Significance
Sialic acid (Sia) residues are essential monosaccharides in mammals and confer multiple biological functions. Their precise generation is important for both structure–function studies and biotechnological applications. We describe a unique technology that enables the controlled generation of protein sialylation in Nicotiana benthamiana. The plant engineering approach relies on a combination of endogenous glycan deconstruction and the introduction of human sialylation capabilities. An arrangement of transgenic and transient expression modules resulted in the targeted synthesis of Sia structures in three different linkage types, reaching a polymerization degree exceeding 40 residues (polySia). Importantly, the obtained functional activities of polySia point to novel biotherapeutic applications. Our results highlight the exceptional flexibility of the plant-based expression platform for engineering complex posttranslational protein modifications.
Keywords: protein polysialylation, Nicotiana benthamiana, recombinant proteins, glycoengineering, in planta sialylation
Abstract
Sialic acids (Sias) are abundant terminal modifications of protein-linked glycans. A unique feature of Sia, compared with other monosaccharides, is the formation of linear homo-polymers, with its most complex form polysialic acid (polySia). Sia and polySia mediate diverse biological functions and have great potential for therapeutic use. However, technological hurdles in producing defined protein sialylation due to the enormous structural diversity render their precise investigation a challenge. Here, we describe a plant-based expression platform that enables the controlled in vivo synthesis of sialylated structures with different interlinkages and degree of polymerization (DP). The approach relies on a combination of stably transformed plants with transient expression modules. By the introduction of multigene vectors carrying the human sialylation pathway into glycosylation-destructed mutants, transgenic plants that sialylate glycoproteins in α2,6- or α2,3-linkage were generated. Moreover, by the transient coexpression of human α2,8-polysialyltransferases, polySia structures with a DP >40 were synthesized in these plants. Importantly, plant-derived polySia are functionally active, as demonstrated by a cell-based cytotoxicity assay and inhibition of microglia activation. This pathway engineering approach enables experimental investigations of defined sialylation and facilitates a rational design of glycan structures with optimized biotechnological functions.
In mammals, glycoproteins and glycolipids are frequently terminated with sialic acid (Sia) residues. These negatively charged sugar residues (i.e., N-acetylneuraminic acid) play essential roles in many aspects of life: e.g., in cell–cell interactions, in cell signaling, and for protein stability (1, 2). A unique feature of Sia is that, unlike other sugars, it often forms homo-oligomers or polymers, with its most complex form polysialic acid (polySia) reaching a degree of polymerization up to 400 (3). This sugar polymer is remarkable in that it plays multiple roles across different species from bacteria to humans. Its exceptional physicochemical properties provide the polySia with a large exclusion volume that physically inhibits interactions mediated by polySia-containing molecules, as shown for the major polySia carrier in humans, the neural cell adhesion molecule, NCAM (1, 2, 4). Recent studies demonstrated the effect of polySia in a variety of basic biological processes, like neural cell regeneration and antiinflammatory processes (5). Furthermore, the role of polySia in extraneural tissues and cells has also been described, revealing novel roles of polySia in protection and repair/regeneration and in immunological processes (recently reviewed by refs. 1, 2, 5, and 6). Notably, chemical or in vitro polySia engineering has made a substantial impact on pharmacokinetic properties of recombinant glycoprotein therapeutics (7, 8). These examples demonstrate a manifold functional impact of polySia and highlight the great potential for the development of novel polySia-dependent therapeutics.
The enormous structural diversity that is immanent in the mammalian sialom complicates understanding the biological function of individual sialylated glycan structures. Advanced technologies to generate and probe diverse sialylated glycoproteins are necessary not only to further elucidate the biological significance of sialic acid-containing molecules but also to pursue medicinal and industrial applications. For the recombinant expression of sialylated proteins, preferentially mammalian/human cells are used because they are able to cap N-glycans (e.g., β1,4-linked galactose) with α2,3-linked and α2,6-linked Sia, the latter representing the prevalent variant in human cells (1). Although such cells produce properly sialylated glycoproteins, they have several limitations, like glycan heterogeneity and the incapability to produce distinct glycoforms on demand, that hamper further investigation on the biological impact of sialylation and the development of optimized therapeutic proteins. Substantial efforts have been devoted to genetic engineering of mammalian cells and other organisms to expand their glycosylation capacity and to improve or alter glycosylation, including sialylation (9, 10). Despite impressive recent achievements, designed sialylation strategies are largely elusive.
Plants are increasingly being recognized as an interesting expression system for human glycoproteins, not least due to their ability to produce glycoproteins with homogenous N-glycan structures. Although significant parts of the N-glycosylation machinery are conserved between plants and human cells, plants lack essential parts of the glycosylation repertoire that contribute to the glycan diversity typically observed in mammalian cells. For example, plants lack a number of glycosyltransferases that are mainly responsible for the diversification and elongation of glycans, including branching, galactosylation, and sialylation. Also, plants lack the machinery for biosynthesis of the nucleotide sugar CMP-sialic acid and its transport from the cytoplasm to the Golgi apparatus, where it serves as the donor substrate for different sialyltransferases. Nevertheless, various knock-in and knock-out/knock-down studies have demonstrated the enormous flexibility of plants to synthesize complex human-type glycosylation on demand (11). A prominent example is the generation of a Nicotiana benthamiana (N. benthamiana) glycosylation mutant (∆XTFT) that synthesizes human type N-glycans, namely GnGn structures (GlcNAc2Man3GlcNAc2) at high homogeneity (12). The superior efficiency of glycoengineered monoclonal antibodies (mAbs) produced in these plants has recently been highlighted by ZMapp, the mAb mixture developed for the treatment of Ebola virus-infected patients (13). In addition, the introduction of entire biosynthetic pathways in a transient manner allowed the in planta production of mono- and biantennary α2,6-sialylated N-glycans on therapeutically interesting proteins (14, 15). The approach required the coordinated expression of six foreign glycosylation proteins in different subcellular compartments of N. benthamiana plants. Despite this remarkable achievement, the transient expression methodology is prone to batch-to-batch inconsistencies. A transgenic approach that confers stable pathway engineering would be a reliable alternative to overcome these shortcomings and would lay the basis for elusive engineering approaches like polysialylation.
Here, we focused on the modulation of the plant N-glycosylation pathway for the synthesis of complex sialylated structures by a targeted combination of transgenic and transient expression modules. We used the glycosylation mutant ∆XTFT for the transgenic introduction of the mammalian sialylation pathway (comprising six mammalian proteins), which resulted in ∆XTFTSia plants synthesizing mono- and bisialylated structures in either α2,6- or α2,3-linkage. Subsequently two human polysialyltransferases (ST8Sia-II and ST8Sia-IV) were transiently delivered to ∆XTFTSia by agroinfiltration. The two polysialyltransferases transferred Sia residues in planta to a recombinantly coexpressed Ig5FN1 module of the human neural cell adhesion molecule (NCAM). High performance liquid chromatography (HPLC) of fluorescently labeled polySia chains liberated from Ig5FN1 demonstrated the synthesis of polySia chains that in length extended 40 residues. Functional activities of polySia were determined by cell-based assays. Plant-derived polySia inhibits cytotoxicity of free histones and exhibits attenuation of nitric oxide production of LPS-stimulated microglia cells.
Results
Stable Engineering of an N-Glycan Processing Pathway Toward the Formation of Defined Sialylated Structures (∆XTFTSia).
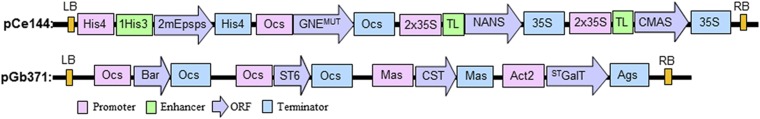
To test the capability of N. benthamiana ∆XTFT for tolerance of N-glycan augmentation/diversification toward sialylated structures, we pursued the generation of transgenic ∆XTFT plants that stably express six mammalian proteins involved in sialylation (14). A library of various promoter–terminator constructs was generated and evaluated by a transient expression approach. To this end, two binary vectors were generated (16): one for the expression of the proteins necessary for the synthesis of the activated nucleotide sugar precursor CMP-Sia (pCe144: GNE, NANS, and CMAS) and one for the expression of proteins necessary for synthesis of the N-glycan acceptor substrate (β1,4-galactosylated N-glycans), Golgi transport, and transfer of sialic acid to nascent glycoproteins (pGb371: STGalT, CST, and ST) (Fig. S1 and Table S1). A mutated version of UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE), a key enzyme for the biosynthesis of CMP-sialic acid (17), was generated. The mutated GNE variant (GNER263L) lacks the binding site for the feedback inhibition and resulted in the synthesis of four times higher amounts of CMP-Sia (1 µmol⋅g−1 vs. 0.25 µmol⋅g−1 fresh weight when native GNE was used). Both vectors (pCe144, pGb371) were cotransformed into the ∆XTFT genome, and different primary transformants that were genomic PCR-positive for all six foreign genes were used to assess their sialylation capacity. For this purpose, recombinant glycoproteins were transiently expressed and subsequently subjected to N-glycan analysis by liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS). Plants that produced recombinant proteins with large amounts of sialylated structures were brought to a homozygous stage, resulting in a previously unidentified plant-based expression platform (∆XTFTSia).
Fig. S1.
Schematic presentation of the multigene vectors used for leaf disk transformation of N. benthamiana ∆XTFT. 1His3, first intron from the histone H3 of Arabidopsis thaliana; 2mEpsps, double-mutant 5-enol-pyruvylshikimic acid 3-phosphate synthase from Zea mays conferring resistance to glyphosate; 35S, cauliflower mosaic virus (CaMV) 35S promoter or terminator; Act2, actin 2 promoter from A. thaliana; Ags, agropin synthase terminator; Bar, glufosinate ammonium resistance gene (Basta); CMAS, Homo sapiens CMP-N-acetylneuraminic acid synthase; CST, mouse CMP-sialic acid transporter; GNEMUT, mouse UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase harboring a point mutation (GNER→L); His4, histone H4 gene promoter or terminator from A. thaliana; LB, left border; Mas, manopine synthase promoter or terminator; NANS, H. sapiens N-acetylneuraminic acid phosphate synthase; Ocs, octopine synthase promoter or terminator; ORF, open reading frame; RB, right border; ST6, rat α2,6-sialyltransferase; STGalT, human β1,4-galactosyltransfease fused to the cytoplasmic tail, transmembrane domain and stem region of the rat α2,6-sialyltransferase; TL, translational enhancer 5′ UTR from tobacco etch virus.
Table S1.
Primers used in this study
| Primer | Restriction | Sequence (5′–3′) |
| Epsps F1 | AvrII/NcoI | cgcgttaatacctaggccatgggccatgg |
| Epsps R1 | NcoI/AvrII | cgcgccatggcccatggcctaggtattaa |
| GNEmut F | — | agcaaggagatggttctagtgatgcggaagaag |
| GNEmut R | — | cttcttccgcatcactagaaccatctccttgct |
| AscImut F | — | ccataaattctagaggcgcatcgcggccgctcc |
| AscImut R | — | ggagcggccgcgatgcgcctctagaatttatgg |
| ST3 F1 | XbaI | tatatctagaatggtcagcaagtcccgctggaa |
| ST3 R1 | BamHI | tataggatcctcagaaggacgtgaggttcttga |
| ST8Sia-II F1 | XbaI | tatatctagaatgcagctgcagttccggagc |
| ST8Sia-II R1 | BglII | tataagatctttacttttcgaactgcggatggctccacgtggccccatcgcactggc |
| ST8Sia-II R2 | BglII | tataagatctcgtggccccatcgcactggc |
| ST8Sia-IV F1 | XbaI | tatatctagaatgcgctccattaggaagag |
| ST8Sia-IV R1 | BamHI | tataggatccttacttttcgaactgcggatggctccattgctttacacactttcc |
| ST8Sia-IV R2 | BamHI | tataggatccttgctttacacactttcc |
Restriction sites are in italics, and Strep-tag sequences are in bold. —, no restriction.
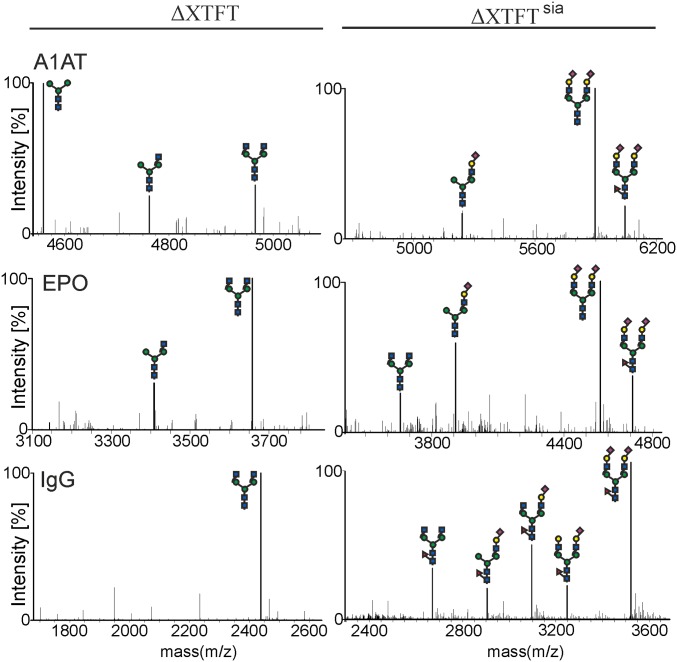
To test the sialylation capability of ∆XTFTSia in detail, we analyzed three human glycoproteins that are naturally sialylated at different intensities: i.e., erythropoietin (EPO), α1-antitrypsin (A1AT), and IgG. Although purified IgG and EPO exhibit GnGn structures when expressed in ∆XTFT, A1AT carries mainly Man3GlcNAc2 N-glycans due to an endogenous β-hexosaminidase activity in plants (18) (Fig. 1). All three glycoproteins were efficiently sialylated when expressed in ∆XTFTSia (Fig. 1). Note that, for IgG sialylation, Arabidopsis thaliana α1,3-fucosyltransferase (FUT11) was transiently coexpressed because core α1,3-fucosylation enhances sialylation of the Fc glycans, which are naturally inefficiently sialylated (19). Transgenic ∆XTFTSia plants stably express rat α2,6-sialyltransferase (ST6) and synthesize α2,6-linked Sia as previously shown using a transient expression approach (14). To test whether α2,3-linked Sia can be synthesized in planta on a recombinant glycoprotein, ST6 was replaced by a construct that expresses human α2,3-sialyltransferase (ST3). Indeed, transient coexpression of ST3, with pCe144, β1,4-galactosyltransferase, and the CMP-sialic acid transporter, resulted in the synthesis of sialylated N-glycan structures on EPO (Fig. S2). The sialylation efficiencies of ST6 and ST3 are comparable; both enzymes transiently sialylate up to 90% of the acceptor substrate. Importantly, besides a considerable decrease in fertilization with reduced seed yield, transgenic ∆XTFTSia plants do not exhibit any obvious morphological or developmental changes compared with ∆XTFT or N. benthamiana WT. Likewise, recombinant glycoprotein production was not hampered by extensive genetic engineering and glycosylation pathway modifications, indicating the generation of a robust plant-based platform for the production of glycoproteins with defined terminal sialic acid residues.
Fig. 1.
Recombinant human glycoproteins are capped with terminal sialic acid in transgenic ΔXTFTsia plants. Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of selected tryptic glycopeptides from A1AT (Asn83, K/ A70ADTHDEILEGLNFNLTEIPEAQIHEGFQELLR101), EPO (Asn83, R/G77QALLVNSSQPWEPLQLHVDK97), and IgG (Asn297, R/E293EQYNSTYR301) expressed in N. benthamiana ΔXTFT and ΔXTFTsia. Glycan diagrams were drawn using the Consortium for Functional Glycomics symbols (www.functionalglycomics.org). A1AT, human α1-antitrypsin; EPO, human erythropoietin; IgG, human Ig 1 (monoclonal antibody).
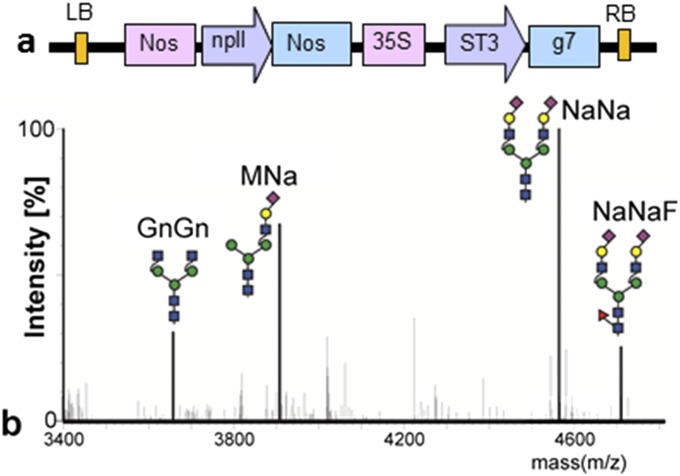
Fig. S2.
Expression of human erythropoietin (EPO) decorated with α2,3-sialylated N-glycans. (A) Schematic presentation of the binary vector used to express the full-length human α2,3-sialyltransferase (ST3). ST3 and EPO (16) vectors were coexpressed in ΔXTFT, along with pCe144 (Fig. S1), the CST, and GalT vectors (14). (B) Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of EPO tryptic glycopeptide 2 (Asn83, G77QALLVNSSQPWEPLQLHVDK97). Glycan diagrams were drawn using the Consortium for Functional Glycomics symbols (www.functionalglycomics.org). 35S, cauliflower mosaic virus (CaMV) 35S promoter; g7, Agrobacterium gene 7 terminator; LB, left border; Nos, nopaline synthase promoter or terminator; npII, neomycin phosphotransferase II gene conferring resistance to kanamycin; RB, right border; ST3, human alpha-N-acetyl-neuraminide alpha-2,3-sialyltransferase (NM_006278).
Human Polysialyltransferases Are Functional When Transiently Expressed in ∆XTFTSia.
Sialic acid residues are usually not extended further with other monosaccharides, except with another sialic acid. Sialyltransferases of the mammalian ST8Sia family catalyze oligo- and polysialylation of glycoproteins through transfer of α2,8-sialic acids from CMP-sialic acid to the nonreducing ends of α2,6- or α2,3-Sia acceptors (20, 21). If the degree of polymerization (DP) exceeds 8 (DP >8), such structures are regarded as polySia chains and can be as long as DP >400 (3). PolySia is a prominent structural feature on the termini of N- or O-glycans of a very small number of mammalian proteins (3). In mammals, ST8Sia polysialyltransferases (polySTs) are type II membrane proteins that reside in the Golgi. Two human polyST members have been characterized, ST8Sia-II and ST8Sia-IV (21, 22), and are responsible for the polysialylation of NCAM. PolySTs are themselves modified by autopolysialylation with α2,8-linked polysialic acid chains (23). Although autopolysialylation is not a prerequisite for polyST enzymatic activity, it enhances NCAM polysialylation (24).
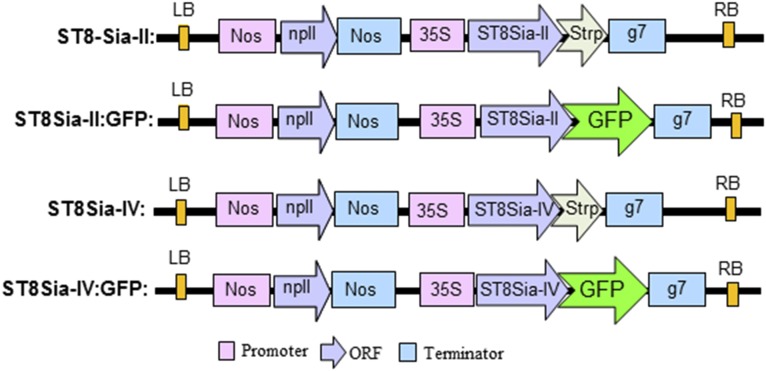
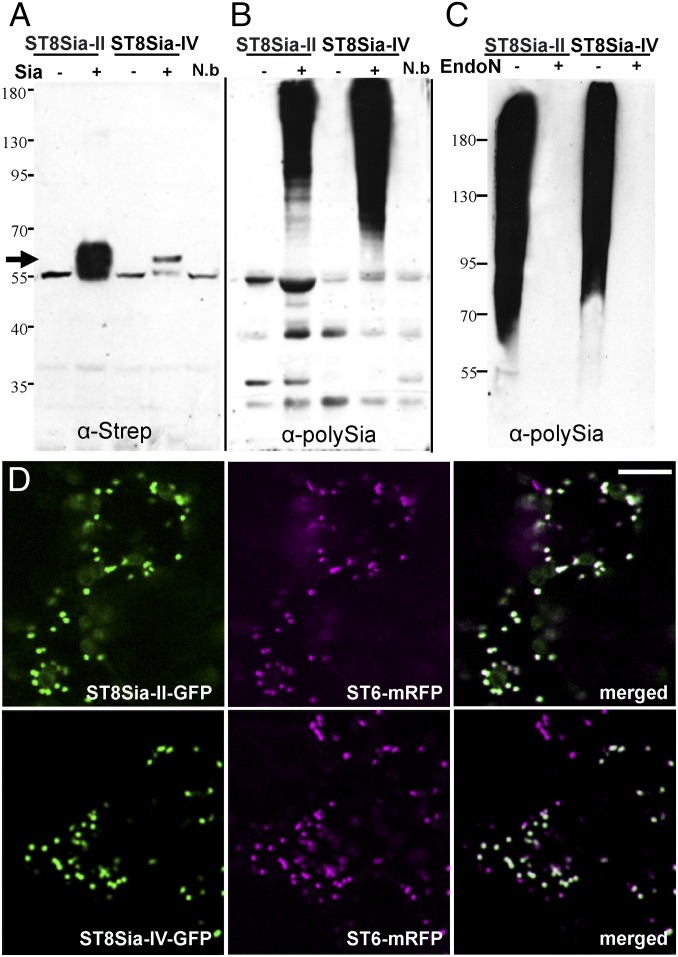
Here, we examined the capability of polySTs to polysialylate glycoproteins in planta when transiently expressed in ∆XTFTSia. Appropriate plant expression vectors coding for Strep-tagged human ST8Sia-II and ST8Sia-IV were generated (Fig. S3 and Table S1) and transiently expressed in N. benthamiana leaves by agroinfiltration. PolyST expression was monitored in total protein extracts by immunoblotting using Strep-specific antibodies. A specific signal was obtained at the expected size (i.e., 58 kDa) for both enzymes expressed in ∆XTFTSia (Fig. 2A). Interestingly the recombinant polySTs could not be detected in ∆XTFT (of WT) plants that lacked sialylation capacity, indicating the requirement of sialylation for protein stability. Because the autopolysialylation capacity of the enzymes is well documented (23–25), we examined the presence of polySia structures on ST8Sia-II and -IV expressed in ∆XTFTSia by Western blotting using a polySia-specific antibody. Although strong signals were obtained on total protein extracts at the high molecular weight range in plants expressing polySTs, none were detected when the respective expression constructs were absent (Fig. 2B). Endoneuraminidase-N (EndoN) digestion, which specifically cleaves α2,8-linked polySia, abolishes the signal indicating the presence of polySia structures in ∆XTFTSia infiltrated with polySTs (Fig. 2C). No polySia-specific signals were obtained when endogenous plant total soluble proteins were analyzed (Fig. S4A), indicating that they are not substrates for polysialylation. Subcellular localization of the recombinant enzymes was determined by live-cell confocal microscopy of ΔXTFTSia leaf epidermal cells expressing ST8Sia-II and ST8Sia-IV fused to GFP (ST8Sia-II-GFP and ST8Sia-IV-GFP) (Fig. S3). Fluorescent protein fusions were transiently coexpressed with the Golgi marker ST6-mRFP (26) in ΔXTFTSia and analyzed 3 d postinfiltration. The punctate fluorescence of both ST8Sia-II-GFP/ST8Sia-IV-GFP (Fig. 2D) and of ST6-mRFP (Fig. 2D) shows significant colocalization (Fig. 2D) (Fig. 2D and Fig. S4B), indicating localization of polyST fusions in the Golgi, the expected subcellular site for their action.
Fig. S3.
Schematic presentation of the binary vectors used in this investigation to transiently express polysialyltransferases in N. benthamiana ∆XTFT and ∆XTFTSia. Human polyST cDNAs were cloned in binary vectors with a C-terminal tag either for assessing expression with anti-Strep antibodies (Strp) or study protein subcellular localization by confocal laser scanning microscopy (GFP). 35S, cauliflower mosaic virus (CaMV) 35S promoter; g7, Agrobacterium gene 7 terminator; GFP, green fluorescent protein; Strp, Strep-tag (WSHPQFEK peptide); LB, left border; Nos, nopaline synthase promoter or terminator; npII, neomycin phosphotransferase II gene conferring resistance to kanamycin; RB, right border; ST8Sia-II, human alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 (NM_006011.3); ST8Sia-IV, human alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 (NM_005668.5).
Fig. 2.
Polysialyltransferases are active in N. benthamiana leaves and located in the Golgi. (A) Western blot analysis of total proteins extracted from ΔXTFT (Sia −) and ΔXTFTSia (Sia +) infiltrated with ST8Sia-II and ST8Sia-IV constructs (Fig. S3). Approximately 50–100 µg of proteins were loaded per lane. Apparent protein molecular masses are indicated in kilodaltons. Specific signals at ∼58 kDa were detected using anti-Strep antibodies (arrow) in ΔXTFTSia. Total proteins extracted from noninfiltrated leaves (N.b) served as negative control. In all samples a 55-kDa protein was visible, which most probably corresponds to rubisco, the most abundant protein in plant leaves. (B) Immunoblotting using the anti-polySia antibody mAb735. (C) Immunoblotting using mAb735; samples were treated with (+) or without (−) endoneuraminidase N (EndoN). (D) Subcellular localization of the recombinant enzymes was determined by live-cell imaging of ΔXTFTSia leaf epidermal cells expressing ST8Sia-II and ST8Sia-IV fused to GFP (ST8Sia-II-GFP and ST8Sia-IV-GFP) (Fig. S3). Fluorescent protein fusions were transiently coexpressed with the Golgi marker ST6-mRFP in ΔXTFTSia and analyzed 3 d postinfiltration. The punctate fluorescence of both ST8Sia-II-GFP and ST8Sia-IV-GFP (green) and of ST6-mRFP (magenta) shows significant colocalization (white, merged). (Scale bar: 6.5 µm.) The integrity of the GFP fusions was determined by immunoblotting with anti-GFP antibodies (Fig. S4B).
Fig. S4.
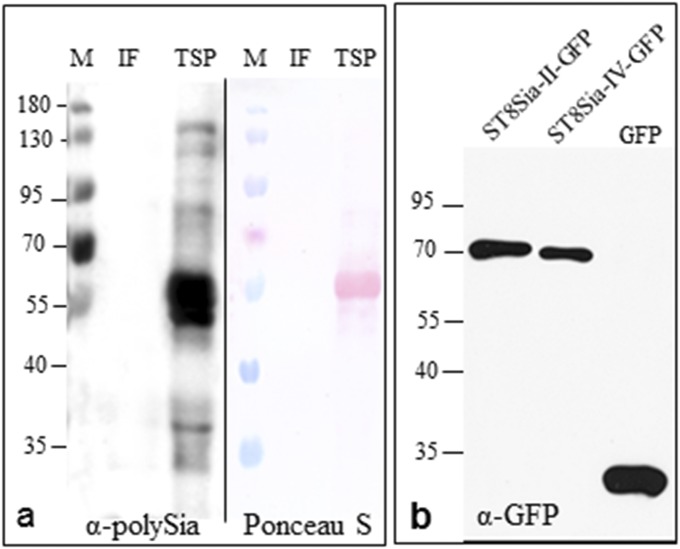
Western blot analysis of polysialyltransferase transiently expressed N. benthamiana. (A) The high molecular weight proteins detected with antibodies against polySia (αPSA, mAb735) on total proteins extracted from ΔXTFTSia leaves (as seen in Fig. 2 B and C) reflect autopolysialylation of ST8Sia-II or ST8Sia-IV. A similar smeared signal is not detected in intracellular fluid (IF) or in total soluble proteins (TSPs). The strong signal at 55 kDa in TSP corresponds to unspecific binding of the mAb735 to the plant rubisco protein. Rubisco is also visualized by Ponceau staining. (B) Immunoblot analysis of total protein extracts from ΔXTFTSia expressing ST8Sia-II-GFP and ST8Sia-IV-GFP using anti-GFP (α-GFP) antibodies shows the integrity of the fusion proteins; free GFP (<35 kDa) is detected only on the control sample. Apparent protein molecular masses are indicated in kilodaltons (M).
The Recombinantly Expressed Ig5FN1 Module of NCAM Is Polysialylated in Vivo in Glycoengineered Plants.
The Ig5FN1 module of NCAM is a well-characterized target for polysialylation (27). Thus, this module was used as a reporter for in planta polysialylation. The Ig5 domain carries three N-glycosylation sites (ASN4–ASN6), with sites 5 and 6 being decorated with polySia structures (28) (Fig. S5A). A secreted version of Ig5FN1 (Fig. S5B) was expressed in ∆XTFTSia and ∆XTFT leaves. Upon inspection of the intercellular fluid (IF) (representing the secretome of a plant cell) on SDS/PAGE and subsequent Coomassie Brilliant Blue staining, a strong signal was visible at position 35 kDa, corresponding to the glycosylated Ig5FN1 polypeptide (Fig. 3A). The band was excised from the gel and analyzed by LC-ESI-MS. Glycopeptide analysis revealed the presence of peaks corresponding to GnGn or bisialylated structures (NaNa: Neu5Ac2Gal2GlcNAc2Man3GlcNAc2) depending on the expression host (∆XTFT or ∆XTFTSia) (Fig. 3A). For the in planta synthesis of polySia structures, Ig5FN1 was coexpressed with polySTs in ∆XTFTSia. In addition, mouse core α1,6-fucosyltransferase (FUT8) was coexpressed to achieve core α1,6-fucosylation (29) because this glycan residue positively influences the synthesis of polySia structures on Ig5FN1 (30). The presence of polySia structures was first monitored by Western blotting of IF resident proteins using the polySia-specific antibody. Strong signals at high molecular weight were visible in samples coexpressing polySTs, Ig5FN1, and FUT8 whereas none were visible when one of these proteins was not coexpressed, indicating specific addition of polySia to Ig5FN1 but not to native plant proteins (Fig. 3B). The signal was abolished after EndoN digestion, pointing to the presence of polySia structures on Ig5FN1 (Fig. 3B). In a next step, the degree of polymerization was analyzed. High performance liquid chromatography (HPLC) of fluorescently labeled polySia chains liberated from IF-derived Ig5FN1 demonstrated the synthesis of polySia chains exceeding a length of 40 Sia residues (Fig. 3C). Notably, no differences were observed in Ig5FN1 expression levels whether expressed in ∆XTFT or ∆XTFTSia with or without polySTs, demonstrating that the generated transgenic expression platform, in combination with the transient expression modules, is highly suitable for the in vivo generation of this carbohydrate polymer on recombinant proteins.
Fig. S5.
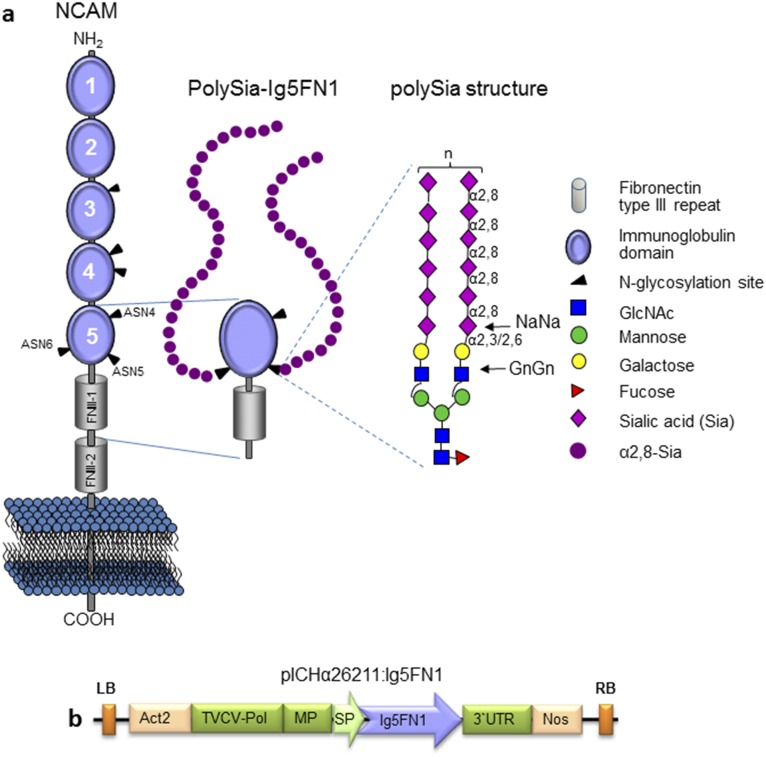
Schematic presentation of NCAM, polysialylated Ig5FN1 module, and its expression vector. (A) Schematic presentation of NCAM and polysialylated Ig5FN1 module. NCAM is a member of the Ig family of cell adhesion molecules with five Ig-like modules (Ig1 to Ig5) and two fibronectin type III repeats (FNIII-1 and FNIII-2) in the extracellular domain. The six N-glycosylation sites (ASN1 to -6) are indicated by black arrowheads on Ig3, Ig4, and Ig5. The polySia enzymes (ST8Sia-II and ST8Sia-IV) recognize an acidic surface on the FN1 domain and selectively transfer a homopolymer of α2,8-linked sialic acid residues (polySia) onto the adjacent Ig5 domain (ASN5 and ASN6) to form polySia structures with >90 monomer units. PolySia chains are linked to complex N-glycans terminally sialylated in α2,3- or α2,6-linkage (NaNa, bisialylated structures). (B) A plant viral-based transient expression system was used for recombinant expression of NCAM-Ig5FN1 module. cDNA (594 bp, amino acids 414–611) was cloned into the magnICON vector pICHα26211. The recombinant protein was targeted for secretion using the barley α-amylase signal peptide present in the vector. The resulting construct (pICHα26211:Ig5FN1) was transferred into Agrobacteria and delivered to plant leaves by agroinfiltration. 3′ UTR, 3′-untranslated region from the tobacco mosaic virus; Act2, Arabidopsis actin 2 promoter; Ig5FN1, two domains from the human NCAM molecule corresponding to the Ig5 and first fibronectin type III repeat. cDNA sequence (GenBank accession no. KU052570) was codon-optimized for expression in N. benthamiana; LB, left border; MP, movement protein from TMV; Nos, nopaline synthase gene terminator; RB, right border; TVCV-Pol, RNA-dependent RNA polymerase from turnip vein-clearing virus; SP, barley α amylase signal peptide.
Fig. 3.
Ig5FN1 is polysialylated when expressed in glycoengineered N. benthamiana. (A) Expression of Ig5FN1 was monitored by SDS/PAGE and Coomassie Brilliant Blue staining of intercellular fluid (IF) derived from ΔXTFT and ΔXTFTSia. A strong band at ∼35–40 kDa corresponds to the glycosylated Ig5FN1 polypeptide. Protein bands ≤35 kDa are associated with agrobacterial infection. IF from infiltrated leaves with Agrobacteria carrying an “empty” plasmid served as a negative control (C). Apparent protein molecular masses are indicated in kDa (M). Glycan analysis of IF-derived Ig5FN1 was performed by LC-ESI-MS of tryptic glycopeptides. The spectra for ASN5 (R/D38GQLLPSSNYSNIK51) are shown. (B) Western blot analysis using mAb735 of IF extracted from ΔXTFTSia infiltrated with (lane 1) Ig5FN1, (lane 2) Ig5FN1 coexpressed with human core αI,6-fucosyltransferase (FUT8), (lane 3) Ig5FN1 coexpressed with both ST8Sia-II and -IV (polySTs), and (lane 4) Ig5FN1 coexpressed with FUT8 and polySTs. As a control sample, (lane C) polySTs were coexpressed with FUT8 in the absence of Ig5FN1. Sample (4) was incubated without (−) and with (+) EndoN. (C) To determine the degree of polymerization (DP), purified polysialylated Ig5FN1 was labeled with 1,2-diamino-4,5-methylenedioxybenzene (DMB) and separated by HPLC. The degree of polymerization is given for selected peaks.
PolySia Generated in Plants Is Functional in Cell-Based in Vitro Assays.
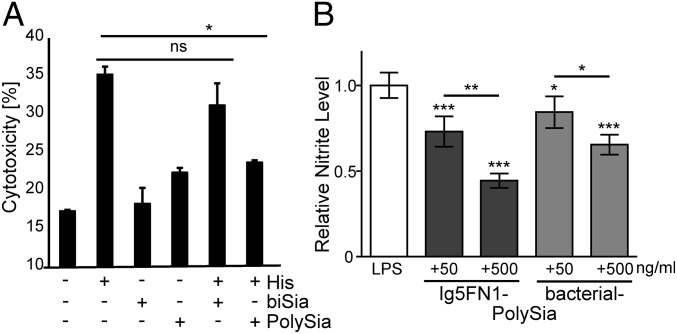
Previous findings have demonstrated that α2,8-linked Sia-polymers can abolish the cytotoxic effect of extracellular histones as seen for both bacterial colominic acid and soluble polySia from NCAM (31, 32). Here, a cytotoxicity inhibition assay against extracellular histone-mediated cytotoxicity of neutrophil extracellular traps (NETs) was performed to determine plant-derived polySia-mediated biological activity. Human umbilical vein endothelial cells (HUVECs) (with or without histone treatment) were cultured in the presence of IF-containing bisialylated Ig5FN1 or polySia-Ig5FN1, and activities were determined by a lactate dehydrogenase (LDH) cytotoxicity assay. Indeed, cytotoxicity induced by extracellular histones could be significantly decreased upon polySia-Ig5FN1 treatments whereas no significant effect was detected with bisialylated Ig5FN1 (Fig. 4A). In a second approach, affinity-purified polySia-Ig5FN1 was tested for its capability to inhibit microglia activation, another activity mediated by polySia structures (33). When present in BV-2 microglial cells, plant-produced polySia-Ig5FN1 was at least as potent as free soluble polySia in reducing the lipopolysaccharide (LPS)-induced production of nitric oxide (Fig. 4B), a pharmacological target and key regulator of the inflammatory response (34).
Fig. 4.
Plant-produced polySia is functionally active in cell-based inhibition assays. (A) Histone-mediated cytotoxicity was determined by its exposure to HUVECs. In parallel, the cytotoxicity was analyzed in the presence of IF-containing bisialylated Ig5FN1 (biSia) or polysialylated Ig5FN1 (polySia). All values are means of four independent experiments. The statistical evaluation was performed by Student´s t test (unequal variances, two-tailed). Significance levels are indicated by n.s. (nonsignificant), *P > 5%. (B) Nitric oxide production of BV-2 microglia cultured for 24 h in the presence of lipopolysaccharide (LPS, 1 µg⋅mL−1) and immunopurified polySia-Ig5FN1 or bacterial-polySia with corresponding concentrations of polySia, as indicated. Values are mean relative nitrite levels ± SD from ≥3 independent replicates for each condition. One-way ANOVA indicated significant effects (P < 0.0001), and Tukey’s post hoc test was applied. ***, **, *, significant differences against controls or between selected groups with P < 0.001, 0.01, or 0.5.
Discussion
Given their exposed position on protein-bound glycans, sialic acids are important mediators of cellular interactions, including cell signaling and specific immune responses (35). Moreover, for recombinant glycoprotein therapeutics, the positive effect of increased sialylation on pharmacokinetic properties is well-known (36).
In this study, we introduce a flexible approach for the generation of targeted sialylation. We demonstrate that whole plants well tolerate not only glycan deconstruction (∆XTFT) but also stable genomic integration of an entire glycosylation pathway. Importantly, both engineering approaches could be combined (∆XTFTSia), and the resultant glycosylation capacity remained stable at least for three generations, the time period evaluated thus far. Future studies will need to address potential challenges during extensive upscaling of glycoprotein production, including gene stability and glycosylation consistency. Interestingly, neither vegetative growth behavior of engineered plants nor the expression of recombinant proteins was affected by the extensive glycan engineering approach. Note that a reduction of seed production was observed in XTFTSia lines, for reasons that are not currently understood. It seems that sialylation of endogenous proteins interferes with the reproductive development of N. benthamiana. Certainly the sole presence of an engineered CMP-Sia pool in plants does not affect seed production, as demonstrated for Arabidopsis (37), and N. benthamiana transformed with only the three proteins needed for the synthesis of CMP-Sia. Nevertheless, in terms of the biotechnological applicability of ∆XTFTSia, this phenomenon should not provide a major limitation.
Efficient sialylation has been achieved in glycoengineered yeasts (38). This approach, which required the elimination of four yeast genes and introduction of 14 heterologous genes, provides certainly a suitably system for the expression of sialylated proteins. However, due to differences in protein folding and secretion, some complex, multimeric proteins might give higher yields in plants.
We demonstrate a rapid way to produce sialic acid in α2,3-linkage or α2,6-linkage, simply by the exchange of the two corresponding STs. The generation of “pure” Sia linkage-forms provides an advantage over mammalian cell systems because they either do not synthesize α2,6-linked Sia (e.g., CHO cells) or generate mixtures of both forms (most human cells). Although the impact of Sia linkage is often not clear, α2,6-sialylation is the preferred form in human serum IgG and shows increased effector functions compared with α2,3-linked variants (39). The ability to generate both linkages will allow a more precise investigation of their possible impacts.
Importantly, we demonstrate that ∆XTFTSia mutants are appropriate hosts for the generation of so far largely unexplored glycan structures: i.e., polySia. By the transient overexpression of human polySTs, the synthesis of polySia structures exceeding DP 40 was achieved. Interestingly, polySTs displayed autopolysialylation, and the presence of polyST proteins could only be detected when the entire sialic acid pathway is coexpressed. The impact of polySia on polyST activities is not entirely clear. One study claims that autopolysialylation is not essential for the enzymes’ activity (23); however, other experiments point to its importance for efficient polysialylation of NCAM (24, 40). Our results indicate that (autopoly) sialylation positively affects the stability of the two polySTs in planta. Similar to plant expression, human polySTs are autopolysialylated when expressed in COS-1 cells but do not polysialylate any endogenous proteins, highlighting the protein specificity of polysialylation (24). By contrast, bacterial polySTs are more promiscuous than the protein-specific mammalian enzymes (41). Together with advances in creating mutated bacterial polyST versions that allow for the synthesis of oligo- and polysaccharides of defined size (42), the bacterial polySTs are promising candidates to further optimize the platform toward in planta site-specific synthesis of defined polySia on many different recombinant glycoproteins. In contrast to autopolysialylation of polySTs, Ig5FN1 required core fucose for polysialylation. It seems that this core glycan residue induces conformational modifications, thereby influencing the processing of glycan structures, an observation also noticed for the glycosylation modulation of the IgG-Fc N-glycan (19). Selective polysialylation determined by specific core residues has been reported for glycoproteins expressed in CHO cells (30). Nevertheless, others have shown that distinct types of glycans are not required for polyST activity (1).
We show, by two independent cell-based assays, the functional activity of plant-derived polySia structures. PolySia produced in planta blocked cytotoxicity of free histones and attenuated the nitric oxide production of LPS-stimulated microglia cells. These results are in line with findings on the impact of polySia structures recently reviewed (2). The physical and biological characteristics of polySia may confer novel functions to these molecules, such as protection of host cells during inflammatory processes (5) or promoting cell migration required for regeneration (43), to name two. These activities of protein-independent polySia have great potential for the development of novel carbohydrate-dependent therapeutics. Likewise, improving the efficacy of therapeutic proteins (e.g., erythropoietin and buturylcholinesterase) using polySia was demonstrated by chemical conjugation and in vitro approaches (7, 8, 44). Clinical trials of polySia-conjugated recombinant proteins are ongoing, indicating the pharmaceutical relevance of polySia (reviewed in ref. 44). The polySia-dependent approach is particularly interesting because a related method, namely the attachment of polyethylene glycol (PEGylation) to components, raised concerns in therapeutic applications, due to the lack of biodegradability and immunogenicity (44).
Collectively, our results underscore the importance of diverse sialic acid modifications and the demand for expression systems that can provide the generation of defined sialylated glycoproteins not only for structure–function studies but also for therapeutic applications. The plant-based expression platform reported here should enable a targeted design of sialylation for many mammalian glycoproteins, which will enable the experimental evaluation of the biological function of defined sialo-forms and will allow rational design of optimized glycan-dependent therapeutics.
Experimental Procedures
See SI Experimental Procedures for descriptions of procedures used for cloning of expression constructs, transient recombinant protein expression, glycosylation analysis by LC-ESI-MS, immunoblotting, subcellular localization by confocal laser scanning microscopy, and analysis of polysialylation by HPLC. In addition, protocols used for testing biological activity of plant-derived polySia are also provided.
SI Experimental Procedures
Multigene Binary Vectors for Nicotiana benthamiana Stable Transformation.
The triple gene vectors pC144 and pG371 containing the expression cassettes for the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine-kinase (GNE), N-acetylneuraminic acid phosphate-synthase (NANS), CMP-Neu5Ac synthetase (CMAS), CMP-Neu5Ac transporter (CST), β1,4-galactosyltransfease fused to the cytoplasmic tail, transmembrane domain, and stem region of the α2,6-sialyltransferase (STGalT), and α2,6-sialyltransferase (ST6) were described previously (16). Here, we have modified these vectors to use them for cotransformation of the N. benthamiana ΔXTFT glycosylation mutant (12).
We introduced in pC144 an expression cassette encoding the glyphosate-resistant 5-enolpyruvoylshikimate-3-phosphate synthase (EPSPS) gene for glyphosate-tolerance (kindly supplied by Koen Wetering, Bayer CropScience, Ghent, Belgium). Annealed Epsps F1/R1 primers (Table S1) were cloned into pC144 to insert AvrII-NcoI restriction sites. These sites were used to introduce the epsps expression cassette as an AvrII- NcoI fragment (Fig. S1, pCe144). Also, a GNE expression cassette was replaced by a mutated version to prevent feedback inhibition. A point mutation on the GNE gene (R263L, GNER→L) was introduced in pSAT1-GNE (16) using the QuikChange II XL Site-directed Mutagenesis Kit (Stratagene) and the primers GNEmut F/R (Table S1), according to the manufacturer’s instructions. This pSAT1-GNER→L expression cassette was assembled in pCe144 as an AscI-AscI fragment replacing the existing one. Also, an expression cassette for glufosinate ammonium resistance (Basta) was transferred into pG371 (Fig. S1, pGb371). Basta resistance cassette was excised from the pPZP-RCS2-bar vector (GenBank accession no. DQ005454) as an AscI-AscI fragment. Because the AscI site was already used to clone in the ST6 expression cassette in pG371 (16), we mutated one of the sites as described above using the primers AscImutF/R (Table S1). Both Ce144 and Gb371 constructs were transformed into Agrobacterium tumefaciens strain UIA143.
Binary Vectors for Transient Expression of Sialyltransferases in N. benthamiana.
cDNA from the human α2,3-sialyltransferase (IMAGE clone IRAD p970E0336D; Life Sciences Source Bioscience) was PCR amplified with primer pair ST3 F1/R1 (Table S1) and digested with XbaI/BamHI and cloned into the binary vector pPT2M (45), digested the same way. cDNA sequences of two human polysialyltransferases ST8Sia-II (IMAGE clone IRCMp5012E1027D) and ST8Sia-IV (IMAGE clone IRATp970A1079D) (both Life Sciences Source Bioscience) were amplified with a C-terminal Strep-tag using primer pairs ST8Sia-II F1/R1 and ST8Sia-IV F1/R1, respectively (Table S1). Resulting PCR products were digested with XbaI/BglII (ST8Sia-II) or XbaI/BamHI (ST8Sia-IV) and cloned into pPT2M digested with XbaI/BglII or XbaI/BamHI. The resulting vectors pST3, pST8Sia-II, and pST8Sia-IV (Figs. S2 and S3) were transformed into A. tumefaciens strain UIA143.
Viral-Based Vectors for Transient Expression of NCAM Module Ig5FN1.
A cDNA fragment (594 bp) corresponding to the NCAM Ig5FN1 module (amino acids 414–611, UniProtKB P13591) was synthesized codon optimized for N. benthamiana with flacking BsaI sites (GenBank accession no. KU052570) and cloned into a BsaI-digested TMV-based Magnicon vector containing α-amylase signal peptide for secretion (pICHα26211) (46).
Plant Material and Plant Transformation.
Agrobacterium-mediated leaf disk transformation of N. benthamiana ∆XTFT was performed by a standard protocol (47). After selection with Basta (3 mg⋅mL−1) and glyphosate (200 µM), transgenic plantlets were screened by PCR for the genomic insertion of the six mammalian genes. Positive plants were propagated for homozygosity (∆XTFTSia). N. benthamiana ∆XTFT and ∆XTFTSia plants were grown in a growth chamber at 22 °C with a 16 h light/8 h dark photoperiod.
Transient Protein Expression.
Agro-infiltration experiments were carried using 4- to 5-wk-old plants. The procedure was recently described in detail (48). For modulation of the N-glycosylation profiles toward sialylation or polysialylation, recombinant proteins were either expressed in ∆XTFTSia or coexpressed in ∆XTFT with the necessary constructs (Figs. S1–S3) (14, 29). Agrobacteria were infiltrated using optical density (OD600) 0.05–0.1. Protein expression was monitored 3–5 d postinfiltration.
Subcellular Localization by Confocal Laser Scanning Microscopy.
To perform subcellular localization studies, ST8Sia-II and ST8Sia-IV were C-terminally fused to green fluorescent protein (GFP). ST8Sia-II and ST8Sia-IV cDNA fragments were PCR amplified using primer pairs ST8Sia-II F1/R2 and ST8Sia-IV F1/R2, respectively (Table S1), digested XbaI/BglII (ST8Sia-II) or XbaI/BamHI (ST8Sia-IV) and cloned into the binary plasmid p20 (14). The resulting vectors ST8Sia-II-GFP and ST8Sia-IV-GFP were transformed into Agrobacteria and infiltrated in N. benthamiana ∆XTFTSia plants. ST6-mRFP (26) was used as a Golgi marker in colocalization studies. Expression was monitored 3 d postinfiltration using a Leica SP5 II confocal laser scanning microscope. Dual-color imaging of GFP- and mRFP-expressing cells was performed simultaneously using a 488-nm argon laser line and a 561-nm helium/neon laser line. The images obtained were processed in Adobe Photoshop CS6 with minimal manipulation.
Protein Extraction and Immunoblotting.
Total soluble proteins were extracted in 1:2 wt/vol extraction buffer (100 mM Tris, 1 mM EDTA, 500 mM NaCl, 40 mM ascorbic acid). Total proteins were extracted the same way in extract buffer containing 1% vol/vol Triton X-100. Secreted proteins were collected from the intracellular fluid (IF) as described previously (49). Proteins were fractionated in 8% or 12% (vol/vol) SDS/PAGE under reducing conditions, and gels were either stained with Coomassie Brilliant Blue or used for immunoblotting. Western blotting was carried out using tag-specific antibodies (1:5,000 mouse anti-Strep-tag, IBA2-1507; IBA GmbH; and 1:2,000 mouse anti-GFP, TP401; AMS Biotechnology (Europe) Ltd) and anti-polySia antibodies (1:750 dilution anti-polySia mAb735) (50). Detection was performed using HRP-conjugated anti-mouse-IgG A2554, diluted 1:10,000 (Sigma Aldrich). Clarity Western enhanced chemiluminescence reagents (Bio-Rad Life Science) were used as substrates. For endoneuraminidase digestion, 50 µL of protein extracts (250 µg) were incubated on ice with 0.5 µg of EndoN (51) for 30 min.
N-Glycan Analyses.
Site-specific N-glycan compositions of recombinantly expressed proteins were analyzed by liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS) using a buffered solvent system. The method for a comprehensive glycoprotein characterization via glycopeptide generation and analysis was recently described in detail (52). In brief, purified or IF-derived recombinant proteins was separated by reducing SDS/PAGE, Coomassie stained, and excised from the gel. Upon S-alkylation and tryptic or tryptic/GluC digestion, fragments were eluted from the gel with 50% (vol/vol) acetonitrile and separated on a reversed phase column (150 × 0.32-mm BioBasic-18; Thermo Scientific) with a gradient from 1% to 70% (vol/vol) acetonitrile. Glycopeptides were analyzed with a Q-TOF Ultima Global mass spectrometer (Waters). Spectra were summed and deconvoluted for identification of glycoforms. Glycan diagrams were drawn using the Consortium for Functional Glycomics symbols (www.functionalglycomics.org).
Sialic Acid (N-Acetylneuraminic Acid) Analysis by HPLC with Fluorescence Detection.
Sialic acids were derivatized by reaction with 1,2-diamino-4,5-methylenedioxybenzene (DMB) (53). Resulting fluorescent products (quinoxalinones) were detected with a flow-through fluorimeter (emission and excitation wavelengths of 448 nm and 373 nm, respectively) after separation by high performance liquid chromatography (HPLC) on a Shimadzu NexeraX2 System. Homogenized samples were subjected to acid hydrolysis with 2 M acetic acid at 80 °C for 2.5 h. Samples were centrifuged, the supernatants dryed in a speedvac concentrator, and resolving aliquots were taken and dried again. Released Sia were labeled with DMB and analyzed via RP-HPLC [Thermo ODS Hypersil, 5 µm, C18, 250 × 4 mm; 1 mL/min, with a linear gradient from 30% to 42% Eluent B [70% (vol/vol) 100 mM NH4Ac, 30% (vol/vol) AcCN; Eluent A, H2O] in 12 min; the HPLC system was a NexeraX2 with RF-10AXL fluorescence detector].
Analysis of Polysialylation by HPLC.
Determination of the polysialic acid (polySia) chain-length in secreted Ig5FN1 was done by HPLC of 1,2-diamino-4,5-methylenedioxybenzene (DMB)-derivatized polySia. To this end, polysialylated Ig5FN1 was isolated using inactivated EndoN coupled to tosyl-activated magnetic dynabeads M-280 (Invitrogen) as described previously (54). Dried samples were solved in 80 µL of DMB reaction buffer and incubated for 24 h at 11 °C on the shaker followed by the addition of 20 µL of 1 M NaOH to stop the reaction as previously described by several groups (55–57).
Fluorescently labeled polySia chains were separated by HPLC on the anion-exchange column DNA-Pak PA-100 (Fisher Thermo Scientific and Dionex) and visualized by a fluorescence detector set at 372 nm for excitation and 456 nm for emission. Milli-Q water (E1) and 4 M ammonium acetate buffer (E2) were used for the elution by a flow of 1 mL/min performed by the following gradient: T0 min = 0% (vol/vol) E2, T5 min = 3% (vol/vol) E2, T15 min = 8% (vol/vol) E2, and T60 min = 18% (vol/vol) E2.
Histone-Mediated Cytotoxicity.
HUVECs were cultured in endothelial cell growth medium (ECGM) (Promocell) containing 10% (vol/vol) FCS (Thermo Fisher Scientific) at 37 °C, 5% CO2. For the following experiments, cells were seeded out on a 96-well plate with 25,000 cells per well. After 24 h, HUVECs were incubated overnight in 100 µL of DMEM without phenol red (Thermo Fisher Scientific) in the absence or presence of histones (100 µg⋅mL−1; Sigma-Aldrich). In parallel, HUVECs were treated with 10 µL of IF containing bisialylated Ig5FN1 or polysialylated Ig5FN1. The cytotoxicity was determined with the lactate dehydrogenase (LDH) cytotoxicity assay (Roche Applied Science) according to the instructions of Roche Applied Science.
Nitric Oxide Production by LPS-Induced BV-2 Microglia.
Cultures of murine BV-2 microglia (58) (kindly provided by Gerd Bicker, University of Veterinary Medicine Hannover, Hannover, Germany) were grown in DMEM with 2.5% FCS and 1% penicillin/streptomycin (all Thermo Scientific). Then, 8 × 104 cells were seeded in 96-well plates, and, after adherence (2 h), lipopolysaccharide (LPS) from Escherichia coli (1 µg⋅mL−1; Sigma) was added for 24 h together with polySia (colominic acid; Sigma) or polySia-Ig5FN1 as indicated. Nitric oxide release was determined by detection of the stable breakdown product nitrite in the cell culture supernatants using the colorimetric Griess assay as described previously (33), but modified by adding 2% dapsone {4-[(4-aminobenzene)sulfonyl]aniline} to enhance sensitivity and by detection at 540 nm against 620 nm as a reference wavelength (59).
Acknowledgments
We thank Pia Gattinger, Thomas Hackl, and Michaela Bogner (Department of Applied Genetics and Cell Biology, University of Natural Resources and Life Sciences) for excellent technical support. This work was supported by Deutsche Forschungsgemeinschaft Grant GA 1755/1-2; by the von Behring-Roentgen-Stiftung (S.P.G.); by the Austrian Research Promotion Agency (FFG) and Icon Genetics GmbH in the frame of Laura Bassi Centres of Expertise PlantBioP (Grant 822757); by Austrian Science Fund (FWF) Grants L575-B13, TRB242 B20, and W1224-B09; and by a grant from the Austrian Federal Ministry of Transport, Innovation and Technology (bmvit).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence for the NCAM Ig5FN1 domain codon optimized for Nicotiana benthamiana reported in this paper has been deposited in the GenBank database (accession no. KU052570).
See Commentary on page 9404.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604371113/-/DCSupplemental.
References
- 1.Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94(2):461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colley KJ, Kitajima K, Sato C. Polysialic acid: Biosynthesis, novel functions and applications. Crit Rev Biochem Mol Biol. 2014;49(6):498–532. doi: 10.3109/10409238.2014.976606. [DOI] [PubMed] [Google Scholar]
- 3.Sato C, Kitajima K. Disialic, oligosialic and polysialic acids: Distribution, functions and related disease. J Biochem. 2013;154(2):115–136. doi: 10.1093/jb/mvt057. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt H, Mühlenhoff M, Gerardy-Schahn R. Polysialylation of NCAM. Adv Exp Med Biol. 2010;663:95–109. doi: 10.1007/978-1-4419-1170-4_6. [DOI] [PubMed] [Google Scholar]
- 5.Shahraz A, et al. Anti-inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci Rep. 2015;5:16800. doi: 10.1038/srep16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiermaier E, et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science. 2016;351(6269):186–190. doi: 10.1126/science.aad0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindhout T, et al. Site-specific enzymatic polysialylation of therapeutic proteins using bacterial enzymes. Proc Natl Acad Sci USA. 2011;108(18):7397–7402. doi: 10.1073/pnas.1019266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilyushin DG, et al. Chemical polysialylation of human recombinant butyrylcholinesterase delivers a long-acting bioscavenger for nerve agents in vivo. Proc Natl Acad Sci USA. 2013;110(4):1243–1248. doi: 10.1073/pnas.1211118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs PP, Callewaert N. N-glycosylation engineering of biopharmaceutical expression systems. Curr Mol Med. 2009;9(7):774–800. doi: 10.2174/156652409789105552. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol. 2015;33(8):842–844. doi: 10.1038/nbt.3280. [DOI] [PubMed] [Google Scholar]
- 11.Strasser R, Altmann F, Steinkellner H. Controlled glycosylation of plant-produced recombinant proteins. Curr Opin Biotechnol. 2014;30:95–100. doi: 10.1016/j.copbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Strasser R, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castilho A, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem. 2010;285(21):15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loos A, et al. Expression and glycoengineering of functionally active heteromultimeric IgM in plants. Proc Natl Acad Sci USA. 2014;111(17):6263–6268. doi: 10.1073/pnas.1320544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castilho A, et al. Generation of biologically active multi-sialylated recombinant human EPOFc in plants. PLoS One. 2013;8(1):e54836. doi: 10.1371/journal.pone.0054836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bork K, Reutter W, Weidemann W, Horstkorte R. Enhanced sialylation of EPO by overexpression of UDP-GlcNAc 2-epimerase/ManAc kinase containing a sialuria mutation in CHO cells. FEBS Lett. 2007;581(22):4195–4198. doi: 10.1016/j.febslet.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Castilho A, et al. Proteolytic and N-glycan processing of human α1-antitrypsin expressed in Nicotiana benthamiana. Plant Physiol. 2014;166(4):1839–1851. doi: 10.1104/pp.114.250720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilho A, et al. Processing of complex N-glycans in IgG Fc-region is affected by core fucosylation. MAbs. 2015;7(5):863–870. doi: 10.1080/19420862.2015.1053683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida Y, Kojima N, Kurosawa N, Hamamoto T, Tsuji S. Molecular cloning of Sia alpha 2,3Gal beta 1,4GlcNAc alpha 2,8-sialyltransferase from mouse brain. J Biol Chem. 1995;270(24):14628–14633. doi: 10.1074/jbc.270.24.14628. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama J, Angata K, Ong E, Katsuyama T, Fukuda M. Polysialic acid, a unique glycan that is developmentally regulated by two polysialyltransferases, PST and STX, in the central nervous system: From biosynthesis to function. Pathol Int. 1998;48(9):665–677. doi: 10.1111/j.1440-1827.1998.tb03967.x. [DOI] [PubMed] [Google Scholar]
- 22.Scheidegger EP, Sternberg LR, Roth J, Lowe JB. A human STX cDNA confers polysialic acid expression in mammalian cells. J Biol Chem. 1995;270(39):22685–22688. doi: 10.1074/jbc.270.39.22685. [DOI] [PubMed] [Google Scholar]
- 23.Mühlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. Autocatalytic polysialylation of polysialyltransferase-1. EMBO J. 1996;15(24):6943–6950. [PMC free article] [PubMed] [Google Scholar]
- 24.Close BE, et al. The polysialyltransferase ST8Sia II/STX: Posttranslational processing and role of autopolysialylation in the polysialylation of neural cell adhesion molecule. Glycobiology. 2001;11(11):997–1008. doi: 10.1093/glycob/11.11.997. [DOI] [PubMed] [Google Scholar]
- 25.Close BE, Colley KJ. In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J Biol Chem. 1998;273(51):34586–34593. doi: 10.1074/jbc.273.51.34586. [DOI] [PubMed] [Google Scholar]
- 26.Schoberer J, et al. Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic. 2010;11(11):1429–1444. doi: 10.1111/j.1600-0854.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Close BE, et al. The minimal structural domains required for neural cell adhesion molecule polysialylation by PST/ST8Sia IV and STX/ST8Sia II. J Biol Chem. 2003;278(33):30796–30805. doi: 10.1074/jbc.M305390200. [DOI] [PubMed] [Google Scholar]
- 28.Nelson RW, Bates PA, Rutishauser U. Protein determinants for specific polysialylation of the neural cell adhesion molecule. J Biol Chem. 1995;270(29):17171–17179. doi: 10.1074/jbc.270.29.17171. [DOI] [PubMed] [Google Scholar]
- 29.Castilho A, et al. Rapid high yield production of different glycoforms of Ebola virus monoclonal antibody. PLoS One. 2011;6(10):e26040. doi: 10.1371/journal.pone.0026040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima N, Tachida Y, Tsuji S. Alpha 1,6-linked fucose affects the expression and stability of polysialic acid-carrying glycoproteins in Chinese hamster ovary cells. J Biochem. 1998;124(4):726–737. doi: 10.1093/oxfordjournals.jbchem.a022173. [DOI] [PubMed] [Google Scholar]
- 31.Saffarzadeh M, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulm C, et al. Soluble polysialylated NCAM: A novel player of the innate immune system in the lung. Cell Mol Life Sci. 2013;70(19):3695–3708. doi: 10.1007/s00018-013-1342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werneburg S, Mühlenhoff M, Stangel M, Hildebrandt H. Polysialic acid on SynCAM 1 in NG2 cells and on neuropilin-2 in microglia is confined to intracellular pools that are rapidly depleted upon stimulation. Glia. 2015;63(7):1240–1255. doi: 10.1002/glia.22815. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 35.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott S, et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21(4):414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]
- 37.Castilho A, et al. Construction of a functional CMP-sialic acid biosynthesis pathway in Arabidopsis. Plant Physiol. 2008;147(1):331–339. doi: 10.1104/pp.108.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton SR, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313(5792):1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 39.Lin CW, et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc Natl Acad Sci USA. 2015;112(34):10611–10616. doi: 10.1073/pnas.1513456112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mühlenhoff M, Manegold A, Windfuhr M, Gotza B, Gerardy-Schahn R. The impact of N-glycosylation on the functions of polysialyltransferases. J Biol Chem. 2001;276(36):34066–34073. doi: 10.1074/jbc.M101022200. [DOI] [PubMed] [Google Scholar]
- 41.Willis LM, Gilbert M, Karwaski MF, Blanchard MC, Wakarchuk WW. Characterization of the alpha-2,8-polysialyltransferase from Neisseria meningitidis with synthetic acceptors, and the development of a self-priming polysialyltransferase fusion enzyme. Glycobiology. 2008;18(2):177–186. doi: 10.1093/glycob/cwm126. [DOI] [PubMed] [Google Scholar]
- 42.Keys TG, et al. Engineering the product profile of a polysialyltransferase. Nat Chem Biol. 2014;10(6):437–442. doi: 10.1038/nchembio.1501. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya A, et al. Polysialic acid/neural cell adhesion molecule modulates the formation of ductular reactions in liver injury. Hepatology. 2014;60(5):1727–1740. doi: 10.1002/hep.27099. [DOI] [PubMed] [Google Scholar]
- 44.Bader RA, Wardwell PR. Polysialic acid: Overcoming the hurdles of drug delivery. Ther Deliv. 2014;5(3):235–237. doi: 10.4155/tde.13.153. [DOI] [PubMed] [Google Scholar]
- 45.Strasser R, et al. Molecular basis of N-acetylglucosaminyltransferase I deficiency in Arabidopsis thaliana plants lacking complex N-glycans. Biochem J. 2005;387(Pt 2):385–391. doi: 10.1042/BJ20041686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol. 2005;23(6):718–723. doi: 10.1038/nbt1094. [DOI] [PubMed] [Google Scholar]
- 47.Horsch RB, et al. A simple and general method for transferring genes into plants. Science. 1985;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- 48.Loos A, Castilho A. Transient glyco-engineering of N. benthamiana aiming at the synthesis of multi-antennary sialylated proteins. Methods Mol Biol. 2015;1321:233–248. doi: 10.1007/978-1-4939-2760-9_17. [DOI] [PubMed] [Google Scholar]
- 49.Castilho A, et al. N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans. Glycobiology. 2011;21(6):813–823. doi: 10.1093/glycob/cwr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frosch M, Görgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: Isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobsson E, Schwarzer D, Jokilammi A, Finne J. Endosialidases: Versatile tools for the study of polysialic acid. Top Curr Chem. 2015;367:29–73. doi: 10.1007/128_2012_349. [DOI] [PubMed] [Google Scholar]
- 52.Gruber C, Altmann F. Site-specific glycosylation profiling using liquid chromatography-tandem mass spectrometry (LC-MS) Methods Mol Biol. 2015;1321:407–415. doi: 10.1007/978-1-4939-2760-9_27. [DOI] [PubMed] [Google Scholar]
- 53.Hara S, Takemori Y, Yamaguchi M, Nakamura M, Ohkura Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem. 1987;164(1):138–145. doi: 10.1016/0003-2697(87)90377-0. [DOI] [PubMed] [Google Scholar]
- 54.Kaese M, et al. Polysialylation takes place in granulosa cells during apoptotic processes of atretic tertiary follicles. FEBS J. 2015;282(23):4595–4606. doi: 10.1111/febs.13519. [DOI] [PubMed] [Google Scholar]
- 55.Sato C, Inoue S, Matsuda T, Kitajima K. Development of a highly sensitive chemical method for detecting alpha2-->8-linked oligo/polysialic acid residues in glycoproteins blotted on the membrane. Anal Biochem. 1998;261(2):191–197. doi: 10.1006/abio.1998.2718. [DOI] [PubMed] [Google Scholar]
- 56.Inoue S, Lin SL, Lee YC, Inoue Y. An ultrasensitive chemical method for polysialic acid analysis. Glycobiology. 2001;11(9):759–767. doi: 10.1093/glycob/11.9.759. [DOI] [PubMed] [Google Scholar]
- 57.Galuska CE, Maass K, Galuska SP. Mass spectrometric analysis of oligo- and polysialic acids. Methods Mol Biol. 2015;1321:417–426. doi: 10.1007/978-1-4939-2760-9_28. [DOI] [PubMed] [Google Scholar]
- 58.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2-3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 59.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]