Abstract
Background
The US Food and Drug Administration has not approved a treatment for cocaine addiction, possibly due in part to the fact that repeated cocaine use results in dysregulation of multiple neurotransmitter systems, including glutamate and dopamine, and an emergence of increased negative affective states and heightening motivation to take cocaine despite negative consequences. We used a combination therapy approach to assess whether modulation of both glutamate and dopamine transmission would reduce the motivation to self-administer cocaine compared to modulation of either system alone.
Methods
The metabotropic glutamate 2/3 receptor agonist, LY379268, and the monoamine releaser, phenmetrazine, were used to assess their individual and combined ability to decrease the reinforcing efficacy of cocaine because they modulate glutamate and dopamine levels, respectively. Cocaine breakpoints and cocaine intake was assessed, using a progressive ratio schedule, at baseline in three groups based on dose of cocaine (0.19, 0.38, 0.75 mg/kg/infusion), and following LY379268 (0.03 or 0.30 mg/kg; i.p.), phenmetrazine (25 mg/kg/day; osmotic minipump), and a combination of the two drugs.
Results
LY379268 and phenmetrazine alone reduced breakpoints for all doses of cocaine. The combination of the two drugs showed a concerted effect in reducing breakpoints for all doses of cocaine, with the lowest dose of cocaine reduced by as much as 70%.
Conclusions
These data support combination therapy of dopamine and glutamate systems as an effective means to reduce the motivation to take cocaine since a combination of drugs can address neurobiological dysfunction in multiple neurotransmitter systems compared to therapies using single drugs.
Keywords: combination therapy, LY379268, mGluR2/3 agonist, phenmetrazine, dopamine releaser, cocaine self administration
Graphical Abstract
1. INTRODUCTION
Drug addiction, including addiction to cocaine, is often defined as a chronically relapsing disorder comprising of compulsive drug seeking and inability to control intake. These features describe cocaine addiction because these are relatively consistent behavioral characteristics in an otherwise complicated pathology. In fact, the complex nature of cocaine addiction is perhaps a major reason why there is no effective therapy to combat cocaine addiction to date. Specifically, the lack of effective treatment may be due in part to the fact that there may be multiple predisposing factors which heighten the motivation to take cocaine, any one of which may be sufficient to induce relapse. Indeed, repeated cocaine use results in neuroadaptive changes to a number of overlapping and distinct brain circuits.
For example, extended and/or repeated exposure to cocaine results in long lasting dysregulation of metabotropic glutamate receptor 2/3 (mGluR2/3) function in the nucleus accumbens (NAc) and prefrontal cortex (PFC; Xi et al., 2002; Beveridge et al., 2011), which are located presynaptically and function as glutamate autoreceptors in the NAc. Therefore, activation of mGluR2/3 decreases presynaptic glutamate release (Conn and Pin, 1997; Cartmell and Schoepp, 2000; Pinheiro and Mulle, 2008). However, in rats with a history of cocaine exposure, mGluR2/3 function is blunted in the NAc (Xi et al., 2002; Xie and Steketee, 2009), suggesting reduced autoreceptor regulation and increased glutamate release. This would result in increased excitability of the PFC glutamatergic projections to the NAc and extend glutamatergic signaling, an effect which has been shown to enhance stress-induced reinstatement of cocaine seeking (Caffino et al., 2015). Based on previous studies, it is possible that rectifying dysregulated glutamate transmission would lead to an overall reduction in the motivation to seek cocaine (Baptista et al., 2004; Adewale et al., 2006; Hao et al., 2010).
In addition to glutamate transmission, mesolimbic dopamine dysfunction has been repeatedly observed in animals with a history of cocaine self-administration (Siciliano et al., 2015; Wilhun et al., 2015; Ferris et al., 2011). A wealth of research shows that disruption of the mesolimbic dopamine circuit plays a critical role in a phenotype expressing anhedonia (Siciliano et al., 2015). Moreover, it is often suggested that animals escalate cocaine intake in order to offset reductions in dopamine function that occur after repeated exposure (Norman et al., 2011). Indeed, the biological basis for the efficacy of dopamine agonists for the treatment of cocaine addiction is rooted in the drug’s ability to increase extracellular dopamine tone and serve as replacement therapy for cocaine. For example, continuous infusion of amphetamine via an osmotic mini-pump has been shown to reduce cocaine intake on a fixed-ratio 1 (FR1) schedule of reinforcement and reduce the motivation to self-administer cocaine as measured by a progressive-ratio (PR) schedule of reinforcement (Chiodo et al., 2008; Chiodo and Roberts, 2009; Zimmer et al., 2014).
Both glutamate and dopamine transmission have been shown to play crucial roles in regulating cocaine seeking in animals with a history of cocaine use. Therefore, the purpose of this study was to examine two compounds that have been shown to modulate glutamate and dopamine transmission for their individual and combined ability to reduce the motivation to self-administer cocaine as measured by a PR schedule of reinforcement. We chose to study the mGluR2/3 agonist, LY379268, given the fact that mGluR2/3 receptors are altered following cocaine self-administration. We also chose to study the monoamine releaser, phenmetrazine, given its ability to increase dopamine tone in a manner similar to amphetamine combined with the fact that in the clinic it can be administered orally via its prodrug, phendimetrazine, which exhibits relatively low abuse potential compared to other proposed agonist therapies like amphetamine (Bolin et al., 2016). Finally, we chose to investigate the combined effects of these compounds given increasing interest in the ability of combination therapy to reduce self-administration to a greater extent than monotherapy in addition to the fact that these drugs have been proposed to reduce cocaine self-administration, in part, through distinct mechanisms.
2. MATERIAL AND METHODS
2.1 Subjects
Male, Sprague-Dawley rats (325 – 350 g; Harlan Laboratories, Indianapolis, IN) were used for cocaine self-administration (N=26) and locomotor (N=8) experiments. Animals were maintained on a 12:12 light/dark cycle (0300 hours – lights off and 1500 hours – lights on) and were given access to food and water ad libitum throughout the entire experiment and during self-administration sessions. All rats were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
2.2 Cocaine self-administration
The detailed procedures for catheter implantation into the jugular vein have been described previously (Richardson and Roberts, 1996). Following the surgery, rats were housed individually in stainless steel custom-made experimental chambers (30×30×30 cm) 24 hours per day/7 days per week. Animals were maintained on a 12:12 hour light/dark cycle and self-administration occurred during the active/dark phase (between 0900 hours and 1500 hours).
All rats were trained to self-administer cocaine on an FR1 schedule of reinforcement prior to switching to a PR reinforcement schedule. During training, each lever press resulted in the intravenous delivery of 1.5 mg/kg/infusion cocaine (free base) over 4 seconds. Immediately following each lever press, the lever retracted and a stimulus light above the lever was activated for 20 s to signal a time-out period. The session ended after 40 cocaine injections or at the end of six hours, whichever occurred first. Typically under these conditions, rats acquired a stable pattern of cocaine self-administration and will take all 40 injections in a session within one week. Once the animal reached the maximum number of injections allowed during a single six hour session (40 injections), they were required to self-administer 40 injections for five consecutive days.
Once five days of 40 injections were completed, rats were switched to a PR schedule of reinforcement during which number of required lever presses per injection increased progressively once the previous ratio was met. On this schedule, the response requirement was increased for each consecutive infusion in the following sequence: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc. The breakpoint was defined as the number of infusions earned before a one hour period elapsed without completion of the current ratio (Richardson and Roberts, 1996). Decades of previous research and observation demonstrate that a one hour cut off is sufficient to determine breakpoints since this provides enough time to reach very high rates of responding and breakpoints did not differ when additional time was allowed (Richardson and Roberts, 1996). Separate groups of rats self-administered 0.75 mg/kg/infusion (n=6), 0.375 mg/kg/infusion (n=8), or 0.1875 mg/kg/infusion (n=12) cocaine. Rats’ responding was maintained on the PR schedule with their respective doses of cocaine until the end of the experiments.
2.3 Experimental paradigm
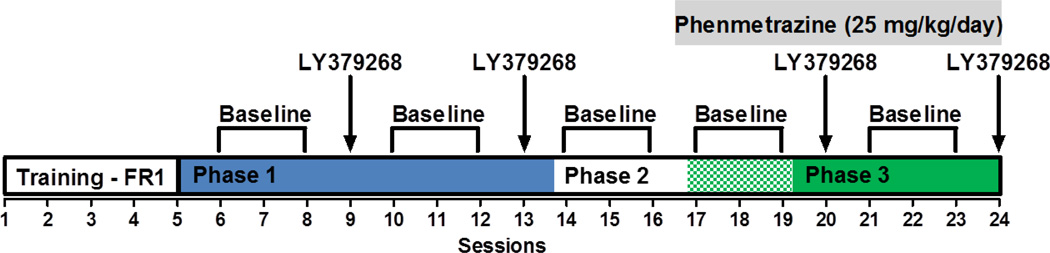
The general procedure for each experiment was the same for each dose of cocaine and the experimental timeline is presented in Figure 1. This experiment had three phases: Phase one with LY379268 alone, Phase two with Phenmetrazine alone, and Phase three with a combination of LY379268 and Phenmetrazine. This study used a within subject experimental design as all animals completed all three phases of the experiment. Details of each phase are below:
Figure 1.
Schematic of the experimental paradigm. Each session following training assessed breakpoints on a progressive-ratio schedule of reinforcement using cocaine (0.1875, 0.375, 0.75 mg/kg/infusion) across three phases based on treatment. Phase 1 (blue shaded) tested the effects of LY379268 (0.03 or 0.30 mg/kg, counterbalanced) alone, Phase 2 (non-shaded/white) allowed time for breakpoints to stabilize after the last LY379268 dose, Phase 2 (green and white checker shaded) tested the effects of phenmetrazine alone, and Phase 3 (green shaded) tested the combined effects of LY379268 and phenmetrazine on cocaine breakpoints. Osmotic mini-pump delivered phenmetrazine (25 mg/kg/day). FR1, fixed ratio 1; PR, progressive ratio.
2.3.1 Phase One (Fig. 1, blue shaded area)
Once baseline breakpoints were stable (less than 10% variation across three consecutive days), rats were administered one of the two doses of LY379268 (0.03 mg/kg or 0.3 mg/kg, i.p) and 30 mins later a PR cocaine self-administration session was started. The doses used for LY379268 in the current study were selected because these doses do not affect feeding behavior in our experimental environment (Crawford et al., 2013), but show effects on other schedules of cocaine self-administration in other laboratories (Peters and Kalivas, 2006; Justinova et al., 2015). On subsequent days, breakpoints were recorded until they again reached a stable baseline with no treatment, following which, rats were administered with the second dose of LY379268 that they had not yet received (Phase One dose order counterbalanced).
2.3.2 Phase Two
Following Phase One, breakpoints were recorded daily until they were stabilized (less than 10% variation across three consecutive days; Fig. 1, unshaded area). Rats were then implanted with a subcutaneous osmotic minipump (Model 2001; Alzet; Cupertino, CA) containing phenmetrazine (25 mg/kg/day) 18 hours prior to beginning the self-administration session. This dose was selected based on the findings in a previous study (Czoty et al., 2015) from our group showing similar effects across several doses of phenmetrazine (25 and 50 mg/kg/day, respectively) on other schedules of cocaine self-administration. Therefore we chose to use the lower dose (25 mg/kg/day) used in that study. Breakpoints were then recorded daily until they were stabilized (Fig. 1, green and white checkered area). Since minipumps provided stable drug infusion over seven days, these minipumps were replaced at the end of day seven with no break in daily self-administration session for animals that required more than seven days to reach stable breakpoint.
2.3.3 Phase Three
After completion of Phase Two, the Phase One procedures using LY379268 were repeated but this time in the presence of phenmetrazine minipumps (Fig. 1, green shaded area).
2.4 Locomotor behavior
In order to test for nonspecific locomotor effects of these drugs that could influence self-administration behavior, the individual and combined effects of LY379268 and phenmetrazine on locomotor activity were assessed in an open field activity assay and total distance traveled was recorded. Rats were habituated to the room containing locomotor assessment chambers for 30 mins prior to being placed in the open field box. Baseline locomotor behavior was recorded for 90 mins, followed by a challenge injection (saline or LY379268: 0.03 or 0.30 mg/kg; i.p.) and an additional 90 mins recording. All rats received saline and the two doses of LY379268 in a counterbalanced order. In order to test the impact of phenmetrazine plus LY379268 on locomotor behavior, the same open field assay was used with identical parameters however, this was conducted in presence of an osmotic minipumps containing phenmetrazine (25 mg/kg/day) implanted subcutaneously the day prior to locomotor assessment.
2.5 Drugs
Cocaine HCl (National Institute on Drug Abuse Drug Supply Program), LY379268 (RTI International, Raleigh, NC), (+)-phenmetrazine hemifumarate (RTI International, Raleigh, NC) were dissolved in sterile saline. Cocaine solutions that were delivered intravenously were passed through a 22 µm microfilter.
2.6 Statistical analysis
All statistical analyses were conducted and graphs were created using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Two-way analysis of variance (ANOVA) was conducted to compare drug effects on cocaine self-administration across the three doses of cocaine and locomotor activity to test non-specific effects of LY379268 and phenmetrazine. Two-way ANOVAs were followed by planned comparisons to compare dose effects of LY379268 in the presence and absence of phenmetrazine and the effects of phenmetrazine alone. One-way ANOVAs followed by planned comparisons were used to analyze individual and combined effects of LY379268 and phenmetrazine separately in the three different doses of cocaine used in this study. All data are reported as mean ± standard error of the mean. The significance level (alpha) for all statistical measures was set at p < 0.05.
3. RESULTS
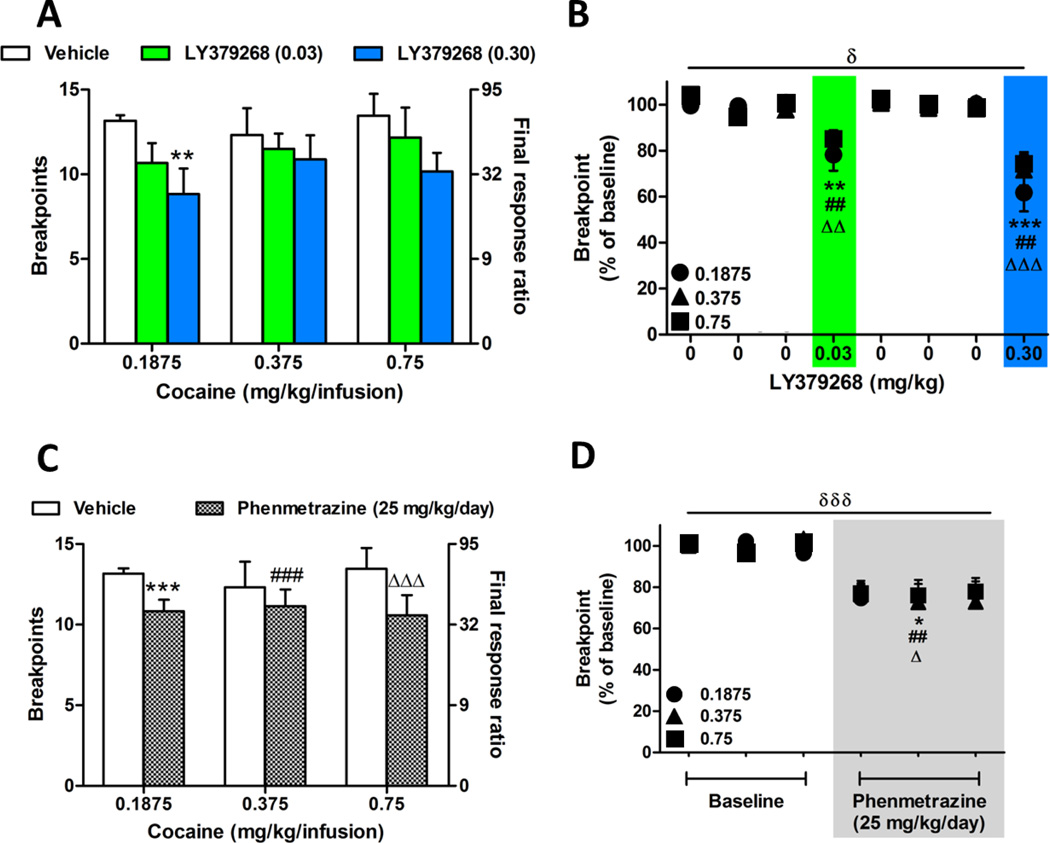
3.1 LY379268 decreases cocaine breakpoints
A single injection of LY379268 (0.03 mg/kg and 0.3 mg/kg) significantly and dose-dependently decreased breakpoints on a PR schedule of self-administration (Fig. 2A and B; Table 1). A two-way mixed ANOVA comparing the LY379268 doses across the three cocaine doses revealed an overall main effect of LY379268 on breakpoints (Fig. 2A; F(2,16) = 10.77; p < 0.001). Bonferoni comparisons revealed a significant decrease in breakpoints when LY379268 (0.30 mg/kg) was administered to rats self-administering cocaine at the 0.1875 mg/kg/infusion dose (p < 0.01). As shown in Table 1, when data are expressed as a percent of baseline, a two-way mixed ANOVA revealed a significant overall effect of LY379268 over the three doses of cocaine (Fig. 2B; percent change in breakpoints; F(1,25) = 6.18; p < 0.05). Planned comparisons revealed that the lower dose of LY379268 (0.03 mg/kg) significantly decreased cocaine breakpoints at 0.1875 mg/kg/infusion (Fig. 2B; circle; p < 0.01), 0.375 mg/kg/infusion (Fig. 2B; triangle; p < 0.01), and 0.75 mg/kg/infusion (Fig. 2B; square; p < 0.01). Similarly, the higher dose of LY379268 (0.30 mg/kg) significantly attenuated cocaine self-administration at the 0.1875 mg/kg/infusion (Fig. 2B; circle; p < 0.001), 0.375 mg/kg/infusion (Fig. 2B; triangle; p < 0.01), and 0.75 mg/kg/infusion (Fig. 2B; square; p < 0.001) doses.
Figure 2.
Effects of LY379268 and phenmetrazine alone on cocaine self-administration. (A) Effects of the lower (0.03 mg/kg; green) and higher (0.30 mg/kg; blue) doses of LY379268 relative to saline injection on increasing doses of cocaine. (B) LY379268-induced decrease in breakpoints shown in Panel A expressed as a percent of baseline. (C) Phenmetrazine minipumps (25 mg/kg/day; gray bars) significantly reduced breakpoints at each dose of cocaine during self-administration compared to saline minipumps. (D) Phenmetrazine-induced (shaded area) reduction in breakpoint across three cocaine doses in Panel C expressed as a percent of baseline. Number of animals: A, B (0.1875 mg/kg/infusion, n=12; 0.375 mg/kg/infusion, n=8; 0.75 mg/kg/infusion, n=6); C, D (0.1875 mg/kg/infusion, n=6; 0.375 mg/kg/infusion, n=8; 0.75 mg/kg/infusion, n=6). Main effect of treatment on cocaine self-administration: δp<0.05, δδδp<0.001. Post-hoc comparison of LY379268 or phenmetrazine: *p< 0.05, **p<0.01, ***p<0.001 at 0.1875 mg/kg; #p<0.05, ##p<0.01, ###p<0.001 at 0.375 mg/kg cocaine; Δp<0.05, ΔΔp<0.01, ΔΔΔp<0.001 at 0.75 mg/kg cocaine. All doses of cocaine are mg/kg/infusion. Data points are expressed as mean±SEM. Note: In panels A and C, bars are plotted for linear breakpoints on left y-axis and corresponding final response ratios on log scale are plotted on right y-axis for comparison.
Table 1.
Summary of individual and combined ability of LY379268 and Phenmetrazine to decrease breakpoints for three doses of cocaine during self-administration.
| Cocaine Dose (mg/kg/infusion) |
LY379268 (0.03 mg/kg) |
LY379268 (0.30 mg/kg) |
Phenmetrazine (25 mg/kg/day) |
LY379268+Phen (0.03 + 25) |
LY379268+Phen (0.30 + 25) |
|---|---|---|---|---|---|
| 0.1875 | 18.9** | 32.9*** | 17.7* | 63.3*** | 67.1*** |
| 0.375 | 15.5** | 21.7** | 18.6** | 48.9*** | 55.1*** |
| 0.75 | 9.69** | 24.5** | 21.4** | 35.7** | 49.3** |
Note: Data are expressed as a percent decrease in baseline responding and significance pertains to comparisons with baseline responding with no treatment drugs. Phen, phenmetrazine.
p<0.05,
p<0.01,
p<0.001.
3.2 Phenmetrazine reduces cocaine breakpoints
Following administration of the second dose of LY379268, stable baseline self-administration was reestablished for each rat. Osmotic minipumps were then implanted and rats were allowed to self-administer again to investigate the effects of phenmetrazine (25 mg/kg/day) delivered via osmotic mini-pump on breakpoints. Phenmetrazine significantly attenuated cocaine breakpoints (Fig. 2C; F(1,16) = 107.6; p < 0.001). Bonferonni comparisons showed that phenmetrazine significantly attenuated breakpoints for each all three cocaine doses (p < 0.001). For data expressed as a percent of baseline, a two-way mixed ANOVA revealed an overall effect of phenmetrazine on breakpoints (Fig. 2D; Table 1; percent change in breakpoints; F(1,19) = 29.56; p < 0.001). Continuous administration of phenmetrazine (25 mg/kg/day) significantly reduced cocaine self-administration at 0.1875 mg/kg/infusion (Fig. 2D, circle; p < 0.05), 0.375 mg/kg/infusion (Fig. 2D, triangle; p < 0.01), and 0.75 mg/kg/infusion (Fig. 2D, square; p < 0.05).
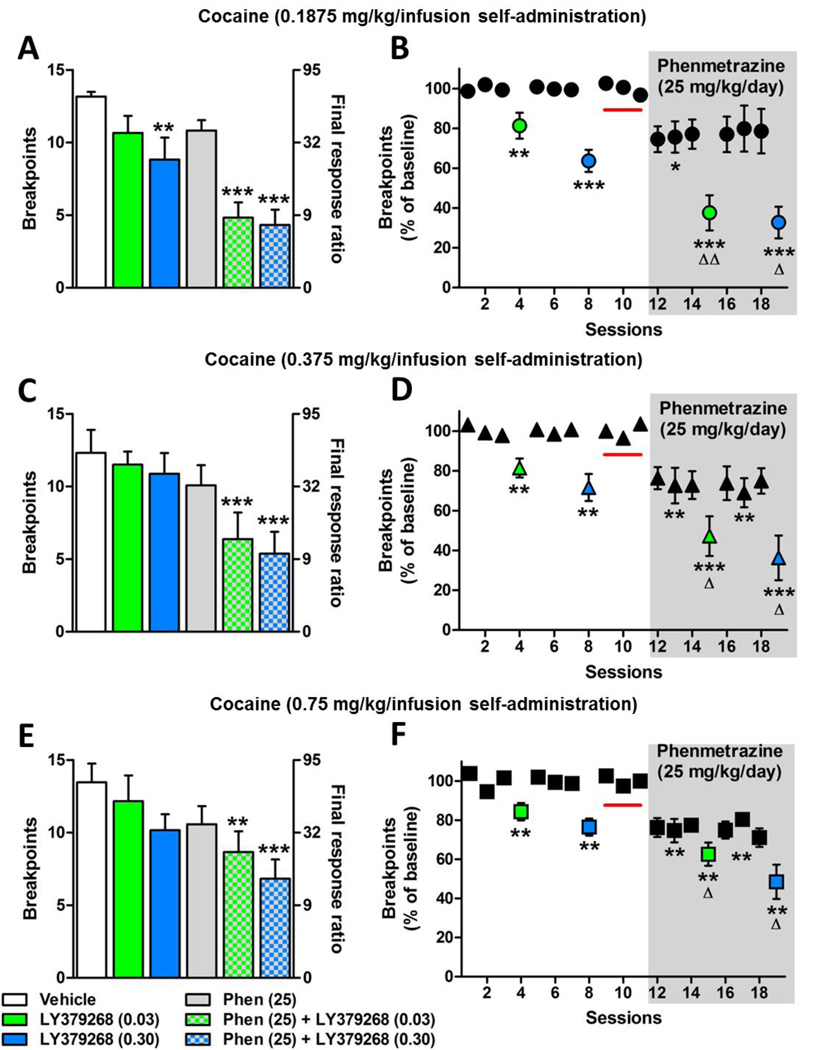
3.3 The combined effects of LY379268 and Phenmetrazine
After baseline breakpoints were established with the phenmetrazine minipump, the LY379268 injection protocol was repeated in order to examine the combined effect of LY379268 and phenmetrazine on breakpoints. To be as conservative as possible for these analyses, the individual effects of each treatment were included in the ANOVA despite having been analyzed in the previous sections. For the group self-administering 0.1875 mg/kg/infusion dose of cocaine, a one-way repeated measures ANOVA revealed a significant effect of treatment (Fig. 3A; F(5,30) = 11.52; p < 0.001). Tukey’s comparison showed that breakpoints decreased significantly following the LY379268 (0.30 mg/kg; p < 0.01), phenmetrazine + LY379268 (0.03 mg/kg; p < 0.001) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments. Similarly, when these data were expressed as percent of baseline (Table 1), a repeated measures one-way ANOVA revealed a significant effect of treatment (Fig. 3B; F(8,45)= 13.82; p < 0.0001). Tukey’s comparison showed that breakpoints decreased significantly following the LY379268 (0.30 mg/kg; p < 0.001), phenmetrazine + LY379268 (0.03 mg/kg; p < 0.001) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments when compared to the baseline breakpoints during phenmetrazine alone. Additionally, Tukey’s comparison revealed that phenmetrazine administration alone and phenmetrazine + LY379268 administration decreased cocaine self-administration for both doses when compared with the pre-minipump baseline (Fig. 3B; p < 0.001) and phenmetrazine + either dose of LY379268 showed an augmented decrease in breakpoints compared to the effect of LY379268 alone (Fig. 3B; 0.03 mg/kg, p < 0.01; 0.30 mg/kg, p < 0.05). The effect of the two drugs was a 65% decrease in breakpoints for the lower dose (0.03 mg/kg) of LY379268 and a 70% decrease in breakpoints at the higher dose (0.30 mg/kg) compared to a 20% – 35% decrease with 0.03 mg/kg and 0.30 mg/kg LY379268, respectively, and a 25% decrease with phenmetrazine (25 mg/kg/day) alone. This reduction in breakpoints observed following the drug combinations exceeded the summation of the effects of either drug alone. These effects are summarized in Table 1.
Figure 3.
The individual and combined effects of phenmetrazine (shaded/gray area) and LY379268 (0.03 mg/kg, green; 0.30 mg/kg, blue) on breakpoints during progressive-ratio responding for cocaine expressed as raw breakpoints (left column; A, C, E) and as a percent of baseline (right column; B, D, F). In panels A, C, and E, bars are plotted for linear breakpoints on left y-axis and corresponding final response ratios on log scale are plotted on right y-axis for comparison. Number of animals: A, B (n=6); C, D (n=8); E, F (n=6). Red line signifies baseline for phenmetrazine alone and combined treatment. *p<0.05, **p<0.01, ***p<0.001, comparison to respective baseline breakpoints. Δp<0.05, ΔΔp<0.01, combined treatment compared to the respective LY379268 dose. Data points are expressed as mean±SEM.
For animals in the 0.375 mg/kg/infusion cocaine group, a one-way ANOVA revealed a significant effect of treatment (Fig. 3C; F(5,36) = 4.21; p < 0.001). Tukey’s comparison showed that breakpoints decreased significantly following the phenmetrazine + LY379268 (0.03 mg/kg; p < 0.001) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments. Similarly, when these data were expressed as percent of baseline, a one-way ANOVA revealed a significant effect of treatment (Fig. 3D; F(8,63) = 12.24; p < 0.001). Tukey’s comparison showed that breakpoints decreased significantly following the LY379268 (0.3 mg/kg; p < 0.05), phenmetrazine + LY379268 (0.03 mg/kg; p < 0.001) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments when compared to the phenmetrazine treatment baseline. Additionally, Tukey’s comparison revealed that phenmetrazine + LY379268 administration decreased cocaine self-administration for both doses when compared with the pre-minipump baseline (points above red line in Fig. 3D; p < 0.0001) and phenmetrazine + either dose of LY379268 showed an augmented decrease in breakpoints compared to the effect of LY379268 alone (Fig. 3D; 0.03 mg/kg, p < 0.01; 0.30 mg/kg, p < 0.05). These effects are summarized in Table 1.
For the group administering the 0.75 mg/kg/infusion dose of cocaine, a one-way ANOVA revealed a significant effect of treatment (Fig 3E; F(5,30) = 2.98; p < 0.05). Tukey’s comparison showed that breakpoints decreased significantly following the phenmetrazine + LY379268 (0.03mg/kg; p < 0.01) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments. Similarly, when these data were expressed as percent of baseline, a one-way ANOVA revealed a significant effect of treatment (Fig. 3F; F(8,63) = 10.82; p < 0.0001). Tukey’s comparison showed that breakpoints decreased significantly following the LY379268 (0.30 mg/kg; p < 0.01), phenmetrazine + LY379268 (0.03 mg/kg; p < 0.001) and phenmetrazine + LY379268 (0.30 mg/kg; p < 0.001) treatments when compared to the phenmetrazine baseline. Additionally, Tukey’s comparison revealed that phenmetrazine + LY379268 administration decreased cocaine self-administration for both doses when compared with the pre-minipump baseline (points above red line in Fig. 3F; p < 0.0001) and phenmetrazine + either dose of LY379268 showed an augmented decrease in breakpoints compared to the effect of LY379268 alone (Fig. 3F; 0.03 mg/kg, p < 0.05; 0.30 mg/kg, p < 0.05). These effects are summarized in Table 1.
3.4 LY379268 and phenmetrazine combinations significantly reduced cocaine intake
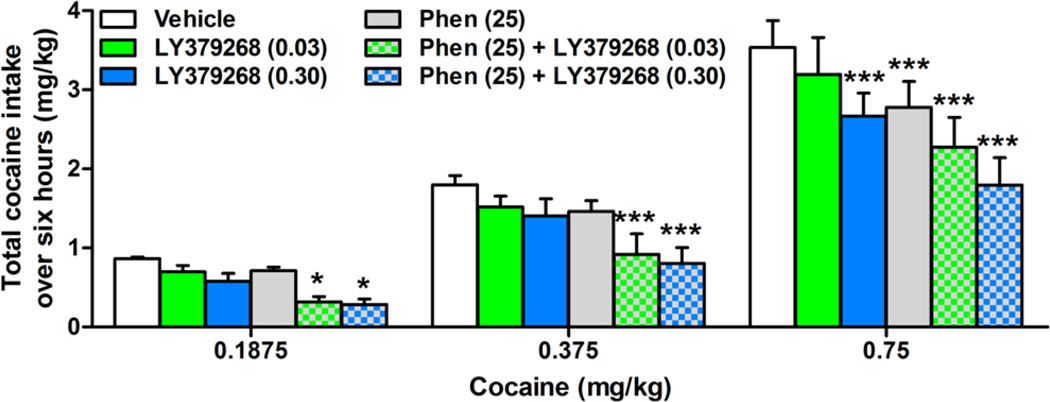
The average total cocaine intake (± SEM) during self-administration for each of the three doses of cocaine were as follows: 0.86 ± 0.02 mg (0.1875mg/kg/infusion); 1.80 ± 0.12 mg (0.375mg/kg/infusion); 3.54 ± 0.34 mg (0.75mg/kg/infusion). A two-way mixed ANOVA revealed an interaction between treatment and cocaine dose (Fig. 4; F(10,80) = 2.29; p < 0.05), a main effect of cocaine dose (F(2,16) = 28.8; p < 0.001), and a main effect of treatment (F(5,16) = 26.32; p < 0.001). Bonferroni’s analyses revealed that phenmetrazine + LY379268 (0.03 and 0.30 mg/kg) significantly decreased cocaine intake at all three doses of cocaine by nearly 50% (p < 0.05), but the effects of LY379268 (0.30 mg/kg) and phenmetrazine when administered alone significantly decreased cocaine intake only at the highest dose of cocaine (0.75 mg/kg/infusion; p < 0.05).
Figure 4.
Effect of LY379268 and phenmetrazine alone and in combination on cocaine intake (mg). The highest dose of LY379268 (0.30 mg/kg; blue) alone and phenmetrazine (25 mg/kg/day; gray) alone decreased total cocaine intake significantly at the highest dose of cocaine (0.75 mg/kg). Combining the two drugs (checkered bars) significantly decreased total cocaine intake at all three doses of cocaine *p<0.05; ***p<0.001 relative to saline/vehicle. N=20.
3.5 Effects of LY379268 and phenmetrazine on locomotor activity
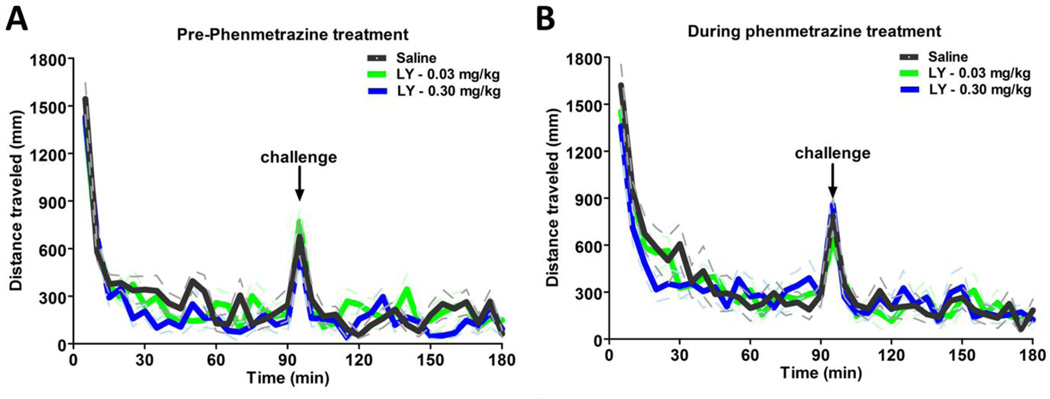
In order to verify that changes in breakpoints were not due to generalized decreases in locomotor activity, an open field locomotor activity assay was used to test the combined effects of these drugs on locomotion (n=8). Neither dose of LY379268 (0.03 and 0.30 mg/kg), both alone (Fig. 5A) and in the presence (Fig. 5B) of phenmetrazine minipumps, altered locomotor activity as compared with saline in the absence (Fig. 5A) and presence (Fig. 5B) of phenmetrazine. Additionally, phenmetrazine alone did not significantly affect locomotor behavior.
Figure 5.
The individual and combined effects of LY379268 and phenmetrazine on locomotor behavior. (A) Administration of LY379268 (0.03 mg/kg, blue; 0.30 mg/kg, green) did not affect locomotor behavior relative to saline (black) injection prior to phenmetrazine mini-pump implantation. (B) Administration of phenmetrazine via subcutaneous osmotic mini-pump in combination with LY379268 did not alter locomotor behavior relative to saline. N=8.
4. DISCUSSION
The goal of the current study was to examine both the individual and combined effects of LY379268 and phenmetrazine treatment on breakpoints and intake in rats responding under a PR schedule of cocaine self-administration. Administration of LY379268 (0.03 mg/kg and 0.30 mg/kg) prior to cocaine self-administration sessions decreased cocaine breakpoints at all three cocaine doses studied in a dose-related manner when data are normalized to their respective baselines or analyzed individually. Similarly, continuous treatment of phenmetrazine alone via an osmotic minipump significantly reduced cocaine self-administration for all three doses of cocaine under these same conditions, confirming previous observations by this group (Czoty et al., 2015). However, the reduction of breakpoints for all three doses of cocaine by combination of phenmetrazine and LY379268 was significantly more pronounced than that produced by the individual treatments. The doses of LY379268, phenmetrazine, and their combination that reduced cocaine self-administration had no effect on locomotor activity. Therefore, the reduction in breakpoints observed cannot be explained by putative decreases in general motoric disturbances or activity.
4.1 mGluR2/3 receptor activation attenuates cocaine breakpoints
LY379268 decreased breakpoints for cocaine in a manner that was sensitive to both the dose of LY379268 and the dose of cocaine administered by the animal. Despite the dose of cocaine influencing treatment effects, we failed to observe a dose-responsiveness of cocaine dose at baseline. This lack of dose-response is consistent with previous published reports using the exact training protocol and equipment (Morgan et al., 2006). Notably, in experiments that were designed specifically to address the effect of training protocol on breakpoints for cocaine, Morgan et al (2006) showed that the training protocol used in our current manuscript (their “Group D”) led to identical final ratios to ours with little to no dose-responsiveness between 0.19 and 0.75 mg/kg/infusion; the same doses used in the current manuscript (c.f., their Figure 4). Outlining the mechanism for the effects of training protocol on breakpoints exceed the purpose of this study and is an area of active research, but it is interesting to note that cocaine dose still influences the efficacy of pharmacological interventions despite our training protocol blunting the dose-responsiveness of cocaine at baseline. We conclude from this finding that the efficacy of pharmacological interventions can be assessed despite a lack of cocaine dose-response under baseline conditions.
Evidence suggests that mGluR2/3 receptors are heavily implicated in both anxiety-like behavior (Grillon et. al., 2003; Muly et al., 2007) and drug addiction (Peters and Kalivas, 2006; Hao et al., 2010), and are altered as a function of cocaine intake (Beveridge et al., 2011). For example, activation of mGluR2/3 results in anxiolytic effects in both fear potentiated startle (FPS) and elevated plus maze (EPM) in rodents as well as attenuated panic symptoms induced by CO2 challenge in humans (Grillon et al., 2003; Schoepp et al., 2003; Helton et al., 1998). The anxiolytic effects of mGluR2/3 agonists are exacerbated in animals with cocaine experience, and these effects have been linked to the repeated cocaine-induced alterations in mGluR2/3 expression and function in the prefrontal cortex and nucleus accumbens (Hao et al., 2010; Xi et al., 2002). For example, LY379268 reduces anxiety-like behavior only in animals with a history of extended access cocaine self-administration (Aujla et al., 2008). Additionally, studies have shown that mGluR2/3 activation attenuates cue-induced reinstatement of cocaine seeking (Baptista et al., 2004; Adewale et al., 2006) and decreases breakpoints on PR reinforcement schedules in animals with escalated cocaine (Hao et al. 2010) and methamphetamine intake (Crawford et al., 2013). The current findings are consistent with these previous studies given our observation of reduced breakpoints at both the lower (0.03 mg/kg) and higher (0.30 mg/kg) doses of LY379268 used in the current study. Interestingly, LY379268 failed to affect responding for food on a PR, suggesting the possibility of a specific effect on cocaine-maintained responding (Crawford et al., 2013).
4.2 Phenmetrazine alone is sufficient to reduce cocaine breakpoints
Previous studies have shown that continuous treatment with amphetamine via an osmotic mini-pump result in decreased cocaine intake in rodents and monkeys (Negus and Mello, 2003; Chiodo et al., 2008; Chiodo and Roberts, 2009; Czoty et al., 2010; 2011; 2015). Like cocaine, amphetamine increases monoamine levels in the NAc albeit via a distinct mechanism; cocaine blocks the reuptake of monoamines while amphetamine releases monoamines via reverse transport. Human self-administration studies (Greenwald et al., 2010; Rush et al., 2010) and clinical trials (Mooney et al., 2009) show that amphetamine treatment decreases cocaine intake. Similar to amphetamine, phenmetrazine acts as a monoamine reverse transport releaser, however in contrast to amphetamine, phenmetrazine when given orally in the form of its prodrug phendimetrazine has relatively low abuse liability (Bolin et al., 2016). Moreover, heart rate measures in human cocaine users have shown that phendimetrazine is safe and tolerable across a threefold span of doses alone and when combined with cocaine (Bolin et al., 2016; Stoops et al., 2016).
Congruent with reduced breakpoints and cocaine intake following continuous administration of phenmetrazine observed in the currents study, previous studies have shown that continuous administration of phenmetrazine resulted in reduced cocaine self-administration on a FR schedule in rodents and monkeys with no change in food self-administration (Negus et al., 2009; Banks et al., 2013c). Phenmetrazine has also been shown to decrease choice for cocaine in the context of an alternative food reinforcer (Negus et al., 2009; Banks et al., 2013a). Most pertinently, we report similar results to a recent study which examined the individual effects of phenmetrazine on cocaine self-administration under a PR schedule (Czoty et al., 2015). These studies are consistent with the present study and show that phenmetrazine alone is sufficient to reduce motivation to self-administer cocaine. Interestingly, similar to LY379268, phenmetrazine administration does not reduce food maintained responding in animals (Czoty et al., 2015). It should be noted that a recent study in human cocaine abusers showed that phendimetrazine did not reduce hypothetical demand for cocaine or the subject-rated effects of sampled cocaine (Stoops et al., 2016) in an experimental setting. Comparing the effects of phenmetrazine in rodents under a PR schedule of reinforcement in the current study with lack of effects of phendimetrazine on hypothetical demand for cocaine in human cocaine users is difficult. However, Stoops et al. (2016) noted that differences in phendimetrazine efficacy between animal models and their study in humans could be attributed to reductions that are specific to the reinforcing effects of cocaine among other factors.
4.3 LY379268 and phenmetrazine combinations decrease reinforcing efficacy of cocaine
There is increased interest in using combination pharmacotherapy to treat substance use disorders. Clinical studies have shown attenuation of cocaine seeking following combination treatments (see Stoops and Rush, 2014 for review). A combination therapy approach in which two medications are combined has many advantages over monotherapies. First, combining medications can reduce the side effects of either medication alone because some combinations may allow for lower doses to be used. For example, one preclinical study showed that the combination of low doses of metyrapone (corticosterone synthesis inhibitor) and oxazepam (benzodiazepine) reduced cocaine responding and prevented problematic side effects of the constituent compounds (Goeders et al., 2008). Second, combining two medications may result in additive and potentially synergistic effect of the two drugs; i.e., the drugs in combination will have a greater reduction in stimulant use than the each drug alone. For example, a recent study showed that a dual stimulant-opioid pharmacotherapy was more effective in attenuating cocaine self-administration than each drug alone (Smith et al., 2014). Moreover, a clinical study showed that bromocriptine and desipramine in combination decreased cocaine withdrawal symptoms greater than bromocriptine alone (Giannini and Billett, 1987). More extensive experimentation, perhaps using isobolograms, would need to be performed on the drug combination used in the current study in order to fully establish the additive or synergistic effect of their combination. This is particularly apparent since the combination at the lowest dose of cocaine resulted in an effect size that was greater than the sum of the effect sizes of the individual drugs, whereas the combination effect size at the two higher doses of cocaine appeared to equal the sum of the two individual effect sizes. Third, clinical work seems to be progressing more towards symptom treatment to combat substance use disorders, rather than monotherapy designed to “cure” substance use disorders (Stoops and Rush, 2014). Cocaine addicts exhibit psychiatric comorbidities that include anxiety and anhedonia. Specifically, cocaine addiction is associated with an inability (or decreased ability) to experience pleasure from natural rewards (i.e., anhedonia) during periods of withdrawal and abstinence (Koob, 2013). For example, animals withdrawn from cocaine exhibit less seeking of natural rewards such as sucrose (Barnea-Ygael et al., 2014). Much of the work on cocaine induced anhedonia, however, stems from studies measuring brain stimulation reward (BSR) using intracranial self-stimulation (ICSS) of ascending mesolimbic dopamine circuits in rodents. Repeated exposure to, and withdrawal from, chronic cocaine increases BSR thresholds in rats and mice (Kenny et al., 2003; Stoker and Markou, 2011) indicating reduced brain reward function during cocaine abstinence. Shifts in BSR thresholds are sensitive to drugs that modulate dopamine function. For example, acute exposure to indirect dopamine agonists like cocaine reduces BSR thresholds indicating of an augmented brain reward activity and catecholamine releasers are known to decrease BSR thresholds in rats that express anhedonic-like behavior (Prins et al., 2012).
We used a combined therapy approach using drugs that have previously been shown in rodents not only to reduce anxiety-like and anhedonic-like behavior, but acutely reverse long-term deficits circuits known to be altered by cocaine use. The current results show that a LY379268 and phenmetrazine combination reduced cocaine intake to levels ranging from what would be expected for additive effects at a minimum (for 0.375 and 0.75 mg/kg/infusion cocaine) to levels that exceed addition of the two drugs alone (for 0.1875 mg/kg/infusion cocaine). Again, more extensive investigation would need to be performed to outline the nature of the relationship between the two treatments when used in combination.
Notably, phenmetrazine appears to be more effective when combined with LY379268 in reducing cocaine breakpoints compared to an alternative approach of simply increasing the dose of phenmetrazine alone. Indeed, Czoty et al. (2015) showed that the ability of phenmetrazine to reduce cocaine breakpoints are near maximal at the dose used in the current study (25 mg/kg/day), such that combining phenmetrazine with LY379268 is far more effective than reducing breakpoints with increasing doses of phenmetrazine. This observation also underscores the premise that we are likely manipulating two systems that are relatively distinct sources of variance that contribute to the motivation to self-administer cocaine (either brain systems or comorbidities). In other words, the concerted effects of LY379268 and phenmetrazine combinations suggests that this drug combination works through two distinct mechanisms to reduce the reinforcing efficacy of cocaine and that modulating both mechanisms in concert may be more effective than an attempt to modulate a single system with higher doses of drug.
In light of the possibility that we are modulating two systems, we would also predict that other drugs which alter glutamate transmission might work in combination with phenmetrazine to reduce cocaine breakpoints. Recent studies have shown that mGluR2/3 positive allosteric positive modulators, which have enhanced receptor subtype selectivity, decrease cocaine intake in dependent animals (Dhanya et al., 2014). Using compounds with mGluR subtype selectivity is potentially useful as mGluR 2 and 3 show differential function and location. For example, mGluR2 are predominantly located on presynaptic terminals of glutamatergic neurons (Schoepp, 2001) and activation of these receptors results in neurodegeneration (Corti et al., 2007). On the other hand, mGluR3 are mainly located postsynaptically and on glial cells (Riedel et al., 2003; Schoepp, 2001). Activation of mGluR3 results in neuroprotection (Corti et al., 2007; Durand et al., 2013). Additionally, previous studies have also shown that mGluR5 inhibition reduces reinstatement of cocaine seeking (Kumaresan et al., 2009; Wang et al., 2013), likely through direct inhibition of these excitatory receptors on medium spiny neurons as opposed to inhibition of glutamate release via presynaptic mGluR2/3 activation with LY379268. Additionally, long-lasting dysfunction of accumbal astrocytes, which maintain glutamate homeostasis via glutamate transporters, is linked to reinstatement of drug seeking (Scofield and Kalivas, 2014). Therefore it is possible that any medication that aids in regulating glutamate homeostasis and therefore maintains normal glutamate neurotransmission may be used in combination with phenmetrazine as a pharmacotherapy to treat addiction.
4.4 Conclusions
The current study is part of a growing literature that suggests combination pharmacotherapy allows the use of low doses of two drugs to potentially yield increased efficacy in reducing the motivation to administer cocaine. This is the first study to utilize an mGluR2/3 agonist and a monoamine releaser in combination to test their combined ability to reduce cocaine self-administration. Future studies are required to examine the extent to which the combined effects can be generalized to other drugs that affect the same circuits or neurotransmitters.
HIGHLIGHTS.
-
▪
LY379268, an mGluR2/3 agonist, reduced breakpoints for cocaine by 40%.
-
▪
Phenmetrazine, a dopamine releaser, reduced breakpoints for cocaine by about 25% maximum.
-
▪
The combination of the two drugs showed a supra-additive effect in reducing breakpoints for cocaine by as much as 70%.
-
▪
Neither LY379268 nor phenmetrazine, showed any nonspecific locomotor effects.
Acknowledgments
The author’s would like to thank the investigators/colleagues from the Center for the Neurobiology Addiction Treatment (CNAT) in the Department of Physiology and Pharmacology at Wake Forest School of Medicine for their helpful commentary and L. Thomas for technical support. This work was funded by NIH grants P50 DA006634 (MJF, SRJ), R00 DA031791 (MJF), R01 DA030161 (SRJ), T32 AA007565 (ANK).
Role of funding source: Nothing declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: TJRB, and MJF were responsible for the study concept and design. ANK conducted all the experiments and analyzed these data. BEB synthesized and provided all drugs used. ANK and MJF wrote the manuscript. SRJ provided critical revision of the manuscript and provided important intellectual content. All authors critically reviewed content and approved final version for publication.
Conflict of interest: No conflict declared.
REFERENCES
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav. Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013b;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013c;38:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J. Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Ygael N, Gal R, Zangen A. Chronic cocaine administration induces long-term impairment in the drive to obtain natural reinforcers in high- but not low-demanding tasks. Addict. Biol. 2014 doi: 10.1111/adb.12196. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas P. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Group II metabotropic glutamate receptors in the striatum of non-human primates: dysregulation following chronic cocaine self-administration. Neurosci. Lett. 2011;496:15–19. doi: 10.1016/j.neulet.2011.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, See RE. Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology. 2012;223:179–190. doi: 10.1007/s00213-012-2705-1. [DOI] [PubMed] [Google Scholar]
- Bolin BL, Stoops WW, Sites JP, Rush CR. Abuse potential of oral phendimetrazine in cocaine-dependent individuals: implications for agonist-like replacement therapy. J. Addict. Med. 2016 doi: 10.1097/ADM.0000000000000206. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino L, Calabrese F, Giannotti G, Barbon A, Verheij MM, Racagni G, Fumagalli F. Stress rapidly dysregulates the glutamatergic synapse in the prefrontal cortex of cocaine-withdrawn adolescent rats. Addict. Biol. 2015;20:158–169. doi: 10.1111/adb.12089. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous D-amphetamine treatment in rats. Psychopharmacology. 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo KA, Läck CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology. 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J. Neurosci. 2007;27:8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JT, Roberts DC, Beveridge TJ. The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug Alcohol Depend. 2013;132:414–419. doi: 10.1016/j.drugalcdep.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Effects of chronic d-amphetamine administration on the reinforcing strength of cocaine in rhesus monkeys. Psychopharmacology. 2010;209:375–382. doi: 10.1007/s00213-010-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Tran P, Thomas LN, Martin TJ, Grigg A, Blough BE, Beveridge TJR. Effects of the dopamine/norepinephrine releaser phenmetrazine on cocaine self-administration and cocaine-primed reinstatement in rats. Psychopharmacology. 2015;232:2405–2414. doi: 10.1007/s00213-015-3875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanya RP, Sidique S, Sheffler DJ, Nickols HH, Herath A, Yang L, Dahl R, Ardecky R, Semenova S, Markou A, Conn PJ, Cosford ND. Design and synthesis of an orally active metabotropic glutamate receptor subtype-2 (mGluR2) positive allosteric modulator (PAM) that decreases cocaine self-administration in rats. J. Med. Chem. 2011;54:342–353. doi: 10.1021/jm1012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanya RP, Sheffler DJ, Dahl R, Davis M, Lee PS, Yang L, Nickols HH, Cho HP, Smith LH, D'Souza MS, Conn PJ, Der-Avakian A, Markou A, Cosford ND. Design and synthesis of systemically active metabotropic glutamate subtype-2 and −3 (mGlu2/3) receptor positive allosteric modulators (PAMs): pharmacological characterization and assessment in a rat model of cocaine dependence. J. Med. Chem. 2014;57:4154–4172. doi: 10.1021/jm5000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Carniglia L, Caruso C, Lasaga M. mGlu3 receptor and astrocytes: partners in neuroprotection. Neuropharmacology. 2013;66:1–11. doi: 10.1016/j.neuropharm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol. Biochem. Behav. 2009;91:181–189. doi: 10.1016/j.pbb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocainebut not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacol. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine LR, Morgan CA., 3rd Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology. 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol. Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Heilig M, Carlezon WA., Jr Circumspectives: the replacements. Neuropsychopharmacology. 2015;40:1813–1814. doi: 10.1038/npp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Justinova Z, Le Foll B, Redhi GH, Markou A, Goldberg SR. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3994-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Keys AS, Mark GP, Emre N, Meshul CK. Reduced glutamate immunolabeling in the nucleus accumbens following extended withdrawal from self-administered cocaine. Synapse. 1998;30:393–401. doi: 10.1002/(SICI)1098-2396(199812)30:4<393::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Sher L. Suicidal behavior in a medical professional with comorbid depression and substance use disorder: an educational case report. Int. J. Adolesc. Med. Health. 2015;27:231–233. doi: 10.1515/ijamh-2015-5017. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front. Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav. Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Roberts DC. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2006;31:121–128. doi: 10.1038/sj.npp.1300773. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur. J. Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Mania I, Guo JD, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: correspondence of physiological response and subcellular distribution. J. Comp. Neurol. 2007;505:682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J. Pharmacol. Exp. Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Tabet MR, Tsibulsky VL, Pesce AJ. Competitive dopamine receptor antagonists increase the equiactive cocaine concentration during self-administration. Synapse. 2011;65:404–411. doi: 10.1002/syn.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat. Rev. Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Poindexter A. Appetite suppressant drugs: a controlled clinical comparison of benzphetamine, phenmetrazine, d-amphetamine and placebo. Curr. Ther. Res. Clin. Exp. 1960;2:354–363. [PubMed] [Google Scholar]
- Prins J, Kenny PJ, Doomernik I, Schreiber R, Olivier B, Mechiel Korte S. The triple reuptake inhibitor DOV 216,303 induces long-lasting enhancement of brain reward activity as measured by intracranial self-stimulation in rats. Eur. J. Pharmacol. 2012;693:51–56. doi: 10.1016/j.ejphar.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Radke AK, Gewirtz JC. Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology. 2012;37:2405–2415. doi: 10.1038/npp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendel-Short J. Obesity in childhood: a clinical trial of phenmetrazine. BMJ. 1960;1:703–704. doi: 10.1136/bmj.1.5174.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med. Chem. 2012;4:245–265. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J. Clin. Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20:610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynatic metabotropic glutamate receptor in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J. Neurosci. Methods. 2003;128:103–110. doi: 10.1016/s0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Ferris MJ, Jones SR. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur. J. Neurosci. 2015;42:2091–2096. doi: 10.1111/ejn.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Pitts EG, Walker KL, Lang KC. The effects of amphetamine, butorphanol, and their combination on cocaine self-administration. Behav. Brain Res. 2014;274:158–163. doi: 10.1016/j.bbr.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav. Brain Res. 2011;223:176–181. doi: 10.1016/j.bbr.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Exp. Rev. Clin. Pharmacol. 2014;7:363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Strickland JC, Hays LR, Rayapati AO, Lile JA, Rush CR. Safety and tolerability of intranasal cocaine during phendimetrazine maintenance. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4260-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict. Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J. Pharmacol. Exp. Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology. 2009;203:501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- Zimmer BA, Chiodo KA, Roberts DC. Reduction of the reinforcing effectiveness of cocaine by continuous D-amphetamine treatment in rats: importance of active self-administration during treatment period. Psychopharmacology. 2014;231:949–954. doi: 10.1007/s00213-013-3305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]