, et al. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29(4):574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Prasad K, Beach TG, Hedreen J, Richfield EK. Critical role of truncated α-synuclein and aggregates in Parkinson’s disease and incidental Lewy body disease. Brain Pathol. 2012;22(6):811–825. doi: 10.1111/j.1750-3639.2012.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci USA. 2005;102(6):2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata A, et al. Alpha-synuclein degradation by serine protease neurosin: Implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12(20):2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- 21.Lewis KA, Yaeger A, DeMartino GN, Thomas PJ. Accelerated formation of alpha-synuclein oligomers by concerted action of the 20S proteasome and familial Parkinson mutations. J Bioenerg Biomembr. 2010;42(1):85–95. doi: 10.1007/s10863-009-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis KA, et al. Abnormal neurites containing C-terminally truncated alpha-synuclein are present in Alzheimer’s disease without conventional Lewy body pathology. Am J Pathol. 2010;177(6):3037–3050. doi: 10.2353/ajpath.2010.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishizen-Eberz AJ, et al. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003;86(4):836–847. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 24.Sung JY, et al. Proteolytic cleavage of extracellular secreted alpha-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280(26):25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 25.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanaja SK, Rathinam VAK, Fitzgerald KA. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015;25(5):308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302(5651):1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci USA. 2005;102(6):2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheer JM, Wells JA, Romanowski MJ. Malonate-assisted purification of human caspases. Protein Expr Purif. 2005;41(1):148–153. doi: 10.1016/j.pep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Boxer MB, et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5(5):730–738. doi: 10.1002/cmdc.200900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh M, et al. Potential pharmacological chaperones targeting cancer-Associated MCL-1 and Parkinson disease-associated α-synuclein. Proc Natl Acad Sci USA. 2014;111(30):11007–11012. doi: 10.1073/pnas.1320556111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michell AW, et al. The effect of truncated human alpha-synuclein (1-120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transplant. 2007;16(5):461–474. doi: 10.3727/000000007783464911. [DOI] [PubMed] [Google Scholar]
- 34.Liu CW, et al. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: Implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280(24):22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 35.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 36.McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76(6):550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 37.Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: Evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord. 2007;22(13):1852–1856. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- 38.Blum-Degen D, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;202(1-2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 39.Mogi M, et al. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211(1):13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 40.Mogi M, et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165(1-2):208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 41.Mogi M, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180(2):147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 42.Villoslada P, et al. Immunotherapy for neurological diseases. Clin Immunol. 2008;128(3):294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz M, Butovsky O, Kipnis J. Does inflammation in an autoimmune disease differ from inflammation in neurodegenerative diseases? Possible implications for therapy. J Neuroimmune Pharmacol. 2006;1(1):4–10. doi: 10.1007/s11481-005-9010-2. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83(2):447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 45.Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knoblach SM, et al. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J Neurotrauma. 2002;19(10):1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- 47.Furuya T, et al. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci. 2004;24(8):1865–1872. doi: 10.1523/JNEUROSCI.3309-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullen V, et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol. 2011;69(6):940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 49.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: Role of neuroinflammation. Environ Health Perspect. 2003;111(8):1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klevenyi P, et al. Transgenic mice expressing a dominant negative mutant interleukin-1beta converting enzyme show resistance to MPTP neurotoxicity. Neuroreport. 1999;10(3):635–638. doi: 10.1097/00001756-199902250-00035. [DOI] [PubMed] [Google Scholar]
- 51.Ling Z, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17(1):116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- 52.Gao H-M, et al. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119(6):807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: Role of neuroinflammation. Environ Health Perspect. 2003;111(8):1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klevenyi P, et al. Transgenic mice expressing a dominant negative mutant interleukin-1beta converting enzyme show resistance to MPTP neurotoxicity. Neuroreport. 1999;10(3):635–638. doi: 10.1097/00001756-199902250-00035. [DOI] [PubMed] [Google Scholar]
- 55.Ling Z, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17(1):116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- 56.Du Y, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98(25):14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bassil F, et al. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc Natl Acad Sci USA. 2016;113:9593–9598. doi: 10.1073/pnas.1609291113. [DOI] [PMC free article] [PubMed] [Google Scholar]