Significance
Upon virus infection, host cells detect viral nucleic acids and initiate antiviral innate immune responses by producing type I IFNs and proinflammatory cytokines. As a cytoplasmic sensor for RNA viruses, retinoic acid-inducible gene 1 (RIG-I) recognizes the pathogenic RNAs and activates downstream signaling for initiation of antiviral innate immunity. In this study, we identify RING finger protein 122 (RNF122) as an E3 ligase that directly binds to RIG-I to induce K48-linked ubiquitination of RIG-I and mediate RIG-I degradation, consequently suppressing RIG-I–triggered antiviral innate immune response. RNF122 expression is upregulated by innate stimuli, and then acts as a feedback-negative regulator of RIG-I signaling to restrain type I IFN overproduction. Our study adds insight into the role of E3 ligases in the control of antiviral innate immunity.
Keywords: RIG-I, E3 ligase, RNF122, innate immunity, type I interferon
Abstract
The activation of retinoic acid-inducible gene 1 (RIG-I), a cytoplasmic innate sensor for viral RNA, is tightly regulated to maintain immune homeostasis properly and prevent excessive inflammatory reactions other than initiation of antiviral innate response to eliminate RNA virus effectively. Posttranslational modifications, particularly ubiquitination, are crucial for regulation of RIG-I activity. Increasing evidence suggests that E3 ligases play important roles in various cellular processes, including cell proliferation and antiviral innate signaling. Here we identify that E3 ubiquitin ligase RING finger protein 122 (RNF122) directly interacts with mouse RIG-I through MS screening of RIG-I–interacting proteins in RNA virus-infected cells. The transmembrane domain of RNF122 associates with the caspase activation and recruitment domains (CARDs) of RIG-I; this interaction effectively triggers RING finger domain of RNF122 to deliver the Lys-48–linked ubiquitin to the Lys115 and Lys146 residues of RIG-I CARDs and promotes RIG-I degradation, resulting in a marked inhibition of RIG-I downstream signaling. RNF122 is widely expressed in various immune cells, with preferential expression in macrophages. Deficiency of RNF122 selectively increases RIG-I–triggered production of type I IFNs and proinflammatory cytokines in macrophages. RNF122-deficient mice exhibit more resistance against lethal RNA virus infection, with increased production of type I IFNs. Thus, we demonstrate that RNF122 acts as a selective negative regulator of RIG-I–triggered antiviral innate response by targeting CARDs of RIG-I and mediating proteasomal degradation of RIG-I. Our study outlines a way for E3 ligase to regulate innate sensor RIG-I for the control of antiviral innate immunity.
Innate immunity is the first line of host defense against infection. Activation of innate immune response involves the detection of pathogen-associated molecular patterns (PAMPs) such as microbial nucleic acids, proteins, lipids, and carbohydrates. PAMPs are recognized by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), retinoic acid-inducible gene 1 (RIG-I)–like receptors, NOD-like receptors, and C-type lectin receptors (1, 2). The RIG-I–like receptor RIG-I is a cytoplasmic viral RNA sensor that triggers the signal to induce type I IFNs and proinflammatory cytokine production in response to viral infection. RIG-I protein contains tandem caspase-recruitment domains (CARDs) at N termini, which mediate downstream signaling; a central DExD/H helicase domain with an ATP-binding motif; and a C-terminal region known as the RNA-binding domain (3–7). Upon binding of pathogenic RNA to the helicase domain, RIG-I undergoes a conformational change and is recruited to the mitochondrial antiviral signaling (MAVS) adaptor (8). Downstream from MAVS, TBK1/IKKi, canonical IKKs, and transcription factors IRF3 and NF-κB are activated to stimulate the expression of type I IFNs and proinflammatory cytokines for host defense (9–11). Insufficient IFN production causes chronic infection, whereas excessive IFN leads to pathogenesis of autoimmune and/or inflammatory diseases (12, 13). Thus, precise control of RIG-I signaling is critical for efficient viral clearance without harmful immunopathology. How to timely terminate RIG-I signaling to prevent overproduction of type I IFNs and avoid inflammatory autoimmune disease needs to be investigated. The previously unknown negative regulators of RIG-I signaling remain to be further identified.
Posttranslational modifications, particularly ubiquitination, play critical roles in regulation of RIG-I activity. E3 ubiquitin ligases have been reported to be involved in the regulation of RIG-I signaling (14, 15). For example, E3 ubiquitin ligases TRIM25, Riplet, and MEX3C can mediate K63-linked polyubiquitination of human RIG-I (10, 16–19), thus positively regulating RIG-I–mediated signaling. On the contrary, RING finger protein (RNF) 125 (RNF125) or c-Casitas B lineage lymphoma (c-Cbl) catalyzes K48-linked ubiquitination and promotes proteasomal degradation of RIG-I, thus negatively regulating RIG-I-mediated signaling (20, 21). One question in this field is that whether there are any other previously unknown E3 ligases that can regulate RIG-I–triggered innate response. The critical residue for human RIG-I ubiquitination by these E3 ligases is found not to be the same conserved in mouse. So, which kind of E3 ligases contributing to ubiquitinate mouse RIG-I and regulating RIG-I–mediated antiviral response needs to be further clarified.
The RNF family is one of the major families of E3 ubiquitin ligase, which are involved in many biological processes, including cell cycle progression, DNA damage response, cell signaling, and maintenance of genome stability (22–25). Several RNF proteins have been recently implicated in the regulation of innate immunity (26, 27). To identify the proteins that can directly interact with RIG-I and negatively regulate RIG-I activity, we screened the RIG-I–interacting proteins by immunoprecipitation of endogenous RIG-I from the RNA virus-infected cells followed by multidimensional MS analysis. Among the candidates of RIG-I–interacting proteins, here we identified that the E3 ubiquitin ligase RNF122 could directly bind CARDs of mouse RIG-I and mediate K48-linked ubiquitination of RIG-I, consequently suppressing RIG-I–dependent antiviral responses by promoting proteasomal degradation of RIG-I.
Results
E3 Ubiquitin Ligase RNF122 Interacts with RIG-I.
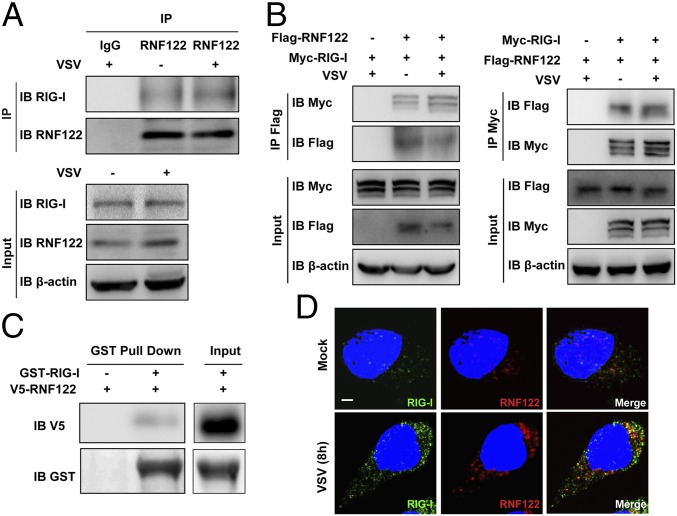
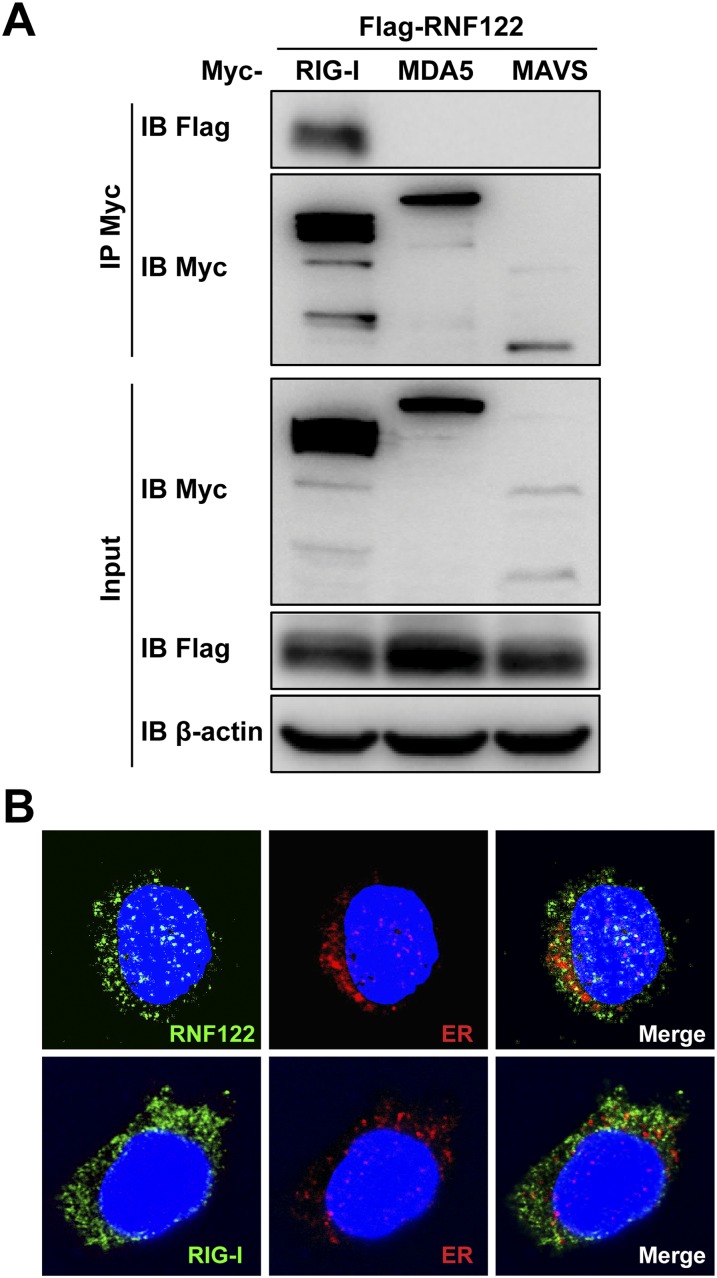
RIG-I–interacting proteins were identified by immunoprecipitation using anti–RIG-I antibody in L929 cells infected with VSV, followed by MS. Among the candidates of RIG-I–interacting proteins, E3 ubiquitin ligase RNF122 attracted our attention. First, we demonstrated that RNF122 could interact with RIG-I in L929 cells with or without VSV infection (Fig. 1A), and also confirmed the interaction of RNF122 with RIG-I in HEK293T cells transfected with Myc-tagged RIG-I and Flag-tagged RNF122 (Fig. 1B). Moreover, in vitro GST pull-down assay confirmed the direct interaction of RNF122 with RIG-I (Fig. 1C). Whereas RNF122 failed to interact with RIG-I downstream signaling molecule MAVS and another RNA sensor MDA5 (Fig. S1A). Immunofluorescence imaging showed the colocalization of RNF122 and RIG-I with or without VSV infection in the cytoplasm of mouse peritoneal macrophages (Fig. 1D), although a part of RNF122 protein is localized to the endoplasmic reticulum (ER) apparatus (Fig. S1B), as described in previous studies (28). The findings demonstrate that RNF122 directly binds to RIG-I, which inspired us to investigate the potential function of RNF122 in RIG-I–triggered antiviral innate response.
Fig. 1.
Interaction of RNF122 with RIG-I. (A) L929 cells were infected with VSV for 4 h. Cell lysates were immunoprecipitated by using anti-RNF122 or control IgG antibody, followed by Western blot analysis using the indicated antibodies. (B) The indicated plasmids were transfected into HEK293T cells with or without VSV infection for 4 h. Protein–protein interactions were detected by immunoprecipitation by using the anti-Myc or anti-Flag antibody, followed by Western blot analysis using the indicated antibodies. (C) HEK293T cells were transfected with V5-tagged RNF122 plasmid. After 24 h, cell lysates were incubated with purified GST–RIG-I proteins and subjected to GST pull-down assays. The proteins bound to glutathione–Sepharose beads were analyzed by Western blot with the indicated antibodies. (D) Peritoneal macrophages were infected with or without VSV for 8 h, and then the cells were immunostained with the indicated antibodies and analyzed by fluorescence microscopy. (Scale bar, 5 μm.) Image shown is representative of three independent experiments.
Fig. S1.
Interaction of RNF122 with RIG-I, MDA5, or MAVS. (A) The indicated plasmids were transfected into HEK293T cells. Protein–protein interactions were detected by immunoprecipitation using the anti-Myc antibody, followed by Western blot analysis using the indicated antibodies. (B) Peritoneal macrophages were infected with VSV for 8 h. Cells were then immunostained with anti-RNF122 (green) and the ER marker anticalreticulin (red; Upper), or anti–RIG-I (green) and the ER marker anticalreticulin (red; Lower) and analyzed by fluorescence microscopy. (Magnification, 400×.)
RNF122 Catalyses Its Own Degradation via Self-Ubiquitination.
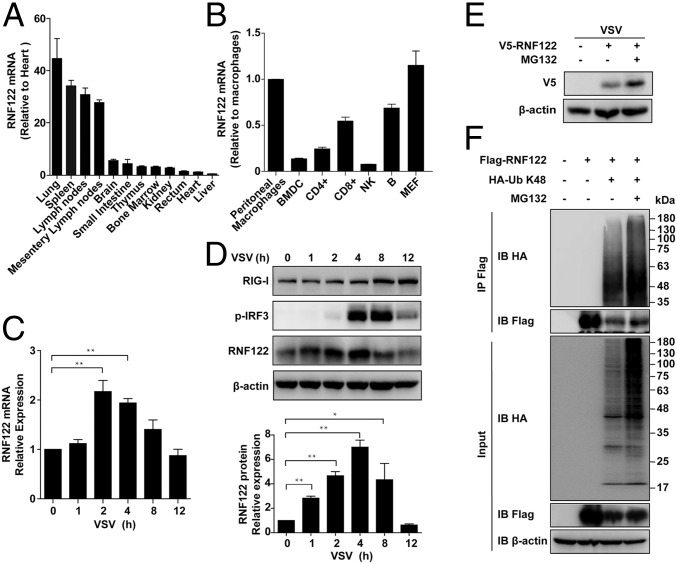
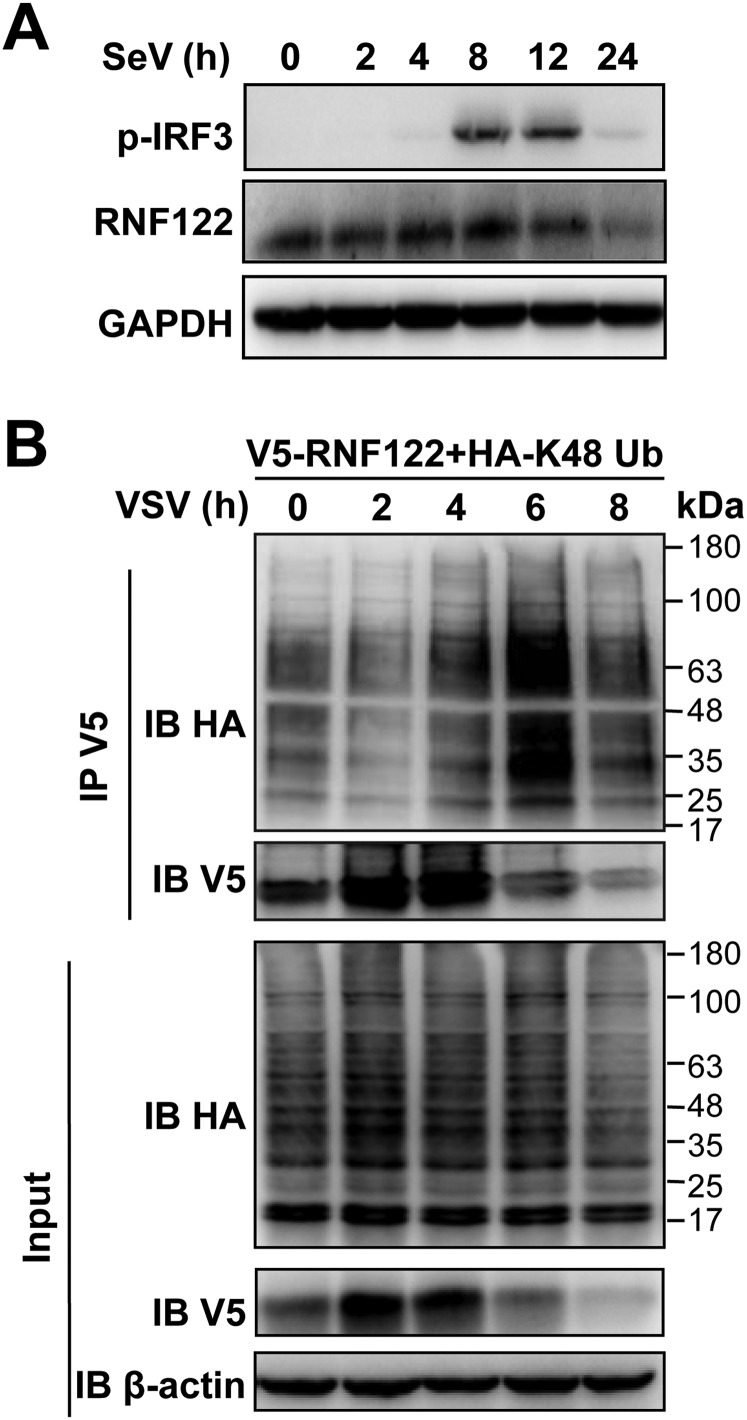
RNF122 contains a transmembrane (TM) domain in the N terminus and RING finger domain in the C terminus, suggesting its potential E3 ubiquitin ligase activity (28). Mouse RNF122 protein shares 97% homology with the human counterpart. RNF122 was widely expressed in mouse tissues, with particularly abundant in immune organs (spleen and lymph nodes; Fig. 2A). RNF122 was also widely expressed in various immune cells, with preferential expression in peritoneal macrophages (Fig. 2B), further indicating its involvement in the immune system. mRNA (Fig. 2C) and protein (Fig. 2D and Fig. S2A) levels of RNF122 were up-regulated within 4 h after RNA virus [VSV or Sendai virus (SeV)] infection. Moreover, the expression of RNF122 was significantly up-regulated in HEK293T cells transfected with RNF122 expression vector in the presence of proteasome inhibitor MG132 (Fig. 2E), consistent with previous observation that RNF122 catalyses its own degradation in a proteasome-dependent manner (28). Ubiquitination analysis demonstrated that RNF122 displayed self-ubiquitination activity, and this activity is increased dramatically by addition of MG132 (Fig. 2F). In addition, we found that K48-linked ubiquitination of RNF122 was significantly up-regulated within 4–8 h after VSV infection in HEK293T cells transfected with RNF122 expression vector. Correspondingly, the expression of RNF122 protein was decreased within 4–8 h after VSV infection (Fig. S2B). These data indicate that RNF122 undergoes self-ubiquitination and subsequent degradation by the proteasome.
Fig. 2.
Identification and characterization of RNF122. (A) RNA from various mouse tissues was extracted and subjected to real-time quantitative PCR (qPCR) analysis to examine the expression profile of RNF122 and GAPDH. Data are mean values ± SD (n = 3). (B) Distribution of RNF122 in various immune cells and MEF cells was determined by using qPCR analysis. Data are mean values ± SD from one experiment representative of three independent experiments. (C and D) Peritoneal macrophages were infected with VSV (multiplicity of infection, 1) for the indicated time. mRNA levels were then analyzed by qPCR (C), and protein levels were analyzed by Western blot (D, Upper). Relative RNF122 levels were determined by using ImageJ (D, Lower). Data are from three independent experiments. Error bars represent SEM (*P < 0.05 and **P < 0.01). (E) HEK293T cells were transfected with V5-tagged RNF122 with or without MG132 treatment for 6 h. Expression of RNF122 was analyzed by immunoblot with indicated antibodies. (F) HEK293T cells were transfected with Flag-RNF122 and HA-K48-Ub with or without MG132 treatment for 6 h. Ubiquitination of RNF122 was analyzed by immunoprecipitation with anti-Flag, followed by Western blot analysis using the indicated antibodies.
Fig. S2.
Expression of RNF122 is regulated by viral infection. (A) Murine peritoneal macrophages were infected with SeV (multiplicity of infection, 0.5) for the indicated time, and the protein level of RNF122 was determined with immunoblot analysis. (B) HEK293T cells were transfected with V5-RNF122 and HA-K48 Ub with VSV infection for the indicated time. Ubiquitination of RNF122 was analyzed by immunoprecipitation with anti-V5, followed by Western blot analysis using the indicated antibodies.
RNF122 Selectively Inhibits RIG-I–Triggered Antiviral Innate Immune Response Against RNA Virus.
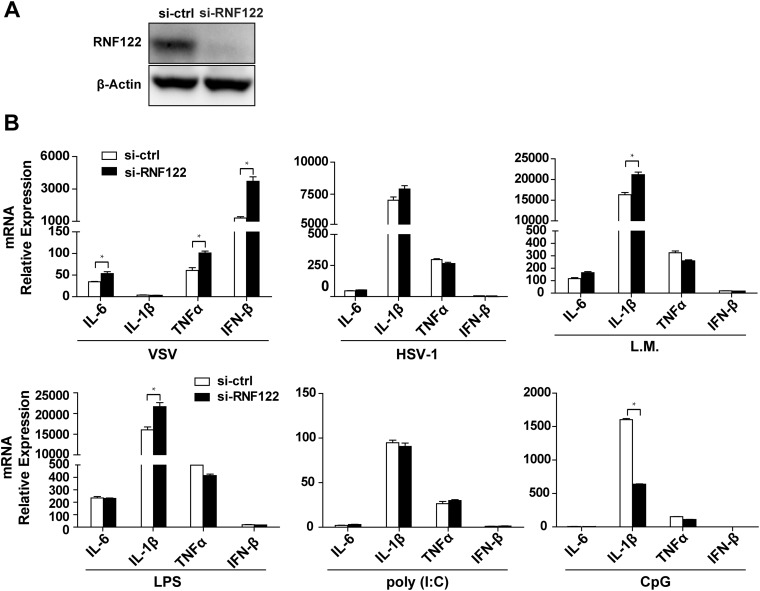
To explore the physiological role of RNF122, we efficiently silenced RNF122 expression in murine peritoneal macrophages (Fig. S3A) and found that knockdown of RNF122 significantly enhanced the expression of IFN-β and proinflammatory cytokines IL-6, IL-1β, and TNF-α in response to infection with RNA virus (i.e., VSV), but not to infection with DNA virus herpes simplex virus 1 (HSV-1), bacteria Listeria monocytogenes, or stimulation with various TLR ligands, such as LPS, poly(I:C), and CpG ODN (Fig. S3B). These data show that RNF122 selectively inhibits innate immune responses against RNA virus infection.
Fig. S3.
RNF122 selectively inhibits RNA virus induced IFN-β and proinflammatory cytokine expression in macrophages. (A) Peritoneal macrophages were transfected with indicated siRNA oligos for 48 h. The protein levels of RNF122 were determined with immunoblot analysis. (B) Peritoneal macrophages were transfected with indicated siRNA oligos for 48 h. Cells were then infected with VSV, HSV, or L. monocytogenes for 8 h or stimulated with LPS, poly(I:C), or CpG for 3 h. Total RNA was isolated and analyzed by using qPCR to determine expression levels of IL-6, IL-1β, TNF-α, and IFN-β. The data shown are the means ± SEM from three independent experiments.
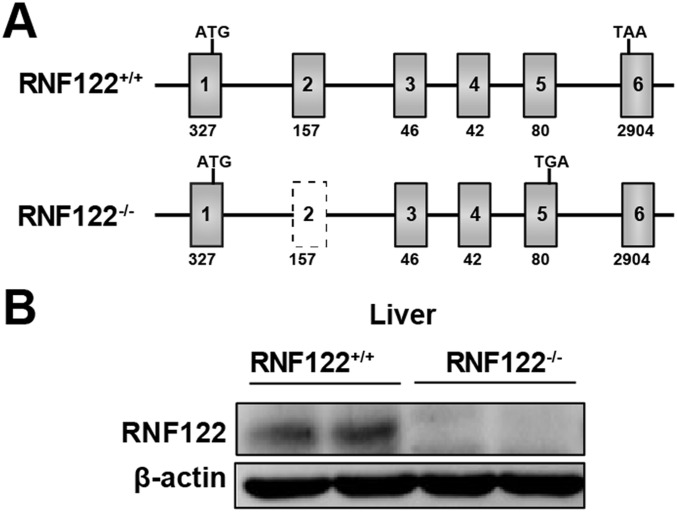
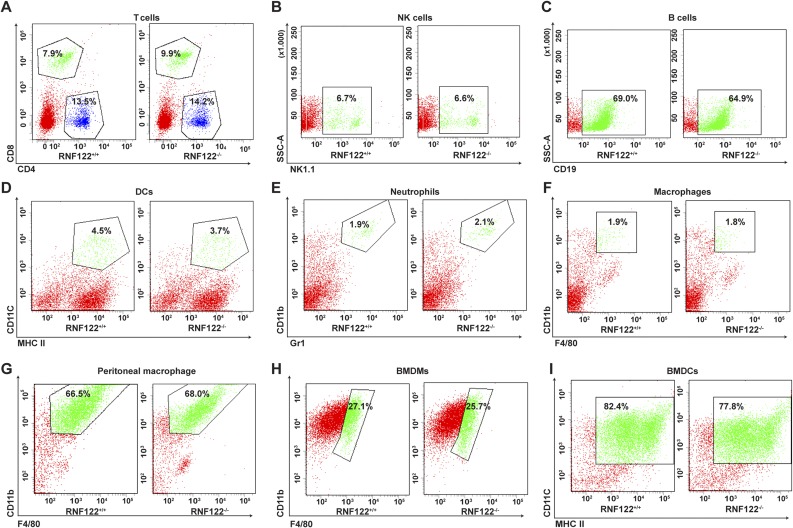
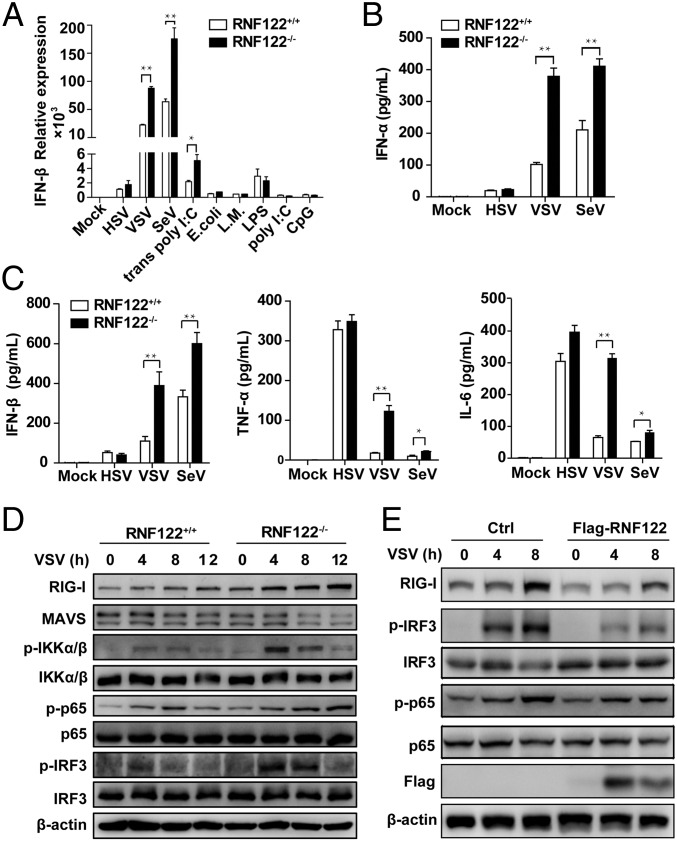
To further examine the significance of RNF122 in antiviral immune responses in vivo, we generated RNF122-deficient (RNF122−/−) mice, which lack exon 2 with the CRISPR/Cas9 system by introducing frame-shift mutated RNF122 mRNA (Fig. S4A). The expression of RNF122 was efficiently deleted in RNF122−/− mice (Fig. S4B). WT and RNF122−/− mice were viable, fertile, and normal in size, and they displayed no gross physical or behavioral abnormalities. Moreover, we observed the similar frequency of T cells (Fig. S5A), natural killer cells (Fig. S5B), B cells (Fig. S5C), dendritic cells (DCs) (Fig. S5D), neutrophils (Fig. S5E), and F4/80+CD11b+ macrophages (Fig. S5F) in the spleen of WT and RNF122−/− mice. WT and RNF122−/− mice had similar proportion of peritoneal macrophages in isolated peritoneal cavity cells (Fig. S5G). The generation of bone marrow-derived macrophages (BMDMs) and conventional bone marrow-derived DCs (BMDCs) had no significant difference in RNF122−/− mice compared with control mice (Fig. S5 H and I). Therefore, RNF122−/− mice have normal myeloid development and macrophage differentiation. Then, we detected the production of cytokines in RNF122−/− macrophages in response to the innate stimuli. Consistent with the phenotype of RNF122 knockdown, deficiency of RNF122 significantly increased mRNA expression of IFN-β in macrophages upon infection with RNA virus (VSV and SeV) and transfection of poly(I:C), but not other innate stimuli (Fig. 3A). Furthermore, RNF122−/− macrophages and mouse embryonic fibroblast (MEF) cells secreted much more type I IFNs (IFN-α and IFN-β), TNF-α, and IL-6 than WT cells in response to infection with VSV and SeV, but not DNA virus or another RNA virus, encyphalomyocarditis virus, which is recognized by MDA5 (Fig. 3 B and C and Fig. S6 A and B). However, RNF122 deficiency did not affect the RIG-I–triggered downstream signaling in conventional BMDCs compared with WT cells after infection with VSV (Fig. S6C), which may be a result of low expression of RNF122 in BMDCs (Fig. 2B). In addition, plasmacytoid BMDCs play important roles in innate antiviral defense, in which TLR7 is the major sensor of RNA viruses. The expression of IFN-β was not altered in RNF122−/− plasmacytoid BMDCs upon VSV or SeV infection (Fig. S6D), suggesting that RNF122 also does not participate in type I IFN production in plasmacytoid BMDCs. These data suggest that RNF122 selectively inhibits RIG-I–triggered antiviral innate response against RNA virus.
Fig. S4.
Efficient deletion of RNF122 in RNF122−/− mice. (A) Schematic illustration of the locations of KO sequence of RNF122 in RNF122−/− mice. Mouse genomic RNF122 contains six exons. The start codon and stop codon are indicated respectively. The targeting vector was designed to delete a 157-bp fragment of exon 2, which encodes TM domain, resulting in frame-shift mutation and early termination. (B) The protein level of RNF122 in mouse liver was determined with immunoblot analysis.
Fig. S5.
Normal myeloid development and macrophage differentiation in RNF122−/− mice. (A–F) The percentages of T cells, natural killer (NK) cells, B cells, DCs, neutrophils, and F4/80+CD11b+ macrophages in splenocytes from RNF122+/+ and RNF122−/− mice were analyzed by flow cytometry. (G–I) The similar proportion of peritoneal macrophages in isolated peritoneal cavity cells, and the similar generation of BMDMs and conventional BMDCs from RNF122+/+ and RNF122−/− mice, were observed by flow cytometry analysis. Data shown are representative of three independent experiments.
Fig. 3.
RNF122 selectively inhibits RNA virus infection-induced antiviral innate response in macrophages. (A) Peritoneal macrophages from RNF122+/+ and RNF122−/− mice were infected with HSV, VSV, SeV, E. coli, and L. monocytogenes (L.M.) for 8 h, or stimulated with LPS, poly(I:C), or CpG for 3 h or transfected with poly(I:C) for 8 h. Total RNAs were reversed-transcribed into cDNA and analyzed by qPCR. The results shown are means ± SD (n = 3). (B and C) Peritoneal macrophages from RNF122+/+ and RNF122−/− mice were infected with HSV, VSV, and SeV for 12 h. The concentrations of IFN-α, IFN-β, TNF-α, and IL-6 in the supernatants were measured with ELISA. Data are from three independent experiments. The results shown are means ± SEM (*P < 0.05 and **P < 0.01). (D) Peritoneal macrophages from RNF122+/+ and RNF122−/− mice were infected with VSV for the indicated time, and the cell lysates were subjected to immunoblot with indicated antibodies. (E) RAW264.7 cell line stably expressing Flag-tagged RNF122 was infected with VSV for 4 or 8 h. Cell lysates were analyzed by Western blot using the indicated antibodies.
Fig. S6.
RNF122 selectively inhibits RIG-I–triggered type I IFNs and proinflammatory cytokine expression. MEF cells (A), conventional BMDCs (C), and plasmacytoid DCs (D) from RNF122+/+ and RNF122−/− mice were infected with VSV or SeV for 8 h. (B) Peritoneal macrophages from RNF122+/+ and RNF122−/− mice were infected with encyphalomyocarditis virus (EMCV) for 16 h. Total RNA was isolated and analyzed using qPCR to determine expression levels of IFN-α, IFN-β, IL-6, TNF-α, and IL-1β. The concentrations of IFN-α, IFN-β, IL-6, and TNF-α in the culture supernatants were measured with ELISA. The data shown are the means ± SEM from three independent experiments.
We further investigated the effect of RNF122 on the innate signaling in cells upon RNA viral infection. As shown in Fig. 3D, deficiency of RNF122 significantly increased the phosphorylation of the transcription factors IRF3 and p65 in peritoneal macrophages in response to VSV infection. Correspondingly, overexpression of RNF122 in RAW264.7 cells significantly decreased the levels of phosphorylated IRF3 and p65 upon VSV infection (Fig. 3E). Collectively, these data demonstrate that RNF122 is a selective, negative regulator of the RIG-I signaling cascade and can significantly suppress the antiviral innate response against RNA viral infection.
Deficiency of RNF122 Protects Mice from RNA Virus Infection by Inducing More Type I IFN Production.
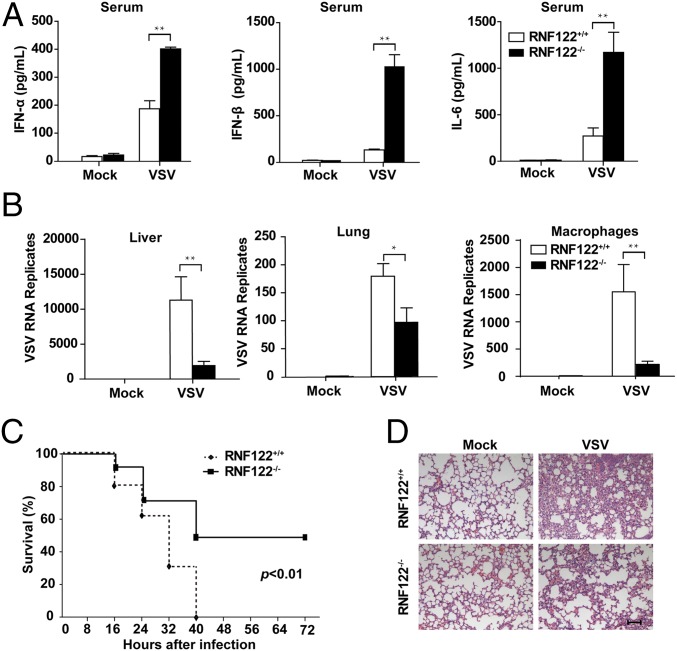
To investigate the function of RNF122 in host antiviral innate response, we challenged RNF122−/− mice with VSV via tail intravenous injection. The levels of type I IFNs (IFN-α and IFN-β) and proinflammatory cytokine IL-6 in sera of RNF122−/− mice were significantly higher than that in control littermates (Fig. 4A). Correspondingly, VSV replication in organs liver and lung and peritoneal macrophages from RNF122−/− mice was significantly reduced compared with WT mice (Fig. 4B). As a result, RNF122−/− mice exhibited longer survival (Fig. 4C) and less infiltration of inflammatory cells in the lungs (Fig. 4D) after lethal VSV infection than control groups. These data show that RNF122−/− mice are more resistant to RNA virus infection by producing more IFNs and inflammatory cytokines, further demonstrating that RNF122 can significantly suppress RIG-I–triggered antiviral innate response.
Fig. 4.
RNF122-deficient mice are more resistant to VSV infection. (A) RNF122+/+ and RNF122−/− mice were injected with VSV via the tail vein. After 20 h, IFN-α, IFN-β, and IL-6 in sera were measured with ELISA (n = 5 mice per genotype). (B) RNF122+/+ and RNF122−/− mice were challenged as in A. At 20 h after infection, VSV replication in the liver, spleen, or peritoneal macrophages were assessed with qPCR (n = 3 mice per genotype). The results shown are means ± SEM (*P < 0.05 and **P < 0.01). (C) RNF122+/+ and RNF122−/− mice (n = 10) were intravenously injected with VSV via tail vein and then monitored every 8 h after infection (P < 0.01). (D) RNF122−/− mice were challenged as in A for 20 h. The lungs were stained with H&E. Images shown are representative of individual mice. (Scale bar, 80 μm.)
TM Domain of RNF122 Interacts with CARDs of RIG-I.
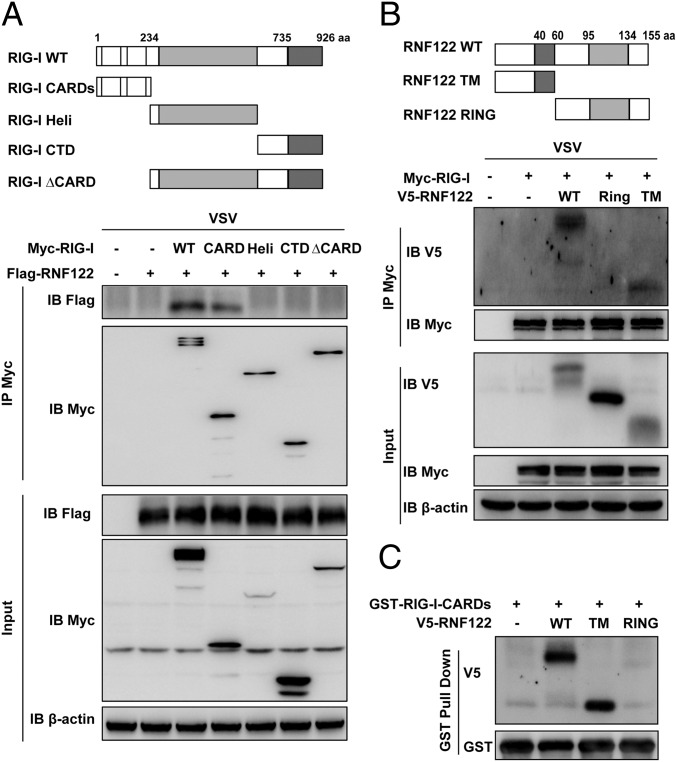
To determine the binding domains for the interaction between RIG-I and RNF122, we analyzed the interactions between Myc-tagged recombinant RIG-I and Flag/V5-tagged recombinant full-length RNF122 and truncation mutants of both. Schematic diagrams of RIG-I, RNF122, and their mutants used are shown in Fig. 5 A and B. Both recombinant full-length and CARDs of RIG-I bound RNF122 (Fig. 5A). In addition, both recombinant full-length and the N-terminal TM domain of RNF122 bound RIG-I (Fig. 5B). The direct interaction of purified GST-CARDs of RIG-I with full-length or TM domain of RNF122 was confirmed by in vitro GST pull-down assay (Fig. 5C). These data indicate that TM domain of RNF122 is responsible for the interaction with CARDs of RIG-I.
Fig. 5.
TM domain of RNF122 associates with the CARDs of RIG-I. (A and B) Schematic structure of RIG-I and RNF122 and the derivatives used in this work (Upper). HEK293T cells were transfected with the indicated plasmids and infected with VSV for 4 h. Cell lysates were immunoprecipitated by using anti-Myc antibody. The precipitates were analyzed by Western blot using indicated antibodies. (C) HEK293T cells were transfected with V5-RNF122 (WT), V5-RNF122-TM, or V5-RNF122-RING plasmids. After 24 h, cell lysates were incubated with purified GST-RIG-I CARDs proteins and subjected to GST pull-down assays. The proteins bound to glutathione–Sepharose beads were analyzed by Western blotting with the indicated antibodies.
RNF122 Induces K48-linked Ubiquitination of RIG-I CARDs.
To investigate whether RIG-I undergoes protein ubiquitination and degradation, we transfected HEK293T cells with vectors expressing for Myc-tagged RIG-I and increasing amounts of V5-tagged RNF122, followed by immunoblot analysis. We found that RNF122 could decrease RIG-I protein level in a dose-dependent manner (Fig. 6A), and RNF122-mediated decrease of RIG-I protein was blocked by the proteasome inhibitor MG132 (Fig. 6B), indicating that RNF122 caused RIG-I degradation via a proteasomal pathway. In contrast, the protein level of other RIG-I–associated proteins, including TRIM25, MEX3C, and Riplet, MAVS, and MDA5 were not affected by RNF122, indicating that RNF122 did not target these molecules for degradation and suppress RIG-I function (Fig. S7).
Fig. 6.
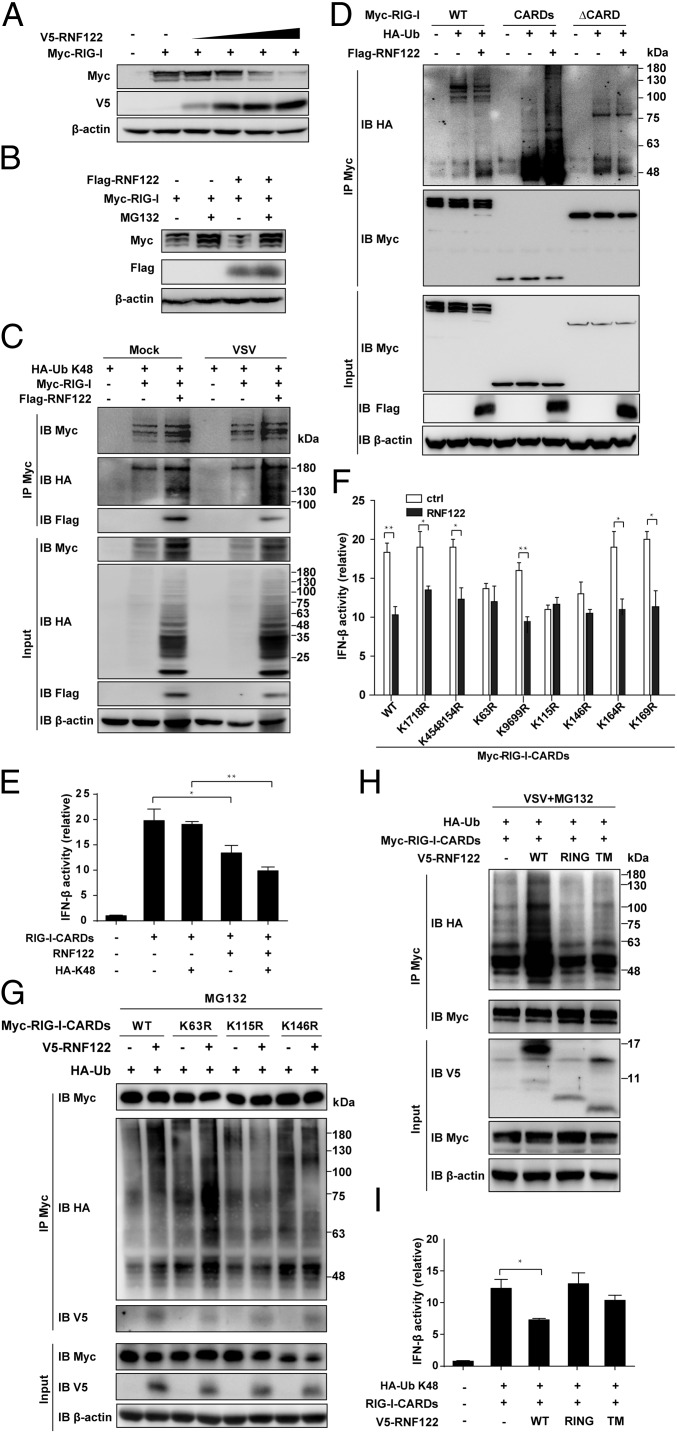
RNF122 promotes proteasomal degradation of RIG-I protein by mediating K48-linked ubiquitination via the residue K115 and K146 of RIG-I. (A) HEK293T cells were transfected with RIG-I plasmid, along with increasing amounts of plasmid expressing RNF122. At 24 h after transfection, the expression of Myc, V5, and β-actin were determined by Western blot. (B) HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 for 8 h. Cell lysates were immunoblotted with the indicated antibodies. (C, D, G, and H) HEK293T cells were transfected with the indicated plasmids. After 24 h, cells were treated with MG132 for 8 h and/or infected with VSV for 4 h. Cell lysates were immunoprecipitated using anti-Myc antibody, followed by Western blot analysis using the indicated antibodies. (E, F, and I) HEK293T cells were analyzed 24 h after transfected with the indicated plasmids together with an Ifnb promoter-driven reporter plasmid, and the luciferase activity was determined. Data are from three independent experiments (mean ± SEM; *P < 0.05 and **P < 0.01).
Fig. S7.
RNF122 does not suppress the expression of TRIM25, MEX3C, Riplet, MAVS, and MDA5. HEK293T cells were transfected with the plasmids of TRIM25 (A), MEX3C (B), Riplet (C), MAVS (D), and MDA5 (E), along with increasing amounts of plasmid expressing RNF122. At 24 h after transfection, the cells were infected with VSV for 4 h. Cell lysates were then immunoblotted by the indicated antibodies.
To investigate the molecular mechanisms of RNF122-mediated degradation of RIG-I, we additionally transfected vectors expressing HA-tagged K48-linked ubiquitin in HEK293T cells, and found that overexpression of RNF122 enhanced the ubiquitination of RIG-I with or without VSV infection (Fig. 6C). These data demonstrate that RNF122 mediates K48-linked ubiquitination of RIG-I.
To determine the ubiquitination domain of RIG-I by RNF122, we transfected vectors expressing HA-tagged K48-linked ubiquitin and Myc-tagged full-length (WT) RIG-I or truncated RIG-I that contained only CARDs or lacked the CARDs (ΔCARD) in HEK293T cells. Immunoprecipitation analysis of ubiquitin demonstrated that the ubiquitination of WT or CARDs of RIG-I, but not ΔCARD of RIG-I, was strongly enhanced by overexpression of RNF122 (Fig. 6D). By using luciferase reporter assay of the Ifnb promoter, we found that overexpression of RNF122 inhibited the Ifnb promoter activity in HEK293T cells expressing CARDs of RIG-I. Moreover, overexpression of K48-linked ubiquitin further enhanced the inhibitory effect of RNF122 on Ifnb promoter activity (Fig. 6E). These findings suggest that RNF122 induces ubiquitination of CARDs of RIG-I by K48-mediated linkage.
Lys115 and Lys146 of RIG-I Are the Sites for RNF122-Mediated Ubiquitination.
RIG-I contains 15 lysine residues in its CARDs domain. To determine the target residues of RIG-I CARDs domain that are ubiquitinated by RNF122, we mutated individual lysine residues in the CARDs to arginines. Overexpression of RNF122 inhibited the activity of Ifnb promoter in HEK293T cells expressing mutant RIG-I CARDs with the K17/18R, K45/48/154R, K96/99R, K164R, or K169R substitution, but not K63R, K115R, or K146R substitution (Fig. 6F). Immunoprecipitation with anti–Myc-RIG-I CARDs and immunoblot analysis of ubiquitin demonstrated that only mutations of the arginine at K115 or K146 completely blocked the ubiquitination of RIG-I by RNF122 via K48-mediated linkage (Fig. 6G). These data indicate that Lys115 and Lys146 are the important sites for the ubiquitination of RIG-I CARDs targeted by RNF122.
Finally, we determined which domain of RNF122 mediated the ubiquitination of RIG-I. Overexpression of full-length RNF122, but not RING finger domain or TM domain, led to more polyubiquitination of CARDs of RIG-I (Fig. 6H), and inhibited the activity of Ifnb promoter in HEK293T cells expressing RIG-I-CARDs (Fig. 6I). These data suggest that the degradation of RIG-I is dependent on full-length RNF122.
Discussion
Recognition of viral PAMPs through the actions of cellular PRRs triggers the antiviral innate responses to limit viral replication and active adaptive immunity (29). In particular, RIG-I is a key PRR that can detect virus-derived RNAs in the cytoplasm during infection with a variety of viruses, such as influenza virus and hepatitis C virus, which are closely related to human disease pathogenesis (30). Activation of RIG-I results in an antiviral response necessary to suppress virus spread. Limiting the overactivation of RIG-I signaling, however, is essential to prevent the host from the inappropriate response and inflammatory injury. Several regulatory mechanisms that suppress RIG-I activity have been described. For example, the E3 ubiquitin ligases RNF125 and c-Cbl catalyze the attachment of K48-linked polyubiquitin chains to RIG-I (20, 21), targeting it for proteasomal degradation and thereby inhibiting the antiviral immune response and inflammation. However, considering the mechanisms of the same substrate recognized by multiple E3 ligases are highly varied, it is necessary to further define the modification of RIG-I with ubiquitin proteins. Here, we screened RIG-I–related E3 ubiquitin ligases and identified RNF122 as a negative regulator in RIG-I–triggered type I IFN induction pathway.
RNF122 is widely expressed in various mouse organs and prominent in immune-related organs and cells. Of note, RNF122 is very unstable as a result of its self-ubiquitination and degradation in a proteasome-dependent manner. The biological role of E3 ligase self-ubiquitination has also been implicated in regulating the ligases’ activity and the recruitment of substrates with ubiquitin-binding properties, as shown for TRAF6 and NEDD4 (31–33). Also, binding to the substrate or other proteins can protect the ligase from self-destruction. RNF122, for example, is reported to be stabilized by the interaction with calcium-modulating cyclophilin ligand (28). Furthermore, E3 ligases may have external ligases that target them for degradation, but not autocatalytic destruction, as previously described for Ring1B targeted by E6-AP (34) and the Cbl ligases targeted by Nedd4 and Itch (35). Notably, RNF122 expression was up-regulated upon RNA virus infection despite being subjected to its own degradation, which implicates the important role of tightly regulated RNF122 in innate immune response to pathogen infection or inflammation. By searching the Gene Expression Omnibus databases, we found that RNF122 expression increased dramatically in macrophages in response to LPS stimulation (accession no. GSE9509). Moreover, RNF122 expression is up-regulated in Con A-induced fulminant hepatitis model (accession no. GSE17184). Thus, it is possible that RNF122 may participate in the pathological development of hepatitis. However, the identification of definite roles for RNF122 in these pathogenic conditions will require further experiments.
RNF122 is a member of the RNF family. RNFs are present in eukaryotic cells, and the majority of them act as E3 ubiquitin ligases. Emerging evidence has suggested that E3 ligases control the stability, trafficking, and activity of proteins that are involved in many aspects of cellular and physiological processes. We showed that RNF122 promoted RIG-I degradation and inhibited RIG-I–induced type I IFN overproduction by mediating K48-linked ubiquitination of mouse RIG-I Lys-115 and Lys146 residues, which are conserved in human. However, it remains unclear why the K63R affects the RNF122-mediated inhibition of RIG-I–dependent Ifnb promoter activation but undergoes relatively normal ubiquitination. It is possible that the mutation of lysine 63 influences the interaction of RIG-I with other downstream signaling molecules.
Thus, RNF122 may be implicated in human diseases ranging from autoimmune injury to inflammatory diseases. RNF122 may be a potential target to be activated for therapeutic approach to the control of inflammatory diseases. Besides RNF122, several other E3 ubiquitin ligases that target RIG-I for ubiquitination have been identified. Riplet has been shown to mediate the K63-linked polyubiquitination of the C-terminal region of RIG-I. In addition, TRIM25 and MEX3C have both been shown to mediate the K63-linked ubiquitination of RIG-I CARDs at lysine 172, 99, or 169, respectively (10, 16–19). Different E3 ubiquitin protein ligases mediate different ubiquitination sites of RIG-I, indicating that the coordinated regulation of these molecules is required for the RIG-I–mediated antiviral immune responses.
RNF122 as an anomalistic PA-TM-RING protein composes two conserved domains, the TM domain and the RING-finger domain, lacking the signal peptide sequence and PA domain (28). Interestingly, TM domain alone mediates the interaction of RNF122 with RIG-I CARDs, but its E3 ubiquitin ligase activity is noted to be dependent on the RING finger domain, which potentially explains the degradation of RIG-I dependence on full-length RNF122. However, how these two domains fit together to ubiquitinate substrate will require further investigation.
In conclusion, we identified RNF122 as a RIG-I–interacting protein that suppressed type I IFN production by promoting K48-linked ubiquitination and degradation of RIG-I. The discovery that RNF122 is a negative regulator in the sensing of RNA virus provides insight into the mechanism of regulation of cellular antiviral innate response and inflammation.
Materials and Methods
Animals.
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Chinese Academy of Medical Sciences (ILAS-GC-2015-002). RNF122−/− mice were generated as described in SI Materials and Methods.
RNAi.
siRNAs against mouse RNF122 or negative control were from Invitrogen. Cells were transfected with siRNA by using RNAiMAX reagent (Invitrogen) via a transfection procedure according to the manufacturer’s instructions. siRNA of RNF122 sequences are CCAACAAGUCCUGCUCAAUTT and AUUGAGCAGGACUUGUUGGTT.
GST Pull-Down Assay.
GST and GST-RIG-I WT and GST-RIG-I CARDs were expressed in BL21DE Escherichia coli cells and purified by using previously described methods (36). The GST pull-down assay was performed as described previously (37).
Flow Cytometry.
Cells were collected and stained with monoclonal antibodies as described previously (38). Data were obtained on a BD FACSAria II system and analyzed with FACSDiva software (BD Biosciences).
Statistical Analysis.
A two-tailed Student t test was used to analyze statistical significance between two-group comparisons. The statistical significance of survival curves were compared with the generalized Wilcoxon test. The value P < 0.05 was considered statistically significant. Additional methods are described in SI Materials and Methods.
SI Materials and Methods
Generation and Identification of RNF122−/− Mice.
C57BL/6J mice were obtained from the Institute of Zoological Sciences at the Chinese Academy of Medical Sciences (Beijing, China). By using CRISPR/Cas9-mediated genome editing, we established RNF122-deficient (RNF122−/−) mice on a C57BL/6J background. In brief, a mixture of plasmids encoding mCas9 and sgRNAs containing RNF122 A and RNF122 B were injected into fertilized eggs. The transgenic fertilized eggs were planted into pseudopregnant mice. The oligonucleotides for sgRNAs are as follows: A, GGCACTACCTACAAGCTACCTGG; B, GGCATAAAGGATATTGAGCAAGG. Mouse pups genomic DNA was extracted from the tails of 7-d-old mice using phenol-chloroform and recovered by alcohol precipitation to detect the mutations. RNF122 was detected with a primer set as 5′-CAATGGCAGGGACCTTGAAG-3′ and 5′-GAGAACTATCAGCACCAACAGAACG-3′. All mice were bred in specific pathogen-free conditions. Six- to 8-wk-old littermate mice were used in the experiment. Major procedures were blinded. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Chinese Academy of Medical Sciences (ILAS-GC-2015-002).
Cells, Viruses, and Reagents.
The RAW264.7, L929, and HEK293T cell lines (American Type Culture Collection) were maintained in DMEM (HyClone) supplemented with 10% (vol/vol) FCS (Invitrogen). Peritoneal macrophages were prepared as described previously (21). For stable transfection, RAW264.7 macrophages were transfected with FuGENE HD reagents (Promega), and then selected with 1,000 ng/mL G418 (Invitrogen) and pooled for further experiments. BMDMs, BMDCs, and Flt3 ligand-induced plasmacytoid DCs were generated as previously described (16, 38). MEF cells were isolated from WT or RNF122−/− embryos (day 13.5). LPS, CpG ODN, and poly (I:C) were from Sigma-Aldrich. MG132 was from Calbiochem. SeV, HSV-1 virus, Listeria monocytogenes, and Escherichia coli 0111:B4 were obtained and cultivated as described previously (21).
Plasmids and Antibodies.
The Myc-tagged full-length RIG-I and RIG-I mutation plasmids, as well as HA-tagged ubiquitin expression plasmids, were constructed as described previously (21). Recombinant vectors encoding WT or mutant RNF122 (National Center for Biotechnology Information reference NM_175136.2) were constructed by PCR-based amplification of cDNA from mouse peritoneal macrophages and then subcloned into the pcDNA3.1-Flag and pcDNA3.1-v5 eukaryotic expression vectors, respectively. Antibody to RNF122 (sc-98113) was obtained from Santa Cruz. Antibody to RIG-I (635202) was obtained from BioLegend. Antibody to calreticulin (ab22683) was obtained from Abcam. Antibodies to RIG-I (D14G6), MAVS (4983), IRF3 phosphorylated at Ser396 (4D4G), IRF3 (D83B9), p65 phosphorylated at Ser536 (3031), p65 (8242), HA-Tag (6E2), Myc-Tag (71D10; HRP conjugate), Flag (2368), anti-GAPDH (3H12), and anti–β-actin (6D1) were used as previously described (21).
Quantitative RT-PCR.
For quantitative PCR, total RNA was extracted by using an RNeasy mini kit (Qiagen) according to the instructions of the manufacturer. RNA (1 µg) was reverse-transcribed using reverse transcriptase (Takara). A Light Cycler (Roche) and SYBR RT-PCR kit (Takara) were used for quantitative real-time RT-PCR analysis. Data were normalized to GAPDH expression. The sequences of the primers for RNF122 are 5′-CATGGTCATCTTCGGCACAG-3′ (forward) and 5′-CATGGAAGCACGCCTAGTTC-3′ (reverse). The sequences of the primers for GAPDH, IL-6, TNF-α, IL-1β, IFN-α, and IFN-β were used as described previously (21).
Immunofluorescence Assay and Confocal Microscopy.
Macrophages plated on glass coverslips in 24-well plates were infected with or without VSV for the indicated time. Then, cells were operated and labeled with rabbit anti-RNF122 antibody (Santa Cruz), rat anti–RIG-I antibody (BioLegend), and/or mouse anticalreticulin antibody (Abcam) before the addition of Alexa Fluor 488-conjugated and Alexa Fluor 594-conjegated secondary Ab (Life Technology). The coverslips were finally examined under a Zeiss LSM 510 confocal microscope (Carl Zeiss).
ELISA.
The concentration of TNF-α, IL-6, IFN-α, and IFN-β in the supernatants or serum was measured by ELISA kits (R&D Systems; PBL Interferon Source) according to the manufacturer’s instructions.
Immunoprecipitation and Immunoblot Analysis.
Cells were lysed in lysis buffer (Pierce) supplemented with protease inhibitors mixture (Merck). Immunoprecipitation experiments were performed as described previously (39). The supernatant samples or cell extracts were subjected to SDS/PAGE. Immunoblotting was performed as described previously (21).
Assays of Dual-Luciferase Reporter Gene Expression.
For luciferase assays, HEK293T cells were cotransfected with appropriate amounts of luciferase reporter plasmid, Renilla luciferase reporter vector, Myc-RIG-I-CARDs expressing plasmid, HA-Ub-K48, and V5-RNF122 expressing plasmid. At 24 h after transfection, cells were infected with VSV for 4 h, and then luciferase activity was measured by using the dual-luciferase reporter assay kit (Promega) according to the manufacturer’s instructions in a Lumat LB 9507 luminometer (Berthold).
Acknowledgments
This work was supported by National Key Basic Research Program of China Grants 2013CB530503 and 2013CB944903; National Natural Science Foundation of China Grants 31390431, 81550024, and 81230074; and National Mega Project of China Grants 2016ZX10002024 and 2013ZX10002002-002-002.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604277113/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13(9):817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang F, et al. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479(7373):423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowalinski E, et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147(2):423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, et al. Structural insights into RNA recognition by RIG-I. Cell. 2011;147(2):409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang QX, Chen ZJ. Structural insights into the activation of RIG-I, a nanosensor for viral RNAs. EMBO Rep. 2011;13(1):7–8. doi: 10.1038/embor.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peisley A, Wu B, Xu H, Chen ZJ, Hur S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature. 2014;509(7498):110–114. doi: 10.1038/nature13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Li X, et al. Methyltransferase Dnmt3a upregulates HDAC9 to deacetylate the kinase TBK1 for activation of antiviral innate immunity. Nat Immunol. 2016;17(7):806–815. doi: 10.1038/ni.3464. [DOI] [PubMed] [Google Scholar]
- 10.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 11.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16(1):35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 13.McGlasson S, Jury A, Jackson A, Hunt D. Type I interferon dysregulation and neurological disease. Nat Rev Neurol. 2015;11(9):515–523. doi: 10.1038/nrneurol.2015.143. [DOI] [PubMed] [Google Scholar]
- 14.Medvedev AE, Murphy M, Zhou H, Li X. E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses. Immunol Rev. 2015;266(1):109–122. doi: 10.1111/imr.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol Med. 2011;3(9):513–527. doi: 10.1002/emmm.201100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuniyoshi K, et al. Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity. Proc Natl Acad Sci USA. 2014;111(15):5646–5651. doi: 10.1073/pnas.1401674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284(2):807–817. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- 18.Oshiumi H, et al. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8(6):496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, et al. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36(6):959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arimoto K, et al. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci USA. 2007;104(18):7500–7505. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152(3):467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Lipkowitz S, Weissman AM. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer. 2011;11(9):629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattiroli F, et al. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150(6):1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Koo BK, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 25.Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci USA. 2015;112(24):7548–7550. doi: 10.1073/pnas.1508113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong B, et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity. 2009;30(3):397–407. doi: 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, et al. The E3 ubiquitin ligase Nrdp1 ‘preferentially’ promotes TLR-mediated production of type I interferon. Nat Immunol. 2009;10(7):744–752. doi: 10.1038/ni.1742. [DOI] [PubMed] [Google Scholar]
- 28.Peng Z, Shi T, Ma D. RNF122: A novel ubiquitin ligase associated with calcium-modulating cyclophilin ligand. BMC Cell Biol. 2010;11:41. doi: 10.1186/1471-2121-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehwinkel J, Reis e Sousa C. RIGorous detection: Exposing virus through RNA sensing. Science. 2010;327(5963):284–286. doi: 10.1126/science.1185068. [DOI] [PubMed] [Google Scholar]
- 31.Sorrentino A, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10(10):1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 32.Xia ZP, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461(7260):114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woelk T, et al. Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol. 2006;8(11):1246–1254. doi: 10.1038/ncb1484. [DOI] [PubMed] [Google Scholar]
- 34.Zaaroor-Regev D, et al. Regulation of the polycomb protein Ring1B by self-ubiquitination or by E6-AP may have implications to the pathogenesis of Angelman syndrome. Proc Natl Acad Sci USA. 2010;107(15):6788–6793. doi: 10.1073/pnas.1003108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnifico A, et al. WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. J Biol Chem. 2003;278(44):43169–43177. doi: 10.1074/jbc.M308009200. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, et al. E3 ubiquitin ligase CHIP facilitates Toll-like receptor signaling by recruiting and polyubiquitinating Src and atypical PKCzeta. J Exp Med. 2011;208(10):2099–2112. doi: 10.1084/jem.20102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, et al. K33-linked polyubiquitination of Zap70 by Nrdp1 controls CD8(+) T cell activation. Nat Immunol. 2015;16(12):1253–1262. doi: 10.1038/ni.3258. [DOI] [PubMed] [Google Scholar]
- 38.Xia M, et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity. 2013;39(3):470–481. doi: 10.1016/j.immuni.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, et al. Rhbdd3 controls autoimmunity by suppressing the production of IL-6 by dendritic cells via K27-linked ubiquitination of the regulator NEMO. Nat Immunol. 2014;15(7):612–622. doi: 10.1038/ni.2898. [DOI] [PubMed] [Google Scholar]