The article by Wälti et al. (1) in PNAS is a milestone in biomedicine. It describes in atomic detail fibrils that form the plaques in diseased brains, first observed by Alois Alzheimer 110 y ago. These extracellular fibrils are now known to be composed of amyloid-β, a peptide of various lengths between 38 and 43 residues, proteolytically cleaved from the cell-surface membrane protein called amyloid precursor protein (APP). Amyloid-β(1–42) is more cytotoxic than its cousin, shorter by two residues, amyloid-β(1–40), for which structures have been known for several years (2). Because of its role in the etiology of Alzheimer’s disease, the structure of amyloid-β(1–42) has been eagerly awaited, and now two independent studies simultaneously describe identical, disease-relevant fibrils: the report by Wälti et al. (1) is from the laboratories of Meier and Riek at Eidgenössiche Technische Hochschule Zurich and Böckmann at Lyon, and the other is from Griffin at the Massachusetts Institute of Technology and Linse at the University of Lund, Sweden (3).
These structures are determined largely from distance constraints between pairs of isotopically labeled atoms measured by solid-state NMR. Computer-aided energy minimization of the amyloid-β(1–42) peptide chains yields a “bundle” of atomic models, each obeying the distance constraints. That is, the final structure is a constrained set of closely related models, rather than a single structure as yielded by a high-resolution X-ray determination or a direct image produced by cryo-EM. Although it is not possible to be certain that there is no other model that also fits the same NMR constraints, alternative models become increasingly improbable as the number of distance constraints grows. Confidence in the amyloid-β(1–42) structure is reinforced by the essentially identical results of the two independent studies. For the Wälti et al. (1) structure, the rmsd of backbone atoms of the 10 lowest-energy atomic models within the bundle is 0.89 Å, and for the Colvin et al. (3) structure it is 0.71 Å.
Structure of Amyloid-β(1–42)
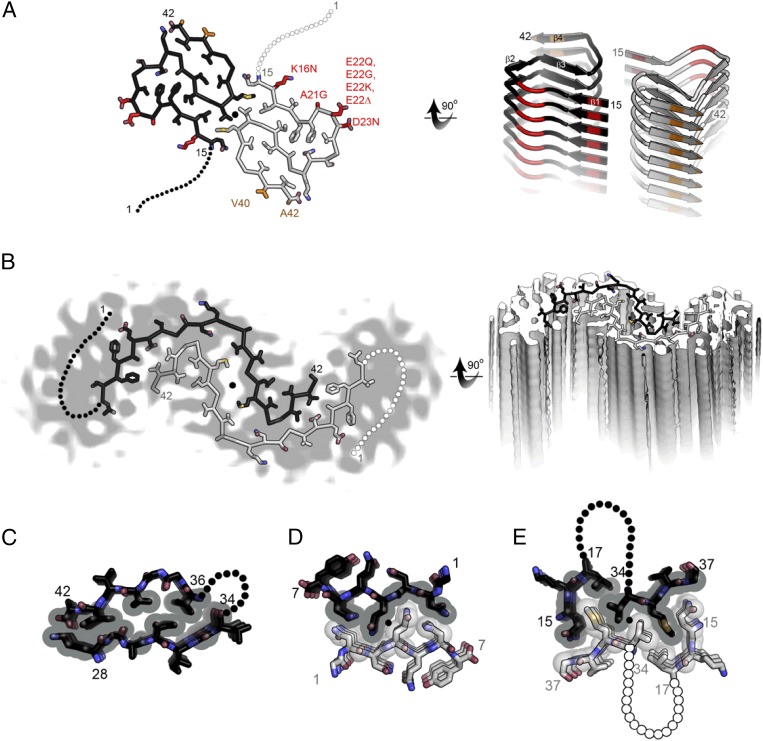
The structure (Fig. 1A) reveals two amyloid-β(1–42) chains, essentially flat, each shaped like a double horseshoe (similar to the letter S), with the two double horseshoes meeting around a twofold axis. The hydrophilic N terminus of residues 1–14 is poorly ordered, but residues 15–42 of each double horseshoe are arranged into four short β-strands that stack up and down the fibril into parallel, in-register β-sheets. That is, each stack of double horseshoes forms a thread, and the two threads twist around each other to form a two-stranded “protofilament.” Glycine residues permit sharp turns at the “corners” of the horseshoes. As in the amyloid-like protofilaments formed from short segments of amyloid proteins (4, 5), backbone hydrogen bonds between amide groups link the layers of the fibrils, and asparagine side-chains form ladders of hydrogen bonds up and down the fibrils. These lines of dipolar amide groups polarize each other, increasing stability (6). Another similarity is that water molecules are excluded from the core of the protofilament by the tight mating between pairs of the β-sheets, a structural motif known as a steric zipper. Each of the horseshoes is stabilized by hydrophobic interactions among apolar residues buried in the core and from the entropy increase arising from freeing water from the apolar residues. Charged residues are solvent-exposed. The twofold symmetric interaction between double horseshoes around the protofilament axis is a hydrophobic interface. So the stability of these amyloid protofilaments arises from both polarized hydrogen bonding and hydrophobic interactions.
Fig. 1.
Comparison of two molecular models for amyloid-β(1–42) fibrils. (A) The near atomic resolution model determined by solid-state NMR by Wälti et al. (1). Two double-horseshoe–shaped molecules of amyloid-β(1–42) are shown (black and gray) related by a twofold axis (marked by a circle), which runs down the center of the fibril. The N-terminal 14 residues are disordered; one possible conformation is shown here by dotted lines. Many of the known familial mutations are carried by residues located on the outer surface (red). The surface hydrophobic patch formed by residues V40 and A42 (orange) may explain the greater rate of secondary nucleation by the 1–42 species compared with 1–40 (3). (B). The lower resolution (5–7 Å) model determined by Schmidt et al. (7) by cryo-EM is another polymorph of amyloid-β(1–42), also with two molecules per layer, related by a twofold axis, and also with a poorly ordered N terminus. The gray color represents a slice through the cryo-EM map. The two molecules appear to be related by a homo-steric zipper type of bonding. In both A and B the models are viewed down the fibril axis on the left and nearly perpendicular to the fibril axis on the right. (C) A hetero-zipper in amyloid-β(1–42) fibrils from Wälti et al. (1). (D) A homo-zipper from GNNQQNY (4). (E) A noncontiguous homo-zipper from Wälti et al. (1). Dotted lines represent intervening residues.
The amyloid-β structures reveal two elements of fibril architecture not observed previously by crystallography. The first is that three of the sheet–sheet interfaces in amyloid-β are similar to the steric zippers of crystalline amyloid fibrils but are hetero-zippers, formed by two different β-sheets. Most of the crystalline steric zippers are homo-zippers, formed by two copies of the same sheet, usually around a twofold axis of symmetry. That is, the pairs of sheets mated by dry interfaces in amyloid-β protofilaments are composed of distinct sequences (e.g., sheets composed of amyloid-β residues 28-KGAIIGL-34 mate with sheets composed of residues 36-VGGVVIA-42) (Fig. 1C). Symmetry enhances steric zipper formation because contacts on one side of the symmetry axis are duplicated on the opposite side (Fig. 1D). The sheet–sheet hetero-zippers in amyloid-β exclude water, and exhibit the same quantitative measures of interaction as homo-steric zippers: They bury similar amounts of surface area (∼150–200 Å2 per pair of strands), and mate with high shape complementarity (>0.6).
The second new element of fibril architecture in the amyloid-β structure is interaction of noncontiguous segments of sequence about the protofilament axis. That is, the symmetrically related segments are not single strands, but instead are two separate sequence segments: the “corner” of a horseshoe (residues 34–37) and a noncontiguous segment (residues 15–17) (Fig. 1E). The area buried in this interface (224 Å2 per pair segment) is greater than any of the individual sheet–sheet interfaces within the protofilament and the shape complementarity is just somewhat lower (0.57), indicating this interface stabilizes fibril assembly. The discovery of this noncontiguous steric zipper adds a new dimension of complexity to the repertoire of structural features present in amyloid fibrils.
Polymorphs of Amyloid-β(1–42)
The nearly identical, newly determined structures of amyloid-β(1–42) appear distinctly different from a 2015 cryo-EM structure at 5–7 Å resolution of the same molecule (Fig. 1B) (7). In the cryo-EM structure, there are also two amyloid-β(1–42) molecules per layer of the protofilament, also related by a twofold axis. Around the twofold axis, the two molecules appear to be bound to each other by a homo-steric zipper interaction, in which side-chains from the two chains interdigitate, contributing to the stability. These distinctly different fibril structures illustrate the phenomenon of amyloid polymorphism. In amyloid polymorphs, molecules of identical sequence assemble as fibrils that differ at both the atomic and morphological level. NMR studies have detected polymorphic structures also of amyloid-β(1–40) (8, 9). It seems likely that amyloid polymorphism underlies the mysterious behavior of prion strains.
For both structure determinations (1, 3), a crucial constraint was obtained from measuring the mass per unit length of the fibrils. This number reveals how many amyloid-β molecules per layer there are along the fibril, and is determined by STEM (scanning tunneling electron microscopy). The STEM measurements on both fibrils were performed by Joseph Wall of Brookhaven National Laboratory in Upton, New York, who coauthors both papers. As far as we are aware, this facility is the only accessible one world-wide for such measurements and remains an indispensable resource to enable continuing studies of amyloid structure.
Both new papers (1, 3) note the fascinating implications of two independent studies of amyloid-β(1–42), taking different preparative paths, converging to the same fibril polymorph. Wälti et al. (1) used recombinantly expressed amyloid-β, and prepared homogeneous fibrils by repeated seeding of dissolved amyloid-β(1–42) with preformed fibrils. In contrast, Colvin et al. (3) used synthesized amyloid-β(1–42) and achieved uniform fibrils without seeding. Additionally, details of pH, peptide concentration, and salt concentration differed in their separate preparations of fibrils, yet the resulting polymorph is the same. Colvin et al. suggest that this means the resulting polymorph may be the thermodynamically most stable polymorph of amyloid-β(1–42). Wälti et al. (1) suggest that, although this polymorph is not a biologically evolved structure, it has undergone a primitive form of selection through three seeding operations. This process seems to result in a structure that is particularly “fit” for its pathologic function of spreading throughout the brain and contributing to damage. Indeed, spreading of amyloid-β fibrils throughout the brain is well established (10). Dilute brain extracts of amyloid-β from Alzheimer’s disease patients when infused intracerebrally into APP-transgenic mice stimulate formation and spreading of plaques in the brains of these animals. The two papers (1, 3) raise the question of whether it is this particular fibril structure that is responsible for spreading, and if so, what features of the fibril endow it with this deadly property.
Connection of the Structure to Disease
Although the new structure (1, 3) does not immediately suggest the toxic mechanism of amyloid-β(1–42), it does suggest avenues of approach to this question. Several mutations associated with early-onset forms of Alzheimer’s disease cluster around positions 22 and 23 in the amyloid-β sequence. These positions lie at a solvent-exposed bulge in the corner of one of the horseshoes (Fig. 1A). Because this loop is exposed, it appears that these various disease-enhancing mutations can be accommodated in the new structure. A next step may be to model the mutant forms, perhaps offering clues about how these mutations enhance disease. Colvin et al. (3) also note that the new information about the outer surfaces of the protofilament may shed light on the process of secondary nucleation, in which monomers and fibril surfaces cooperate in producing toxic species (11). Colvin et al. (3) suggest that the hydrophobic strip formed by residues V40 and A42 that runs down the outer surface of the protofilament could enhance secondary nucleation. Because this small cluster includes a residue, A42, not present in amyloid-β(1–40), it could explain the greater toxicity of amyloid-β(1–42).
There is evidence that the structure of amyloid-β(1–42) is relevant to Alzheimer’s disease. It has long been known that the 1–42 fragment of APP is the major amyloid-β species in the amyloid plaques of Alzheimer’s disease patients (12), and it aggregates more rapidly than the 1–40 fragment (13); the extensive hydrophobic cores in amyloid-β(1–42) may account for this. Wälti et al. (1) strengthen the connection of the new structure to Alzheimer’s disease by a dot blot analysis of their fibrils, using monoclonal antibodies prepared in the laboratory of Charles Glabe at the University of California, Irvine. Wälti et al. (1) found that their NMR fibrils are positive to fibril-specific antibodies, which bind intracellular deposits and senile plaques in brains of human Alzheimer’s disease patients, and are negative for fibril-specific antibodies that are not able to detect intracellular deposits and senile plaques in human Alzheimer’s patients.
Whereas the new structure may assist in the development of drugs against the accumulation of amyloid-β in the brain plaques, the role of amyloid-β in the development of Alzheimer’s disease remains a question of intense investigation. Accumulation of amyloid-β itself seems insufficient to drive cognitive decline: There is no correlation between the amount of amyloid-β plaques and cognition, and much amyloid-β has been found in the brains of elderly persons who are cognitively normal (14, 15). Recent experiments with mice indicate that development of pathology depends also on the conversion of the protein tau to amyloid form (16). The role of amyloid-β may be to facilitate the conversion of tau.
Footnotes
The authors declare no conflict of interest.
See companion article on page E4976.
References
- 1.Wälti MA, et al. Atomic resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc Natl Acad Sci USA. 2016;113:E4976–E4984. doi: 10.1073/pnas.1600749113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paravastu AK, Leapman RD, Yau W-M, Tycko R. Molecular structural basis for polymorphism in Alzheimer’s beta-amyloid fibrils. Proc Natl Acad Sci USA. 2008;105(47):18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colvin MT, et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J Am Chem Soc. July 14, 2016 doi: 10.1021/jacs.6b05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson R, et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawaya MR, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 6.Tsemekhman K, Goldschmidt L, Eisenberg D, Baker D. Cooperative hydrogen bonding in amyloid formation. Protein Soc. 2007;16(4):761–764. doi: 10.1110/ps.062609607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M, et al. Peptide dimer structure in an Aβ(1-42) fibril visualized with cryo-EM. Proc Natl Acad Sci USA. 2015;112(38):11858–11863. doi: 10.1073/pnas.1503455112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307(5707):262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 9.Petkova AT, Yau W-M, Tycko R. Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry (Mosc) 2006;45(2):498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70(4):532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SIA, et al. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci USA. 2013;110(24):9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roher AE, et al. Beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrett JT, Berger EP, Lansbury PT. The C-terminus of the beta protein is critical for seeding of amyloid formation. Biochemistry. 1993;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 14.Katzman R, et al. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 15.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 16.Li T, et al. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nat Commun. July 4, 2016 doi: 10.1038/ncomms12082. [DOI] [PMC free article] [PubMed] [Google Scholar]