Significance
We previously demonstrated that the microtubule (MT) motor protein kinesin-1 slides and transports MTs themselves in addition to its established role in organelle transport. However, the physiological importance of MT sliding has not been determined. Here, we identified the mechanism of kinesin-1–based MT sliding and generated a sliding-deficient Drosophila mutant. Additionally, we generated a chimeric motor that actively slides MTs but cannot transport organelles. Using these tools, we demonstrated that MT sliding is an essential biological process, with key roles in both axon and dendrite outgrowth. Because the C-terminal MT-binding site of kinesin-1, which is essential for sliding, is highly conserved in vertebrates and invertebrates, we postulate that MT sliding is important for nervous system development in many organisms.
Keywords: kinesin-1, microtubules, Drosophila, axon outgrowth, dendrite outgrowth
Abstract
The plus-end microtubule (MT) motor kinesin-1 is essential for normal development, with key roles in the nervous system. Kinesin-1 drives axonal transport of membrane cargoes to fulfill the metabolic needs of neurons and maintain synapses. We have previously demonstrated that kinesin-1, in addition to its well-established role in organelle transport, can drive MT–MT sliding by transporting “cargo” MTs along “track” MTs, resulting in dramatic cell shape changes. The mechanism and physiological relevance of this MT sliding are unclear. In addition to its motor domain, kinesin-1 contains a second MT-binding site, located at the C terminus of the heavy chain. Here, we mutated this C-terminal MT-binding site such that the ability of kinesin-1 to slide MTs is significantly compromised, whereas cargo transport is unaffected. We introduced this mutation into the genomic locus of kinesin-1 heavy chain (KHC), generating the KhcmutA allele. KhcmutA neurons displayed significant MT sliding defects while maintaining normal transport of many cargoes. Using this mutant, we demonstrated that MT sliding is required for axon and dendrite outgrowth in vivo. Consistent with these results, KhcmutA flies displayed severe locomotion and viability defects. To test the role of MT sliding further, we engineered a chimeric motor that actively slides MTs but cannot transport organelles. Activation of MT sliding in KhcmutA neurons using this chimeric motor rescued axon outgrowth in cultured neurons and in vivo, firmly establishing the role of sliding in axon outgrowth. These results demonstrate that MT sliding by kinesin-1 is an essential biological phenomenon required for neuronal morphogenesis and normal nervous system development.
Neurons are the basic unit of the nervous system, forming vast networks throughout the body that communicate using receptor-ligand machinery located in long cellular projections called axons and dendrites. Learning how these processes form is key to understanding the early development and pathology of the nervous system. Microtubules (MTs) and actin microfilaments have been implicated in neurite outgrowth, with many studies focusing on the growth cone at the tip of the axon. Previous models suggest that the driving forces for neurite outgrowth are MT polymerization and the treadmilling of F-actin (1, 2). However, other studies demonstrate that F-actin is dispensable to outgrowth and neurites extend even in the absence of F-actin (3–5).
Our group has found that the motor protein kinesin-1 can rearrange the MT network by sliding MTs against each other (6). We have shown that kinesin-1 is required for MT sliding in cultured neurons and kinesin-1 depletion inhibits both neurite outgrowth and regeneration (7, 8). Additionally, we have observed MT sliding in axons as well as MTs pushing on the axon tip (9). Recent studies from other groups have also implicated MT translocation in axon extension and dendritic organization (10–12). Based on these studies, we hypothesize that kinesin-1 drives neurite extension by sliding MTs against the plasma membrane. Consistent with this model, kinesin-1 depletion in vivo has severe phenotypes in the nervous system. Knockout of kinesin-1 in Drosophila results in locomotion defects and eventual death during the larval stages due to nervous system dysfunction (13, 14). However, because kinesin-1 engages in both MT sliding and organelle transport, it is hard to determine which phenotypes are caused by a deficiency in MT sliding. To test the role of MT sliding in neurite outgrowth directly, we set out to create a sliding-deficient kinesin-1 mutant that can dissociate the roles of kinesin-1 in sliding and organelle transport.
A previous study demonstrated that human kinesin-1 heavy chain (KHC) contains an additional MT-binding site besides the motor domain (15). Biochemical studies mapped this site to positively charged residues at the extreme C terminus of the heavy chain (16–18). Based on these studies, we hypothesized that KHC engages in MT sliding by binding one MT with its C-terminal MT-binding site while walking along a second MT using its motor domain. Here, we demonstrate that this C-terminal MT-binding site is required for MT sliding and introduce a sliding-deficient KHC mutant, which allowed us to determine the physiological role of MT sliding. We introduced this sliding-deficient mutant into the endogenous Khc locus and demonstrated that MT sliding is required for Drosophila nervous system development. We also engineered a chimeric motor protein containing the motor domain of kinesin-3 fused to the extreme C terminus of kinesin-1, including its MT-binding site. This motor is able to slide MTs, but it cannot transport organelles. Expressing this chimeric motor in our MT sliding-deficient KHC flies rescued axon outgrowth phenotypes both in cultured neurons and in vivo, further demonstrating the importance of sliding in neurodevelopment.
Results
Kinesin-1 Slides MTs Using Its C-Terminal–Binding Site.
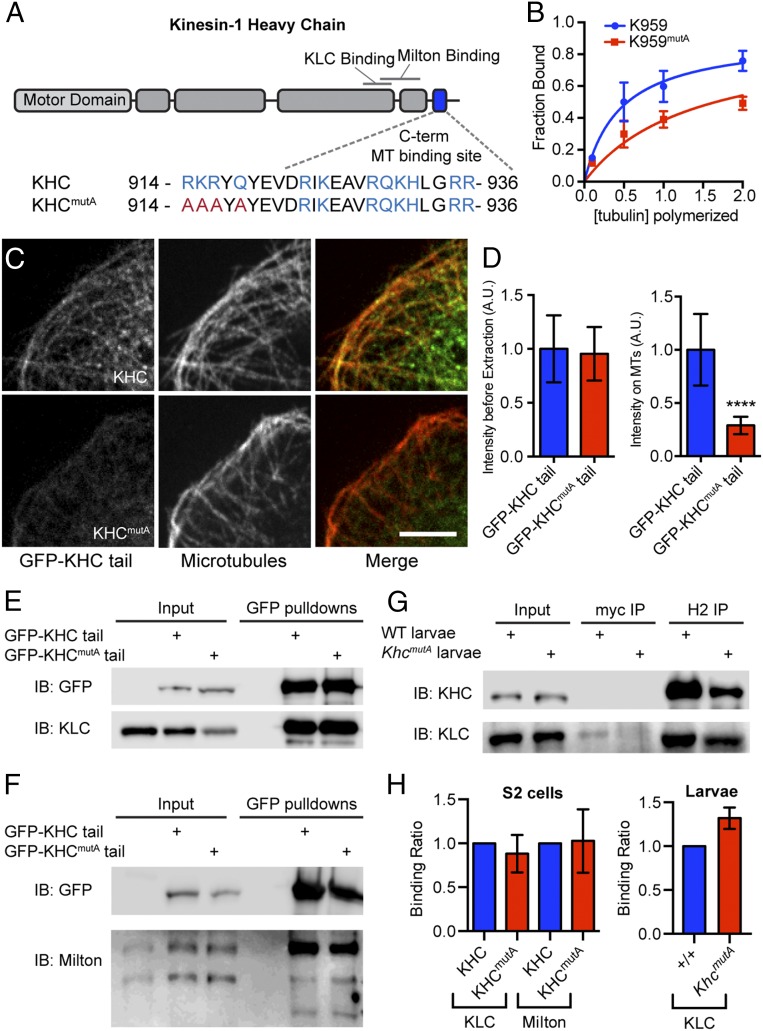
We have previously shown that kinesin-1 slides cytoplasmic MTs in Drosophila and mammalian cells (6, 7). We hypothesized that kinesin-1 engages in MT sliding by binding one MT using the MT-binding site at the extreme C terminus of the heavy chain (15–17) while the motor domain walks on another MT. To test this hypothesis, we mutated the C-terminal MT-binding site of KHC based on a previous study on human kinesin-1 (17). We mutated four residues (R914A, K915A, R916A, and Q918A) in the C-terminal MT-binding site, generating KHCmutA (Fig. 1A). To test whether these alanine mutations reduced MT binding, we purified short kinesin-1 fragments (amino acids 844–959) with or without alanine mutations (K959 and K959mutA, respectively) from Escherichia coli. We found that K959 bound MTs with a submicromolar affinity [Kd = 0.45 ± 0.08 μM (average ± SD)], whereas K959mutA bound with a significantly weaker affinity (Kd = 1.37 ± 0.16 μM) (Fig. 1B). To test whether KHCmutA affected MT affinity in cells, we generated Drosophila S2 cell lines expressing GFP-tagged wild-type KHC or KHCmutA tails (amino acids 345–975). As described previously (15), GFP-KHC tail decorated MTs in S2 cells and remained bound to MTs after extraction of soluble proteins with detergent (Fig. 1C). Conversely, significantly more GFP-KHCmutA tail was extracted from the cells after detergent treatment, demonstrating that KHCmutA has diminished MT binding in cells (Fig. 1 C and D).
Fig. 1.
Mutation of the C-terminal MT-binding site of KHC reduces affinity for MTs but does not affect cargo adapter binding. (A) Schematic diagram of KHC depicting the C-terminal MT-binding site and binding sites for cargo adapters (KLC and Milton). The amino acid sequences of the C-terminal MT-binding site and a quadruple-alanine mutation (KHCmutA) are depicted. (B) Binding curves of purified wild-type or KHCmutA tail fragments (amino acids 844–959; called K959 and K959mutA, respectively) with MTs in vitro. K959 binds MTs with Kd = 0.44 ± 0.08 μM, and K959mutA tail binds with Kd = 1.37 ± 0.16 μΜ (both represent average ± SD). These values were obtained from three independent experiments. Error bars indicate SEM. (C) Triton-extracted and fixed Drosophila S2 cells expressing GFP fusions of either wild-type KHC or KHCmutA tail (residues 345–975) and stained for α-tubulin. Note that KHC tail extensively decorates MTs, whereas KHCmutA tail is mostly removed by detergent extraction. (Scale bar, 5 μm.) (D) Intensity of GFP tail before extraction and after extraction along MTs. GFP-KHCmutA tail displayed significantly less accumulation along MTs. Before extraction: GFP-KHC tail, n = 29; GFP-KHCmutA tail, n = 49; after extraction: GFP-KHC tail, n = 39; GFP-KHCmutA tail, n = 45. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% confidence interval (CI). A.U., arbitrary units. (E and F) Pull-down of control, GFP-KHC tail, and GFP-KHCmutA tail from S2 cells stably expressing the constructs. KLC and mitochondrial adapter Milton both bind to GFP-KHC tail and GFP-KHCmutA tail. Multiple bands in the anti-Milton blot represent different splice isoforms. IB, immunoblot. (G) Coimmunoprecipitation of KHC from extracts of +/+ and KhcmutA larvae. Negative control immunoprecipitations (IPs) were performed using a myc monoclonal antibody and KHC IPs were performed using a KHC monoclonal antibody, H2. Both wild-type KHC and KHCmutA pulled down KLC. (H) Quantification of cargo adapter binding. The ratio of cargo adapter to KHC signal was quantified from more than three pull-down or IP experiments for each condition. The affinity of KLC or Milton for KHC was comparable in control and KHCmutA extracts from either S2 cells or larval extracts. Error bars indicate 95% CI.
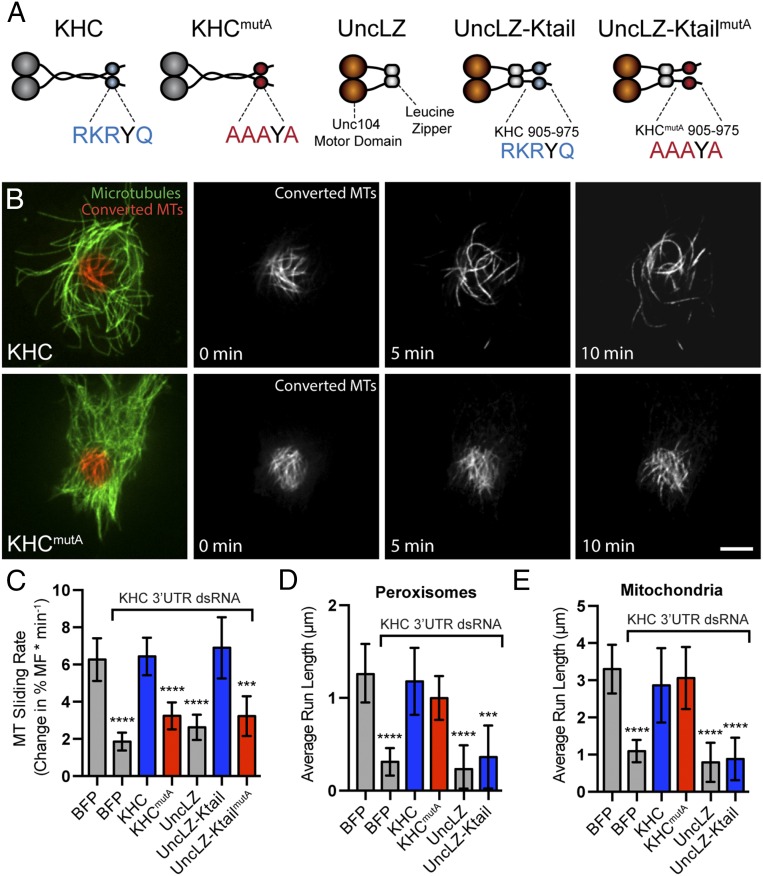
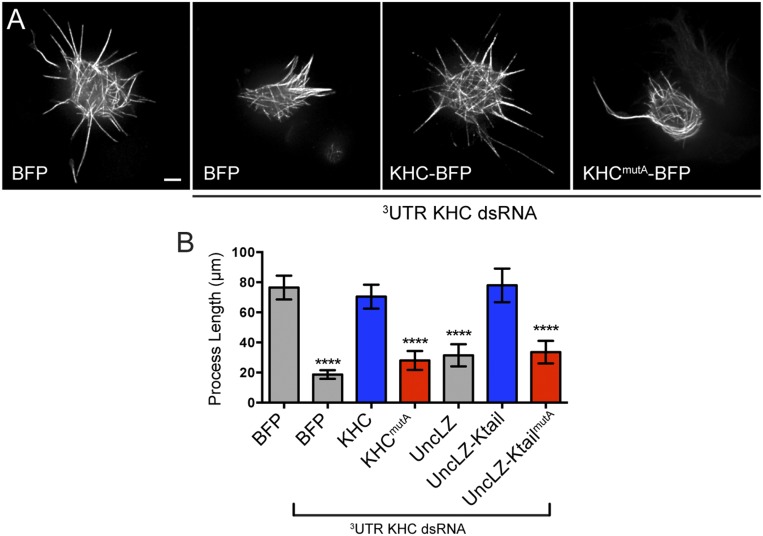
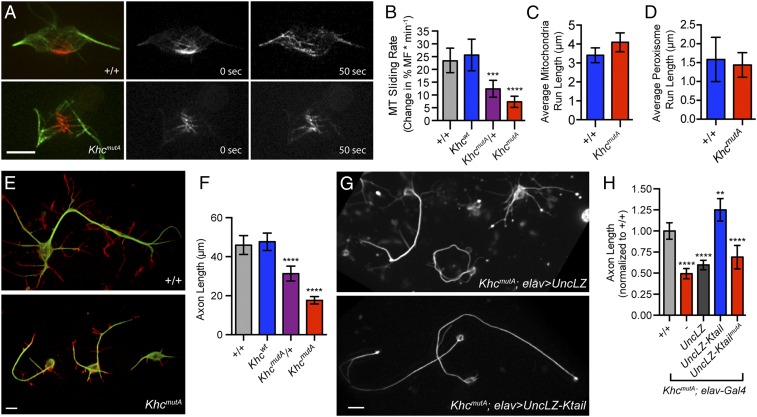
To test whether the C-terminal MT-binding site of KHC is necessary for MT sliding, we assayed MT sliding using α-tubulin tagged with a tandem dimer of the photoconvertible probe EOS (tdEOS-tubulin) (9, 19), which changes emission spectra from green to red upon exposure to UV light (20). To quantify MT sliding, we expressed tdEOS-tubulin in Drosophila S2 cells, photoconverted a small subset of MTs from green to red, and imaged converted MTs in the red channel. To test whether KHCmutA can slide MTs, we depleted endogenous KHC with dsRNA targeting its 3′-UTR and cotransfected KHCmutA and tdEOS-tubulin. KHCmutA was tagged with blue fluorescent protein (BFP) so that we could assess expression (21). BFP-tagged wild-type KHC was used as a control for these experiments. We found that KHCmutA displayed greatly reduced MT sliding compared with control (Fig. 2 B and C and Movie S1). To test further if KHCmutA is sliding-deficient, we assayed a MT sliding-based activity. Kinesin-1–dependent sliding has been shown to drive the formation of cellular processes in S2 cells after F-actin depolymerization (6, 22). We quantified the length of processes generated by either KHC or KHCmutA. In agreement with the MT sliding data, KHCmutA generated significantly shorter processes compared with KHC (Fig. S1 A and B). These results demonstrate that the C-terminal MT-binding site of KHC is necessary for MT sliding in S2 cells.
Fig. 2.
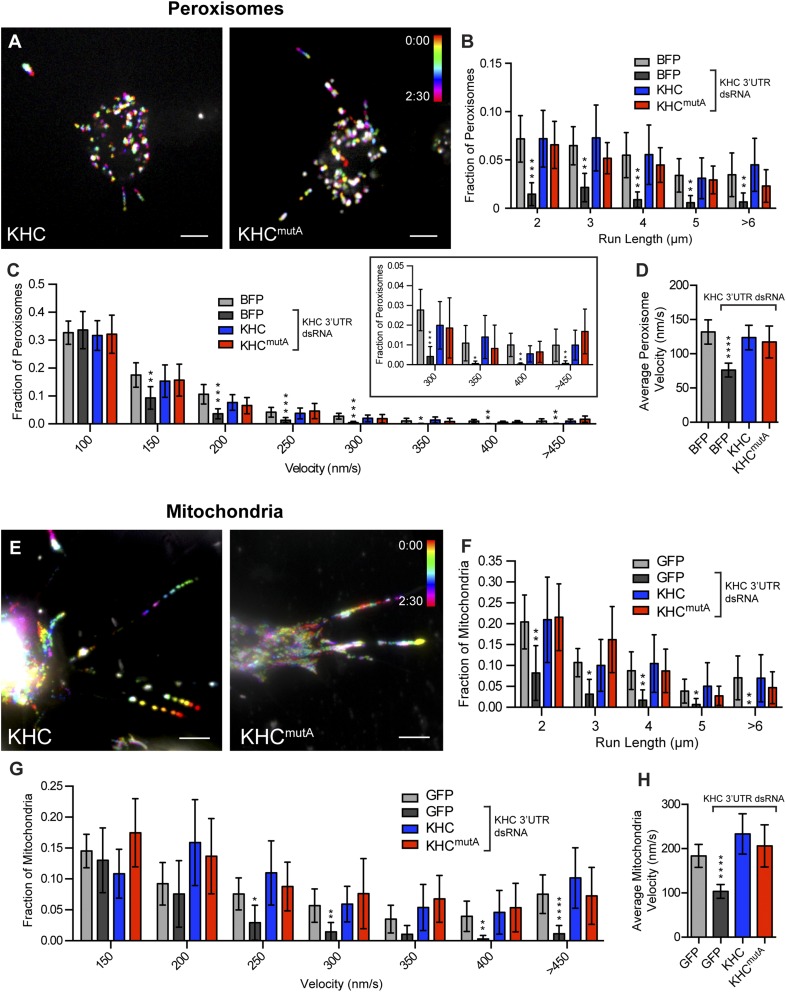
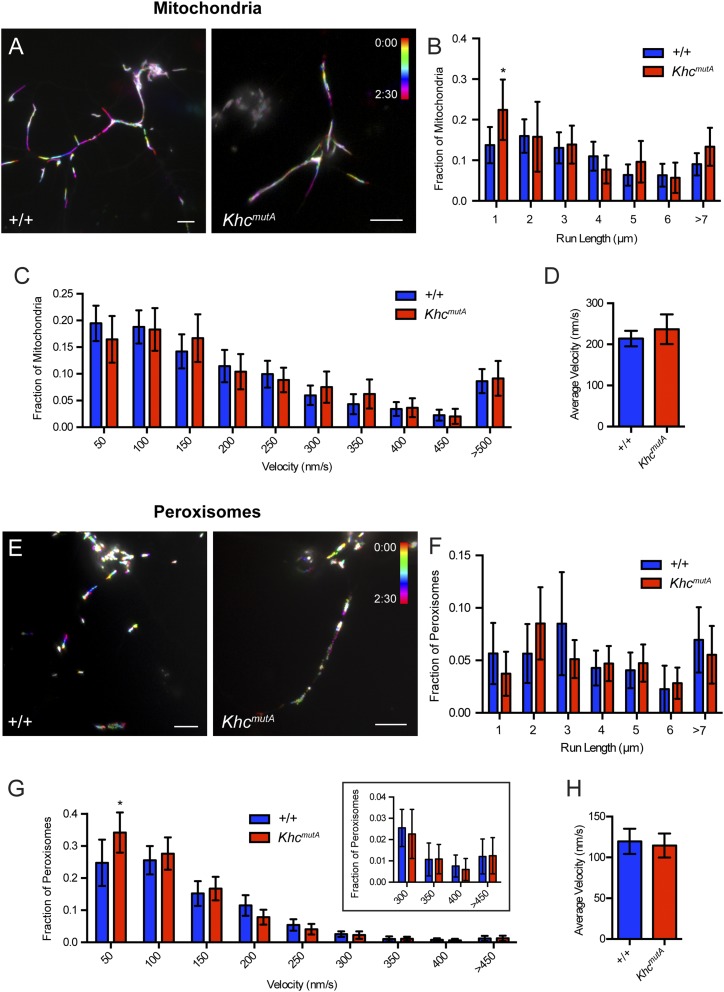
KHCmutA cells displayed impaired MT sliding but normal mitochondria and peroxisome transport. (A) Schematic diagrams of constructs expressed after endogenous KHC knockdown. (B) S2 cells expressing photoconvertible tdEOS-tagged α-tubulin and KHC or KHCmutA. Endogenous KHC was depleted by dsRNA targeting the 3′-UTR region. Photoconverted MT segments are depicted in red, and unconverted MTs are depicted in green. Photoconverted segments were tracked over time. Note that KHCmutA moves MTs slower than wild-type KHC (Movie S1). (Scale bar, 5 μm.) (C) MT sliding was quantified by segmenting MTs and determining the gross rate of MT movement outside of the initial converted zone (more detail is provided in Materials and Methods). KHCmutA displayed significantly reduced MT sliding activity compared with KHC. A chimeric motor containing the motor domain of kinesin-3/Unc104 fused to the C terminus of KHC (amino acids 905–975) rescued sliding, whereas KHCmutA alanine substitutions in this construct prevented this rescue (Movie S2) BFP, n = 35; BFP + KHC dsRNA, n = 29; KHC, n = 38; KHCmutA, n = 46; UncLZ, n = 17; UncLZ-Ktail, n = 25; UncLZ-KtailmutA, n = 27. **P = 0.001, ***P = 0.0006, ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (D and E) Average peroxisome and mitochondria run length was assayed after expression of each construct. KHC and KHCmutA were both able to rescue cargo transport after endogenous KHC knockdown (Movies S3 and S4). Chimeric motors were unable to engage in peroxisome and mitochondria transport. (D) BFP, n = 41; BFP + KHC dsRNA, n = 50; KHC, n = 30; KHCmutA, n = 37; UncLZ, n = 13; UncLZ-Ktail, n = 13. ***P = 0.0003, ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (E) GFP, n = 33; GFP + KHC dsRNA, n = 42; KHC, n = 21; KHCmutA, n = 17; UncLZ, n = 17; UncLZ-Ktail, n = 16. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI.
Fig. S1.
Mutation of the C-terminal MT-binding site of kinesin-1 inhibits process formation in Drosophila S2 cells (related to Fig. 2). (A) Micrographs of S2 cells expressing tdEOS-tubulin and BFP, KHC-BFP, or KHCmutA-BFP. Endogenous kinesin-1 was depleted by 3′-UTR KHC dsRNA where indicated. Note that replacement of KHC with KHCmutA resulted in reduced process formation. (Scale bar, 5 μm.) (B) Quantification of total process length per cell for each tested construct. KHCmutA cells displayed a significantly reduced process length compared with KHC cells. UncLZ-Ktail–expressing cells could rescue process formation after endogenous KHC knockdown; however, UncLZ and UncLZ-KtailmutA could not. BFP, n = 83; BFP + KHC dsRNA, n = 87; KHC, n = 71; KHCmutA, n = 39; UncLZ, n = 43; UncLZ-Ktail, n = 40; UncLZ-KtailmutA, n = 38. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% confidence interval.
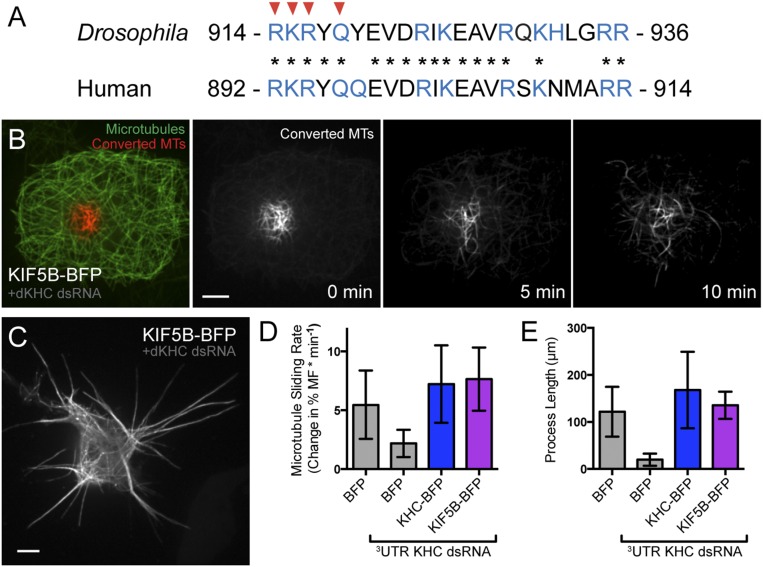
Next, we wanted to test whether this C-terminal MT-binding site was sufficient to slide MTs when coupled to a motor domain. We used a chimeric motor protein consisting of the motor domain of Unc104/kinesin-3 and a leucine zipper for dimerization (called UncLZ) (23). We fused this chimeric motor to the C terminus of KHC (amino acids 905–975), including the MT-binding site, creating UncLZ-Ktail (Fig. 2A). We found that UncLZ-Ktail was as effective at MT sliding as endogenous kinesin-1. Introduction of the quadruple-alanine mutations to the UncLZ-Ktail construct (UncLZ-KtailmutA) significantly reduced the ability of the chimera to slide MTs (Fig. 2C and Movie S2). We also found that after F-actin depolymerization, UncLZ-Ktail could generate processes comparable to control, whereas UncLZ and UncLZ-KtailmutA could not (Fig. S1B). Overall, these data demonstrate that the C-terminal MT-binding site of KHC is a necessary and sufficient binding site that allows kinesin-1 to bind and slide MTs in Drosophila S2 cells. This mechanism is likely conserved in mammals because the MT-binding site itself is highly conserved (Fig. S2A) and expression of human KHC rescues MT sliding and process formation in Drosophila S2 cells depleted of endogenous kinesin-1 (Fig. S2 B–E).
Fig. S2.
Human kinesin-1 engages in MT–MT sliding in Drosophila S2 cells. (A) Comparison of the C-terminal MT-binding sites in Drosophila KHC and human KHC (KIF5B). Red arrowheads indicate residues mutated in KHCmutA, and asterisks indicate conserved residues between Drosophila and human. (B) Photoconversion experiment of an S2 cell expressing tdEOS-tubulin and KIF5B-BFP. Endogenous kinesin-1 was depleted by dsRNA against the 3′-UTR. Immediately after photoconversion, unconverted MTs are depicted as green and converted MTs are depicted as red. Time-lapse imaging of converted MTs demonstrates that KIF5B robustly slides MTs. (Scale bar, 5 μm.) (C) S2 cell expressing tdEOS-tubulin and KIF5B-BFP after cytochalasin D treatment and endogenous kinesin-1 depletion. Note that the cell forms numerous long processes. (Scale bar, 5 μm.) (D) Quantification of MT sliding in control, KHC-BFP, and KIF5B-BFP–expressing S2 cells. KIF5B slides MTs as efficiently as Drosophila KHC. BFP, n = 10; BFP + KHC dsRNA, n = 10; KHC-BFP, n = 9; KIF5B-BFP, n = 11. Error bars indicate 95% confidence interval (CI). (E) Quantification of process formation in control, KHC-BFP, and KIF5B-BFP–expressing S2 cells. KIF5B generates processes as efficiently as Drosophila KHC. BFP, n = 9; BFP + KHC dsRNA, n = 10; KHC-BFP, n = 9; KIF5B-BFP, n = 16. Error bars indicate 95% CI.
KHCmutA Does Not Affect Organelle Transport or Adapter Binding in S2 Cells.
To determine whether KHCmutA supports normal cargo transport, we first tested whether KHCmutA binds the known cargo adapters kinesin-1 light chain (KLC; used for transport of many kinesin-1 cargoes) (24) and Milton (used as an adapter for mitochondria transport) (25). Using S2 cell lines stably expressing GFP-tagged KHC or KHCmutA tail, we performed GFP pull-downs and probed pelleted proteins using antibodies against these adapters. We quantified the ratio of adapter to KHC signal and found that KHCmutA maintained normal affinity for both KLC and Milton (Fig. 1 E, F, and H). Additionally, to determine if similar interactions occurred in vivo, we immunoprecipitated KHC or KHCmutA from flies (a description of flies expressing KHCmutA is provided in Genomic Replacement of Khc with KhcmutA Reduces Drosophila Viability) and found that both interacted with KLC equivalently (Fig. 1 G and H).
Although KHCmutA was able to bind cargo adapters, we wondered whether this mutant could transport organelles. We assayed the transport of two kinesin-1–dependent cargoes: peroxisomes, which use KLC to bind KHC (26), and mitochondria, which use the adapter Milton (25). We monitored peroxisome transport using a GFP-tagged peroxisome-targeting sequence (GFP-SKL) (27) and mitochondria transport using a fluorescent dye (MitoTracker Red). KHC and KHCmutA both rescued peroxisome and mitochondria transport in S2 cells after dsRNA depletion of endogenous KHC (Fig. S3 A and E and Movies S3 and S4). We quantified the average run length (Fig. 2 D and E) and velocity (Fig. S3 D and H) of peroxisomes and mitochondria (details are provided in Materials and Methods). No significant difference could be detected between organelles transported by KHC and KHCmutA. Additionally, KHCmutA maintained a normal distribution of organelle run lengths and velocities compared with wild-type KHC (Fig. S3 B, C, F, and G). These results demonstrate that KHCmutA supports normal transport of peroxisomes and mitochondria. We also tested the ability of the sliding-competent UncLZ constructs to transport peroxisomes and mitochondria. Expression of UncLZ or UncLZ-Ktail did not rescue peroxisome or mitochondria run lengths after kinesin-1 knockdown (Fig. 2 D and E), indicating that organelle transport and MT sliding are two independent functions of kinesin-1. Overall, these data demonstrate that KHCmutA is able to bind and transport peroxisomes and mitochondria, suggesting that the mutation impairs MT sliding but has no effect on KLC-dependent transport or mitochondria transport.
Fig. S3.
KHCmutA-expressing S2 cells support normal peroxisome and mitochondria transport. (A) Representative temporal projections of time-lapse movies of peroxisome transport in GFP-SKL– and KHC-BFP– or KHCmutA-BFP–expressing S2 cells. Different frames were color-coded (color legend) and then projected together such that moving peroxisomes are depicted as a multicolored trajectory, whereas immotile peroxisomes are white. Note the presence of multicolored trajectories in both KHC- and KHCmutA–expressing cells, indicating that peroxisomes are actively moving. (Scale bars, 5 μm.) (B) Frequency distribution of peroxisome run lengths. All active trajectories (defined as >0.5-μm span and lasting longer than four frames) were binned according to length and divided by the total number of both motile and immotile peroxisomes to determine the distribution of peroxisome run lengths. Depletion of endogenous KHC by dsRNA significantly reduced all measured run lengths. Both KHC and KHCmutA rescued this phenotype. (C) Frequency distribution of peroxisome velocities. All peroxisome velocities were binned and divided by the total number of peroxisomes to determine the distribution of peroxisome velocities. Depletion of endogenous KHC by dsRNA significantly reduced velocities ≥150 nm⋅s−1. Both KHC and KHCmutA rescued this phenotype. The boxed graph depicts the last four bins, with a smaller range on the y axis. (D) Quantification of average peroxisome velocity. The average velocity of each peroxisome trajectory lasting longer than four frames was measured. Depletion of endogenous KHC by dsRNA significantly reduced the average peroxisome velocity. Both KHC and KHCmutA could rescue this phenotype. Sample sizes for peroxisome experiments: BFP, n = 28 cells, 870 particles; BFP + KHC dsRNA, n = 25 cells, 883 particles; KHC, n = 16 cells, 656 particles; KHCmutA, n = 23 cells, 931 particles. Error bars indicate 95% CI. (E) Representative temporal projections of time-lapse movies of mitochondria transport in KHC-BFP– or KHCmutA-BFP–expressing S2 cells, stained with MitoTracker Red. Note the presence of multicolored trajectories in both KHC- and KHCmutA-expressing cells, indicating that mitochondria are actively moving. (Scale bars, 5 μm.) (F) Frequency distribution of mitochondria run lengths. All active trajectories (defined as >0.5-μm span and lasting longer than four frames) were binned according to length and divided by the total number of mitochondria to determine distribution of mitochondria run lengths. Depletion of endogenous KHC by dsRNA significantly reduced all measured run lengths. Both KHC and KHCmutA rescued this phenotype. (G) Frequency distribution of mitochondria velocities. All mitochondria velocities were binned and divided by the total number of mitochondria to determine the distribution of mitochondria velocities. Depletion of endogenous KHC by dsRNA significantly reduced velocities ≥250 nm⋅s−1. Both KHC and KHCmutA rescued this phenotype. (H) Quantification of average mitochondria velocity. The average velocity of each mitochondria trajectory lasting longer than four frames was measured. Depletion of endogenous KHC by dsRNA significantly reduced the average peroxisome velocity. Both KHC and KHCmutA could rescue this phenotype. Sample sizes for mitochondria experiments: GFP, n = 16 cells, 402 particles; GFP + KHC dsRNA, n = 18 cells, 283 particles; KHC, n = 18 cells, 176 particles; KHCmutA, n = 15 cells, 273 particles. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Mann-Whitney test. Error bars represent 95% CI.
Genomic Replacement of Khc with KhcmutA Reduces Drosophila Viability.
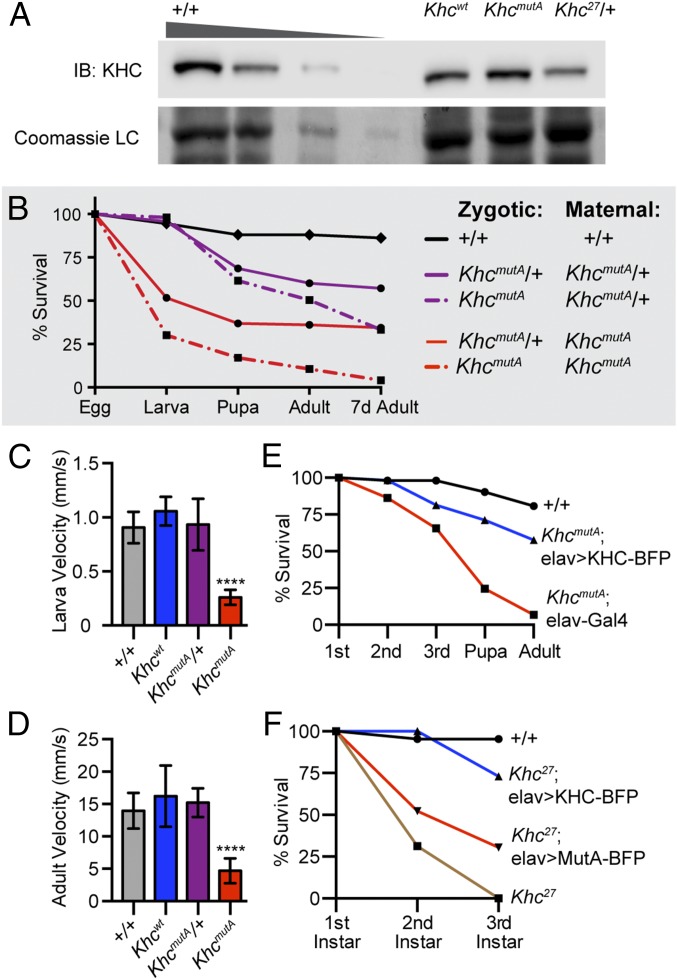
Kinesin-1–null phenotypes are thought to be due to organelle transport defects in the nervous system (28); however, MT sliding has not been examined. To test the role of MT sliding, we generated genomic-replacement KHCmutA flies, called KhcmutA, using genome engineering (29) and φC31 site-specific recombination (30, 31). The endogenous kinesin-1 locus was replaced by a genomic kinesin-1 construct containing the MT-binding site mutations (KHCmutA) (Fig. S4 A and B). As a control, wild-type kinesin-1 was also knocked-in to the endogenous locus, generating Khcwt; this line is viable and fertile, and displays no obvious phenotypes. We determined by Western blot that KHC levels in KhcmutA flies are comparable to Khcwt and control flies (Fig. 3A), demonstrating that the knock-in method does not perturb KHC levels. We next assayed viability in homozygous KhcmutA flies. KhcmutA offspring from KhcmutA/+ parents displayed 49.6% lethality before adulthood (Fig. 3B). However, because KHC is maternally loaded in embryos (13, 14), KhcmutA/+ mothers deposit wild-type kinesin-1 mRNA and proteins into KhcmutA embryos, potentially allowing many to survive throughout development. To observe earlier effects of KhcmutA, we eliminated the maternal contribution of wild-type KHC by crossing KhcmutA females to KhcmutA/+ males. Strikingly, 89.4% of KhcmutA offspring died before adulthood, with the strongest effect in the embryo (Fig. 3B). These results suggest that MT sliding by both maternal and zygotic KHC is important for normal development.
Fig. S4.
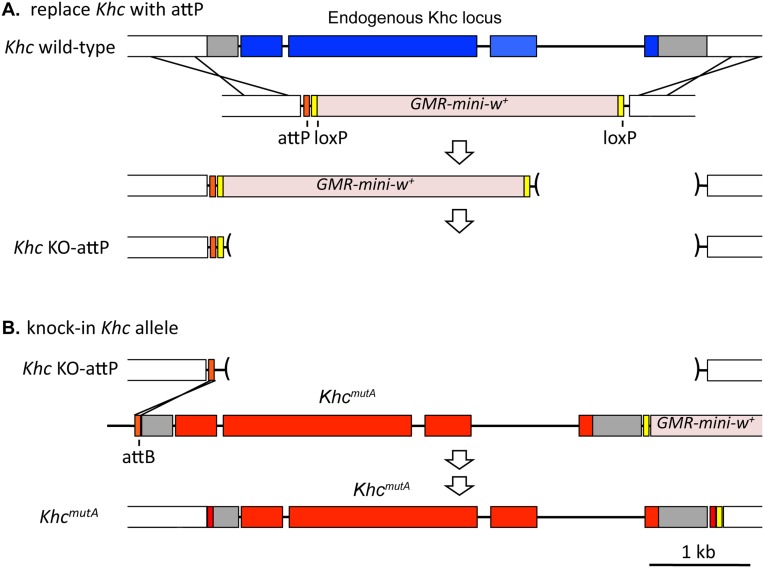
Engineering the Khc gene to facilitate the knock-in of designer Khc alleles. (A) Homologous recombination was used to knock out the endogenous wild-type Khc and replace it with an attP site, generating KhcKO-attP. The donor template also contained a cassette with GMR-mini-w+ flanked by loxP sites, which was used to identify the gene replacement. GMR-mini-w+ was subsequently removed by Cre-loxP recombination. (B) To generate KhcmutA, a plasmid containing the KhcmutA allele was injected into KhcKO-attP embryos expressing ϕC31 integrase, which mediated recombination between the attP site in the Khc locus and an attB site in the plasmid. The plasmid also contained GMR-mini-w+ and a loxP site, which facilitated the removal of both the mini-w+ marker and additional exogenous plasmid sequence following integration.
Fig. 3.
Mutation of the C-terminal MT-binding site of kinesin-1 resulted in locomotion and viability defects in Drosophila. (A) Western blot comparing KHC protein levels in +/+, Khcwt, KhcmutA, and Khc27/+ flies. Protein extract from +/+ flies was loaded at 20 μg, 10 μg, 5 μg, and 2 μg, whereas other genotypes were loaded at 20 μg. Samples were probed with a KHC antibody and stained with Coomassie for a loading control (LC). Khcwt (genomic wild-type KHC knock-in) and KhcmutA (genomic KHCmutA knock-in) flies displayed similar KHC levels to +/+. As a control, Khc27/+ fly extracts were probed; these extracts contained 50% KHC level, as expected. Western blot intensity (average ± SD; A.U.): 20 μg +/+, 1.00 ± 0.00; 10 μg +/+, 0.48 ± 0.30; 5 μg +/+, 0.19 ± 0.19; 2.5 μg +/+, 0.02 ± 0.03; Khcwt, 0.92 ± 0.24; KhcmutA, 0.88 ± 0.05; and Khc27/+, 0.47 ± 0.09. (B) Survival curve of KhcmutA and KhcmutA/+ flies from homozygous or heterozygous parents. KhcmutA flies from homozygous parents displayed the strongest lethality phenotype. +/+, n = 109; KhcmutA/+ (zygyotic; zyg) KhcmutA/+ (maternal; mat), n = 488; KhcmutA (zyg) KhcmutA/+ (mat), n = 250; KhcmutA/+ (zyg) KhcmutA (mat), n = 122; KhcmutA (zyg) KhcmutA (mat), n = 123. (C and D) Motility rates of third-instar larvae and adult flies. KhcmutA animals moved significantly slower than wild-type and heterozygous animals (Movies S5 and S6). (C) +/+, n = 14; Khcwt, n = 12; KhcmutA/+, n = 19; KhcmutA, n = 19. (D) +/+, n = 13; Khcwt, n = 18; KhcmutA/+, n = 20; KhcmutA, n = 21. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (E) Expression of wild-type KHC in neurons partially rescued KhcmutA lethality. +/+, n = 52; KhcmutA, elav-Gal4, n = 73; KhcmutA, elav > KHC-BFP, n = 59. (F) Expression of wild-type KHC in neurons delayed lethality in KHC protein-null Khc27 larvae (blue line). Expression of KHCmutA was not as effective (red line). All Khc27 animals died before pupation regardless of KHC expression. +/+, n = 43; Khc27, n = 15; Khc27, elav > KHC-BFP, n = 37; Khc27, elav > KHCmutA-BFP, n = 23.
Interestingly, KhcmutA/+ flies from the same crosses also displayed reduced viability (39.9% died from KhcmutA/+ mothers and 63.9% died from KhcmutA mothers). Because kinesin-1 molecules contain two heavy chains, many kinesin-1 dimers in KhcmutA/+ animals are likely KHC/KHCmutA heterodimers. The lethality observed in KhcmutA/+ animals may suggest that KHCmutA can exert a dominant-negative effect and that mixed KHC/KHCmutA heterodimers cannot bind MTs as efficiently as wild-type homodimers. Alternatively, this effect may be caused by earlier patterning defects caused by the maternally contributed KhcmutA.
Previous studies reported that kinesin-1–null larvae and temperature-sensitive mutant adults display locomotion defects (13). To determine if MT sliding contributes to these defects, we assayed crawling and walking velocities in KhcmutA larvae and adults (32). KhcmutA larvae displayed severe locomotion defects, with crawling velocities significantly slower than control larvae (Fig. 3C and Movie S5). Some larvae displayed a classic tail-flipping locomotion defect, caused by dorsal-ventral paralysis that disrupts normal peristaltic muscle contractions (28). KhcmutA adults also moved significantly slower than control (Fig. 3D and Movie S6). These results suggest that loss of kinesin-1–based MT sliding causes locomotion defects.
Our laboratory has demonstrated that kinesin-1 is required for neurite outgrowth and axon regeneration in cultured primary neurons (7, 8), prompting us to test whether KhcmutA lethality is caused by defects in the nervous system. Our strategy was to rescue MT sliding in neurons using the Gal4/UAS tissue-specific expression system and then to assay viability. For these experiments, we generated third-chromosome transgenes encoding UASp-KHC-BFP or UASp-KHCmutA-BFP. Expression of wild-type KHC in the nervous system using the pan-neuronal driver elav (33, 34) partially rescued the viability defect in KhcmutA flies (Fig. 3E), suggesting neuronal MT sliding is important in vivo. However, because the rescue was partial, MT sliding is likely important in other tissues as well. In support of this idea, an accompanying study demonstrates the role of MT sliding in cytoplasmic streaming and polarity determination during Drosophila oogenesis (35). We also tested whether we could rescue lethality in Khc27 flies, a kinesin-1–null allele (14). Neuronal expression of wild-type KHC, but not KHCmutA, delayed lethality in Khc27 larvae (Fig. 3F); however, all Khc27 animals died before pupation, regardless of transgenic expression. Together, these data highlight the importance of MT sliding in the nervous system.
KhcmutA Neurons Exhibit Decreased MT Sliding and Axon Outgrowth but Maintain Normal Peroxisome and Mitochondria Transport.
We next tested whether KhcmutA flies displayed reduced MT-sliding rates as demonstrated in Drosophila S2 cells (Fig. 2). We assayed MT-sliding rates in neurons because neuronal MT sliding appears to be important for survival (Fig. 3 E and F). To avoid the effect of maternally loaded KHC, we isolated and cultured neurons from dissociated brains of third-instar larvae (8), when maternal load is minimal (13). To assay MT sliding, MTs were photoconverted in the cell bodies of newly cultured neurons expressing elav > tdEOS-tubulin (7). KhcmutA neurons displayed significantly reduced MT sliding compared with control (Fig. 4 A and B and Movie S7). KhcmutA/+ neurons exhibited an intermediate sliding rate, supporting the idea that KhcmutA might reduce MT sliding through dominant-negative inhibition (Fig. 4B).
Fig. 4.
Neurons from KhcmutA larvae displayed reduced MT sliding and axon length but maintained normal mitochondria and peroxisome transport. (A) Primary neurons from +/+ and KhcmutA larvae expressing elav > tdEOS-tagged α-tubulin. Photoconverted MTs are depicted in red, and unconverted MTs are depicted in green. Note that fewer MTs leave the photoconverted zone in the KhcmutA neuron (Movie S7). (Scale bar, 5 μm.) (B) Quantification of MT sliding activity. KhcmutA homozygous and heterozygous neurons displayed reduced MT sliding compared with control neurons. +/+, n = 41; Khcwt, n = 31; KhcmutA/+, n = 26; KhcmutA, n = 27. ***P = 0.0012, ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (C and D) Mitochondria and peroxisome transport was assayed in primary neurons using a mitochondria dye, MitoTracker Red, or a transgene, UASp-GFP-SKL. No significant difference in average mitochondria or peroxisome run lengths was observed between +/+ and KhcmutA (Movies S8 and S9). Mitochondria: +/+, n = 51; KhcmutA, n = 44. Peroxisomes: +/+, n = 23; KhcmutA, n = 31. Error bars indicate 95% CI. (E and F) Neurons were fixed and stained 48 h after plating. MTs are depicted in green, and F-actin is depicted in red. KhcmutA neurons displayed significantly shorter axons than control. KhcmutA/+ neurons displayed an intermediate phenotype. +/+, n = 78; Khcwt, n = 83; KhcmutA/+, n = 92; KhcmutA, n = 121. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (Scale bar, 5 μm.) (G and H) UncLZ or UncLZ-Ktail expression was driven in KhcmutA neurons by elav-Gal4. Neurons were fixed and stained using an antibody against α-tubulin 48 h after plating. Axon length was measured and normalized to control neurons. Expression of UncLZ-Ktail rescued axon outgrowth in KhcmutA neurons and resulted in axons significantly longer than control neurons. Expression of UncLZ or UncLZ-KtailmutA in KhcmutA neurons did not rescue axon length. +/+, n = 104; KhcmutA: elav-Gal4, n = 120; KhcmutA: elav > UncLZ, n = 168; KhcmutA: elav > UncLZ-Ktail, n = 89; KhcmutA: elav > UncLZ-KtailmutA, n = 90. **P = 0.0036, ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (Scale bar: G, 5 μm.)
To test whether mutant KHC can maintain normal organelle transport in KhcmutA neurons, we assayed mitochondria transport in cultured neurons using MitoTracker Red. No significant difference was observed between mitochondria run lengths and velocities in control and KhcmutA neurons (Fig. 4C, Fig. S5 A–D and Movie S8). Additionally, we wanted to test whether KHC from KhcmutA flies could interact with KLC, which acts as a general adapter for many different kinesin-1 cargoes. We immunoprecipitated wild-type KHC and KHCmutA from +/+ and KhcmutA larval extracts. We probed extracts with an anti-KLC antibody and found that both wild-type and mutant KHC interacted with KLC (Fig. 1F). Furthermore, we assayed peroxisome transport in cultured neurons isolated from control and KhcmutA larvae expressing elav > GFP-SKL. No significant difference was observed between peroxisome run lengths and velocities in control and KhcmutA neurons (Fig. 4D, Fig. S5 E–H, and Movie S9). These results demonstrate that KhcmutA flies display defects in MT sliding but maintain normal peroxisome and mitochondria transport.
Fig. S5.
KhcmutA neurons support normal peroxisome and mitochondria transport. (A) Representative temporal projection of time lapses of +/+ and KhcmutA primary neurons stained with MitoTracker Red. Different frames are color-coded (color legend) and projected together such that a spectrum of color indicates movement and white particles are immotile. Note that both +/+ and KhcmutA neurons display colored trajectories, indicating that mitochondria are actively moving during the 2.5-min time lapse. (Scale bars, 5 μm.) (B) Distribution of mitochondria run lengths in primary neurons. Active movement events were defined as having a >0.5-μm span and lasting longer than eight frames. These run lengths were binned based on length and divided by the total number of particles to determine the distribution of mitochondria run lengths. No significant difference was observed between +/+ and KhcmutA neurons, except for a moderately significant increase in mitochondria run lengths binned at 1 μm. (C) Frequency distribution of mitochondria velocities. All mitochondria velocities were binned and divided by the total number of mitochondria to determine the distribution of velocities. No significant difference was observed between +/+ and KhcmutA at any velocity. (D) Quantification of average mitochondria velocity. The average velocity of each mitochondria trajectory lasting longer than four frames was measured. No significant difference was observed between +/+ and KhcmutA neurons. Sample sizes for mitochondria experiments: +/+, n = 52 neurons, 662 particles; KhcmutA, n = 35 neurons, 362 particles. *P < 0.05; Mann–Whitney test. Error bars indicate 95% CI. (E) Representative temporal projection of time lapses of +/+ and KhcmutA primary neurons expressing elav > GFP-SKL. Different frames are color-coded (color legend) and projected together such that a spectrum of color indicates movement and white particles are immotile. Note that both +/+ and KhcmutA neurons display colored trajectories, indicating that peroxisomes are actively moving during the 2.5 min time lapse. (Scale bars, 5 μm.) (F) Distribution of peroxisome run lengths in primary neurons. Active movement events were defined as having a >0.5-μm span and lasting longer than eight frames. These run lengths were binned based on length and divided by the total number of particles to determine the distribution of peroxisome run lengths. No significant difference was observed between +/+ and KhcmutA neurons. (G) Frequency distribution of peroxisome velocities. All peroxisome velocities were binned and divided by the total number of peroxisomes to determine the distribution of peroxisome velocities. KhcmutA neurons displayed a modest but significant increase in velocities binned at 50 nm⋅s−1. No significant difference could be observed at any other velocity. The boxed graph depicts four binned velocities with a different y-axis scale. *P < 0.05; Mann–Whitney test. (H) Quantification of average peroxisome velocity. The average velocity of each peroxisome trajectory longer than eight frames was measured. No significant difference was observed between +/+ and KhcmutA neurons. Sample sizes for peroxisome experiments: +/+, n = 23 neurons, 683 particles; KhcmutA, n = 28 neurons, 567 particles. Error bars indicate 95% CI.
Based on our previous studies (6–9, 36), we hypothesized that kinesin-1–based MT sliding drives axon outgrowth. Although kinesin-1 has been implicated in axon outgrowth previously (37, 38), the mechanism has not been elucidated and the role of MT sliding is not yet clear. To test our hypothesis of sliding-based outgrowth directly, we cultured KhcmutA neurons for 48 h before fixing and staining for MTs and F-actin. Strikingly, KhcmutA neurons displayed significantly shorter axons compared with control neurons (Fig. 4 E and F), suggesting that MT sliding is required for axon outgrowth. KhcmutA/+ neurons also displayed an intermediate axon outgrowth phenotype, consistent with the intermediate sliding and lethality phenotypes observed previously (Figs. 3B and 4B).
To test our hypothesis of sliding-based axon outgrowth further, we generated transgenic flies containing the Unc104/kinesin-3 chimeric motor, UncLZ-Ktail, which slides MTs but cannot transport organelles (Fig. 2). We found that expression of UncLZ-Ktail in KhcmutA neurons rescued the shortened axon phenotype and generated axons that were even longer than control (Fig. 4 G and H). Expression of the control chimera, UncLZ, or mutated chimera, UncLZ-KtailmutA, did not rescue axon outgrowth in KhcmutA neurons. These experiments demonstrate that kinesin-1–based MT sliding drives axon outgrowth in Drosophila neurons.
KhcmutA Animals Exhibit Reduced MT Sliding and Axon Outgrowth Defects in Vivo.
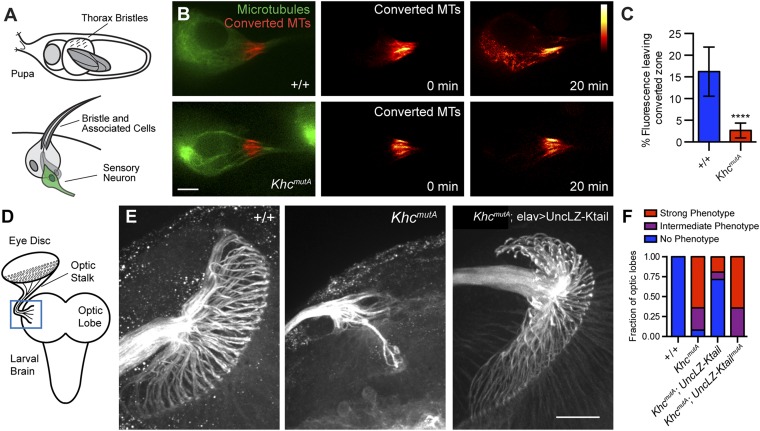
Although MT sliding has been observed in tissue culture cells and cultured neurons, no study has yet demonstrated that MT sliding can occur in an animal. This lack of demonstration is likely because photoconversion experiments in vivo are difficult and MT sliding is only observed during active outgrowth (7). To test whether sliding occurs in neurons in vivo, we imaged sensory neurons expressing the photoconvertible probe, tdMaple3-tubulin (35), driven by neur-Gal4 (39) in the anterior and posterior dorsocentral macrochaetae of intact pupae (40) (Fig. 5A). To visualize MTs during a stage of active axon outgrowth when MT sliding occurs, we imaged these sensory neurons 20–22 h after puparium formation, during the initial axon growth stage. MTs were photoconverted at the proximal axon to image MT sliding. We found robust MT sliding in these neurons during 20-min time lapses (Fig. 5B and Movie S10). To test if this MT sliding was kinesin-1–based, we imaged MT sliding in sensory neurons in KhcmutA animals. KhcmutA neurons in intact pupae exhibited greatly reduced MT sliding compared with control (Fig. 5B). Due to low signal and high background, the in vivo images of MTs were challenging to analyze using our standard method. To overcome this obstacle, we quantified the percentage of fluorescence leaving the initial converted zone after photoconversion as an indirect measure of MT sliding. We found that significantly more fluorescence left the initial converted zone in control neurons compared with KhcmutA neurons (Fig. 5C). It should be noted that these results quantify total photoconverted tdMaple3-tubulin signal. Although the majority of tdMaple3-tubulin is likely incorporated in MTs, these measurements cannot be directly compared with the quantification of MT sliding shown in Figs. 2 and 4. Overall, these results demonstrate that robust MT sliding occurs in vivo and that kinesin-1 drives this sliding using the same MT-binding site as has been characterized in S2 cells and in cultured neurons.
Fig. 5.
Neurons in intact KhcmutA animals displayed reduced MT sliding and axon defects in the optic lobes. (A) Diagram of Drosophila pupa and bristle structure. Large sensory bristles called macrochaetae coat the surface of the Drosophila pupa (Top). These structures each contain a sensory neuron (green, Bottom), whose dendrite innervates the base of the bristle and whose axon grows deep into the thoracic ganglion. (B) MT sliding was assayed in macrochaetae sensory neurons expressing neurA101 > tdMaple3-tubulin. Photoconverted MTs are depicted in red, and unconverted MTs are depicted in green. A red heat map was used to display converted MTs at 0 and 20 min. Note that KhcmutA neurons display drastically less MT sliding (Movie S10). (Scale bar, 5 μm.) (C) Percentage of fluorescence leaving the initial converted zone was quantified for +/+ and KhcmutA sensory neurons. Significantly less signal left the converted zone in KhcmutA neurons compared with control. +/+, n = 20; KhcmutA, n = 10. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (D) Diagram of dissected third-instar larval brain. The optic stalk, which contains axons of photoreceptors neurons, connects the eye imaginal disc to the optic lobe of the brain. This interface was imaged (blue box). (E) Third-instar larval brains were fixed and stained with anti-Futsch antibody (a neuronal MT-associated protein). Axon terminals at the optic lobe display an extensive umbrella-like pattern in control larvae. This pattern is lost in a majority of KhcmutA larvae. Expression of the chimeric motor UncLZ-Ktail, capable of MT sliding but not organelle transport, rescued this phenotype in many KhcmutA animals. The chimera containing the KHCmutA mutation (UncLZ-KtailmutA) was unable to rescue this phenotype. (Scale bar, 20 μm.) (F) Graph depicts the fraction of optic lobes displaying no phenotype (blue), a strong phenotype (red), or an intermediate phenotype (purple). +/+, n = 10; KhcmutA, n = 25; KhcmutA: elav > UncLZ-Ktail, n = 21; KhcmutA: elav > UncLZ-KtailmutA, n = 14.
We next tested whether the decrease in MT sliding observed in KhcmutA animals resulted in axon phenotypes in vivo. We examined the classic photoreceptor axon-targeting pattern in the optic lobes of third-instar larvae. Axons extend from the cell bodies of photoreceptor neurons in the eye disc and converge together into the optic stalk. The axons encounter the lamina of the optic lobes and form a retinotopic pattern (41) (Fig. 5D). We dissected brains from third-instar KhcmutA larvae and fixed and stained for Futsch, a neuron-specific MT-associated protein, to reveal the axon patterning of the photoreceptor neurons (Fig. 5E). Compared with the control, we found that KhcmutA photoreceptor neurons displayed a severe phenotype: Axons targeting to the lamina were mostly lost (Fig. 5E). The optic stalk in KhcmutA was also dramatically thinner compared with control, suggesting that many axons were severely shortened and could not even reach the optic stalk. These phenotypes were highly penetrant and seen in a majority of KhcmutA larvae (Fig. 5F). We attempted to rescue this phenotype by expressing the Unc104/kinesin-3 chimera (UncLZ-Ktail), which can slide MTs but cannot transport organelles. We found that neuronal expression of UncLZ-Ktail in KhcmutA animals rescued axon outgrowth and patterning of photoreceptor neurons in a majority of animals (Fig. 5 E and F). The thinning of the optic stalk was also rescued, suggesting that more axons were able to extend out from the eye disc. Conversely, expression of UncLZ-KtailmutA in KhcmutA larvae did not rescue this axon innervation defect. These results demonstrate that kinesin-1–based MT sliding is required for axon outgrowth not only in culture but also in vivo.
KhcmutA Larvae Display No Synaptotagmin Aggregates and Mild Cysteine String Protein Aggregates in Segmental Nerves.
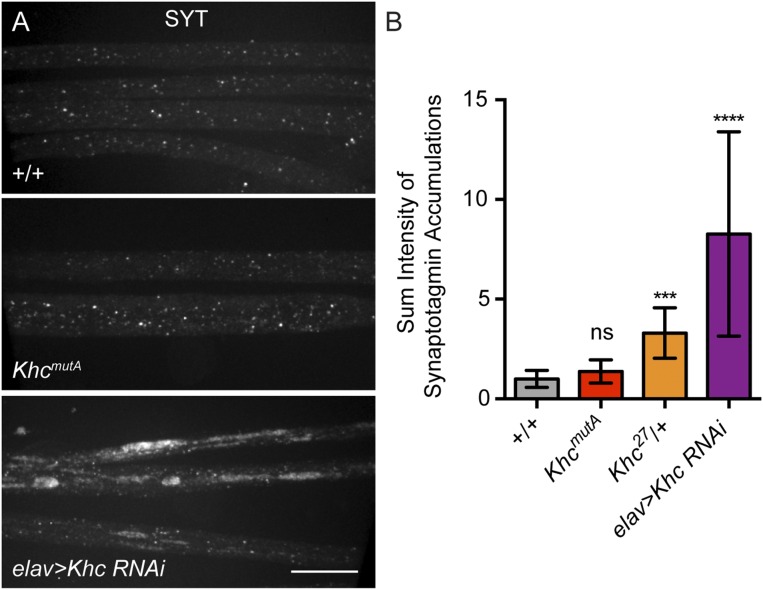
Previous studies have observed swellings along the axon shafts of the segmental nerve motor neurons in Khc mutants (28). These swellings contain accumulations of membrane organelles, such as synaptotagmin (SYT), cysteine string protein (CSP), and mitochondria, which are unable to move to the axon tip in the absence of kinesin-1. We wondered if similar vesicle aggregates could be observed in KhcmutA animals. As a control, we stained the segmental nerves of control larvae and KHC-depleted larvae (elav > Khc RNAi) using a SYT antibody. We found, as previously reported, that KHC depletion (elav > Khc RNAi) resulted in numerous large aggregates of SYT in segmental nerves (28, 42). Conversely, both +/+ and KhcmutA larvae displayed no observable SYT aggregates in the segmental nerves (Fig. S6A). These results were quantified by measuring the sum pixel intensity of SYT aggregates in each genotype (Fig. S6B).
Fig. S6.
KhcmutA larvae display no SYT accumulations in segmental nerves. (A) Representative micrographs of segmental nerves of third-instar larvae, stained with an antibody against SYT. Depletion of KHC (elav > Khc RNAi) resulted in large accumulations of SYT in segmental nerves. Control and KhcmutA larvae displayed no observable SYT accumulations. (Scale bar, 20 μm.) (B) Sum pixel intensity quantification of SYT accumulations. Although elav > Khc RNAi and kinesin-1–null allele heterozygote (Khc27/+) displayed significantly increased SYT accumulation, no difference was observed between +/+ and KhcmutA animals. +/+, n = 10; KhcmutA, n = 11; Khc27/+, n = 8; elav > Khc RNAi, n = 11. ***P < 0.001, ****P < 0.0001; Mann–Whitney test. ns, not significant. Error bars indicate 95% CI.
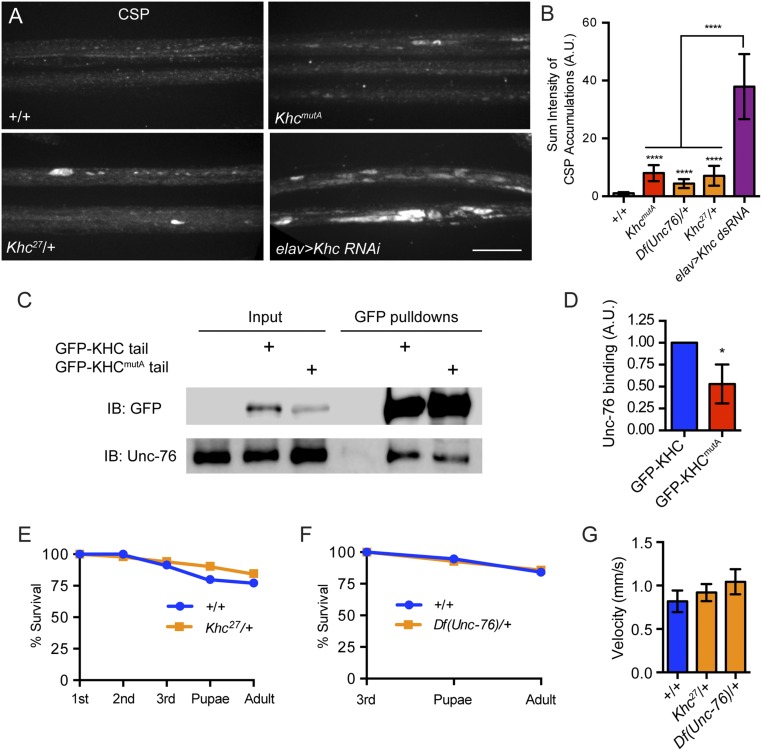
Next, we stained segmental nerves using a CSP antibody. As expected, based on previous studies, CSP robustly accumulated in segmental nerves after kinesin-1 depletion in elav > Khc RNAi larvae (28). In contrast, the segmental nerves of KhcmutA larvae displayed mild accumulation of CSP, whereas control larvae displayed no accumulations (Fig. S7 A and B). Because the kinesin-1–binding protein Unc-76 is important for CSP transport (43), we wondered if the quadruple-alanine mutation in KhcmutA affected the binding site of Unc-76, which is near the C-terminal MT-binding site and IAK domain (44). To test this possibility, we performed pull-downs using GFP-KHC tail and GFP-KHCmutA tail S2 cell lines. We found that although GFP-KHCmutA tail interacted with Unc-76, this binding was decreased to ∼50% of the wild-type level (Fig. S7 C and D). We attempted to recapitulate the KhcmutA CSP phenotype by decreasing either kinesin-1 or Unc-76 protein levels using protein-null heterozygotes, Khc27/+ and Df(Unc-76)/+. We found that many Khc27/+ and Df(Unc-76)/+ animals displayed mild CSP accumulations (Fig. S7B). Khc27/+ animals also displayed mild SYT accumulations, which were not observed in KhcmutA larvae (Fig. S6). Importantly, both Khc27/+ and Df(Unc-76)/+ animals display no significant viability or locomotion defects (Fig. S7 E–G), although they display mild CSP accumulations in segmental nerves. KhcmutA animals, on the other hand, display both viability and locomotion defects (Fig. 3). These results suggest that the mild CSP accumulations observed in KhcmutA animals are likely not responsible for the observed phenotypes.
Fig. S7.
KhcmutA larvae display mild CSP accumulations in segmental nerves, but this defect cannot account for the strong KhcmutA phenotypes. (A) Representative micrographs of segmental nerves of third-instar larvae, stained with an antibody against CSP. Kinesin-1 depletion (elav > Khc dsRNA) results in striking accumulations of CSP in segmental nerves, which are not observed in control. KhcmutA and Khc27/+ larvae display mild CSP accumulations. (Scale bar, 20 μm.) (B) Sum pixel intensity quantification of CSP accumulations. All genotypes display statistically significant CSP accumulations compared with control larvae. However, KhcmutA animals display an intermediate phenotype that is significantly different from elav > Khc dsRNA. Unc-76 and KHC protein-null heterozygotes, Df(Unc-76)/+ and Khc27/+, also display intermediate phenotypes. +/+, n = 14; KhcmutA, n = 15; Df(Unc76)/+, n = 17; Khc27/+, n = 12; elav > Khc RNAi, n = 12. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. A.U., arbitrary units. (C) Pull-down of control, GFP-KHC tail, and GFP-KHCmutA tail from S2 cells stably expressing the constructs. Unc-76 binds to both KHC and KHCmutA, but shows lowered affinity for KHCmutA. IB, immunoblot. (D) Quantification of Unc-76 binding. The ratio of Unc-76 to KHC signal was quantified from more than three pull-down experiments. Unc-76 displayed 50% of the normal affinity for KHCmutA. Error bars indicate SD. *P < 0.05; Mann–Whitney test. (E) Survival curve of +/+ and Khc27/+ animals. No difference was detected in viability between +/+ and Khc27/+ animals. +/+, n = 35; Khc27/+, n = 52. (F) Survival curve of +/+ and Df(Unc-76)/+ females. Because Unc-76 is X-linked, female heterozygotes were selected to perform the experiment. Female third-instar larvae from both genotypes were identified based on their gonad size. No difference was detected in viability between +/+ and Df(Unc-76)/+ females. +/+, n = 19; Df(Unc-76)/+, n = 14. (G) Motility of third-instar larvae. Both Khc27/+ and Df(Unc-76)/+ larvae moved at rates similar to control larvae. +/+, n = 16; Khc27/+, n = 20; Df(Unc-76)/+, n = 15. Error bars indicate 95% CI.
MT Sliding Is Required for Dendritic Arborization.
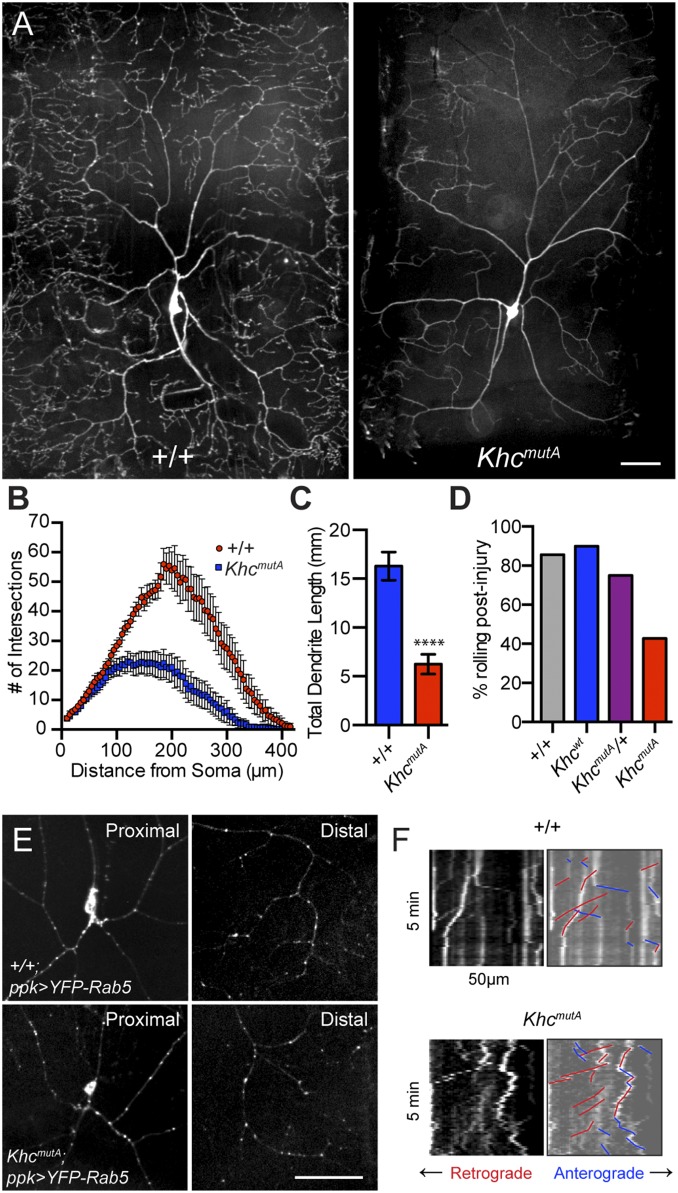
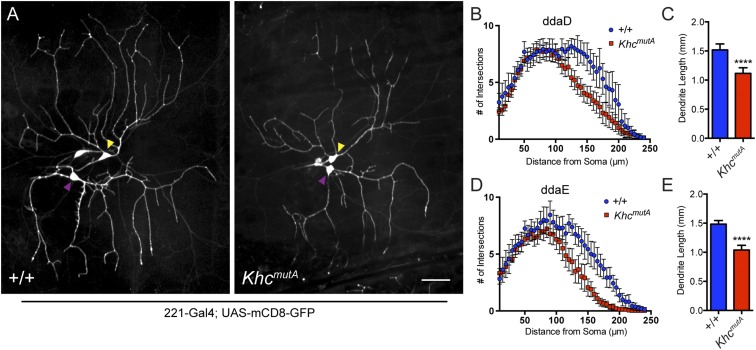
A previous study has implicated kinesin-1 in dendritic branching in class IV dendritic arborization (da) sensory neurons (45). These neurons display complex arborization patterns and are easily imaged in intact third-instar larvae. Kinesin-1 and dynein-based transport of Rab5 vesicles was implicated in dendritic branching, but we wondered if MT sliding played a role in dendrite outgrowth. We generated KhcmutA flies expressing a GFP membrane marker in class IV neurons (ppk > mCD8-GFP) to image the overall morphology of the neurons. Class IV da neurons displayed a striking phenotype in KhcmutA larvae: The dendritic trees were greatly reduced with less arborization (Fig. 6A). Sholl analysis (46) demonstrated that KhcmutA arbors contain significantly fewer branches (Fig. 6B); additionally, the total dendrite length of KhcmutA neurons was reduced (Fig. 6C). Although KhcmutA larvae were slightly smaller than control, this finding could not account for the difference observed in dendritic arbors, because the size of abdominal segments in KhcmutA larvae was only 9.6% (segment A1) or 14.4% (segment A6) smaller than control when measured from anterior to posterior. We also examined the dendritic trees of class I neurons using 221-Gal4. We observed reduced branching and shortened dendrites in class I da neurons in KhcmutA larvae (Fig. S8 A–E), suggesting that MT sliding may be a general mechanism for dendritic branching.
Fig. 6.
MT sliding contributes to dendritic arborization in Drosophila da neurons. (A) Class IV da neurons imaged in segment A6 of control and KhcmutA larvae. Note the reduced arborization in the KhcmutA larva. (Scale bar, 50 μm.) (B) Sholl analysis indicated that KhcmutA neurons contain less complex dendritic trees. (C) Total dendrite length is also reduced in KhcmutA neurons compared with control. +/+, n = 16; KhcmutA, n = 24. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI. (D) Stereotypical dorsal-ventral rolling behavior was assayed in Drosophila larvae after mechanical stimulation. A total of 42.9% of KhcmutA larvae responded to this stimulation, whereas 85.7% and 90% of +/+ and Khcwt larvae responded, respectively. +/+, n = 7; Khcwt, n = 10; KhcmutA/+, n = 12; KhcmutA, n = 28. (E) Rab5 vesicles were visualized in class IV da neurons in +/+ and KhcmutA larvae using a transgene (UASp-YFP-Rab5). Rab5 vesicles were detected in proximal and distal dendrite arbors in +/+ and KhcmutA larvae. +/+, n = 13; KhcmutA, n = 12. (Scale bar, 50 μm.) (F) Time-lapse images of Rab5 vesicles near the cell body of class IV da neurons were acquired in live +/+ and KhcmutA larvae. Kymographs of YFP-Rab5 time lapses from +/+ and KhcmutA larvae suggest that both genotypes support Rab5 transport. Retrograde transport events were colored red, and anterograde transport events were colored blue (Movie S11). +/+, n = 7; KhcmutA, n = 7.
Fig. S8.
MT sliding by kinesin-1 contributes to dendritic outgrowth in class I da neurons. (A) Class I da neurons in wild-type and KhcmutA larvae expressing 221-Gal4, UAS-mCD8-GFP. Yellow arrowheads indicate cell bodies of class I dorsal dendritic arborization D (ddaD) neurons, and purple arrowheads indicate cell bodies of class I dorsal dendritic arborization E (ddaE) neurons. (Scale bar, 50 μm.) (B and D) Sholl analysis of KhcmutA ddaD and ddaE neurons. KhcmutA neurons displayed significantly reduced dendritic branching in distal regions of their dendritic trees. Error bars indicate 95% CI. (C and E) KhcmutA class I neurons displayed reduced total dendritic length compared with control. +/+ ddaD, n = 16; KhcmutA ddaD, n = 30; +/+ ddaE, n = 16; KhcmutA ddaE, n = 31. ****P < 0.0001; Mann–Whitney test. Error bars indicate 95% CI.
We wondered if these defects in dendritic branching had an effect on sensory processing and behavior. Class IV da neurons have been reported to detect noxious thermal (47) and mechanical stimuli (48, 49), triggering larvae to engage in escape behaviors to evade predators such as parasitoid wasps (50, 51). Previous studies have shown that noxious mechanical stimulation to the middle segments of third-instar larvae results in a reproducible, dorsal-ventral rolling escape behavior (51). Using this rolling behavior as an output, we assayed nociception in KhcmutA larvae. Although a majority of +/+ and Khcwt larvae responded to mechanical stimulation, less than half of KhcmutA larvae responded (Fig. 6D). This finding suggests that the reduction in dendritic arborization seen in KhcmutA class IV da neurons partially desensitizes the larvae to noxious stimuli.
Next, we assayed the distribution of Rab5 vesicles in class IV da neurons to determine whether their transport was impaired, which might explain the reduced arborization. We found Rab5 vesicles in the distal branches of both control and KhcmutA larvae (Fig. 6E), suggesting no defects in Rab5 transport caused by KHCmutA. This result is in contrast to the phenotypes observed in Khc-null animals, where class IV da neurons display very few Rab5 vesicles in distal dendrites and increased accumulation of Rab5 vesicles near the cell body (45). This accumulation of Rab5 vesicles near the cell body results in a striking proximal branching phenotype, which was also not observed in KhcmutA larvae. Time-lapse imaging of Rab5 transport in living, intact larvae also demonstrated that both anterograde and retrograde transport of Rab5 vesicles was unimpaired in KhcmutA animals (Fig. 6F and Movie S11). Altogether, these results demonstrate a previously unidentified role for kinesin-1–based MT sliding in dendritic arborization in vivo.
Discussion
For decades, it has been known that kinesin-1 plays an important role in the nervous system. In vertebrates, knockout of the major kinesin-1 gene, KIF5B, results in embryonic lethality (52). In flies, kinesin-1–null mutants exhibit synaptic dysfunction, locomotion defects, paralysis, and eventual death before reaching pupal stages (13, 14). Kinesin-1 mutation results in an accumulation of synaptic proteins, such as SYT, in swellings along the axon shaft (28). These swellings contain both retrograde and anterograde motors and cargoes, and they are thought to act as traffic jams, blocking fast retrograde and anterograde transport (42). Axonal swellings in kinesin-1 mutants have been compared with pathological swellings observed in human diseases such as amyotrophic lateral sclerosis and spinal muscular atrophy, and were therefore hypothesized to be the primary cause of kinesin-1 mutant lethality. However, contrary to this traffic jam model, live imaging demonstrated that cargoes could robustly move through axonal swellings (53), suggesting that kinesin-1 mutant lethality may be more complicated than general defects in cargo transport.
We have previously shown that in addition to its role in cargo transport, kinesin-1 can transport MTs, resulting in MT sliding (6). These sliding MTs push on the plasma membrane and extend axons in cultured neurons during initial outgrowth and during regeneration after injury (7, 8). Because kinesin-1 is important for both fast axonal transport and MT-driven neurite outgrowth, the cause of early neurological phenotypes in kinesin-null animals is unclear. To determine the role of MT sliding, we needed to identify the mechanism of kinesin-1–based MT sliding and generate a mutant with reduced MT sliding.
Our hypothesis was that the nonmotor MT-binding site in the C terminus of kinesin-1 (15) acts as an MT adapter, allowing kinesin-1 to bind one MT and carry it as a cargo along the second MT, causing MT sliding. This C-terminal MT-binding site has been extensively characterized biochemically (16, 17), but its biological role was not clear. In this study, we created a mutation in the C-terminal MT-binding domain of KHC by substituting four charged residues with alanines (RKRYQ to AAAYA, called KHCmutA). In agreement with the biochemical data on human KHC, this mutant has reduced affinity for MTs in vitro and in cells. Here, we demonstrate, for the first time to our knowledge, that the C-terminal MT-binding domain of KHC is required for cytoplasmic MT sliding. KHCmutA displayed significantly reduced MT sliding compared with wild-type KHC. Importantly, the ability of KHCmutA to bind many cargo adapters and transport cargoes was similar to wild-type KHC. This finding is in agreement with a study in Caenorhabditis elegans, which demonstrated that a similar mutation in the C-terminal MT-binding site of kinesin-1 did not affect mitochondria transport (11).
To determine the role of MT sliding in vivo, we replaced the genomic locus of kinesin-1 with our quadruple-alanine mutant, generating KhcmutA flies. We found that a majority of homozygous KhcmutA animals died before adulthood and larvae displayed locomotion defects, as has been described in kinesin-1 mutants (13). Interestingly, mutation of many kinesin-1 cargo adapters, such as KLC, also results in locomotion defects (54). We speculate that defects in organelle transport may affect delivery of neurotransmitter-containing vesicles, whereas defects in MT sliding may affect neuronal wiring. Either of these defects will impair neuronal communication, potentially resulting in similar phenotypic outputs: locomotion defects and eventual lethality. In support of this idea, depletion of the MT-sliding regulator, Pavarotti/MKLP1, results in locomotion and viability defects (9).
Inhibition of MT sliding also resulted in additional phenotypes. KhcmutA adults are smaller, walk slowly, have trouble eclosing from the pupal case, and incessantly rub their legs together. The lethality observed in these flies seems to be largely caused by neuronal phenotypes, because neuron-specific expression of wild-type KHC greatly alleviated KhcmutA lethality. Additionally, we were able to delay lethality in kinesin-1-null Khc27 larvae by neuronal expression of wild-type KHC, but not KHCmutA. Khc27 larvae with neuronal wild-type KHC expression appeared to have no progressive paralysis or locomotion defects as seen in kinesin-1–null larvae. However, late third-instar larvae, which had previously moved vigorously and appeared healthy, abruptly became paralyzed and died. We suspect that this phenotype could be based on a muscle defect, suggesting that MT sliding is also important in nonneuronal tissue. This idea is consistent with a recent study demonstrating the importance of MT sliding and MAP4 regulation during muscle cell differentiation (55).
Interestingly, KhcmutA/+ flies, which contain one wild-type copy and one mutant copy of KHC, display intermediate phenotypes: Flies have reduced viability, shorter axons in cultured neurons, and significantly reduced MT sliding. Because kinesin-1 molecules contain two heavy chains, many kinesin-1 molecules in KhcmutA/+ flies are likely wild-type KHC and KHCmutA heterodimers, with one wild-type and one deficient MT-binding site. Therefore, the defects observed in KhcmutA/+ flies suggest that KHC and KHCmutA heterodimers cannot slide MTs as efficiently as wild-type KHC, and that the C-terminal MT-binding sites on both heavy chains are required for MT sliding. Because MT binding by kinesin-1 is electrostatic, a reduced number of binding sites should lower the binding affinity and could add an additional layer of regulation to MT sliding in the cell. One caveat for these experiments, however, is that the mothers of KhcmutA/+ flies were either homozygous or heterozygous KhcmutA. It is possible that early developmental defects caused by maternal KhcmutA could be responsible for some of the observed phenotypes instead of a dominant-negative effect.
Previously, we have reported kinesin-1–based MT sliding in tissue culture cells and culture neurons (6, 7, 9). Here, we demonstrate that robust MT sliding occurs in neurons in vivo. This sliding is kinesin-1–dependent and shares the same mechanism as sliding in S2 cells and cultured neurons. Additionally, we have reported that kinesin-1 is required for neurite outgrowth and axon regeneration in cultured neurons (7, 8). Here, we directly test the hypothesis that MT sliding by kinesin-1 drives axon outgrowth. KhcmutA neurons displayed significantly shorter axons in culture and in vivo, suggesting that MT sliding drives axon outgrowth. Expression of an engineered chimeric motor, which can slide MTs but cannot support organelle transport, in KhcmutA neurons rescued axon outgrowth defects in culture and in vivo. These provocative results demonstrate that MT sliding by kinesin-1 is essential for axon outgrowth.
In this study, we have attempted to separate the roles of kinesin-1 in cargo transport and MT–MT sliding. Mutation of kinesin-1’s C-terminal MT-binding site (KHCmutA) resulted in significantly reduced MT sliding in Drosophila S2 cells, primary cultured neurons, and sensory neurons in intact pupae. We have demonstrated that KHCmutA displayed normal affinity for the primary cargo adapter, KLC, as well as for the mitochondrial adapter, Milton. KHCmutA maintains normal peroxisome and mitochondria transport in both Drosophila S2 cells and primary cultured neurons. No defects in SYT transport were detected in segmental nerves in vivo, and Rab5 vesicle distribution and transport were maintained in class IV da neurons in KhcmutA larvae. However, mild accumulation of CSP was observed in the segmental nerves of KhcmutA animals, raising the possibility that defects in CSP transport might contribute to some KhcmutA phenotypes. However, we also observed mild accumulation of CSP in other genotypes [Khc27/+ and Df(Unc-76)/+], which did not result in locomotion and viability defects. However, we cannot rule out the possibility that CSP accumulation in segmental nerves may contribute to some KhcmutA phenotypes.
Finally, we demonstrate a previously unidentified role of MT sliding in dendritic outgrowth in da sensory neurons. KhcmutA larvae display reduced dendritic branching and total dendrite length in class I and IV da neurons, suggesting that MT sliding is important for arborization. This result is consistent with a previous study in C. elegans, where the authors demonstrate that mutations in the C terminus of the kinesin-1 ortholog, unc-116, result in dendritic phenotypes in vivo (11). A unc-116 truncation mutant lacking the C terminus, including the MT-binding site, displayed axon-like MT polarity in dendrites, as well as mislocalization of axonal cargoes to dendrites. Additionally, the authors observe the same phenotype using a mutation of the C-terminal MT-binding site analogous to the KHCmutA mutation. Consistent with our data, their MT-binding site mutant also rescues mitochondria transport. The authors speculate that kinesin-1/unc-116 transports MTs to organize dendritic MT arrays.
Because of the high conservation of the C-terminal MT-binding site of kinesin-1 and important nervous system phenotypes observed in both Drosophila and C. elegans, we speculate that MT sliding plays an important physiological role in all organisms. Consistent with this idea, we have observed MT sliding in vertebrate cells (6). Furthermore, we demonstrated that human kinesin-1, KIF5B, can slide MTs and generate cellular projections in Drosophila S2 cells depleted of endogenous kinesin-1, suggesting KIF5B also engages in sliding. Future studies of kinesin-1–driven MT sliding are warranted in mammalian systems and may provide new insight into the early formation and pathology of the human nervous system.
Materials and Methods
Additional information is available in the detailed SI Materials and Methods.
MT Sliding Analysis.
A custom Java-based Fiji plug-in was developed to quantify MT sliding rates using the following methodology. Time-lapse movies of photoconverted microtubules were bleach-corrected and thresholded to detect MTs. The initial photoconverted zone was identified, and the number of pixels corresponding to MTs was measured in total or outside the initial zone for each frame. The motile fraction of MTs was calculated for each frame by the following equation: %MF = MTsoutside_initial_zone/MTstotal. These values were then plotted against time, and the slope of this graph was calculated for the initial linear section (identified by the highest R2 value of a linear regression containing at least four data points). This slope represents the gross MT sliding rate in each cell with the units: Change in % Motile Fraction * min−1.
To measure MT sliding in bristle neurons in vivo, a Fiji plug-in, StackReg (>Translation), was used to align the position of the neurons, reducing the effect of small movements of the animal during imaging. Then, another Fiji plug-in, Bleach Correction (>Histogram), was used to minimize the bleaching effect of the red signal. Finally, the fluorescence intensity outside the initial photoconverted zone was measured at 0 min and 20 min. The percentage of fluorescence leaving the converted zone was used to indicate the level of MT sliding in these neurons, which was determined by the following equation: (Fluorescenceoutside_20min − Fluorescenceoutside_0min)/Fluorescencetotal_20min.
Organelle Transport Analysis.
Peroxisome transport and mitochondria transport were quantified by the particle-tracking software DiaTrack 3.04 (56). To determine average run length, all active transport events (particle run lengths) were defined as having a span of >0.5 μm and lifetime of longer than four frames. All run lengths in each time-lapse movie were summed and divided by total number of particles (both motile and immotile), resulting in the average run length per cell. To determine the distribution of run lengths, run lengths in each cell were binned with a bin width of 1 μm using Prism 6 (GraphPad) and divided by the total particle count, resulting in the fraction of run lengths in each bin. To determine average particle velocities, particles were tracked for at least four frames and their velocity was calculated; the average velocity of all particles was then determined per cell (including slow and immotile particles). To determine the distribution of particle velocities, individual velocities were binned with a bin width of 50 nm⋅s−1 on Prism 6 and divided by the total count, resulting in the fraction of velocities in each bin.
SI Materials and Methods
Fly Stocks.
Flies were maintained at room temperature on regular cornmeal food, supplemented with dry active yeast. To collect embryos for survival curves, crosses were flipped overnight to black agarose plates (50% apple juice, 1% agarose, 0.3% activated charcoal, 0.4% propionic acid). The addition of apple juice and yeast is essential to maintain optical female fecundity and to supply developing larvae with food. Stocks used in this study include UASp-tdEOS-αtub84B (no. 51313; Bloomington Stock Center) (7); elav-Gal4 (a gift from C. Doe; University of Oregon, Eugene, OR); ppk-Gal4, UASt-mCD8-GFP and 221-Gal4, UASt-mCD8-GFP (gifts from M. Rolls; Penn State University, University, Park, PA); UASp-GFP-SKL [no. 28882; Bloomington Stock Center (donated by J. Brenman)]; UASp-YFP-Rab5 [no. 24616; Bloomington Stock Center (donated by H. Bellen)]; ppk-Gal4 [no. 32079; Bloomington Stock Center (donated by P. Adler)]; creMos1.hs [no. 1092; Bloomington Stock Center (donated by D. Hartl)]; and Khc27 (a gift from W. Saxton, University of California, Santa Cruz, CA) (14). Stocks generated for this study include KhcKO-attP; Khcwt; KhcmutA; UASp-KHC-BFP (III); UASp-KHCmutA-BFP (III); UASp-UncLZ-BFP (III)/(II); UASp-UncLZ-Ktail-BFP (III)/(II); and UASp-UncLZ-KtailmutA-BFP (III)/(II). Khcwt and KhcmutA flies were generated by φC31 recombination of wild-type KHC or KHCmutA kinesin-1 locus from pGE-attB-GMR-Khcwt or pGE-attB-GMR-KhcmutA constructs into KhcKO-attP flies. Transgene and φC31 constructs were sent to BestGene for injection. UASp transgenes were generated through a standard P element-mediated transformation by BestGene.
Drosophila S2 Cell Culture.
Cells were cultured in Insect-Xpress media (Lonza) at 25 °C. GFP-KHC tail cell lines were generated by cotransfection of pMT-GFP-KHC tail and pCoHygro plasmids at a ratio of 20:1 and cultured with 250 μg/mL hygromycin in Insect-Xpress media for 5 wk. For MT sliding experiments in S2 cells, 750 ng of each KHC construct and 250 ng pMT-tdEOS-tubulin were transfected into 1 × 106 cells using Effectene (Qiagen). For peroxisome transport experiments, KHC constructs and pAC-GFP-SKL were transfected at a ratio of 4:1 using Effectene. Expression of pMT constructs was induced by adding 200 μΜ CuSO4 to the media for 48 h. Endogenous kinesin-1 was depleted by two treatments of 18 μg of dsRNA against the 3′-UTR over a 5-d period (immediately after plating and on day 3; cells were also induced with CuSO4 on day 3 and imaged on day 5).
Recombinant Protein.
K959 and K959mutA were expressed and purified from Escherichia coli BL21 (DE3) cells using TALON metal affinity resin (Clontech) according to standard protocols. MBP was fused to the N terminus of the protein fragments to increase solubility.
Microscopy and Photoconversion.
To image Drosophila S2 cells, cultured neurons, and pupal sensory neurons, a Nikon Eclipse U200 inverted microscope with a Yokogawa CSU10 spinning disk confocal head, Nikon Perfect Focus system, and 100×/1.45-N.A. objective was used. The da neurons were imaged with a 20×/0.75-N.A. objective, and stained larval brains were imaged with a 40×/0.95-N.A. objective. Images were acquired with an Evolve EMCCD (Photometrics) using Nikon NIS-Elements software (AR 4.00.07 64-bit). S2 cells expressing tdEOS-tagged were photoconverted for 6 s using 405-nm light from a light-emitting diode light source (89 North Heliophor), which was constrained to a small circle by an adjustable diaphragm. Cultured neurons expressing tdEOS-tubulin were photoconverted for 10 s, and sensory neurons expressing tdMaple3-tubulin were photoconverted for 15 s. Images were collected every minute for >5 min in S2 cells, every minute for 20 min in pupal sensory neurons, and every 5 s for >1.5 min in cultured neurons. Peroxisome transport in S2 cells was acquired using the same microscope, with a time interval of 5 s for 2.5 min. Mitochondria movies were acquired using a Nikon Eclipse U2000 inverted microscope with a complementary metal-oxide-semiconductor (CMOS) camera, ORCA-Flash4.0 V2 C11440-22CU (Hamamatsu Photonics), and 100×/1.4-N.A. objective. Images were acquired every 5 s for 2.5 min. YFP-Rab5 vesicles were imaged using a Nikon Ti Eclipse microscope equipped with perfect focus (second generation), a Yokogawa CSU-X1 spinning disk confocal head, and a 20×/0.75-N.A. objective. Images were acquired with an iXon EMCCD using Metamorph. Z-series images were acquired and stitched together to determine YFP-Rab5 distribution. Time-lapse movies consist of a single plane imaged every 5 s for 5 min.
Constructs.
The following constructs were generated for this study. pMT-BFP, mTag2-BFP, was inserted into pMT.A by NotI and XbaI. pMT-KHC-BFP, full-length KHC cDNA, was inserted into pMT-BFP by EcoRI and NotI with an N-terminal GGGS linker. pMT-KHCmutA-BFP, a synthetic oligo, was ordered from GeneArt (ThermoFisher Scientific) and encoded amino acids 870–975 with quadruple-alanine point mutations in the MT-binding site (914–918, RKRYQ to AAAYA; KHCmutA); this oligo was inserted into pMT-KHC-BFP using an endogenous MfeI site and NotI. pMT-EmGFP, emerald GFP, was inserted into pMT by KpnI and BamHI with C-terminal GSGPGPEF linker. pMT-EmGFP-KHC, full-length KHC cDNA, was inserted into pMT-EmGFP by EcoRI and NotI. pMT-EmGFP-KHCmutA, full-length KHCmutA cDNA, was inserted into pMT-EmGFP by EcoRI and NotI. pMT-EmGFP-KHC 345–975, KHC cDNA encoding amino acids 345–975, was inserted into pMT-EmGFP by EcoRI and NotI. pMT-EmGFP-KHCmutA 345–975, KHC cDNA encoding amino acids 345–975, was inserted into pMT-EmGFP by EcoRI and NotI. UASp-KHC-BFP, full-length KHC cDNA, was inserted by EcoRI and NotI. mTag2-BFP was inserted by NotI and XbaI. UASp-KHCmutA-BFP, full-length KHCmutA cDNA, was inserted by EcoRI and NotI. pMT-UncLZ-BFP, Caenorhabditis elegans unc-104/kinesin-3 motor domain (amino acids 1–389), fused to a leucine zipper (VKQLEDKVEELASKNYHLENEVARLKKLV) (23) was inserted by KpnI and SpeI into pMT-BFP. pMT-UncLZ-Ktail-BFP, amino acids 905–975 of KHC were inserted into pMT-UncLZ-BFP by SpeI and NotI. pMT-UncLZ-KtailmutA-BFP, amino acids 905–975 of KHCmutA were inserted into pMT-UncLZ-BFP by SpeI and NotI. UASp-UncLZ-BFP, UASp-UncLZ-Ktail-BFP, and UASp-UncLZ-KtailmutA-BFP, inserts from pMT-UncLZ constructs, were cloned by KpnI and NotI into UASp-BFP. pGE-attB-GMR-Khcwt, wild-type genomic kinesin-1 locus in pGE-attB-GMR donor cassette containing attB site for site-specific recombination and GMR-miniwhite+ (GMR-miniw+) flanked by loxP sites. pGE-attB-GMR-KhcmutA, a synthetic oligo gene block was ordered from (Integrated DNA Technologies) encoding parts of KHC exons 3 and 4 with quadruple-alanine mutations in the C-terminal MT-binding site (KHCmutA); this oligo was inserted in pGE-attB-GMR-Khcwt using endogenous XhoI and Rsr II sites. Constructs were generated using standard PCR, restriction enzyme digestion, and DNA ligation techniques.
Genomic Replacement of Kinesin-1 Locus.
To replace the endogenous Khc locus, the KhcKO-attP strain was generated using an ends-out gene-targeting approach as previously described (29, 31). The endogenous Khc gene was replaced with an attP site through homologous recombination using a donor template. The donor contained targeting homology arms, GMR-mini-w+ flanked by loxP sites, and an attP site, which facilitates the knock-in of designer Khc alleles. This approach resulted in the deletion of the entire Khc gene (upstream flanking sequence: 5′-AGAATCAAACTAAAGAGACCTT-3′, downstream flanking sequence: 5′-TCACTTTTGTTTTTCCTGTCC-3′). GMR-mini-w+ was removed by crossing to flies expressing Cre recombinase (y1 w67c23; snaSco/CyO, P{w[+mC] = Crew}DH1, stock no. 1092; Bloomington Drosophila Stock Center). As a control, an integration plasmid (pGE-attBGMR) (29) containing wild-type Khc was injected (BestGene) into KhcKO-attP embryos and integrated at the Khc locus through ϕC31-mediated recombination. The resulting Khcwt flies are homozygous-viable and fertile, and display no apparent phenotypes. To generate the KhcmutA strain, KhcKO-attP embryos were injected with a construct containing the quadruple-alanine microtubule binding site mutation. Western blot analysis of fly extracts confirmed that KhcmutA is expressed in adult flies at levels comparable to Khcwt and endogenous KHC.
Nociception Assay.
The nociception assay was performed in accordance with previous protocols (47), with some modifications. Wandering third-instar larvae were collected, placed in a cell strainer, and immersed in water such that their ventral sides could still touch the bottom on the dish. Noxious mechanical stimulation was delivered by sharply prodding the middle abdominal segments with one side of no. 5 forceps. Larvae were scored as positively responding to stimuli if at least one dorsal-ventral rolling response was induced after two attempts.
Optic Lobe Staining.
Optical lobes were dissected from third-instar larva in 1× PBS and fixed in 4% (wt/wt) formaldehyde (methanol-free) diluted in PBT (1× PBS, 0.1% Triton X-100) for 20 min according to standard protocols; optical lobes were then washed five times with PBTB (1× PBS, 0.1% Triton X-100, 0.2% BSA) for 10 min and blocked in 5% (vol/vol) normal goat serum-containing PBTB for 1 h to block nonspecific antibody binding. Samples were then incubated with primary anti-Futsch antibody (1:100 of the concentrate antibody, 22C10; Developmental Studies Hybridoma Bank) at 4 °C overnight followed by five 10-min washes with PBTB, incubated with secondary anti-mouse FITC antibody (0.1 mg/mL) at 4 °C overnight, and washed with PBT 10 min for five times before mounting.
Segmental Nerve Staining.
Third-instar larvae were filleted based on a previous protocol (57). Larvae were pinned dorsal-side up onto Sylgard dishes (Sylgard 184 Silicone Elastomer Kit; Dow Corning) in 1× PBS, with one pin in the tail between the posterior spiracles and a second pin above the brain. Using spring scissors, an incision perpendicular to the body axis was made at the posterior of the larva, followed by a long incision along the dorsal midline stopping at the brain. The body walls were stretched and pinned down with two pins on each side. Internal organs were removed, being careful to not sever the segmental nerves or damage the brain. Fillets were fixed with 3.7% (wt/wt) formaldehyde in 1× PBS for 20–40 min at room temperature according to standard protocols. Fillets were washed in 1× PBT twice and blocked in 5% (vol/vol) normal goat serum (SouthernBiotech) in 1× PBT for 1 h to block nonspecific antibody binding. Primary antibodies (CSP, 1:150; SYT, 1:100) were incubated at 4 °C overnight, followed by four 10-min 1× PBS washes and incubation with tetramethylrhodamine isothiocyanate (TRITC) conjugated anti-mouse secondary antibodies (0.1 mg/mL) for 4 h at room temperature. After four 10-min 1× PBT washes, fillets were removed from Sylgard dishes and mounted between a slide and coverslip with a small spacer and in 80% (vol/vol) glycerol to provide structural support and prevent evaporation during imaging.
Supplementary Material
Acknowledgments
We thank Dr. Chris Q. Doe (University of Oregon), Dr. Melissa Rolls (Penn State University), Dr. William M. Saxton (University of California, Santa Cruz), and the Bloomington Stock Center (NIH P40OD018537) for fly stocks. The mouse monoclonal anti-Futsch (22C10) antibody contributed by Seymour Benzer (California Institute of Technology), CSP (DCSP-2, 6D6) monoclonal antibody contributed by Seymour Benzer, and SYT (3H2 2D7) monoclonal antibody contributed by Kai Zinn (California Institute of Technology) were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the Department of Biology, University of Iowa. We also thank the Center for Advanced Microscopy at Northwestern University and Constadina Arvanitis for assistance in imaging YFP-Rab5 vesicles in class IV da neurons. The research reported here was supported by the National Institute of General Medical Science of the NIH under Award R01GM052111 (to V.I.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522416113/-/DCSupplemental.
References
- 1.Zhang XF, Hyland C, Van Goor D, Forscher P. Calcineurin-dependent cofilin activation and increased retrograde actin flow drive 5-HT-dependent neurite outgrowth in Aplysia bag cell neurons. Mol Biol Cell. 2012;23(24):4833–4848. doi: 10.1091/mbc.E12-10-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3(3):a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn KC, et al. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76(6):1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283(5409):1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 5.Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984;99(6):2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolly AL, et al. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci USA. 2010;107(27):12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr Biol. 2013;23(11):1018–1023. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu W, Lakonishok M, Gelfand VI. Kinesin-1-powered microtubule sliding initiates axonal regeneration in Drosophila cultured neurons. Mol Biol Cell. 2015;26(7):1296–1307. doi: 10.1091/mbc.E14-10-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Castillo U, Lu W, Winding M, Lakonishok M, Gelfand VI. Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Curr Biol. 2015;25(2):200–205. doi: 10.1016/j.cub.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roossien DH, Lamoureux P, Miller KE. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J Cell Sci. 2014;127(Pt 16):3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- 11.Yan J, et al. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. eLife. 2013;2:e00133. doi: 10.7554/eLife.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roossien DH, Lamoureux P, Van Vactor D, Miller KE. Drosophila growth cones advance by forward translocation of the neuronal cytoskeletal meshwork in vivo. PLoS One. 2013;8(11):e80136. doi: 10.1371/journal.pone.0080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxton WM, Hicks J, Goldstein LS, Raff EC. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64(6):1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- 14.Brendza KM, Rose DJ, Gilbert SP, Saxton WM. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J Biol Chem. 1999;274(44):31506–31514. doi: 10.1074/jbc.274.44.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navone F, et al. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol. 1992;117(6):1263–1275. doi: 10.1083/jcb.117.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackney DD, Stock MF. Kinesin’s IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat Cell Biol. 2000;2(5):257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- 17.Seeger MA, Rice SE. Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. J Biol Chem. 2010;285(11):8155–8162. doi: 10.1074/jbc.M109.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong YL, Rice SE. Kinesin’s light chains inhibit the head- and microtubule-binding activity of its tail. Proc Natl Acad Sci USA. 2010;107(26):11781–11786. doi: 10.1073/pnas.1005854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlan K, Lu W, Gelfand VI. The microtubule-binding protein ensconsin is an essential cofactor of kinesin-1. Curr Biol. 2013;23(4):317–322. doi: 10.1016/j.cub.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6(2):131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subach OM, Cranfill PJ, Davidson MW, Verkhusha VV. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS One. 2011;6(12):e28674. doi: 10.1371/journal.pone.0028674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kural C, et al. Kinesin and dynein move a peroxisome in vivo: A tug-of-war or coordinated movement? Science. 2005;308(5727):1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 23.Tomishige M, Klopfenstein DR, Vale RD. Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science. 2002;297(5590):2263–2267. doi: 10.1126/science.1073386. [DOI] [PubMed] [Google Scholar]
- 24.Kuznetsov SA, et al. The quaternary structure of bovine brain kinesin. EMBO J. 1988;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173(4):545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci USA. 2004;101(50):17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould SJ, Keller GA, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144(3):1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Zhou W, Dong W, Watson AM, Hong Y. From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci USA. 2009;106(20):8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166(4):1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Zhou W, Watson AM, Jan YN, Hong Y. Efficient ends-out gene targeting in Drosophila. Genetics. 2008;180(1):703–707. doi: 10.1534/genetics.108.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J Vis Exp. 2012;(61):3795. doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos AR, Rosen DR, Robinow SN, White K. Molecular analysis of the locus elav in Drosophila melanogaster: A gene whose embryonic expression is neural specific. EMBO J. 1987;6(2):425–431. doi: 10.1002/j.1460-2075.1987.tb04772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126(2):294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- 35.Lu W, et al. Microtubule–microtubule sliding by kinesin-1 is essential for normal cytoplasmic streaming in Drosophila oocytes. Proc Natl Acad Sci USA. 2016;113:E4995–E5004. doi: 10.1073/pnas.1522424113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Castillo U, Winding M, Lu W, Gelfand VI. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. eLife. 2015;4:e10140. doi: 10.7554/eLife.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karle KN, Möckel D, Reid E, Schöls L. Axonal transport deficit in a KIF5A( -/- ) mouse model. Neurogenetics. 2012;13(2):169–179. doi: 10.1007/s10048-012-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992;117(3):595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76(3):477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 40.Simpson P, Woehl R, Usui K. The development and evolution of bristle patterns in Diptera. Development. 1999;126(7):1349–1364. doi: 10.1242/dev.126.7.1349. [DOI] [PubMed] [Google Scholar]
- 41.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. 2011;21(1):76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Martin M, et al. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10(11):3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gindhart JG, et al. The kinesin-associated protein UNC-76 is required for axonal transport in the Drosophila nervous system. Mol Biol Cell. 2003;14(8):3356–3365. doi: 10.1091/mbc.E02-12-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176(1):11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satoh D, et al. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10(10):1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 46.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein painless is important for thermal nociception but not mechanical nociception. PLoS One. 2012;7(1):e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauthner SE, et al. Balboa binds to pickpocket in vivo and is required for mechanical nociception in Drosophila larvae. Curr Biol. 2014;24(24):2920–2925. doi: 10.1016/j.cub.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20(5):429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson JL, Tsubouchi A, Tracey WD. Larval defense against attack from parasitoid wasps requires nociceptive neurons. PLoS One. 2013;8(10):e78704. doi: 10.1371/journal.pone.0078704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang RY, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17(24):2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka Y, et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93(7):1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 53.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17(4):2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gindhart JG, Jr, Desai CJ, Beushausen S, Zinn K, Goldstein LS. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141(2):443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mogessie B, Roth D, Rahil Z, Straube A. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. eLife. 2015;4:e05697. doi: 10.7554/eLife.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallotton P, Olivier S. Tri-track: Free software for large-scale particle tracking. Microsc Microanal. 2013;19(2):451–460. doi: 10.1017/S1431927612014328. [DOI] [PubMed] [Google Scholar]
- 57.Halachmi N, Nachman A, Salzberg A. Visualization of proprioceptors in Drosophila larvae and pupae. J Vis Exp. 2012;(64):3846. doi: 10.3791/3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.