Protein-based biologics have revolutionized the treatment of many diseases, but low efficacy, occasional undesirable side effects, and rapid clearance from circulation limit their full potential. The majority of pharmaceutically relevant proteins are N-linked glycosylated, and their sugar moieties have significant impact on their folding, assembly, solubility, serum and shelf half-life, and functionality (1). Thus, one approach to enhance the potency, safety, and stability of therapeutic proteins is glycoengineering, altering protein-associated carbohydrate to achieve the desirable protein properties. The challenge is to develop biological systems that can consistently produce glycoproteins with homogeneous glycans on demand. The availability of such systems will lead to breakthroughs in two fronts: (i) elucidating the contribution of sugar moieties for various biological functions and (ii) developing novel biologics with tailor-made glycosylation based on their functional needs. In PNAS, Kallolimath et al. (2) describe their efforts in bringing one such host system to reality.
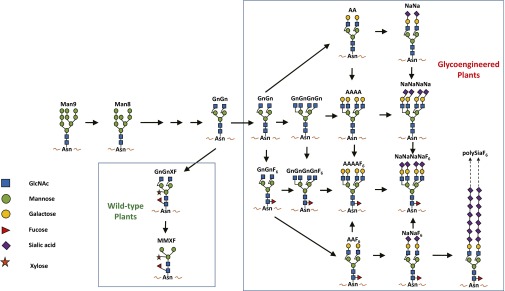
Unlike the protein backbone that is synthesized based on a defined template, sugar chains are assembled enzymatically. As a result, these chains are diverse in both the number and the linkage patterns of the sugar units. In addition, different host cells may modify the same protein with different sugar structures. Protein N-linked glycosylation involves the addition of an oligosaccharide (Man9) to an asparagine residue on a nascent polypeptide. In eukaryotes, the transfer and initial processing of Man9 start in the endoplasmic reticulum to form Man8, which is further processed in Golgi compartments to form complex N-glycans (3). The early steps of N-glycan processing up to the formation of the intermediate of GnGn are well preserved among most eukaryotic cells (Fig. 1). However, processing beyond this point differs significantly, leading to the formation of different complex N-glycoforms (3). In mammalian cells, there is a large diverse population of glycoenzymes for extensive elongation of the GnGn substrate, giving rise to numerous different N-glycans (3). The majority of pharmaceutical companies currently use Chinese hamster ovary (CHO) cells to produce human biologics. CHO cells generally produce N-glycans with structures similar to their human counterparts (4). Because of its large glycome however, CHO cell-derived glycoproteins exhibit substantial glycan heterogeneity, precluding the ability to generate distinct glycoforms that could be used in comparative studies of specific biological effects (4). Furthermore, N-glycan processing in CHO cells is prone to environmental variation and is difficult to control during bioprocessing (4). CHO cells also add sialic acid in a different linkage and do not produce bisecting GlcNAc structures that are common in human glycoproteins (1). This has led to CHO cell glycoengineering efforts to control their glycosylation capacity. However, the overall success of glycoengineering in CHO cells has been relatively modest, especially in producing homogeneous defined N-glycoforms (4). This and the lack of success by chemical synthesis have encouraged the development of alternative systems that can produce distinct human glycoforms on demand.
Fig. 1.
Major N-glycan structures produced in wild-type and glycoengineered plants.
Plants have been explored as a platform for producing biologics with the expectation that they will offer high production speed and scalability and improved safety (5). The most exciting aspect of plant-based systems for biologic development is their amenability for glycoengineering. In contrast to mammals, plant cells have a drastically reduced repertoire of Golgi-located glycoenzymes, and give rise to only two dominant glycan structures, GnGnXF and MMXF (Fig. 1). As a result, unlike CHO cell-derived proteins that carry a mixture of several N-glycans, plant proteins usually bear a single dominant N-glycan structure. GnGnXF and MMXF contain core α1,3-fucose and xylose, which are not present in human glycoproteins (1). Concerns were raised that biologic proteins produced in plants might trigger immune responses leading to production of plant-glycan–specific antibodies that could cause adverse effects. Paradoxically, the limited repertoire of glycoenzymes for N-glycosylation has turned out to be an advantage for plants as a host for generating proteins with homogeneous glycans, in contrast to the large glycome and the resulting glycan heterogeneity that impedes the targeted manipulation of the N-glycosylation pathway in mammalian cells (4). Plants also exhibit a remarkable tolerance toward various glycan manipulations and in response display no major phenotypic changes in growth or development (1). The general approach for plant glycoengineering is to first eliminate nonhuman sugars, and subsequently build human glycoforms by introducing mammalian enzymes. Consequently, a Nicotiana benthamiana line called ΔXF that does not produce plant-specific N-glycans was created by suppressing the expression of two plant glycoenzymes (1). The successful development of ΔXF plants not only eliminates the concern for the immunogenicity of plant-produced glycoproteins, but also demonstrates the plasticity of plants in tolerating the manipulation of their native glycosylation pathway. Moreover, various monoclonal antibodies (mAbs) produced in ΔXF plants have been shown to carry a homogenous (>90%) GnGn N-glycan structure and have significantly enhanced neutralization or antibody-dependent cell-mediated cytotoxicity potency (6, 7). These enhancements were highlighted by ZMapp, a mixture of three anti-Ebola mAbs produced in ΔXF plants. These mAbs have a superior potency to their CHO cell-produced counterparts and were able to rescue 100% of rhesus macaques even when given 5 d after a lethal Ebola challenge (8), leading to ZMapp’s compassionate use in human patients during the 2014 Ebola outbreak.
Subsequently, a series of successes were achieved in plants in producing various defined human N-glycan structures, including α1,6 fucosylated, bisected, and tetra-antennary and bigalactosylated complex N-glycoforms (1). Glycoengineered plants can now produce mAbs with identical N-glycans, differing only in core α1,6-fucose, and overcome CHO cells’ inability to synthesize multiantennary N-glycans. These studies also revealed that fine-tuning the suborganelle localization of the introduced glycoenzymes is crucial for producing the target human glycoforms, as random introduction of mammalian enzymes would interfere with the endogenous glycosylation pathway and produce incomplete or unusual hybrid N-glycans (1). This knowledge has led to the success of producing biantennary sialylated N-glycans by the simultaneous expression and precise targeting of six mammalian glycoenzymes to various subcellular compartments using a transient expression system (9).
To extend this biosynthetic capacity, a research goal has been to produce terminal polysialic acid (polySia) glycoproteins in plants. Forming oligomers is a unique property of sialic acid; polymeric structures can have up to 400 monomeric units (10). PolySia has been shown to play multiple roles in various biological processes, such as cell regeneration and various immunological processes (10). In humans, polySia is found on neural cell adhesion molecules (NCAM), where it plays a vital role in brain development (10). Clinical studies with polysialylated insulin and erythropoietin have shown that polySia is a superb alternative to polyethyleneglycol (PEG) in endowing protein drugs with longer circulatory half-lives and reduced immunogenicity (11). Furthermore, chemically polysialylated antitumor mAb fragments (scFvs) have shown significant increase in bioavailability and tumor uptake, suggesting scFvs with polySia are a viable alternative to whole IgGs (12). The highly hydrophilic nature of polySia renders a hydration pattern similar to PEG, thus creating a protective microenvironment to increase the active life of biologics and prevent them from being recognized by the immune system. Unlike PEG, polySia is a natural sugar that can be metabolized by sialidases, effectively resolving the issue of toxicity associated with PEGylation (10). Unfortunately, the currently polySia conjugation is a process that requires multiple fermentations, in vitro chemical reactions, and product purifications, making the process technically challenging and expensive (10). Developing a host system able to produce N-glycans with polySia would be a more desirable alternative, though challenging.
In PNAS, Kallolimath et al. (2) tackle this challenge and develop a plant-based system that can produce polysialylated N-glycans in a controlled manner. The authors first demonstrate the generation of stable transgenic plants that can produce defined sialylated N-glycan structures by cotransforming six mammalian glycoenzymes into ΔXF plants. A key variation from previous attempts was to use a mutated enzyme with abolished feedback inhibition for the biosynthesis of CMP-sialic acid, allowing a higher level accumulation of this nucleotide sugar precursor. The resultant homozygous plant line, called ΔXFSia, was then used to examine the feasibility of plants for producing polySia structures. Kallolimath et al. (2) show that two human polysialyltransferases (polySTs) are functionally active when transiently expressed in ΔXTSia, resulting in the specific polysialylation of the recombinant polySTs. The authors thoughtfully chose several recombinant proteins in validating the capacity of their newly developed sialylation and polySia host platform. First, the sialylation status of three human glycoproteins that are naturally sialylated at various degrees was examined. Results indicated that all three ΔXFSia plant-produced glycoproteins were efficiently sialylated. Furthermore, Kallolimath et al. (2) demonstrate that both α2,6- and α2,3-linked sialylation can be flexibly accomplished in ΔXFSia plants simply by choosing between α2,6- and α2,3-sialyltransferase. This ability of synthesizing terminal sialic acid with specific linkage on demand is advantageous because CHO cells can at best generate a mixture of both glycoforms. The development of the stable ΔXFSia line also addresses the issue of sialylation variation between production batches in previously developed transient expression systems (9). For polysialylation, Kallolimath et al. (2) examined the Ig5FN1 module of NCAM, the most important and well-studied polysialylated molecule in humans. Their results demonstrate that IgGFN1 is modified with polySia chains of over 40 monomers when coexpressed with polySTs. This is a remarkable achievement, considering that glycoproteins with polySia are among the most complex glycosylated structures found in humans. The authors also observed that the growth and development of the host plants and their robustness in producing recombinant protein biologics are not significantly impacted by this new step of glycoengineering, demonstrating the commercial potential of this platform. Finally, Kallolimath et al. (2) confirmed the functional activities of plant-produced polySia in two cell-based assays that are well characterized for polySia from NCAM (10).
The polySia platform developed by Kallolimath et al. (2) will undoubtedly facilitate both the functional understanding of polySia and the development of polySia-dependent biologics. For example, it is now conceivable to study the impact of sialylation on mAb’s anti-inflammatory property, stability, FcγR binding, effector function, and even pathological consequences, such as antibody-dependent enhancement of infection, which has been suggested for many viral diseases, including Zika infection (13, 14). Even though sialylation has also been achieved in glycoengineered yeast (15), the plant-based system developed here (2) is unrivaled in its control, speed, and flexibility compared with other glycoengineered systems. Combined with the previous glycoengineering efforts of Loos et al. (1), this study (2) provides a full portfolio of glycoengineered plant lines that in theory will allow the production of glycoproteins with various tailor-designed mammalian N-glycans on demand. This ability will have profound impact in several areas, including basic glycobiology research and novel biologic development to improve efficacy, safety, and product consistency for regulatory approval. The flexibility of glycoengineering in plants may even allow the design of biologics with novel N-glycans not found in nature to further improve their performance. In addition, the versatile transient expression system will allow the rapid screening of target N-glycoforms, and the stable transgenic glycoengineered plants will provide the scalable hosts for the production of selected biologics. Thus, glycoengineered plant-based systems have the potential to provide a complete platform for both novel drug development and their large scale manufacturing.
Acknowledgments
The author’s research is supported by NIH–NIAID Grants U01 AI075549 and R33AI101329.
Footnotes
The author declares no conflict of interest.
See companion article on page 9498.
References
- 1.Loos A, Steinkellner H. Plant glyco-biotechnology on the way to synthetic biology. Front Plant Sci. 2014;5:523. doi: 10.3389/fpls.2014.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kallolimath S, et al. Engineering of complex protein sialylation in plants. Proc Natl Acad Sci USA. July 21, 2016;113:9498–9503. doi: 10.1073/pnas.1604371113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A. Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol. 2011;3(6):a005462. doi: 10.1101/cshperspect.a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol. 2015;33(8):842–844. doi: 10.1038/nbt.3280. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Davis KR. The potential of plants as a system for the development and production of human biologics. F1000 Res. 2016;5(912):F1000 Faculty Rev-912. doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai H, et al. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnol J. 2014;12(8):1098–1107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilho A, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem. 2010;285(21):15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colley KJ, Kitajima K, Sato C. Polysialic acid: Biosynthesis, novel functions and applications. Crit Rev Biochem Mol Biol. 2014;49(6):498–532. doi: 10.3109/10409238.2014.976606. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, et al. Polysialylated insulin: Synthesis, characterization and biological activity in vivo. Biochim Biophys Acta. 2003;1622(1):42–49. doi: 10.1016/s0304-4165(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 12.Constantinou A, et al. Site-specific polysialylation of an antitumor single-chain Fv fragment. Bioconjug Chem. 2009;20(5):924–931. doi: 10.1021/bc8005122. [DOI] [PubMed] [Google Scholar]
- 13.Dejnirattisai W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol. June 23, 2016 doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, et al. Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus. PLoS ONE. 2014;9(3):e93541. doi: 10.1371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton SR, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313(5792):1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]