Significance
We demonstrate that protein-R (arginine)-methyltransferase-6 (PRMT6) and its associated histone mark, asymmetric dimethylation of R2 on histone H3 (H3R2me2a), are decreased in the nucleus accumbens (NAc) of mice and rats after repeated cocaine exposure, as well as in the NAc of cocaine-addicted humans. We show that cocaine-induced PRMT6 down-regulation occurs selectively in NAc medium spiny neurons expressing dopamine D2 receptors (D2-MSNs) and serves to protect against cocaine-induced behavioral abnormalities. Furthermore, we provide the first, to our knowledge, genome-wide characterization of H3R2me2a within a specific brain region in vivo, and identify Src kinase signaling inhibitor 1 (Srcin1 or p140Cap) as a key target for this chromatin modification. Srcin1 induction in the NAc after cocaine exposure, which is associated with reduced Src signaling, decreases cocaine reward.
Keywords: histone arginine (R) methylation, drug addiction, medium spiny neurons, ChIP-seq, Src
Abstract
Repeated cocaine exposure regulates transcriptional regulation within the nucleus accumbens (NAc), and epigenetic mechanisms—such as histone acetylation and methylation on Lys residues—have been linked to these lasting actions of cocaine. In contrast to Lys methylation, the role of histone Arg (R) methylation remains underexplored in addiction models. Here we show that protein-R-methyltransferase-6 (PRMT6) and its associated histone mark, asymmetric dimethylation of R2 on histone H3 (H3R2me2a), are decreased in the NAc of mice and rats after repeated cocaine exposure, including self-administration, and in the NAc of cocaine-addicted humans. Such PRMT6 down-regulation occurs selectively in NAc medium spiny neurons (MSNs) expressing dopamine D2 receptors (D2-MSNs), with opposite regulation occurring in D1-MSNs, and serves to protect against cocaine-induced addictive-like behavioral abnormalities. Using ChIP-seq, we identified Src kinase signaling inhibitor 1 (Srcin1; also referred to as p140Cap) as a key gene target for reduced H3R2me2a binding, and found that consequent Srcin1 induction in the NAc decreases Src signaling, cocaine reward, and the motivation to self-administer cocaine. Taken together, these findings suggest that suppression of Src signaling in NAc D2-MSNs, via PRMT6 and H3R2me2a down-regulation, functions as a homeostatic brake to restrain cocaine action, and provide novel candidates for the development of treatments for cocaine addiction.
Repeated cocaine exposure is marked by persistent changes in gene expression within the nucleus accumbens (NAc), a central component of the brain’s reward circuitry (1, 2). Important aspects of cocaine action appear to be mediated by changes in gene transcription via chromatin regulatory mechanisms such as histone acetylation or methylation on Lys (K) residues (3–11). However, in contrast to K methylation, the functional role of histone Arg (R) methylation remains underexplored in addiction models and poorly understood in the brain in general.
The methylation of R residues is catalyzed by the protein R methyltransferase (PRMT) family of enzymes, which can generate different methylated R states with diverse functional consequences, including monomethylarginine (MMA) and dimethylarginine (DMA) residues, the latter of which can be either asymmetric (aDMA) or symmetric (sDMA). PRMTs that catalyze aDMA are designated type I, and those that generate sDMA are designated type II (12, 13). Histone tails are prime targets for these PRMTs, and R methylation induces alterations in chromatin architecture, either condensing or relaxing its structure, thereby creating binding sites for regulatory proteins that contain specialized binding domains (14, 15).
PRMT6, a nuclear enzyme that modifies histone tails, is the primary enzyme responsible for asymmetric dimethylation of R2 on histone H3 (H3R2me2a) in mammalian cells (14, 16). The H3R2me2a mark is thought to be repressive in nature because of its ability to counteract the activator function of the nearby H3K4me3 mark (17, 18). Our group has previously shown a pronounced enrichment of the H3K4me3 mark at key gene promoters in the NAc after repeated cocaine exposure (9). Based on these findings, we sought to determine whether PRMT6 and its associated histone mark, H3R2me2a, play roles in the remodeling of chromatin in the NAc after cocaine exposure.
Results
Regulation of PRMT6 in Rodent Addiction Models and Addicted Humans.
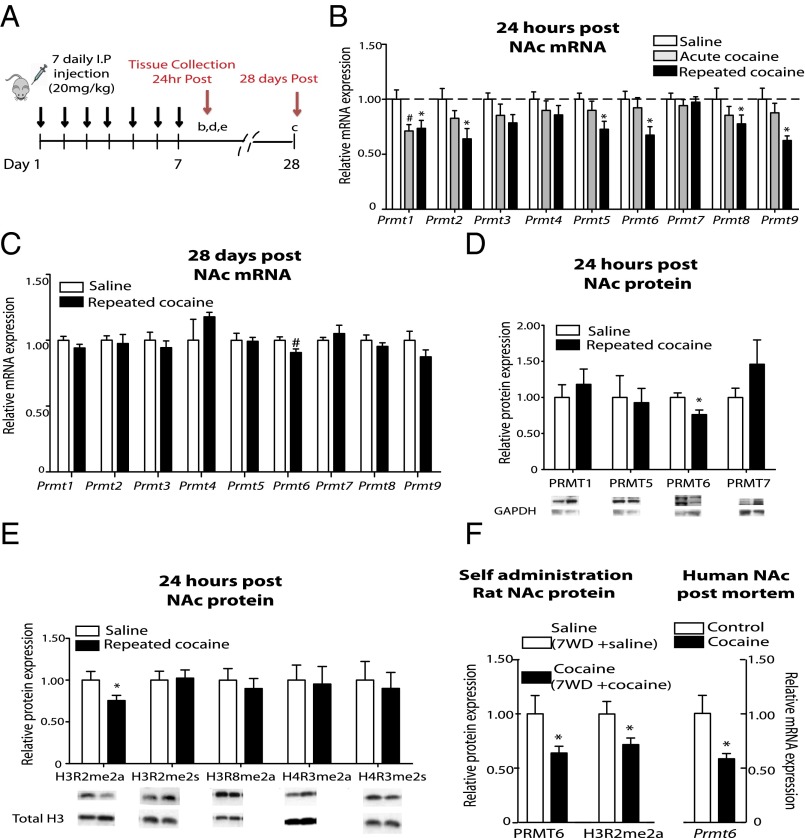
As a first step in assessing the involvement of histone R methylation in cocaine action, we screened the nine mammalian PRMTs in the NAc of animals treated with experimenter-delivered cocaine in their home cage for 1 or 7 d (Fig. 1 A and B). At 24 h after the last i.p. cocaine injection, the mRNA expression levels of several Prmts were dynamically regulated in this brain region (Fig. 1B). Prmt1, which encodes the major type I enzyme that has wide substrate specificity and is responsible for the majority (∼85%) of total protein R methylation in cultured cells (19), was down-regulated by both single and repeated cocaine injections. Prmt2, Prmt5, Prmt6, Prmt8, and Prmt9 (20, 21) were down-regulated by repeated cocaine administration, but not by a single cocaine exposure. Prmt8 is enriched in brain (22), and Prmt6 is the only type I PRMT exclusively located in the nucleus that modifies histone H3 (16, 17, 23).
Fig. 1.
Repeated cocaine administration persistently represses PRMT6 expression in the NAc of mice, rats, and addicted humans. (A) Schematic for i.p. injection of saline or cocaine (20 mg/kg) in mice shown in the other panels. (B) NAc mRNA levels of the nine mammalian PRMTs after seven daily i.p. injections of saline (saline) or cocaine (repeated cocaine) or six daily saline treatments and one cocaine injection (acute cocaine). NAc mRNA levels are compared with the NAc of mice treated with seven daily i.p. saline injections. Shown are NAc mRNA levels of Prmt1 [F(2,69) = 4.36, P = 0.016; saline vs. acute: t(41) = 2.42, P < 0.05; saline vs. repeated: t(52) = 2.57, P < 0.01], Prmt2 [F(2,52) = 4.23, P = 0.02; saline vs. repeated: t (36) = 2.91, P < 0.01], Prmt5 [F(2,70) = 4.62, P = 0.013; saline vs. repeated: t(53) = 3.02, P < 0.01], Prmt6 [F(2,53) = 3.72, P = 0.03; saline vs. repeated: t (36) = 2.53, P < 0.01], Prmt8 [F(2,27) = 3.41, P = 0.049, saline vs. repeated: t (17) = 2.61, P < 0.01], and Prmt9 [F(2,27) = 6.31, P = 0.006; saline vs. repeated: t(27) = 3.49, P < 0.01] at 24 h after the last i.p. injection. n = 9–27. #P < 0.05; *P < 0.01. (C) NAc mRNA levels of PRMT6 [t(30) = 2.77, P = 0.014] at 28 d after the last i.p. injection. n = 14–16. #P < 0.05; Bonferroni correction, *P < 0.006. (D and E) Protein levels at 24 h after seven daily i.p. injections of either cocaine (20 mg/kg) (repeated cocaine) or saline (saline). (D) NAc protein levels of PRMT6 [t(26) = 2.44, P = 0.011). n = 9–18. *P < 0.05. (E) NAc protein levels of H3R2me2a [t(37) = 2.12, P = 0.02]. n = 7–30. *P < 0.05. (F) PRMT6 and H3R2me2a protein levels in rat NAc and Prmt6 mRNA levels in human postmortem NAc after cocaine exposure. Shwon are NAc rat protein levels of PRMT6 [t(14) = 2.51, P < 0.05] and H3R2me2a [t(14) = 2.38, P < 0.05] after 10 d of either saline or cocaine self-administration (SA) at 1 mg/kg/infusion for 2 h (FR1;TO30s), followed by 7 d of withdrawal with same animals given a single SA reexposure session with either saline or cocaine at 24 h after the reexposure test. n = 5–11. Also shown is the NAc human mRNA level of Prmt6 [t(25) = 2.16, P < 0.05]. n = 13–14. *P < 0.05. Data are presented as mean ± SEM.
To determine the persistence of this down-regulation, we examined Prmt mRNA levels in the NAc at 28 d after the last cocaine injection and observed a persistent down-regulation of Prmt6 only (Fig. 1C). To validate the mRNA findings, we characterized cocaine regulation of the protein levels of selected PRMTs in the NAc by Western blot analysis. PRMT6 protein levels were down-regulated in the NAc of mice at 24 h after repeated cocaine injection (Fig. 1D). In contrast, PRMT1, PRMT5, and PRMT7 protein levels were not significantly altered.
We next studied whether cocaine-induced alterations in PRMT expression are associated with global changes in histone R-methylation in the NAc. Using an extensively validated antibody that is selective for H3R2me2a (SI Appendix, Fig. S1) (23), we found that this asymmetric dimethylation modification, which is the major mark deposited by the nuclear localized enzyme PRMT6, was down-regulated in the NAc of mice treated with repeated cocaine injections (Fig. 1E). All other histone R methylation marks were unaffected. In contrast, neither PRMT6 nor H3R2me2a was altered in the prefrontal cortex or dorsal striatum in response to repeated cocaine administration (SI Appendix, Fig. S2).
Next, to validate our findings in a model of contingent cocaine administration, we examined the NAc of rats trained to self-administer cocaine for 10 d that went through a 7-d withdrawal period followed by reexposure to self-administration. In this paradigm, we also observed down-regulation of PRMT6 and H3R2me2a levels in the NAc (Fig. 1F). Furthermore, we examined the NAc of postmortem human brains and found that PRMT6 mRNA levels were decreased in cocaine-addicted subjects compared with matched control subjects (Fig. 1F). Taken together, these results support the hypothesis that the persistent down-regulation of PRMT6 and its associated mark H3R2me2a in the NAc is a consistent feature of cocaine action.
PRMT6 Modulates Addictive-Like Behaviors.
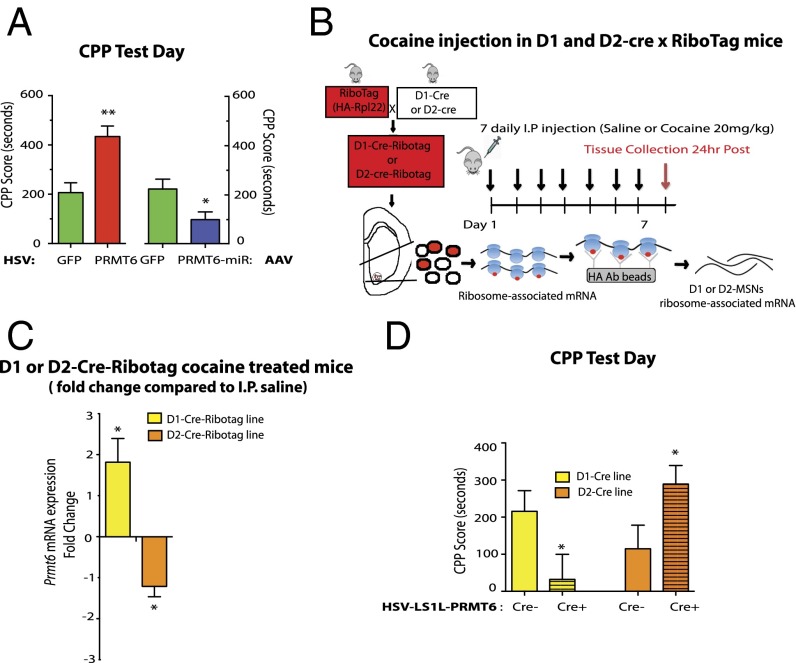
To directly test the hypothesis that cocaine-induced down-regulation of PRMT6 in the NAc is important in controlling behavioral responses to cocaine, we used an unbiased conditioned place-preference (CPP) paradigm, which provides an indirect measure of cocaine reward, and overexpressed PRMT6, or PRMT1, PRMT5, or PRMT7 as comparisons, in the NAc by use of herpes simplex virus (HSV)-mediated gene transfer (SI Appendix, Fig. 3, i, ii). As a control, we showed that overexpression of PRMT6 increased NAc levels of H3R2me2a (SI Appendix, Fig. S3, iv), and that PRMT6 or PRMT1 overexpression did not alter expression levels of the other Prmt isoforms (SI Appendix, Fig. S3, iii). In the CPP paradigm, mice that received intra-NAc HSV-PRMT6 spent more time in the cocaine-paired chamber compared with animals that received HSV-GFP (Fig. 2A), demonstrating that induction of PRMT6 in the NAc increases cocaine reward. In contrast, overexpression of PRMT1, PRMT5, or PRMT7 in the NAc did not alter cocaine CPP (SI Appendix, Fig. S4). To obtain the converse type of evidence, we generated an AAV vector that expresses an miRNA (miR) construct that knocks down PRMT6 when delivered into the NAc (SI Appendix, Fig. S3, v). Mice that received intra-NAc injections of AAV-PRMT6-miR exhibited a decrease in cocaine CPP compared with animals that received AAV-GFP (Fig. 2A). Taken together, these data demonstrate that PRMT6 down-regulation in the NAc, which occurs after repeated cocaine administration, represents a homeostatic adaptation that counters aspects of the addictive phenotype.
Fig. 2.
Cell type-specific effects of PRMT6 in the NAc in cocaine action. (A and D) CPP testing with cocaine (7.5 mg/kg) using intra-NAc delivery of an HSV-PRMT6 construct (A, Left), an AAV-PRMT6-miR construct (A, Right), or an HSV-LS1L-PRMT6 construct (D). (A, Left) PRMT6 overexpression in the NAc increased time spent in the cocaine-paired CPP chamber [t(37) = 3.41, P = 0.0016]. n = 7–27. (A, Right) PRMT6 knockdown in the NAc decreased the time spent in the cocaine-paired CPP chamber [t(46) = 2.46, P = 0.017]. n = 13. (B) RiboTag methodology schematic. RiboTag mice (HA-tagged Rpl22) were crossed to D1-Cre or D2-Cre mice, resulting in expression of the HA-tagged Rpl22 only in cells expressing Cre (D1-Cre-RiboTag or D2-Cre-RiboTag mouse lines). Cell-specific ribosome-associated mRNA was isolated from the NAc of these mice using HA-tagged magnetic beads and then analyzed by qRT-PCR. (C) In D1-Cre-RiboTag and D2-Cre-RiboTag mice, NAc mRNA levels and fold change of Prmt6 at 24 h after seven daily i.p. injections of either cocaine (20 mg/kg) or saline. Shown are Prmt6 NAc mRNA levels in D1-Cre-RiboTag mice [t(21) = 2.09, P = 0.049] compared with saline-treated D1-Cre-RiboTag mice and in D2-Cre-RiboTag mice [t(20) = 2.46, P = 0.02] compared with saline-treated D2-Cre-RiboTag mice at 24 h after the last i.p. cocaine injection. n = 11. (D) Cell type-specific PRMT6 overexpression in D1-MSNs or D2-MSNs in the NAc oppositely regulated the time spent in the cocaine-paired CPP chamber. Shown is cocaine CPP in D1-Cre+ and D2-Cre+ mice and their respective WT littermate controls D1-Cre− and D2-Cre−, all of which were injected intra-NAc with HSV-LS1L-PRMT6. D1-Cre+ vs. D1-Cre−: t(33) = 2.10, P = 0.04; n = 17–18. D2-Cre+ vs. D2-Cre−: t(44) = 2.17, P = 0.03; n = 22–24. Data are presented as mean ± SEM. **P < 0.001, *P < 0.05.
Cell Type-Specific Action of PRMT6 in Cocaine Action.
There are two major populations of NAc MSNs, those expressing predominantly either dopamine D1 receptors (D1-MSNs) or D2 receptors (D2-MSNs), which together compose >95% of all NAc neurons (2). To examine whether Prmt6 down-regulation by cocaine occurs in one or both of these MSN subtypes, we isolated ribosome-associated mRNA transcripts from D1-MSNs or D2-MSNs by extracting the NAc of D1-Cre-RiboTag or D2-Cre-RiboTag mice at 24 h after 7 d of cocaine or saline administration (Fig. 2 B and C) (24). Prmt6 mRNA was decreased in D2-MSNs but increased in D1-MSNs (Fig. 2C), indicating that the down-regulation observed in whole NAc extracts is selective for D2-MSNs. Given that baseline levels of Prmt6 expression are roughly comparable between D1- MSNs and D2-MSNs (SI Appendix, Fig. S5, i), why the decrease in D2-MSNs predominates is unclear. Because we found an opposite regulation of Prmt6 in D1-MSNs vs. D2-MSNs with cocaine, we achieved cell type-specific control over Prmt6 expression by cloning Prmt6 into a Cre-inducible loxP-STOP-loxP HSV vector (25) (HSV-LS1L-PRMT6), which directed PRMT6 overexpression to D1-MSNs or D2-MSNs selectively on injection into the NAc of D1-Cre or D2-Cre mice, expression not seen in WT littermates (SI Appendix, Fig. S3, vi). Overexpression of PRMT6 in D2-MSNs increased cocaine CPP, as observed when PRMT6 was overexpressed in all NAc neurons (Fig. 2A), whereas PRMT6 overexpression in D1-MSNs decreased cocaine CPP (Fig. 2D). Furthermore, overexpression of PRMT6 specifically in D2-MSNs increased cocaine-induced locomotor activity (SI Appendix, Fig. S5, ii). These results demonstrate that cocaine-induced PRMT6 down-regulation in the NAc occurs specifically in D2-MSNs, where it counteracts the rewarding effects of cocaine, thus reinforcing the hypothesis that this MSN subtype is necessary for opposing cocaine use (26).
Decreased H3R2me2a Binding at Target Genes After Repeated Cocaine.
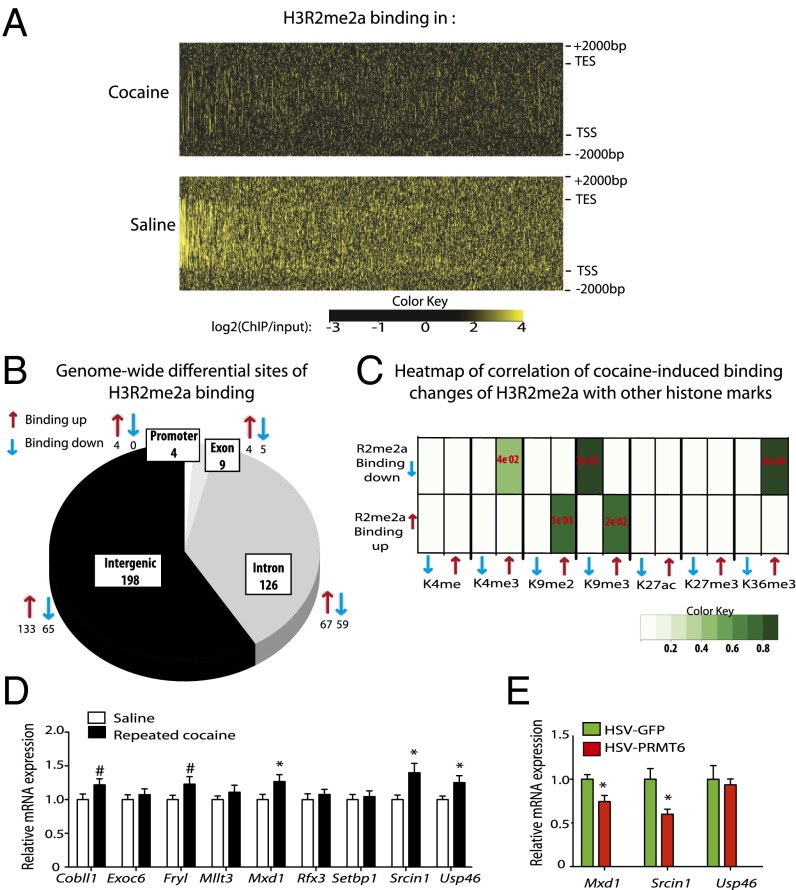
Genome-wide methods have increasingly been used to obtain an unbiased view of chromatin changes in animal models of psychiatric disorders including changes in the NAc in addiction models (4, 9, 11). In nonneural tissues, H3R2me2a has been shown to act as a repressive mark that antagonizes H3K4me3 (17, 18). Previous studies using chromatin immunoprecipitation (ChIP) in human blood and leukemia cells showed that H3R2me2a is distributed within gene bodies and at the 3′ end of genes, regardless of their transcriptional state, whereas it is selectively depleted from active promoters, coincident with the presence of H3K4me3 (18); however, nothing is known about the location of H3R2me2a in brain chromatin.
To investigate the genomic consequences of H3R2me2a down-regulation in the NAc after repeated cocaine administration, we used ChIP-seq with diffReps analysis (27) and captured this mark at specific genomic loci at 24 h after the last drug dose (Datasets S1 and S2). Genome-wide heat map examination of saline-enriched H3R2me2a-binding sites revealed relative enrichment of H3R2me2a within gene bodies with a general depletion of this enrichment after cocaine administration (Fig. 3A). Mapping saline- and cocaine-associated peaks to various genomic locations revealed that under saline and cocaine conditions, H3R2me2a peaks were found in both intergenic (i.e., noncoding) and genic regions, with introns being preferentially marked with H3R2me2a relative to exons (Fig. 3B and SI Appendix, Fig. S7).
Fig. 3.
H3R2me2a is regulated genome-wide in the NAc after repeated cocaine. (A) Averaged genome-wide heatmap profile of three independent biological replicates of H3R2me2a binding under saline (Lower) and cocaine (Upper) conditions. Each line of the x-axis represents a single gene, arranged in order of decreasing H3R2me2a-binding enrichment in saline conditions. The y-axis represents the genomic region from 5′ to 3′ with 2,000 bp upstream of TSSs and downstream of transcription end sites (TESs). (B) Pie chart showing ChIP-seq genome-wide distribution patterns of significant differential H3R2me2a-binding sites in the NAc between saline and cocaine conditions. Arrows represent sites showing a significant cocaine-induced increase (red) or decrease (blue) in H3R2me2a binding. Cocaine induced 337 differential H3R2me2a-binding sites, among which 129 were decreased and 208 were increased with cocaine. (C) Heatmap of correlation between cocaine-induced H3R2me2a differential binding sites (diffReps) and seven other chromatin modification differential binding sites (H3K4me1, H3K4me3, H3K9me2, H3K9me3, H3K27ac, H3K27me3, and H3K36me3). Binding up/down (red/blue arrow) indicates differential binding sites increased/ decreased after cocaine. The shade of green indicates the odds ratio (i.e., observation/expectation) of enrichments. The P values of enrichments were determined using Fisher’s exact test and are labeled in red. (D) NAc mRNA levels of nine genes characterized by a cocaine-induced decrease in H3R2me2a binding and an increase in H3K4me3 binding. NAc mRNA levels are compared with those of saline-treated animals for Cobll1 [t(27) = 1.759, P = 0.0433], Fryl [t(27) = 1.784, P = 0.0662], Mxd1 [t(27) = 2.108, P = 0.022], Srcin1 [t(27) = 2.535, P = 0.008], and Usp46 [t(27) = 2.151, P = 0.02]. n = 14–15. (E) Repression of gene expression in the NAc with local PRMT6 overexpression achieved with intra-NAc infusion of HSV-PRMT6 vs. HSV-GFP controls. Mxd1, t(6) = 2.108, P = 0.0126; Srcin1, t(8) = 2.921, P = 0.0096 compared with respective HSV-GFP. n = 4–5. Data are presented as mean ± SEM. #P < 0.1, *P < 0.05, **P < 0.01.
We next conducted a correlation analysis between H3R2me2a and our previously published datasets (9) for seven other histone modifications in the NAc of mice that received equivalent cocaine or saline treatments (diffReps for H3K4me1, H3K4me3, H3K9me2, H3K9me3, H3K27ac, H3K27me3, and H3K36me3) (Fig. 3C). We found that two histone K methylation marks generally associated with gene activation (H3K4me3 and H3K36me3) showed strong correlations with H3R2me2a down-regulation, whereas two histone K methylation marks generally associated with gene repression (H3K9me2 and H3K9me3) showed strong correlations with H3R2me2a up-regulation. These findings support the hypothesis that H3R2me2a in the NAc is associated with gene repression as observed in nonneural tissues. Considering previous reports that H3R2me2a prevents H3K4me3 deposition at gene promoters in peripheral cells (18), we screened the mRNA expression levels in the NAc of the nine genes characterized by cocaine-induced decreases of H3R2me2a binding and increases of H3K4me3 binding in independent biological replicates (Fig. 3D). Quantitative PCR (qPCR) revealed induction of Cobll1, Mxd1, Srcin1, and Usp46, with a trend toward induction observed for Fryl. To directly link PRMT6 and H3R2me2a to repression of these genes, NAc tissue was collected from animals infused intra-NAc with HSV-GFP or HSV-PRMT6. Compared with animals infused with HSV-GFP, those overexpressing PRMT6 in the NAc showed repression of Mxd1 and Srcin1 (Fig. 3E). Based on recent studies indicating that Srcin1 plays a critical role in regulating synaptic plasticity in the hippocampus and spatial memory (28, 29), and on the fact that Srcin1 is the most highly regulated among the genes that show reciprocal changes in H3R2me2a and H3K4me3, we focused subsequent attention on cocaine regulation of Srcin1.
Regulation of Srcin1 and Src Signaling by Repeated Cocaine.
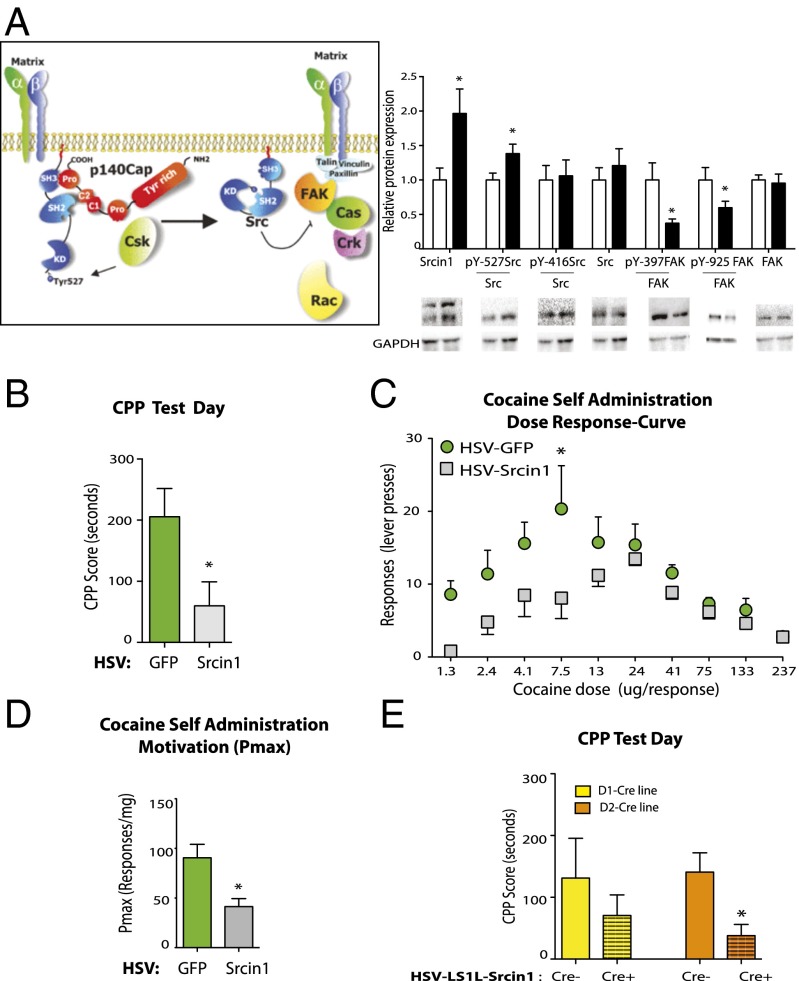
Srcin1 encodes Src kinase signaling inhibitor 1, also called p140Cap, an endogenous inhibitor that constrains the activity of the Src family of protein tyrosine kinases (30). Based on our unbiased discovery of cocaine regulation of Srcin1, we investigated cocaine regulation of Src signaling in the NAc and its behavioral consequences. We found that Srcin1 as well as several other components of the Src signaling cascade, none of which have heretofore been examined in cocaine action, were regulated in the NAc by repeated cocaine administration (Fig. 4A). Levels of Srcin1 protein were up-regulated in the NAc by cocaine in concert with its transcriptional activation. We also verified that cocaine induction of Srcin1 was blocked by overexpression of PRMT6 (SI Appendix, Fig. S8).
Fig. 4.
Srcin1 induction in the NAc opposes cocaine-induced behaviors. (A, Left) p140Cas-associated protein, p140Cap (also known as Srcin1) intracellular signaling cascade (40). (A, Right) NAc protein levels of Srcin1 [t(41) = 2.32, P = 0.013], the inactive (phosphorylated at tyrosine Y residue 527) form of Src (pY527Src) [t(35) = 2.14, P = 0.02], the active (phosphorylated at tyrosine Y residue 416) form of Src (pY416Src), total Src, the phosphorylated at tyrosine 397 focal adhesion kinase (pY397FAK) [t(37) = 2.18, P = 0.02], the Src-dependent phosphorylated at tyrosine 925 FAK (pY925FAK) [t(44) = 2.03, P = 0.02], and total FAK. n = 16–24. (B) Srcin1 overexpression in the NAc decreased the time spent in the cocaine-paired CPP chamber [t(18) = 2.40; P = 0.03]. n = 9–13. (C and D) Overexpression of Srcin1 in the NAc decreases the reinforcing efficacy to self-administer cocaine in rats. Rats were injected intra-NAc with HSV-GFP or HSV-Srcin1 and tested 2 d later for 3 consecutive days. n = 5–6. (C) The threshold procedure paradigm was used to generate dose–response curves for cocaine and determine cocaine reinforcing efficacy. Dose–response curves occurred within a single session, and data were averaged from three sessions and presented as lever press responses vs. dose. Two-way ANOVA; virus effect: F(1, 80) = 19.74, P < 0.0001; dose effect: F(9, 80) = 6.71, P < 0.0001; interaction effect: F(9, 80) = 1.35, P = 0.22; dose GFP vs. Srcin1: t(8) = 3.75, P < 0.01. (D) Overexpression of Srcin1 in the NAc of rats decreases the motivation to self-administer cocaine. Pmax (price at which maximal responding occurs): t(8) = 3.31, *P < 0.05. (E) Cell type-specific Srcin1 overexpression in D2-MSNs in the NAc decreased the time spent in the cocaine-paired CPP chamber. Shown is cocaine CPP in D1-Cre+ and D2-Cre+ mice and their respective WT littermate controls (D1-Cre− and D2-Cre−), all of which were injected intra-NAc with HSV-LS1L-Srcin1. D1-Cre+ vs. D1-Cre−: t(9) = 0.88, P = 0.43; n = 5–6. D2-Cre+ vs. D2-Cre−: t(20) = 2.38, P = 0.04; n = 9–13. Data are presented as mean ± SEM. *P < 0.05.
We next investigated whether cocaine induction of Srcin1 is associated with changes in its downstream target, Src. Repeated cocaine administration increased the phosphorylation of Tyr517-Src, a phosphorylation site associated with inactivation of Src’s tyrosine kinase activity, without altering total Src expression levels in the NAc. In contrast, repeated cocaine administration did not alter the phosphorylation of Tyr416-Src, associated with activation of Src kinase. This cocaine inhibition of Src is associated with decreased phosphorylation of Tyr925 and Tyr397 on focal adhesion kinase (Fig. 4A), a known Src substrate. Mice overexpressing Srcin1 in the NAc (SI Appendix, Fig. S9) showed decreased cocaine CPP (Fig. 4B), as well as decreased cocaine self-administration, using a threshold paradigm that induces behavioral readouts within the time frame of maximal HSV-mediated transgene expression. Srcin1 overexpression in the NAc decreased the reinforcing efficacy of cocaine (Fig. 4C) and the motivation to self-administer the drug (Fig. 4D) (31, 32). Using similar methodology as in Fig. 2D, we investigated the behavioral effects of overexpressing Srcin1 in D1-MSNs vs. D2-MSNs in cocaine CPP by cloning Srcin1 into the Cre-inducible loxP-STOP-loxP HSV vector (HSV-LS1L-Srcin1), which directed Srcin1 overexpression to D1-MSNs or D2-MSNs selectively on injection into the NAc of D1-Cre or D2-Cre mice (SI Appendix, Fig. S10). Overexpression of Srcin1 in D2-MSNs decreased cocaine CPP (Fig. 4E), as observed when Srcin1 was overexpressed in all NAc neurons (Fig. 4B), whereas Srcin1 overexpression in D1-MSNs did not significantly alter cocaine responses (Fig. 4E). These results show that Srcin1 induction in D2-MSNs, like the down-regulation of PRMT6 in D2-MSNs, counteracts the rewarding effects of cocaine, thus reinforcing the hypothesis that this MSN subtype is important for opposing cocaine use.
Discussion
Results of the present study demonstrate down-regulation of PRMT6 in the NAc of mice and rats treated repeatedly with cocaine and of cocaine-addicted humans examined postmortem. Cocaine down-regulation of PRMT6, which is specific to D2 MSNs in this brain region, constitutes a homeostatic response and reinforces the hypothesis that this MSN subtype is necessary for opposing cocaine-addictive behavior. Genome-wide characterization of H3R2me2a, the mark selectively catalyzed by PRMT6, in the NAc revealed that cocaine induces a small set of genes with coincident decreases in H3R2me2a and increases in H3K4me3. The induction of one such gene, Srcin1, in turn revealed cocaine regulation of the Src signaling pathway, with suppression of Src signaling in the NAc seen after repeated cocaine administration. Such Srcin1 induction and suppression of Src signaling in the NAc, like suppression of PRMT6/H3R2me2a, opposes the rewarding effects of cocaine, including self-administration of the drug.
PRMTs regulate multiple biological processes, including DNA transcription, mRNA splicing, and piRNA biogenesis, but very little is known about the regulation of Prmt translation from mRNA into protein. The disconnect between Prmt1, Prmt2, Prmt5, Prmt6, Prmt8, and Prmt9 mRNA vs. protein expression (Fig. 1) requires further study, but could involve mRNA-binding partners capable of influencing Prmt mRNA stability by modifying mRNA translation or degradation rates.
Histone R methylation has been shown to be important in orchestrating changes in gene transcription (14, 15), and previous work has demonstrated that H3R2 methylation by PRMT6 is prevented by H3K4me3, confirming H3R2me2a’s transcriptional repressive role (17, 18). Here we show that cocaine-induced decreases in H3R2me2a binding and increases in H3K4me3 binding promote transcription of homeostatic target genes such as Srcin1. The lack of study of this posttranslational modification to date may be due to the scarcity of PRMT substrates, to the intrinsic stability histone R methylation (which is seen as incompatible with a role in regulating cellular pathways), and to the lack of tools for detecting R methylation. Together with recent investigations (33–35), the present work dispels these earlier impressions, and raises histone R methylation changes in response to repeated cocaine to the rank of other more well-known histone posttranslational modifications implicated in the epigenetic basis of addiction.
The opposite behavioral effect observed on overexpression of PRMT6 in D2-MSNs, which increased cocaine CPP, vs. overexpression of PRMT6 in D1-MSNs, which decreased cocaine CPP (Fig. 2D), is all the more intriguing because we observed the opposite regulation of Prmt6 expression in D1-MSNs vs. D2-MSNs by cocaine as well (Fig. 2C). The literature implicates opposite roles of D1-MSNs vs. D2-MSNs in drug addiction, with D1-MSNs promoting both reward and sensitizing responses to psychostimulants and D2-MSNs dampening these behaviors (36, 37). Therefore, increased PRMT6 levels in D1-MSNs and decreased PRMT6 levels in D2-MSNs after cocaine exposure may constitute a homeostatic response toward a common outcome, both opposing cocaine-addictive behaviors.
Evidence showing that decreased PRMT6 levels in D2-MSNs opposes addictive-like phenotypes (Fig. 2D) suggests that targeting this mechanism may have therapeutic potential. Although several specific PRMT inhibitors have been identified using in vitro screens, these compounds have proven to be of limited use owing to their inability to enter cells or their cytotoxic effects. Through investigation of the location of H3R2me2a, the mark selectively catalyzed by PRMT6, and its mechanism of action via preferential interaction with H3K4me3 after repeated cocaine, we identified Srcin1 and the Src signaling pathway as prospective therapeutic targets that may have less off-site effects. Indeed, after repeated cocaine administration, the Srcin1 gene showed decreased binding of H3R2me2a and increased binding of H3K4me3 in the NAc, and Srcin1 induction in this brain region by cocaine was reversed on PRMT6 overexpression (SI Appendix, Fig. S8). Further investigation of Srcin1 showed that its induction is associated with suppression of the Src signaling pathway (Fig. 4A), and that Srcin1 overexpression in the NAc exerts antiaddictive properties in opposing the rewarding responses to cocaine (Fig. 4 B–D). This action of Srcin1, like that of PRMT6, appears to be specific to D2-MSNs (Fig. 4E).
Taken together, our present findings establish histone R methylation as a key regulator in the induction of Srcin1, a Src signaling repressor that opposes cocaine action. The results thus provide a new approach to identifying therapeutic targets for the treatment of cocaine addiction.
Methods
Animals and Treatments.
Male C57BL/6J mice (The Jackson Laboratory), D1-Cre and D2-Cre mice (on a C57BL/6J background), and RiboTag mice (HA-Rpl22, also on a C57BL/6J background) crossed to D1-Cre or D2-Cre mice were used for the experiments as described. Male Sprague–Dawley rats (Charles River Laboratories) were used. All animal protocols were approved by the Institutional Animal Care and Use Committee of Icahn School of Medicine at Mount Sinai. Cocaine was purchased from Sigma-Aldrich. Repeated cocaine treatment involved seven daily i.p. injections of cocaine at 20 mg/kg unless stated otherwise.
Human Postmortem Brain Tissue.
NAc tissue, obtained from the Quebec Suicide Brain Bank (Institutional Review Board approval from Douglas Mental Health University Institute) was analyzed for control and cocaine-addicted subjects matched for age, postmortem interval, RNA integrity, and pH (38). Informed consent was obtained from all participants.
Stereotaxic Virus Injection and Cannula Implantation.
Human PRMT1, 5, 6, and 7 pcDNA3 plasmids were obtained from Fabio Casadio (Rockefeller University) and cloned into HSV vectors. Gateway cloning technology (Invitrogen) was then used to recombine PRMT6 or Srcin1 into a Cre-inducible loxP-STOP-loxP HSV p1006 (25). Syringe needles (33G) were bilaterally lowered into the NAc to infuse 0.5 μL of virus at a 10° angle (anterior/posterior, + 1.6 mm; medial/lateral, + 1.5 mm; dorsal/ventral, −4.4 mm from bregma).
Conditioned Place Preference.
Mice were conditioned for 2 d to i.p. saline injections in one chamber for 30 min during a morning session and to cocaine injections (7.5 mg/kg i.p.) in the opposite chamber for 30 min during an afternoon session. The day after the second conditioning, the mice were tested for place preferences during a 20-min session where they were allowed to freely explore all chambers. CPP scores represent the difference in the time spent between the cocaine-paired and saline-paired chambers.
Self-Administration of Cocaine.
Rats were implanted with chronic indwelling intrajugular catheters and trained for self-administration as described in Fig. 4.
ChIP, Library Preparation, and ChIP-seq Data Analysis.
Three fully independent biological replicates for saline and cocaine treatments were obtained for H3R2me2a. For each ChIP-seq replicate, bilateral 14-gauge NAc punches were pooled from 10 mice. ChIP-seq data were aligned to the mouse genome (mm9), and only unique reads were retained for analysis. FastQC was applied for quality control, and SAMTools was used to remove potential PCR duplicates. PhantomPeak was applied to estimate the quality and enrichment of the ChIP-seq datasets. All three replicates of each condition were pooled and normalized to 1 million reads. The density of H3R2me2a binding 1–2 kb upstream and downstream of transcription start sites (TSSs) of coding genes in Ensembl annotations were plotted. diffReps (39) was used to compare the differential enrichment of H3R2me2a between saline-treated mice and cocaine-treated mice.
Statistics.
Numerical analyses were performed using GraphPad Prism 5.0. The Student’s t test was used whenever two groups were compared, and one-way ANOVA was performed to determine significance for all other data. Significant main effects (P < 0.05) were analyzed further using post hoc tests.
Supplementary Material
Acknowledgments
We thank Mark Bedford and Alessandra Di Lorenzo (MD Anderson Cancer Center) for helpful advice on PRMT6 and Rosemary Bagot (Icahn School of Medicine at Mount Sinai) for fruitful discussions regarding the manuscript. This work was supported by grants from the National Institute on Drug Abuse (NIDA, K99DA042111, ESC).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE85310).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605045113/-/DCSupplemental.
References
- 1.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Renthal W, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damez-Werno D, et al. Drug experience epigenetically primes Fosb gene inducibility in rat nucleus accumbens. J Neurosci. 2012;32(30):10267–10272. doi: 10.1523/JNEUROSCI.1290-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38(1):94–110. doi: 10.1038/npp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy PJ, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16(4):434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J, et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 2014;15(4):R65. doi: 10.1186/gb-2014-15-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller EA, et al. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat Neurosci. 2014;17(12):1720–1727. doi: 10.1038/nn.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scobie KN, et al. Essential role of poly(ADP-ribosyl)ation in cocaine action. Proc Natl Acad Sci USA. 2014;111(5):2005–2010. doi: 10.1073/pnas.1319703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik WK, Kim S. Studies of the identity of the unknown in protein hydrolysates. Biochem Biophys Res Commun. 1980;97(1):8–16. doi: 10.1016/s0006-291x(80)80126-4. [DOI] [PubMed] [Google Scholar]
- 13.Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol. 1998;61:65–131. doi: 10.1016/s0079-6603(08)60825-9. [DOI] [PubMed] [Google Scholar]
- 14.Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett. 2011;585(13):2024–2031. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gayatri S, Bedford MT. Readers of histone methylarginine marks. Biochim Biophys Acta. 2014;1839(8):702–710. doi: 10.1016/j.bbagrm.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel A, et al. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J Biol Chem. 2002;277(5):3537–3543. doi: 10.1074/jbc.M108786200. [DOI] [PubMed] [Google Scholar]
- 17.Guccione E, et al. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449(7164):933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- 18.Kirmizis A, et al. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449(7164):928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhar S, et al. Loss of the major type I arginine methyltransferase PRMT1 causes substrate scavenging by other PRMTs. Sci Rep. 2013;3:1311. doi: 10.1038/srep01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei H, Mundade R, Lange KC, Lu T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle. 2014;13(1):32–41. doi: 10.4161/cc.27353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tee WW, et al. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010;24(24):2772–2777. doi: 10.1101/gad.606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Sayegh J, Daniel J, Clarke S, Bedford MT. PRMT8, a new membrane-bound tissue-specific member of the protein arginine methyltransferase family. J Biol Chem. 2005;280(38):32890–32896. doi: 10.1074/jbc.M506944200. [DOI] [PubMed] [Google Scholar]
- 23.Di Lorenzo A, Yang Y, Macaluso M, Bedford MT. A gain-of-function mouse model identifies PRMT6 as a NF-κB coactivator. Nucleic Acids Res. 2014;42(13):8297–8309. doi: 10.1093/nar/gku530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra R, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 2013;6:13. doi: 10.3389/fnmol.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110(5):1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock R, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16(5):632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen L, et al. diffReps: detecting differential chromatin modification sites from ChIP-seq data with biological replicates. PLoS One. 2013;8(6):e65598. doi: 10.1371/journal.pone.0065598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaworski J, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61(1):85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Repetto D, et al. p140Cap regulates memory and synaptic plasticity through Src-mediated and citron-N–mediated actin reorganization. J Neurosci. 2014;34(4):1542–1553. doi: 10.1523/JNEUROSCI.2341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8(10):1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun. 2013;4:2720. doi: 10.1038/ncomms3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226(1):113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo A, et al. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol Cell Proteomics. 2014;13(1):372–387. doi: 10.1074/mcp.O113.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylvestersen KB, Horn H, Jungmichel S, Jensen LJ, Nielsen ML. Proteomic analysis of arginine methylation sites in human cells reveals dynamic regulation during transcriptional arrest. Mol Cell Proteomics. 2014;13(8):2072–2088. doi: 10.1074/mcp.O113.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, et al. Arginine methyltransferase 1 in the nucleus accumbens regulates behavioral effects of cocaine. J Neurosci. 2015;35(37):12890–12902. doi: 10.1523/JNEUROSCI.0246-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robison AJ, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33(10):4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen L, Choi I, Nestler EJ, Won KJ. Human transcriptome and chromatin modifications: An ENCODE perspective. Genomics Inform. 2013;11(2):60–67. doi: 10.5808/GI.2013.11.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Stefano P, et al. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007;26(12):2843–2855. doi: 10.1038/sj.emboj.7601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.