Significance
Lipoic acid, an enzyme cofactor in central metabolism and a livestock feed supplement, is produced on an industrial scale by a costly multistep synthesis. Nature makes lipoic acid in one step by the chemically challenging addition of two sulfur atoms to an inert fatty acid chain. The sulfur source in this reaction has been controversial, and its identity has implications for engineering microorganisms to overproduce lipoic acid. Structural characterization of a lipoyl synthase enzyme captured in the middle of catalysis shows unequivocally that the enzyme obtains its sulfur atoms by cannibalizing an iron–sulfur cluster, another ancient and essential cofactor. This result reveals an alternative strategy for sulfur mobilization and an unexpected self-sacrificial role for iron–sulfur clusters in biology.
Keywords: iron–sulfur cluster, radical SAM enzyme, lipoic acid
Abstract
Lipoyl synthase (LipA) catalyzes the insertion of two sulfur atoms at the unactivated C6 and C8 positions of a protein-bound octanoyl chain to produce the lipoyl cofactor. To activate its substrate for sulfur insertion, LipA uses a [4Fe-4S] cluster and S-adenosylmethionine (AdoMet) radical chemistry; the remainder of the reaction mechanism, especially the source of the sulfur, has been less clear. One controversial proposal involves the removal of sulfur from a second (auxiliary) [4Fe-4S] cluster on the enzyme, resulting in destruction of the cluster during each round of catalysis. Here, we present two high-resolution crystal structures of LipA from Mycobacterium tuberculosis: one in its resting state and one at an intermediate state during turnover. In the resting state, an auxiliary [4Fe-4S] cluster has an unusual serine ligation to one of the irons. After reaction with an octanoyllysine-containing 8-mer peptide substrate and 1 eq AdoMet, conditions that allow for the first sulfur insertion but not the second insertion, the serine ligand dissociates from the cluster, the iron ion is lost, and a sulfur atom that is still part of the cluster becomes covalently attached to C6 of the octanoyl substrate. This intermediate structure provides a clear picture of iron–sulfur cluster destruction in action, supporting the role of the auxiliary cluster as the sulfur source in the LipA reaction and describing a radical strategy for sulfur incorporation into completely unactivated substrates.
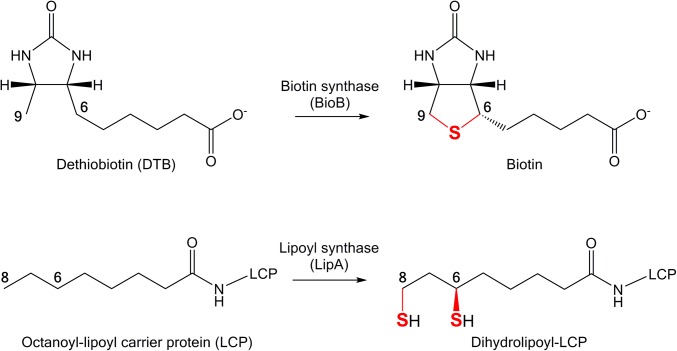
The functionalization of aliphatic carbon centers is widely regarded as one of the most kinetically challenging reactions in nature, a striking example of which is found in the biosynthesis of the lipoyl cofactor, famous for its central role as the “swinging arm” of the pyruvate dehydrogenase enzyme complex. Lipoyl synthase (LipA) generates the lipoyl cofactor by insertion of two sulfur atoms at C6 and C8 of a protein-bound n-octanoyl chain, sites distal from the nearest functionality (1–4). LipA and the closely related biotin synthase (BioB) (Scheme 1) are founding members of the ever-expanding S-adenosyl-l-methionine (AdoMet) radical enzyme superfamily that uses a [4Fe-4S] cluster to reductively cleave the C5′-S bond of AdoMet, generating methionine and a 5′-deoxyadenosyl radical (5′-dA•), a powerful oxidant (5, 6). LipA requires 2 eq AdoMet—one per sulfur insertion—and two sulfur atoms to produce 1 eq lipoyl product through radical-based chemistry (4). One of the most controversial aspects of the LipA and BioB reactions is the source of the sulfur. AdoMet radical enzymes that catalyze sulfur insertion always seem to have an additional iron–sulfur (Fe/S) cluster: a [2Fe-2S] cluster in BioB (7), a [4Fe-4S] cluster in LipA (8), and a [4Fe-4S] cluster in the methylthiotransferases RimO and MiaB (9, 10). These auxiliary clusters in LipA and BioB have been proposed to be cannibalized during turnover to supply the inserted sulfur atom(s) (8, 11–13), a proposal that has not enjoyed universal agreement. Here, we investigate this incredible sulfur insertion chemistry through crystallographic snapshots of LipA from the human pathogen Mycobacterium tuberculosis both in the absence of substrate and at an intermediate stage in the reaction, just after insertion of the C6 sulfur atom but before sulfur insertion at C8.
Scheme 1.
The reactions catalyzed by the AdoMet radical sulfur insertion enzymes (Upper) BioB and (Lower) LipA. The inserted sulfur atoms are marked in red.
Results
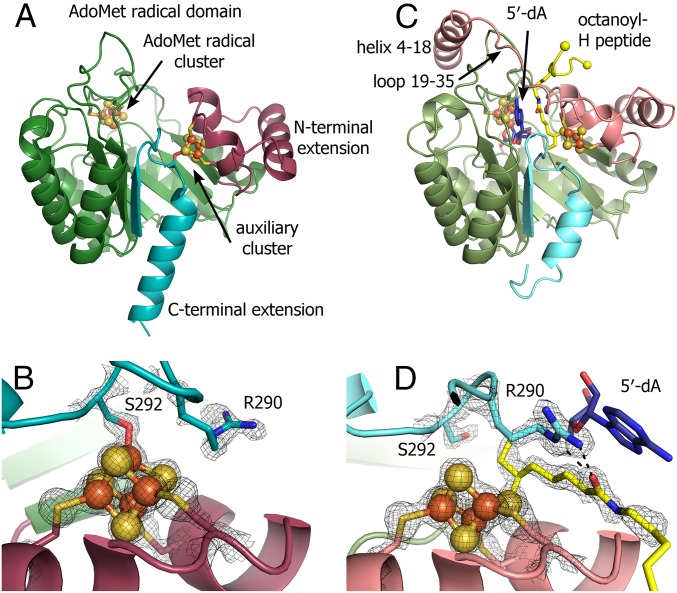
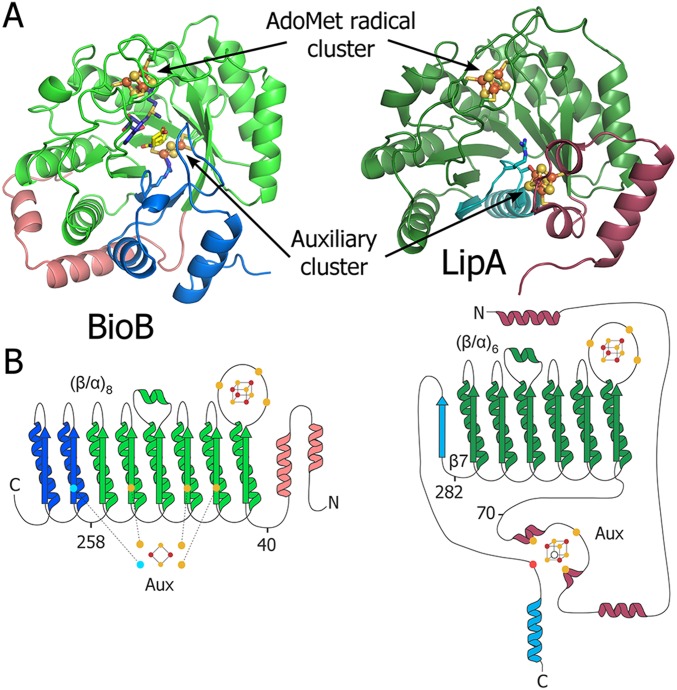
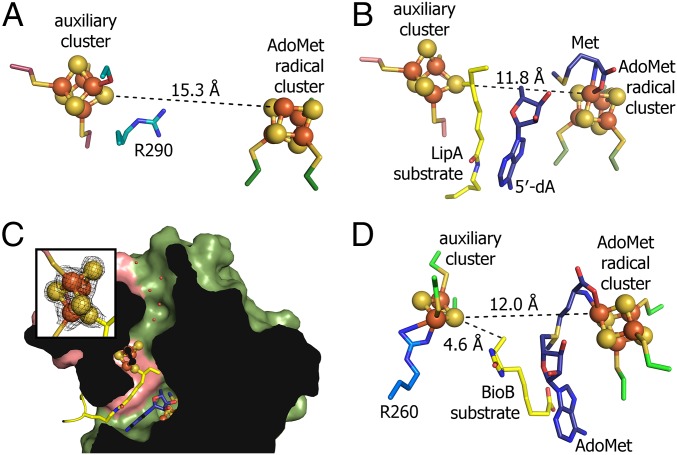
The crystal structure of LipA from M. tuberculosis was determined to 1.64-Å resolution by iron multiwavelength anomalous dispersion phasing (Table S1). The overall fold of LipA consists of a (β/α)6 partial barrel common to most AdoMet radical enzymes (14), an N-terminal α-helical extension, and a C-terminal extension (Fig. 1A and Fig. S1). The [4Fe-4S] cluster that binds AdoMet and is responsible for radical generation is coordinated by three cysteines of the CX3CXΦC motif (Φ denotes an aromatic residue) that is on the AdoMet radical loop between β1 and α1 of the barrel (Fig. S2). The second (auxiliary) [4Fe-4S] cluster is sandwiched between the N- and C-terminal extensions (Fig. 1A). As also observed in a recent structure of LipA from Thermosynechococcus elongatus (15), three of the iron ions of the auxiliary cluster are ligated by cysteines residing in a conserved N-terminal CX4CX5C motif, whereas the fourth iron ion is ligated by S292 found in a conserved C-terminal R(S/T)SΦ motif (Fig. 1B and Fig. S1). R290 of this R(S/T)SΦ motif extends between the two [4Fe-4S] clusters and covers the most exposed face of the auxiliary cluster with the aliphatic portion of its side chain (Fig. 1B). In the absence of substrate, the active site is open and filled with solvent, with a 15.3-Å distance between the two clusters (Fig. 2A). Thus, for the auxiliary cluster to serve as the source of sulfur, displacement of R290 and a conformational tightening of the active site seem to be required.
Table S1.
Data collection and refinement statistics for LipA structures
| Dataset | LipA (2.28-Å resolution) | LipA (1.64-Å resolution) | LipA bound to covalent intermediate | ||
| Peak* | Inflection* | Remote* | |||
| Data collection | |||||
| Space group | P212121 | P212121 | P212121 | P212121 | P21 |
| Cell dimensions | |||||
| a, b, c (Å) | 48.67, 57.81, 116.77 | 48.76, 57.83, 116.70 | 48.66, 57.82, 116.57 | 48.57, 58.52, 98.77 | 81.77, 95.97, 114.33 |
| β (°) | — | — | — | — | 90.46 |
| Wavelength (Å) | 1.7389 | 1.7418 | 0.9795 | 0.9795 | 0.9795 |
| Resolution (Å)† | 50.00–2.93 (2.98–2.93) | 50.00–2.94 (2.99–2.94) | 200.0–2.28 (2.36–2.28) | 100.0–1.64 (1.67–1.64) | 50.00–1.86 (1.89–1.86) |
| Rsym† | 0.100 (0.207) | 0.095 (0.205) | 0.152 (0.458) | 0.098 (0.609) | 0.104 (0.415) |
| I/σI† | 13 (5.5) | 10 (4.0) | 13 (2.4) | 20 (2.2) | 11 (2.5) |
| Completeness (%)† | 94.0 (78.6) | 88.3 (80.9) | 93.1 (66.5) | 99.8 (99.7) | 97.2 (95.3) |
| Redundancy† | 4.0 (3.5) | 2.9 (2.5) | 8.2 (3.6) | 7.5 (6.2) | 3.2 (3.0) |
| Unique reflections | 12,892 | 12,037 | 14,576 | 35,999 | 143,592 |
| Refinement | |||||
| Resolution (Å) | 58.28–2.28 | 50.00–1.64 | 50.00–1.86 | ||
| No. reflections | 26,575 | 35,101 | 143,096 | ||
| Rwork/Rfree | 0.163/0.213 | 0.161/0.197 | 0.160/0.201 | ||
| No. molecules in asymmetric unit | 1 | 1 | 6 | ||
| No. atoms | 2,405 | 2,657 | 16,721 | ||
| Protein | 2,165 | 2,304 | 13,984 | ||
| Ligand/ion | 8 | 9 | 598 | ||
| Water | 216 | 328 | 2,139 | ||
| B factors (Å2) | 34.63 | 22.78 | 19.49 | ||
| Protein | 34.05 | 21.46 | 17.96 | ||
| AdoMet cluster | 30.53 | 18.42 | 11.77 | ||
| Auxiliary cluster | 23.09 | 14.13 | 12.44 | ||
| Substrate peptide‡ | — | — | 29.42 | ||
| 5′-dA | — | — | 11.83 | ||
| Met | — | — | 10.35 | ||
| Other ligand/ion | 38.60 | 27.10 | 21.86 | ||
| Water | 40.85 | 32.32 | 28.82 | ||
| rmsds | |||||
| Bond lengths (Å) | 0.007 | 0.010 | 0.010 | ||
| Bond angles (°) | 1.004 | 1.253 | 1.354 | ||
Each structure corresponds to data collected from a single crystal.
Friedel mates were not merged during scaling.
Statistics for highest resolution shell are in parentheses.
Excludes the inserted sulfur atom.
Fig. 1.
Structural comparisons of substrate-free and intermediate-bound forms of LipA. (A) Structure of substrate-free LipA in ribbon representation with the (β/α)6 AdoMet radical domain (green), N-terminal extension (red), and C-terminal extension (blue). (B) The auxiliary [4Fe-4S] cluster of LipA in the absence of substrate. R290 and S292 of the R(S/T)SΦ motif are shown as sticks. Simulated annealing omit density (2Fo−Fc) is contoured at 2.0σ in black mesh. (C) Structure of LipA after reaction with 1 eq AdoMet in the presence of octanoyl-H peptide substrate (yellow); 5′-dA and methionine are in dark blue. A loop and helix comprising residues 4–30 become ordered when substrate is bound. (D) The auxiliary cluster of LipA with octanoyl-H peptide substrate (yellow) coordinated. Simulated annealing omit density (2Fo−Fc) is contoured at 2.0σ in black mesh.
Fig. S1.
Overall fold of BioB and LipA. (A) Crystal structures of (Left) BioB and (Right) LipA in ribbon representation colored by domain. BioB adopts a (β/α)8 full triosephosphate isomerase (TIM) barrel fold, whereas LipA contains the (β/α)6 partial barrel more common among AdoMet radical enzymes, known as the AdoMet radical core fold. (B) Topology diagrams of (Left) BioB and (Right) LipA. Fe/S clusters are represented in ball and stick form. Fe/S cluster ligands are shown as circles and colored as follows: cyan, arginine; red, serine; and yellow, cysteine.
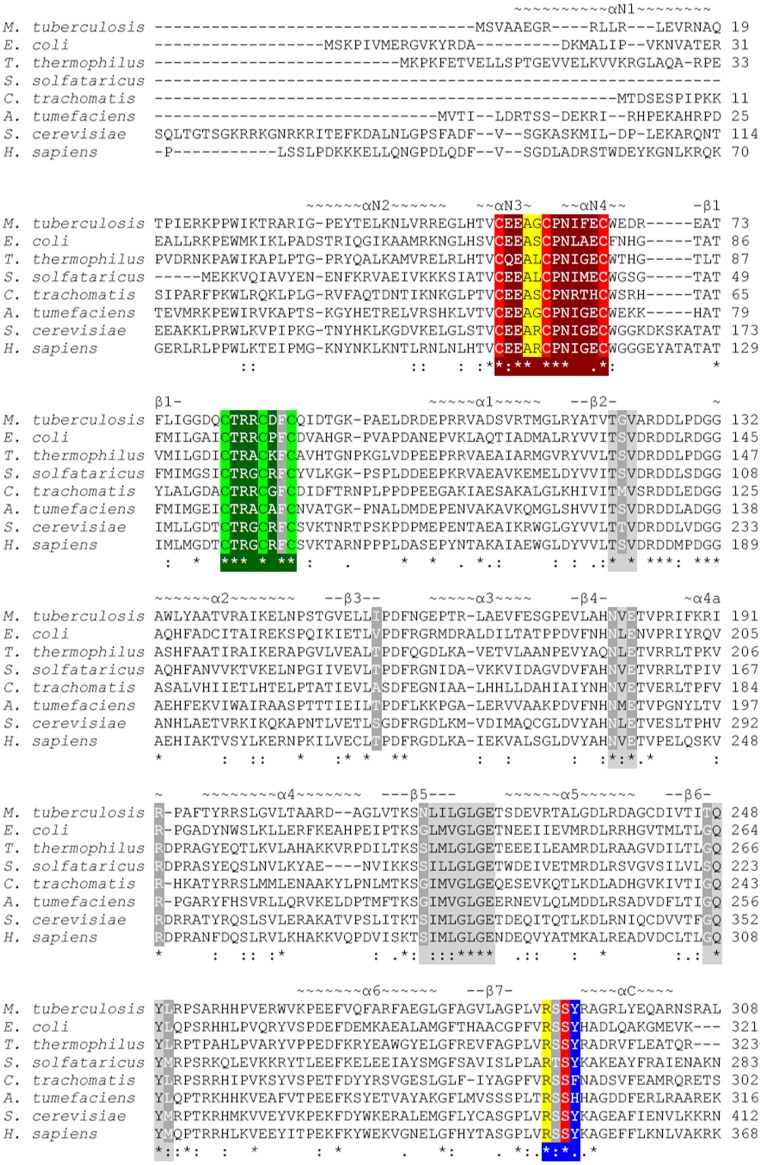
Fig. S2.
Sequence alignment of annotated LipAs from M. tuberculosis, E. coli, Thermus thermophilus, Sulfolobus solfataricus, Chlamydia trachomatis, Agrobacterium tumefaciens, Saccharomyces cerevisiae, and Homo sapiens truncated at residue 308 (M. tuberculosis numbering). Important residues are highlighted as follows: blue, R(T/S)SФ motif; dark gray, residues contacting 5′-dA and methionine; dark green, AdoMet radical CX3CXФC motif; dark red, CX4CX5C motif; light gray, canonical AdoMet binding motifs; light green, AdoMet radical cluster ligands; light red, auxiliary cluster ligands; and yellow, residues contacting the octanoyllysine residue of substrate. Secondary structure is indicated above the sequence, with N standing for N-terminal extension and C standing for C-terminal extension. A corresponding topology diagram is shown in Fig. S1.
Fig. 2.
The active sites of LipA and BioB. (A) Substrate-free LipA with R290 blocking access to auxiliary cluster. (B) Intermediate-bound LipA structure. (C) A cross-section of the intermediate-bound LipA structure showing water-filled cavity created by residues in β1 (E71–T73), β2 (Y117–T119), and the C-terminal helix (S292–R297). Inset depicts simulated annealing omit density (2Fo−Fc) around the auxiliary cluster contoured at 2.0σ. (D) BioB active site (Protein Data Bank ID code 1R30) with a highly conserved arginine residue, R260, ligating the auxiliary [2Fe-2S] cluster. AdoMet ligates the AdoMet radical cluster, whereas dethiobiotin, the substrate, is between AdoMet and the auxiliary cluster.
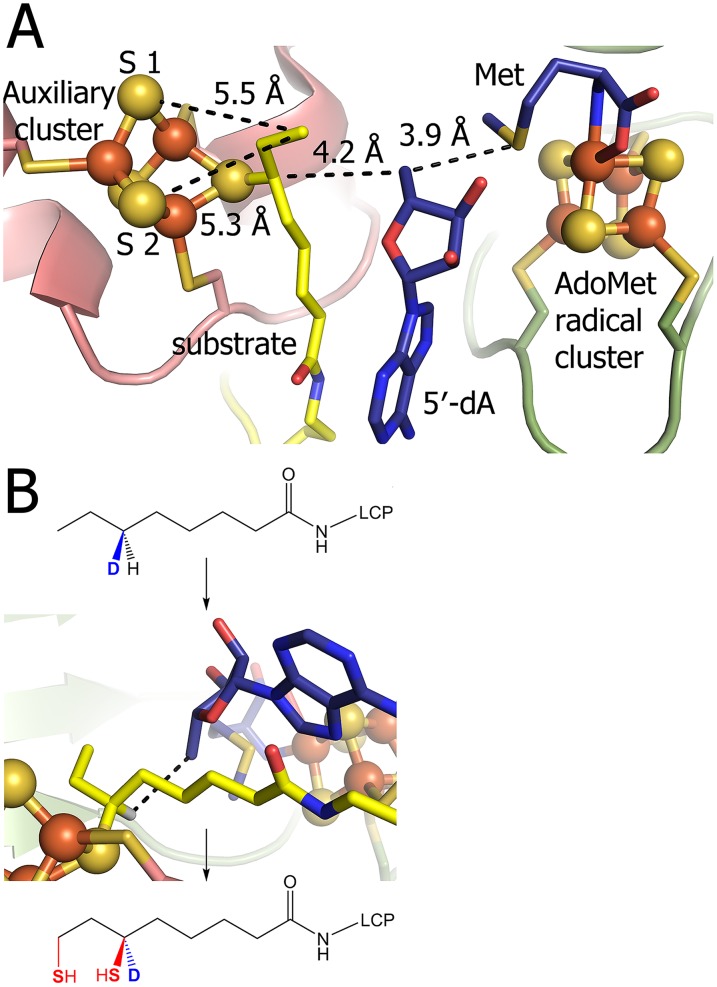
To investigate the mechanism of sulfur insertion by LipA, we crystallographically characterized a stable but kinetically competent reaction intermediate that was previously described by Lanz et al. (16) for Escherichia coli LipA. Briefly, MtLipA was reacted with a synthetic peptide corresponding to eight residues of the M. tuberculosis octanoyl-H protein (sequence ESTKoctSVSD), which supports turnover at the same rate as the full-length protein (17), excess reductant, and 1 eq AdoMet (Scheme 2). Upon reaction under these conditions for 45 min, the resulting protein sample was crystallized, and the crystal structure was determined to 1.86-Å resolution. This “intermediate-bound” structure contains six molecules in the asymmetric unit, all bound with the peptide substrate as well as methionine and 5′-deoxyadenosine (5′-dA), the reductive cleavage products of AdoMet.
Scheme 2.
Proposed catalytic mechanism for LipA. Inserted sulfur atoms are shown in red; species represented by the crystal structures described in the text are shown in blue. The timing of dissociation of the conserved serine ligand, S292, is unknown, but it is shown concomitant with substrate binding. Modified from ref. 16.
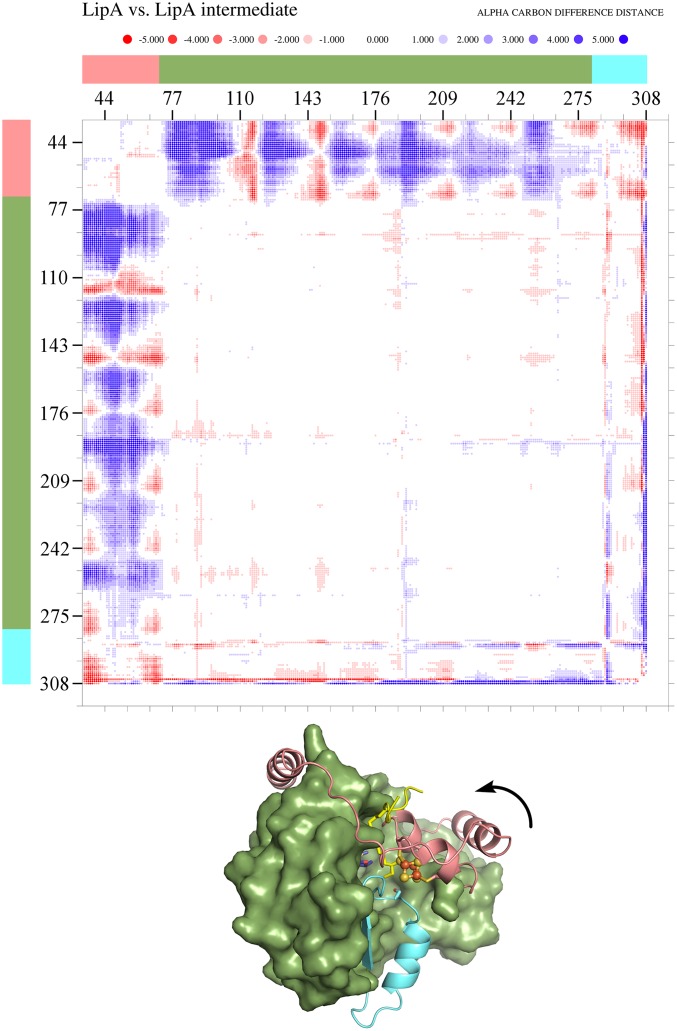
The substrate peptide binds primarily to the N-terminal extension of LipA, such that its octanoyllysine residue inserts into the cavity between the Fe/S clusters (Fig. 1C). It is held in place chiefly by hydrogen bonding contacts involving peptide backbone atoms (Fig. 3). Comparison of the substrate-free and intermediate-bound LipA structures shows a pronounced conformational rearrangement of the enzyme: the N-terminal extension and auxiliary cluster move toward the barrel core (Fig. 1 A and C and Fig. S3), closing off the active site to solvent and decreasing the distance between the clusters to 11.8 Å. Residues 4–30, disordered in the substrate-free structure, are now ordered with residues 4–18 forming a helix adjacent to the AdoMet radical loop and residues 19–35 connecting this helix to the rest of the N-terminal extension (Fig. 1C).
Fig. 3.
Polar interactions between LipA and the octanoyl-H peptide substrate (yellow). Six chains are present in the crystal structure’s asymmetric unit with various degrees of order; the interactions shown are only those present in four or more chains and mediated by zero or one water molecule. The N-terminal extension is in red, the AdoMet radical core is in green, and the C-terminal extension is in cyan.
Fig. S3.
Conformational change in the N-terminal extension of LipA on substrate binding and formation of the covalent intermediate. An α-carbon difference distance matrix created using DDMP (Center for Structural Biology, Yale) shows changes in interresidue distances between the substrate-free and intermediate-bound structures. Pairs of residues that are closer to each other in the LipA intermediate structure are blue dots, whereas those that are closer in the substrate-free structure are red. The N-terminal extension (residues 4–70) is indicated in pink, the AdoMet radical core (residues 71–281) is in green, and the C-terminal extension (residues 282–311) is in cyan. Only the N-terminal extension moves substantially, with the C-terminal extension moving slightly as a result of crystal packing differences. The arrow indicates the direction of N-terminal extension movement on substrate binding and intermediate formation.
The loop in the C-terminal extension that contains the R(S/T)SΦ motif also has been rearranged, and R290 is no longer blocking substrate access to the auxiliary cluster (Fig. 1D). R290 now packs against the octanoyl moiety of the substrate and hydrogen bonds to its acyl carbonyl oxygen (Fig. 1D). The octanoyllysine is additionally secured through hydrogen bonds between its acyl amide and peptidyl amide nitrogens and the carbonyl oxygens of A58 and G59, respectively (Fig. 3), residues that are found in a highly conserved part of LipA sequences between the first and second cysteines of the auxiliary cluster CX4CX5C motif (Fig. S2). Through these interactions, octanoyllysine appears beautifully positioned for radical-based sulfur insertion, in that Met, 5′-dA, and the octanoyl group are all buried away from solvent and all within van der Waals distances of each other (Fig. 2B, Fig. S4, and Table S2). In particular, the C5′ of 5′-dA, which performs the H• abstraction, is 4.2 Å from C6 of the octanoyl substrate. Additionally, the orientation of the octanoyl group with respect to 5′-dA and the auxiliary cluster is consistent with the abstraction of the pro-R H• from C6 (2) and inversion of configuration on sulfur insertion (Fig. 2B and Fig. S4).
Fig. S4.
The active site of chain A of the LipA intermediate-bound structure. (A) Distances between C5′ of 5′-dA and its reaction partners and between C8 of substrate and its possible reaction partners. Other distances are listed in Table S2. (B) The observed stereochemistry of the LipA reaction—abstraction of the pro-R hydrogen atom and inversion of configuration at C6—is reflected in the intermediate-bound structure. Here, a riding hydrogen atom added to C6 is within van der Waals distance of C5′ of 5′-dA.
Table S2.
Selected active site distances (ångströms) in six chains of the LipA intermediate structure
| Chain | C5′ to C6 distance | C5′ to Met S distance | C6–S6 bond | S6–Fe bond 1 | S6–Fe bond 2 | C8 to S distance 1 | C8 to S distance 2 |
| A | 4.20 | 3.87 | 1.79 | 2.30 | 2.37 | 5.31 | 5.54 |
| C | 4.21 | 3.94 | 1.79 | 2.32 | 2.34 | 5.41 | 5.63 |
| E | 4.22 | 3.90 | 1.79 | 2.32 | 2.36 | 5.35 | 5.49 |
| G | 4.13 | 3.89 | 1.78 | 2.39 | 2.32 | 5.32 | 5.69 |
| I | 4.30 | 3.81 | 1.79 | 2.32 | 2.38 | 5.29 | 5.51 |
| K | 4.27 | 3.89 | 1.79 | 2.29 | 2.30 | 5.44 | 5.60 |
| Mean | 4.22 | 3.88 | 1.79 | 2.32 | 2.35 | 5.35 | 5.58 |
| SD | 0.06 | 0.04 | 0.00 | 0.04 | 0.03 | 0.06 | 0.08 |
Consistent with the first half reaction having taken place, we find that the substrate octanoyl group is covalently attached to the auxiliary cluster by a bond between C6 and a cluster sulfur atom (Fig. 1D). S292 is no longer a ligand to the cluster, and the iron atom that was coordinated by S292 in the substrate-free structure is no longer present (Fig. 2C, Inset). Previously, a Mössbauer spectroscopic study revealed the loss of an iron atom from the auxiliary cluster on the timescale of the first sulfur insertion (16), and here, we see which iron is lost, and potentially, how it is lost: a solvent-filled cavity exists on one side of the cluster that extends to the protein surface (Fig. 2C). These structural data also present an explanation of why iron loss could be of mechanistic significance. The presence of this iron atom would block access of the two closest sulfur atoms of the auxiliary cluster (5.5 and 5.3 Å) to C8 of the octanoyl moiety (Fig. S4), hindering the second sulfur insertion.
Discussion
The chemical difficulty of synthesizing the lipoyl and biotin cofactors cannot be overstated. This point is exemplified by the fact that 10–30 tons of biotin sold each year are produced by a chemical synthetic route that is estimated to have as many as 15 steps (18). First, the challenge of regio- and stereoselective C–H bond activation must be overcome, and second, a route for sulfur functionalization must be achieved. Here, we find that the LipA substrate is exquisitely positioned next to 5′-dA for pro-R H• abstraction, allowing for this requisite selectivity. The next step is sulfur functionalization (Scheme 2), which raises the controversial issue of whether an Fe/S cluster is really the source of sulfur. In this respect, the structure of BioB sets the stage, revealing an auxiliary [2Fe-2S] cluster with an atypical arginine ligation near the substrate binding site and the radical AdoMet cluster (Fig. 2D) (19). In this work, we find that substrate binding to LipA induces a large conformational change that shortens the distance between Fe/S clusters (15.3–11.8 Å), such that the agreement with the substrate-bound structure of BioB is now incredible (Fig. 2 B and D). This consistent ∼12-Å distance seems to be ideal for substrate to be packed sufficiently close to the 5′-dA radical for H• abstraction and simultaneously near enough to the auxiliary cluster for sulfur insertion (4.6 Å in BioB). Interestingly, like BioB, there is an unusual ligand to the auxiliary cluster, but the ligand is a serine, not an arginine as it is in BioB. As far as we know, LipA is the only example of serine ligation to a [4Fe-4S] cluster in a WT protein. Serine ligands have been introduced into proteins with [4Fe-4S] clusters by mutagenesis, usually resulting in decreased cluster stability under oxidizing or acidic conditions (20–23). This latter property perhaps explains the choice of serine as a ligand for a [4Fe-4S] cluster that must be labile by design. Although it is not a ligand, arginine 290 of the R(S/T)SΦ motif seems critical for both protection of the auxiliary cluster in the absence of substrate and substrate binding.
Although [3Fe-4S] clusters are often found as dead-end products of oxidative inactivation of [4Fe-4S] enzymes, in LipA, these clusters seem to be reaction intermediates. The strongest support for the catalytic relevance of an intermediary [3Fe-4S] cluster comes from a Mössbauer spectroscopic study that showed that a [3Fe-4S] cluster appears and disappears on the timescale of LipA turnover (16). In particular, under the conditions used for these crystal trials (excess reductant and 1 eq AdoMet), E. coli LipA accumulates a cross-linked species that is stable to gel filtration chromatography and contains 0.9 eq monothiolated peptide and 0.6 eq 3-Fe–containing clusters (Scheme 2) with Mössbauer parameters similar to those of previously described [3Fe-4S] clusters. When reacted with excess AdoMet and reductant, this cross-linked species supports the formation of 0.5 eq lipoyl product in a kinetically competent manner, the Mössbauer signal for the 3-Fe–containing cluster disappears, and signals increase for N/O- and S-coordinated high-spin FeII, corresponding to degradation of the auxiliary cluster. For MtLipA, the analogous cross-linked species supports formation of almost a full equivalent of lipoyl product with a rate similar to that of the overall reaction (17).
One surprising attribute of the cross-linked intermediate is its unusual stability: over 5 d between intermediate generation and crystal growth and for an additional 4 d in crystallo, the partially degraded auxiliary cluster remains intact, and the peptide, 5′-dA, and methionine remain enzyme-bound. It stands to reason that LipA would maximize the sulfur yield from each Fe/S cluster by preventing dissociation of the monothiolated intermediate, but the persistence of 5′-dA and methionine is puzzling, considering that the second sulfur insertion requires their replacement with AdoMet for 5′-dA• generation. Attempts to soak a second molecule of AdoMet into crystals of the LipA intermediate (5 mM AdoMet for 5 d and 10 mM AdoMet for 1 d) do not displace 5′-dA and Met, suggesting that exchange may require a conformational change that is not possible in the crystal. However, cocrystallization of the intermediate-bound species with up to 5 mM AdoMet over several days also fails to exchange 5′-dA and Met for AdoMet, instead producing identical intermediate-bound crystals. These results may indicate that the AdoMet-bound intermediate complex does not crystallize as readily as the 5′-dA and Met-bound complex, that the exchange of 5′-dA and Met for AdoMet is highly unfavorable, or that the dissociation of 5′-dA and Met is slow. Although current data do not support any one explanation, if either of the latter two explanations is correct, exchange may be at least partially rate-limiting in solution-phase experiments with MtLipA.
Overall, these structures corroborate previous biochemical and spectroscopic studies on LipA (3, 4, 8, 13, 16, 17) and support the role of the auxiliary cluster as the sulfur donor (Scheme 2). By showing the octanoyllysine moiety covalently attached to a sulfur atom in the auxiliary cluster, these structural data represent a smoking gun for what has been a truly heated debate about the sulfur source for these reactions. Although these structures may end this debate, there are many exciting questions about LipA that remain: after the first sulfur insertion, how are 5′-dA and Met exchanged for a second equivalent of AdoMet, and which of the two equidistant sulfur atoms is inserted into C8? Perhaps the most enigmatic question regarding the LipA mechanism is the ultimate fate of the degraded auxiliary cluster in vivo. It may be repaired on LipA, removed and replaced by Fe/S cluster assembly machinery, or simply released in the form of free iron and inorganic sulfide ions. The latter process would seem to present a problem for the cell in the form of iron and sulfide toxicity. Given that LipA enzymes exist in a wide range of organisms from pathogens to humans, it is unclear whether there will be a single solution to this question. Certainly, the sulfur insertions en route to production of the lipoyl and biotin cofactors will continue to fascinate for years to come.
Materials and Methods
N-terminally His6-tagged MtLipA was expressed in E. coli BL21(DE3) cells, purified under anaerobic conditions, and reconstituted with iron and sulfide as described previously (16, 17). The MtLipA intermediate was generated by reaction of 200 μM as-isolated MtLipA with 300 μM octanoyl substrate peptide, 180 μM AdoMet, and 2 mM sodium dithionite anaerobically at room temperature for 45 min; intermediate formation was monitored by liquid chromatography-MS of acid-quenched reaction samples (SI Materials and Methods). MtLipA and the MtLipA intermediate were crystallized anaerobically by the vapor diffusion method. Each structure was solved from data collected on a single crystal at beamline ID-C of the Advanced Photon Source. The structure of MtLipA alone was originally solved to 2.28-Å resolution by Fe multiwavelength anomalous dispersion; later, a higher-resolution dataset was collected from a different crystal, and a second structure was solved to 1.64-Å resolution using the first structure as a search model for molecular replacement. This high-resolution MtLipA structure was used as a molecular replacement model to solve the intermediate-bound structure. Details of crystallization, data collection, structure solution, model building, and refinement can be found in SI Materials and Methods.
SI Materials and Methods
Materials.
Isopropanol, Bis⋅Tris propane, sodium dithionite, and Tris(2-carboxyethyl)phosphine (TCEP) were purchased from Sigma Corp. Hepes was from EMD Biosciences, and 2-methyl-2,4-pentanediol was from Alfa Aesar. All other crystallization reagents were purchased from Hampton Research. Mycobacterium tuberculosis octanoyl-H peptide [Glu-Ser-Thr-(N6-octanoyl)Lys-Ser-Val-Ser-Asp] and the external standard peptide for liquid chromatography (LC)-MS analysis (Pro-Met-Ser-Ala-Pro-Ala-Arg-Ser-Met) were synthesized by ProImmune Ltd., and AdoMet was generated from l-methionine, ATP, AdoMet synthetase, and pyrophosphatase as described previously (24). M. tuberculosis octanoyl-H protein was prepared as previously described for Escherichia coli octanoyl-H protein (4, 25). All other chemicals were reagent grade or higher.
Expression and Purification of LipA.
N-terminally hexahistidine-tagged M. tuberculosis LipA was overexpressed in E. coli and purified as described previously (17). Protein denoted “reconstituted” (RC) was further incubated with excess ferric chloride, sodium sulfide, and DTT as described previously (4), whereas protein denoted “as-isolated” (AI) was not. For crystallographic studies, AI and RC LipA were further purified by size exclusion chromatography using an Äkta FPLC System (GE Healthcare) fitted with a HiPrep 16/60 Sephacryl S-200 Column (GE Healthcare) maintained inside an anaerobic chamber and equilibrated in buffer containing 10 mM Hepes, pH 7.5, 150 mM KCl, 5 mM DTT, and 10% (vol/vol) glycerol.
Generation of LipA Intermediate.
In a total volume of 1 mL, 200 µM LipA AI was added to a solution containing 50 mM Hepes, pH 7.5, 300 mM KCl, 10% (vol/vol) glycerol, 300 µM M. tuberculosis octanoyl-H peptide, and 180 µM AdoMet. The reaction was initiated with the addition of 2 mM sodium dithionite. The reaction was incubated at room temperature for 45 min and then, applied to a 10,000 MWCO Amicon Ultra Centrifugal Filter Concentrator (Millipore) for 10–12 min at 7,000 × g to reduce the volume approximately fourfold. The cross-linked enzyme was snap-frozen and stored in liquid nitrogen until ready for use. To monitor reaction progress, aliquots of the reaction mixture were quenched at designated times in 100 mM sulfuric acid containing 4 mM TCEP and 20 µM external peptide standard for LC-MS analysis. A standard curve for analysis by LC-MS was prepared in assay buffer and contained 20 µM external peptide standard, 2 mM TCEP, and 3.9–500 µM octanoyl-peptide. The quantities of lipoyl- and mercapto-octanoyl (i.e., monothiolated) peptide were estimated using the octanoyl peptide standards.
Analysis of Reaction Products by LC-MS.
Assay mixtures were separated on an Agilent Technologies Zorbax Rapid Resolution SB-C18 Column (2.1 × 30 mm; 3.5-μm particle size) equilibrated in 100% (vol/vol) solvent A (0.1% formic acid, pH 2.6) and 0% solvent B (acetonitrile). Solvent B was maintained at 0% for 0.8 min before a gradient of 0 to 19% was applied from 0 to 1.5 min. Solvent B was maintained at 19% from 1.5 to 5 min before returning to 0% from 5 to 6 min. A flow rate of 0.4 mL/min was used throughout the chromatographic procedure. The column was allowed to reequilibrate for 3 min under initial conditions before subsequent sample injections. The external standard peptide elutes at ∼3.1 min under these conditions, whereas the lipoyl, mercapto-octanoyl, and octanoyl peptides elute at 4.7, 4.6, and 5.5 min, respectively.
The external standard, octanoyl, mercapto-octanoyl, and lipoyl peptides were detected by MS2 selected ion monitoring at m/z values of 474.4, 978.5, 1,010.5, and 1,042.5, respectively. Detection of analytes was performed using electrospray ionization in positive mode with the following parameters: a nitrogen gas temperature of 340 °C with a flow rate of 9.0 L/min, a nebulizer pressure of 40 psi, and a capillary voltage of 4,000 V. The fragmentor voltage was optimized for octanoyl peptide at 220 V and used for all derivatives. The fragmentor voltage for the external standard peptide was 135 V.
Crystallization of LipA for 2.28-Å Resolution Structure.
All procedures were performed in a Coy anaerobic chamber maintained in an atmosphere of 95% Ar and 5% H2. LipA RC [8.0 mg/mL in 10 mM Hepes, pH 7.5, 150 mM KCl, 10% (vol/vol) glycerol, 5 mM DTT] was diluted to 7.3 mg/mL in a solution containing 1 mM E. coli octanoyl-H peptide (sequence ESVKoctAASD) and 0.9 mM AdoMet; the protein solution was incubated at room temperature overnight and centrifuged at 2,500 × g for 5 min before crystallization. Crystal trials were performed using the hanging drop vapor diffusion method at room temperature combining 1 µL protein solution with 1 µL precipitant [0.1 M Hepes, pH 7.5, 12% (wt/vol) PEG 10000, 7.5% (vol/vol) 2-methyl-2,4-pentanediol] over a reservoir of 500 µL 0.5 M LiCl. Plate clusters appeared after 1 d and were dragged through Paratone-N to remove solvent before freezing in liquid nitrogen at 4 d.
Crystallization of LipA for 1.64-Å Resolution Structure.
All procedures were performed in a Coy anaerobic chamber maintained in an atmosphere of 95% Ar and 5% H2. LipA RC [8.0 mg/mL in 10 mM Hepes, pH 7.5, 150 mM KCl, 10% (vol/vol) glycerol, 5 mM DTT] was diluted to 7.4 mg/mL in a solution containing 3.3 mg/mL M. tuberculosis octanoyl-H protein [48.5 mg/mL in 50 mM Hepes, pH 7.5, 200 mM KCl, 10% (vol/vol) glycerol] and 1.0 mM AdoMet; the protein solution was incubated at room temperature overnight and centrifuged at 2,500 × g for 5 min before crystallization. Crystal trials were performed using the hanging drop vapor diffusion method at room temperature combining 1 µL protein solution with 1 µL precipitant [0.02 M citric acid, 0.08 M Bis⋅Tris propane, pH 8.8, 16% (wt/vol) PEG 3350] over a reservoir of 500 µL 0.5 M LiCl. A single plate appeared within 5 mo. At 5 mo, the crystal was soaked for <3 min in precipitant and dragged through Paratone-N to remove solvent before freezing in liquid nitrogen.
Crystallization of LipA Intermediate.
Crystallization procedures were performed in an MBraun Anaerobic Chamber maintained in an atmosphere of nitrogen. LipA intermediate [29.5 mg/mL in 50 mM Hepes, pH 7.5, 300 mM KCl, 10% (vol/vol) glycerol] was diluted to 10 mg/mL in 20 mM Hepes (pH 7.5) and 200 mM NaCl; the protein solution was then allowed to stand overnight and centrifuged at 14,100 × g for 5 min before crystallization. Initial crystal trials were performed using the sitting drop vapor diffusion method at 20 °C combining 200 nL protein solution with 200 nL precipitant [0.1 M Bis⋅Tris, pH 6.5, 0.2 M Li2SO4, 25% (wt/vol) PEG 3350] over a reservoir of precipitant. Needle-like crystals appeared after 2 mo and were used to streak seed hanging drop trials at room temperature using the same precipitant; crystals from these hanging drop trials were mixed with precipitant to create a seed stock. Final crystal trials were performed using the sitting drop vapor diffusion method at 20 °C combining 200 nL protein solution with 200 nL precipitant [2% (vol/vol) Tacsimate, pH 7.0, 0.1 M imidazole, pH 7.0, 8% (wt/vol) PEG 3350, 5% (vol/vol) isopropanol] and 50 nL seed stock [LipA intermediate seed crystals in 0.1 M Bis⋅Tris, pH 6.5, 0.2 M Li2SO4, 25% (wt/vol) PEG 3350] over a reservoir of 70 µL 0.5 M LiCl. Rod-shaped crystals appeared after 4 d and were moved to a Coy anaerobic chamber (95% Ar, 5% H2) at 5 d for handling. After 9 d, crystals were soaked in cryoprotectant solution [2% (vol/vol) Tacsimate, pH 7.0, 0.1 M imidazole, pH 7.0, 20% (wt/vol) PEG 3350, 5% (vol/vol) isopropanol] for < 3 min before cryocooling in liquid nitrogen.
Crystallographic Data Collection.
All data were collected at the Advanced Photon Source (Argonne, IL) using Northeastern Collaborative Access Team beamline 24 ID-C at a temperature of 100 K. Indexing, integration, and scaling were performed using the HKL2000 software package (Table S1).
Structure Determination of LipA at 2.28-Å Resolution.
Iron anomalous datasets were collected at peak, inflection, and remote wavelengths (Table S1) using a mini-κ goniometer to collect Bijvoet pairs on the same frame. Multiwavelength anomalous dispersion phasing was performed in autoSHARP (26) using the peak, inflection, and remote datasets; two heavy-atom sites with occupancy >3 were identified, indicating a single molecule in the asymmetric unit with two [4Fe-4S] clusters. Heavy-atom site refinement in autoSHARP yielded experimental phases with an overall figure of merit of 0.57 (acentric)/0.40 (centric) when calculated to 3.9-Å resolution. Anomalous signal extended to roughly 4-Å resolution. Electron density maps were interpretable after solvent flattening using SOLOMON in the autoSHARP interface. These initial maps, cut off at 4-Å resolution, were used to manually build an initial polyalanine model containing several helices, strands, and the two [4Fe-4S] clusters. This initial model was used for phase extension in one step to 2.28-Å resolution, which allowed for residue assignment and placement of sidechains on residues 40–90. A more complete model, including several additional loops and all residue assignments, was built using phenix.AutoBuild (27) against the experimental map with a resolution cutoff of 3.8 Å. After comparing this model with the hand-built model to check for correctness of helix and strand locations, the automatically built model was modified manually in Coot (28) to insert [4Fe-4S] clusters and move protein residues that had been placed into cluster density. The resulting model was refined immediately against the remote dataset at 2.28-Å resolution with no experimental-phase contribution, alternating rounds of refinement in phenix.refine with manual model building in Coot. Ordered solvent was added automatically and checked manually after Rfree reached ∼0.26. Geometric restraint files for the [4Fe-4S] clusters were generated using a 0.72-Å structure of a [4Fe-4S] protein as a guide (Protein Data Bank ID code 3A39) (29) and edited as appropriate to account for noncysteinyl ligation; restraints for d-(+)-DTT were generated using phenix.REEL and used as is.
The final model contains residues 34–311 (of 311); residues 1–33 and the N-terminal hexahistidine tag are disordered. Ramachandran analysis by PROCHECK (30) places 94.9% of residues in the most favored region, 4.2% of residues in the allowed region, no residues in the additionally allowed region, and 0.8% (two residues) in the disallowed region. The residues in the disallowed region, E182 and V289, adopt the well-documented γ-turn backbone conformation that has been added to the allowed regions of more recent Ramachandran validation programs (31). Nonprotein atoms include two [4Fe-4S] clusters, 218 solvent molecules, and a molecule of d-(+)-DTT bound to the AdoMet radical cluster. AdoMet and octanoyl-H peptide are absent from the electron density. The final model was verified using a simulated annealing composite omit map calculated in phenix.AutoBuild.
Determination of a 1.64-Å Resolution LipA Structure.
A native dataset was collected at 0.9795-Å wavelength (Table S1), and the structure was solved by molecular replacement to 3.0-Å resolution using the protein and [4Fe-4S] cluster atoms of the low-resolution LipA structure as a search model, yielding one molecule per asymmetric unit. The initial model was refined at 1.64-Å resolution using phenix.refine and adjusted with Coot as described above, except that the restraints for the AdoMet radical cluster were modified to include a link between a sulfur atom of d-(+)-DTT and the unique (noncysteinyl-ligated) iron of the cluster. The final model includes residues 31–311 (of 311); residues 1–30 and the N-terminal hexahistidine tag are disordered. Ramachandran analysis by PROCHECK places 95.8% of residues in the most favored region, 3.3% of residues in the allowed region, no residues in the additionally allowed region, and 0.8% (two residues) in the disallowed region. As in the lower-resolution structure, the two outlier residues, E182 and V289, adopt canonical γ-turn conformations. Nonprotein atoms include two [4Fe-4S] clusters, 342 solvent molecules, and a molecule of d-(+)-DTT bound to the AdoMet radical cluster. AdoMet and octanoyl-H protein are absent from the electron density. The final model was verified using a simulated annealing composite map calculated in phenix.AutoBuild.
Determination of Intermediate-Bound LipA Structure.
A dataset was collected at 0.9795-Å wavelength (Table S1), and the structure was solved by molecular replacement to 2.5-Å resolution using a truncated version (residues 67–311) of the 1.64-Å resolution LipA structure as a search model, yielding six molecules per asymmetric unit. Rebuilding of residues 4–66 and other manual adjustments to the model were alternated with refinement as described above. Geometric restraint files for malonate and methionine were generated in REEL and used as is; restraints for 5′-dA were a gift from Oliver Smart at Global Phasing, Cambridge, United Kingdom. Restraints for the [4Fe-4S] cluster were obtained as above for the 2.28-Å resolution structure but edited to allow for slightly increased Fe/S bond lengths to the unique (noncysteinyl-ligated) iron as visible in the electron density. Restraints for the [3Fe-4S] cluster cross-linked to the substrate octanoyllysine residue were generated in REEL based on dihydrolipoyllysine (residue code LA2) with iron and sulfur atoms added as appropriate; Fe/S distances and angles in this cluster were restrained to the values used for [4Fe-4S] clusters in the substrate-free LipA structure, except for slightly increased Fe/S bond lengths to the inserted sulfur atom at C6.
In the final model, refined at 1.86-Å resolution, all molecules contain residues 12–305 (of 311) and a substrate octanoyllysine residue covalently linked to a [3Fe-4S] cluster through a sulfur atom on C6. Select molecules also contain residues 4–12 and 306–309 as well as eight residues of the substrate peptide. Ramachandran analysis by PROCHECK places 93.6% of amino acid residues in the most favored region, 5.8% of residues in the allowed region, 0.1% of residues in the additionally allowed region, and 0.4% (seven residues) in the disallowed region, six of which are E182 in γ-turn conformations. All molecules in the asymmetric unit include the following nonprotein atoms: a [4Fe-4S] cluster, a [3Fe-4S] cluster covalently linked to a substrate octanoyllysine residue at C6, 5′-dA, and methionine. The final model also contains 2,140 solvent molecules, 1 malonate ion, 2 chloride ions, 2 imidazole molecules, and 1 isopropanol molecule from the crystallization solution. The final model was verified using a simulated annealing composite omit map calculated in phenix.AutoBuild.
Acknowledgments
This work was supported by NIH Grants R01GM063847 (to S.J.B.) and R01GM103268 (to S.J.B.) and National Science Foundation Grant MCB-0543833 (to C.L.D.). Additionally, C.L.D. and S.J.B. are Howard Hughes Medical Investigators. Additional funding was provided by the Meryl and Stewart Robertson Undergraduate Research Opportunities Program Fund, the Massachusetts Institute of Technology Energy Initiative, and the DeFlorez Endowment Fund. This work is based on research conducted at the Advanced Photon Source (APS) on the Northeastern Collaborative Access Team beamlines, which are supported by National Institute of General Medical Sciences Grant P41GM103403. Use of the APS, an Office of Science User Facility operated for the US Department of Energy (DOE), was supported by US DOE Contract DE-AC02-06CH11357.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 5EXI, 5EXJ, and 5EXK).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602486113/-/DCSupplemental.
References
- 1.Miller JR, et al. Escherichia coli LipA is a lipoyl synthase: In vitro biosynthesis of lipoylated pyruvate dehydrogenase complex from octanoyl-acyl carrier protein. Biochemistry. 2000;39(49):15166–15178. doi: 10.1021/bi002060n. [DOI] [PubMed] [Google Scholar]
- 2.White RH. Stable isotope studies on the biosynthesis of lipoic acid in Escherichia coli. Biochemistry. 1980;19(1):15–19. doi: 10.1021/bi00542a003. [DOI] [PubMed] [Google Scholar]
- 3.Douglas P, Kriek M, Bryant P, Roach PL. Lipoyl synthase inserts sulfur atoms into an octanoyl substrate in a stepwise manner. Angew Chem Int Ed Engl. 2006;45(31):5197–5199. doi: 10.1002/anie.200601910. [DOI] [PubMed] [Google Scholar]
- 4.Cicchillo RM, et al. Lipoyl synthase requires two equivalents of S-adenosyl-l-methionine to synthesize one equivalent of lipoic acid. Biochemistry. 2004;43(21):6378–6386. doi: 10.1021/bi049528x. [DOI] [PubMed] [Google Scholar]
- 5.Lieder KW, et al. S-Adenosylmethionine-dependent reduction of lysine 2,3-aminomutase and observation of the catalytically functional iron-sulfur centers by electron paramagnetic resonance. Biochemistry. 1998;37(8):2578–2585. doi: 10.1021/bi972417w. [DOI] [PubMed] [Google Scholar]
- 6.Frey PA, Hegeman AD, Ruzicka FJ. The radical SAM superfamily. Crit Rev Biochem Mol Biol. 2008;43(1):63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 7.Cosper MM, et al. Characterization of the cofactor composition of Escherichia coli biotin synthase. Biochemistry. 2004;43(7):2007–2021. doi: 10.1021/bi0356653. [DOI] [PubMed] [Google Scholar]
- 8.Cicchillo RM, et al. Escherichia coli lipoyl synthase binds two distinct [4Fe-4S] clusters per polypeptide. Biochemistry. 2004;43(37):11770–11781. doi: 10.1021/bi0488505. [DOI] [PubMed] [Google Scholar]
- 9.Hernández HL, et al. MiaB, a bifunctional radical-S-adenosylmethionine enzyme involved in the thiolation and methylation of tRNA, contains two essential [4Fe-4S] clusters. Biochemistry. 2007;46(17):5140–5147. doi: 10.1021/bi7000449. [DOI] [PubMed] [Google Scholar]
- 10.Lee K-H, et al. Characterization of RimO, a new member of the methylthiotransferase subclass of the radical SAM superfamily. Biochemistry. 2009;48(42):10162–10174. doi: 10.1021/bi900939w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bui BT, et al. Biotin synthase mechanism: On the origin of sulphur. FEBS Lett. 1998;440(1-2):226–230. doi: 10.1016/s0014-5793(98)01464-1. [DOI] [PubMed] [Google Scholar]
- 12.Ugulava NB, Sacanell CJ, Jarrett JT. Spectroscopic changes during a single turnover of biotin synthase: Destruction of a [2Fe-2S] cluster accompanies sulfur insertion. Biochemistry. 2001;40(28):8352–8358. doi: 10.1021/bi010463x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicchillo RM, Booker SJ. Mechanistic investigations of lipoic acid biosynthesis in Escherichia coli: Both sulfur atoms in lipoic acid are contributed by the same lipoyl synthase polypeptide. J Am Chem Soc. 2005;127(9):2860–2861. doi: 10.1021/ja042428u. [DOI] [PubMed] [Google Scholar]
- 14.Dowling DP, Vey JL, Croft AK, Drennan CL. Structural diversity in the AdoMet radical enzyme superfamily. Biochim Biophys Acta. 2012;1824(11):1178–1195. doi: 10.1016/j.bbapap.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmer JE, et al. Structures of lipoyl synthase reveal a compact active site for controlling sequential sulfur insertion reactions. Biochem J. 2014;464(1):123–133. doi: 10.1042/BJ20140895. [DOI] [PubMed] [Google Scholar]
- 16.Lanz ND, et al. Evidence for a catalytically and kinetically competent enzyme-substrate cross-linked intermediate in catalysis by lipoyl synthase. Biochemistry. 2014;53(28):4557–4572. doi: 10.1021/bi500432r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanz ND, et al. Characterization of lipoyl synthase from Mycobacterium tuberculosis. Biochemistry. 2016;55(9):1372–1383. doi: 10.1021/acs.biochem.5b01216. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq PJ. Biotin: A timeless challenge for total synthesis. Chem Rev. 1997;97(6):1755–1792. doi: 10.1021/cr950073e. [DOI] [PubMed] [Google Scholar]
- 19.Berkovitch F, Nicolet Y, Wan JT, Jarrett JT, Drennan CL. Crystal structure of biotin synthase, an S-adenosylmethionine-dependent radical enzyme. Science. 2004;303(5654):76–79. doi: 10.1126/science.1088493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calzolai L, et al. Solution NMR Study of the electronic structure and magnetic properties of cluster ligation mutants of the four-iron ferredoxin from the hyperthermophilic archaeon Pyrococcus furiosus. J Am Chem Soc. 1997;119(40):9341–9350. [Google Scholar]
- 21.Brereton PS, Duderstadt RE, Staples CR, Johnson MK, Adams MWW. Effect of serinate ligation at each of the iron sites of the [Fe4S4] cluster of Pyrococcus furiosus ferredoxin on the redox, spectroscopic, and biological properties. Biochemistry. 1999;38(32):10594–10605. doi: 10.1021/bi990671d. [DOI] [PubMed] [Google Scholar]
- 22.Babini E, et al. A serine to cysteine ligand mutation in the high potential iron-sulfur protein from Chromatium vinosum provides insight into the electronic structure of the [4Fe-4S] cluster. J Am Chem Soc. 1996;118(1):75–80. [Google Scholar]
- 23.Mansy SS, et al. Crystal structure and stability studies of C77S HiPIP: A serine ligated [4Fe-4S] cluster. Biochemistry. 2002;41(4):1195–1201. doi: 10.1021/bi011811y. [DOI] [PubMed] [Google Scholar]
- 24.Iwig DF, Booker SJ. Insight into the polar reactivity of the onium chalcogen analogues of S-adenosyl-l-methionine. Biochemistry. 2004;43(42):13496–13509. doi: 10.1021/bi048693+. [DOI] [PubMed] [Google Scholar]
- 25.Nesbitt NM, et al. Expression, purification, and physical characterization of Escherichia coli lipoyl(octanoyl)transferase. Protein Expr Purif. 2005;39(2):269–282. doi: 10.1016/j.pep.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Vonrhein C, Blanc E, Roversi P, Bricogne G. Automated structure solution with autoSHARP. Methods Mol Biol. 2007;364:215–230. doi: 10.1385/1-59745-266-1:215. [DOI] [PubMed] [Google Scholar]
- 27.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Kusumoto K, Hirano Y, Miki K. Detailed assessment of X-ray induced structural perturbation in a crystalline state protein. J Struct Biol. 2010;169(2):135–144. doi: 10.1016/j.jsb.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26(Pt 2):283–291. [Google Scholar]
- 31.Lovell SC, et al. Structure validation by Calpha geometry: φ, ψ And Cbeta deviation. Proteins. 2003;50(3):437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]