Abstract
Objective
Anxiety disorders are prevalent and cause substantial disability. An important risk factor for anxiety disorders is inhibited temperament, the tendency to be shy and avoid new situations. Inhibited adults have heightened amygdala activation and less flexible engagement of the prefrontal cortex (PFC); however, it remains unknown if these brain alterations are present in inhibited children prior to the onset of anxiety disorders.
Method
Thirty-seven children (18 inhibited, 19 uninhibited), ages 8–10 years, completed a task testing anticipation and viewing of threat stimuli and social stimuli in the magnetic resonance imaging (MRI) scanner. Brain activation and functional connectivity were measured.
Results
During the anticipation of threat stimuli, inhibited children failed to show the robust PFC engagement observed in the uninhibited children. In contrast, when viewing social stimuli, inhibited children had increased medial PFC and dorsolateral PFC activation. Connectivity analyses revealed a pattern of reduced connectivity between prefrontal and limbic regions and among distinct PFC regions in the inhibited group. The medial PFC emerged as a key hub of the altered PFC circuitry in inhibited children.
Conclusion
This study provides new evidence of a neural signature of vulnerability to anxiety disorders. By investigating both anticipation and response to images, we identified that high-risk, inhibited children have widespread alterations in PFC function and connectivity, characterized by an inability to proactively prepare for social threat combined with heightened reactivity to social stimuli. Thus, children at high risk for anxiety show significantly altered prefrontal cortical function and connectivity before the onset of anxiety disorders.
Keywords: inhibited temperament, behavioral inhibition, fMRI, functional connectivity, anticipation
INTRODUCTION
Anxiety disorders are highly prevalent and cause substantial disability and economic burden. One in three Americans will suffer from an anxiety disorder,1 the second leading mental health cause of global disease burden.2 The long-term impact of anxiety disorders is especially pernicious because they have an early onset,1 produce a long course of suffering, and often lead to comorbid depressive and substance use disorders.3–5 Thus, prevention of anxiety disorders has the potential to substantially reduce the overall burden of disease.
For psychiatric disorders, prevention is considered one of the most important, yet still unsolved, problems. Neuroimaging studies of children at risk for developing psychiatric disorders—siblings of children with autism6 or individuals with a schizophrenia prodrome7—show promise for identifying early brain differences associated with risk. For anxiety disorders, a risk phenotype has been well described; however, remarkably little is known about whether there are associated brain alterations. The risk phenotype—inhibited temperament (or behavioral inhibition)—is characterized by shy and cautious responses to novel situations and stimuli. Inhibited children have a significantly increased risk for developing anxiety disorders8–15 and subsequent depression.3 Identifying the neural substrates of inhibited temperament in young children holds promise for elucidating the neurobiological basis of anxiety risk.
To date, neuroimaging studies of inhibited temperament have largely focused on inhibited young adults or older adolescents who were inhibited as children, some of whom have an anxiety disorder (for a review, see16). In these studies, alterations in amygdala function have been well-established, including increased activation,17,18 faster and sustained responses,18–21 and abnormal modulation by attention state.22,23 Inhibited adolescents and young adults show both increased and decreased prefrontal cortex (PFC) function across studies: decreased dorsal anterior cingulate cortex (dACC) and dorsolateral PFC (dlPFC) activation when viewing expected negative social stimuli;22 increased rostral ACC (rACC) and dACC activation when anticipating fear faces;24 and increased dorsomedial PFC, dlPFC, and rACC activity when performing a task that requires cognitive control.25,26 A recent study in inhibited children found heightened dlPFC response during a threat executive-attention task.26 Studies examining risk versus resilience in inhibited temperament have highlighted that greater PFC activity during emotional tasks that require cognitive control predicts resilience to anxiety disorders, suggesting that lack of PFC activity may be associated with anxiety risk.16,24,27 During non-emotional tasks, over-control has also been associated with anxiety risk.28 Finally, in patients with social anxiety disorder, a meta-analysis of functional magnetic resonance imaging (fMRI) studies shows hyperactivity of the amygdala, insula, rACC, mPFC and dlPFC, although it should be noted that the PFC findings are mixed across individual studies and likely reflect task differences.29 Thus, the amygdala and multiple prefrontal cortical regions have been implicated in inhibited temperament, anxiety risk, and social anxiety disorder.
While prior studies have made important contributions to our understanding of the neural basis of inhibited temperament in late adolescents and adults, the age groups studied were largely past the average age of onset of anxiety disorders and thus represented a heterogeneous sample of resilient individuals and individuals with past or current anxiety disorders. Studies in young high-risk children, prior to the onset of anxiety disorders, are critical for disentangling the neural markers of anxiety risk from the neural consequences (i.e., scar markers) of anxiety disorders. In the present study, we examine, for the first time, the neural correlates of anticipatory processing and stimulus viewing in 8- to 10-year-old inhibited children, prior to the development of anxiety disorders. We examined anticipatory processing since anticipatory anxiety is a hallmark of anxiety disorders30 and is associated with activation of the prefrontal cortex, amygdala, and insula.31 During anticipation of negative emotional events, patients with anxiety disorders fail to activate the PFC and have hyperactivity of the amygdala and insula.30–32 Our working model is that during anticipation of fear faces, high-risk inhibited children will have decreased activity in brain regions involved in emotional regulation (ACC, mPFC, dlPFC) and increased activity in limbic-related regions (e.g., amygdala and insula).
METHOD
Participants
Forty children initially participated in this study (20 children with inhibited temperament and a comparison group of 20 children with an uninhibited temperament) and 37 children were in the final analytic sample (18 inhibited, 19 uninhibited). Consistent with the extreme discordant phenotypes approach,33 we compared inhibited children and uninhibited children at the extreme ends to maximize our chances of identifying differences. To obtain pure risk groups (not confounded by existing disorders), children were excluded from the study for having any current or past psychiatric diagnoses, as measured by the Kiddie-Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version (KSADS-PL)34 or having received treatment for anxiety symptoms. Children were also excluded if they had cognitive deficits that might affect task performance (developmental delay, repeating a grade, or receiving special assistance in school), contraindications to MRI scanning, or factors that might affect blood oxygen level-dependent (BOLD) signal (psychotropic medications, history of head injury, major medical or neurological conditions). Intelligence quotient (IQ) was assessed using the Kaufman Brief Intelligence Test.35 Handedness was assessed using the Edinburgh Handedness Inventory.36
There were no significant differences between groups in age, gender, ethnicity, handedness, or IQ (see Table 1). Parents provided informed consent, and children provided informed assent for participation in the study. The Vanderbilt University Institutional Review Board approved this study. Financial compensation was provided.
Table 1.
Characteristics of the Sample
| IT | UT | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | p-value | |
| Demographics | |||||
|
| |||||
| Age (years) | 9.5 | 1.0 | 9.8 | .9 | ns |
| Temperament score (parent report) | 153.8 | 20.2 | 44.7 | 8.5 | < .001 |
| Temperament score (child report) | 122.8 | 19.8 | 91.7 | 42.3 | .005 |
| Temperament score (interviewer rating) | 3.4 | 1.2 | 2.3 | 1.1 | .002 |
| IQ | 116.8 | 9.9 | 116.5 | 12.3 | ns |
|
| |||||
| n | (%) | n | (%) | p-value | |
|
| |||||
| Ethnicity | ns | ||||
| Caucasian | 18 | 81 | 18 | 84 | |
| African-American | 2 | 13 | 2 | 11 | |
| Asian | 1 | 6 | 1 | 5 | |
| Female gender | 10 | 62 | 9 | 47 | ns |
| Right handed | 14 | 88 | 18 | 95 | ns |
|
| |||||
| Clinical Characteristics | Mean | SD | Mean | SD | p-value |
|
| |||||
| Anxiety (parent report) | 20.9 | 8.8 | 3.9 | 3.4 | < .001 |
| Anxiety (child report) | 19.5 | 11.1 | 11.8 | 10.8 | .03 |
| Depression (child report) | 5.7 | 3.2 | 5.4 | 7.4 | ns |
| Hyperactivity (parent report) | 2.9 | 3.2 | 4.0 | 3.1 | ns |
| Social communication skills (parent report) | 3.8 | 3.6 | 2.7 | 1.9 | ns |
Note: IT = inhibited temperament; UT = uninhibited temperament.
Recruitment and Screening
Participants were recruited from the Vanderbilt University Medical Center and surrounding community using flyers, emails, and research recruitment databases. Advertisements were for children who were “quiet,” “cautious,” “shy,” “outgoing,” and general recruitment for a study on “temperament and brain function.” Prior to the first study visit, parents completed a brief online screening, including the Behavioral Inhibition Questionnaire-Parent (BIQ-P),37 a validated measure of childhood inhibited temperament, which has been used for screening in a recent neuroimaging study26 and shows convergent validity with behavioral measures and other measures of social inhibition.37–39 Although four questions in the questionnaire refer to younger age groups, these questions were highly correlated with other items in the scale and therefore were retained as written. Children were selected based on a temperament score ± one standard deviation from the mean based on published norms (inhibited > 123; uninhibited < 59);37 these norms were similar to those identified in children ages 4–1539 and those used in a recent similar neuroimaging study.26
Temperament Measures
During the first study visit, behavioral interaction with an unfamiliar adult was measured, based on prior studies of inhibited temperament (for examples, see 40,41). Children were brought into a room and were told that a “new experimenter was going to come in soon and ask them some questions.” The unknown female experimenter asked a set of standard questions (~15 minutes). Following the interview, the experimenter rated global inhibited temperament and seven other measures (latency to respond, amount of speech, tense or uncomfortable behaviors, positive affect, negative affect, trust, and volume and tone of voice) on a 1–5 Likert scale, based on Ballespí et al.42 Children also completed a self-report of temperament, the Behavioral Inhibition Questionnaire-Child (BIQ-C).39
Psychiatric Symptom Measures
To further characterize participants, both parents and children reported on a number of psychiatric symptom measures, including the Screen for Child Anxiety-Related Disorders,43 Social Phobia and Anxiety Inventory for Children,44 Retrospective Infant Behavioral Inhibition Scale,45 Social Communication Questionnaire,46 Children’s Depression Inventory,47 and Conners’ 3 to measure symptoms of attention-deficit/hyperactivity disorder (ADHD).48
Experimental Design
Cued Anticipation Task
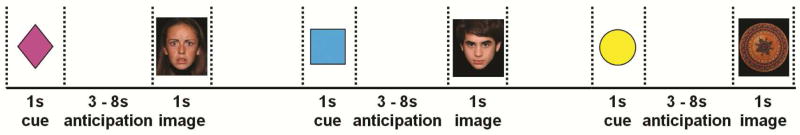
Anticipatory processing was assessed using a cued anticipation task. Children were trained to associate each of three cues (colored shapes) with specific image types (fear face, neutral face, neutral object). Successful learning was confirmed verbally. The test period consisted of four runs (Figure 1). Each trial included: cue (1s); anticipation period (jittered, 3–8s); image (1s); and blank screen (jittered, 3–8s) before the next trial. Each run consisted of eight trials of each type (fear face, neutral face, neutral object) for a total of 24 trials per run and 32 of each trial type across the entire task. Child faces from the National Institute of Mental Health Child Emotional Faces Dataset49 were used. For the control stimuli, neutral objects were used—round non-social objects, the approximate size and shape of faces (i.e., a patterned bowl, a clock)—obtained from several sources, including the International Affective Picture System image set,50 iStockPhoto.com, and publically available images.
Figure 1.
Study design. Note: The task consisted of four runs of 8 fear face, 8 neutral face, and 8 neutral object trials for a total of 24 trials of each type. Each trial consisted of a cue (1s), an anticipation period (3–8s, jittered), and an image (1s). Following each trial, a 3–8s blank screen (jittered) was shown to allow for return to baseline. Fear face, neutral face, and neutral object trials were randomized within runs.
Task Accuracy
To provide a measure of attention to the task, children were asked to press one button during each of the 1s cue and image events. Across all cues and images, children accurately pressed the button within the 1s window 89.7% of the time. To ensure only events where children were paying attention to the task were included, we used a three-step method: 1) participant level: each participant was checked for greater than 50% button press accuracy for each event type across the entire task (fear face cue, neutral face cue, neutral object cue, fear face, neutral face, neutral object); 2 participants with lower than 50% accuracy for any one event type were excluded from the analyses (5% of all participants; 1 inhibited, 1 uninhibited); 2) run level: individual functional runs were excluded for less than 50% button press accuracy across all events (3.3% of total runs); and 3) event level: individual events were excluded if the button was not pressed during the 1s event (7.2% of all remaining events). Thus, only data with an accurate button press were included in the final analyses.
MRI Acquisition
Each child completed a mock MRI scan during the second study visit to acclimate the child to the scanner and improve data quality.51 Data were collected using a 32-channel headcoil on a Philips 3 Tesla scanner. T1-weighted structural data were acquired using the following parameters: 256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap, 2s TR, 22ms TE, 90° flip angle, 1.8 SENSE factor, 240 mm F OV, 3×3 mm in plane resolution. Functional (echo-planar imaging [EPI]) data were acquired using a sequence optimized for the temporal lobe and orbitofrontal cortex with the following parameters: 40 slices, 2.5 mm slice thickness, 0.25 mm gap, and an axial oblique acquisition, tilted 15 degrees, anterior higher than posterior, relative to the intercommisural plane.
MRI Preprocessing
Data preprocessing was performed in Statistical Parametric Mapping – Version 8 (SPM8; http://www.fil.ion.ucl.ac.uk/) implemented in Matlab 2010a software (Version 7.10.0, Mathworks, Inc., Natick, MA). Preprocessing steps included: slice time correction to the middle slice; realignment of functional volumes to mean volume; coregistration of functional and structural scans; normalization of functional scans to EPI template; and smoothing with a 6mm full width at half maximum (FWHM) kernel. For each participant, scans were checked for data quality; functional and structural data were visually inspected for artifacts, coverage of brain regions, and signal dropout. One inhibited participant was excluded for functional data artifacts. Thus the final analytic sample included 18 inhibited and 19 uninhibited participants. Among those included in the analyses, certain functional runs were also excluded for: artifact on visual inspection (1.1%), or an incomplete run in the scanner (2.2%). All participants had at least two runs of data included in the analyses. To control for motion, we used the Robust Weighted Least Squares (rWLS) toolbox.52 rWLS uses standard robust methods to weight the contribution of each volume using the inverse of the variance, thereby reducing the statistical influence of motion outliers without removing data or disrupting the temporal sequence of the data. Overall average maximum displacement due to motion per run was low and there were no significant differences between groups in motion (inhibited [IT]: 1.34 mm translation, .024 radians rotation; uninhibited [UT]: 1.11 mm translation, .019 radians rotation; translation: p = .62; rotation: p = .30; see Supplement 1 for additional details, available online). For all participants with >10mm maximum displacement, the performance of rWLS was reviewed by visually comparing the inverse variance maps across time for each participant.
FMRI Data Modeling
For each participant, a general linear model was created in SPM8 with seven regressors: fear face cue, neutral face cue, neutral object cue, fear face image, neutral face image, neutral object image, and errors. Six contrasts were created for each participant (each condition vs. baseline): fear face anticipation; neutral face anticipation; neutral object anticipation; fear face viewing; neutral face viewing; and neutral object viewing.
Regions of Interest
Analyses were restricted to five regions of interest (ROIs) to focus the analyses on hypothesized regions and reduce the number of overall comparisons. The ROIs were the amygdala, insula, dorsolateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC; see Supplement 1, Method, for definitions, available online). Within each region, data were tested for group voxel-wise differences. Data were cluster-corrected (α < .05) using the AFNI 3dClust function with actual smoothing and 5,000 iterations. Cluster thresholds included (averaged across left and right sides, midline regions were considered bilaterally) with a voxel p-value of .05: amygdala (k = 20; 540 mL), insula (k = 69; 1,863 mL), dlPFC (k = 164; 4,428 mL), mPFC (k = 111; 2,997 mL), and ACC (k = 79; 2,133 mL).
Whole Brain Analyses
To determine if there were any additional regions that showed a temperament x emotion interaction, an exploratory voxel-wise whole brain analysis was conducted. The cluster threshold was calculated using 3dClust using the whole-brain mask image created by SPM, 3x3x3mm voxels, intrinsic smoothing, an FWE rate of .05 and 5,000 iterations. For this analysis, a voxel p-value of .005 and a cluster size of k = 99 (2,673mL) provided an FWE-corrected p-value of .05.
Functional Connectivity
In order to further elucidate key neural networks, follow-up analyses were performed to identify patterns of connectivity with the brain regions that showed significant temperament effects in activation (entire cluster of temperament difference was used as the seed). A general psychophysiological interaction (gPPI) analysis53 was performed with three regressors: the psychological regressor, which was the difference between activation during task, relative to baseline; the physiological regressor, the time series extracted from the seed region; and the interaction of the task and the time series. Since the interaction term in the gPPI is statistically independent from the main effect of task, this analysis is statistically independent from the task findings. The interpretation of the functional connectivity results focused on the inhibited group, based on evidence that the beta estimates do not have an absolute value meaning54 and can only be discussed in relative terms.
Image Rating
Following the MRI scans, children rated the valence of the cues and of a subset of images (10 fear faces, 10 neutral faces, 10 neutral objects). Half of the selected faces were female and half were male. Children were instructed to rate how “happy or sad the pictures made them feel” (1 = very happy; 5 = very sad). Image ratings were accompanied by schematics of each rating (i.e., “1” was presented above a smiley face). The cues and images were presented using Eprime software outside of the scanner. Valence rating data were missing for one uninhibited child due to technical problems.
Data Analysis
Behavioral Data
The behavioral data—button press hit rate (i.e., completed button press during the 1s stimulus), button press reaction time, image valence rating—were analyzed using a general linear model with type (cue/image) and condition (fear face/neutral face/neutral object) as within-subject variables and temperament group as the between-subject variable. As a manipulation check, the image valence rating data were analyzed using a general linear model with type and condition. SPSS (Version 21.0.0.0, IBM Corporation) was used for data analysis with α = .05.
Statistical Analysis
Within-subjects effects of condition were modeled using a flexible factorial model with the within-subjects effects explicitly modeled in SPM8.55 Analyses of variance (ANOVAs) were conducted to test for an interaction of temperament x condition during anticipation and face viewing. To understand how each condition contributed to each interaction, post-hoc analyses were conducted. Effect estimates (percent signal change for fMRI and beta values for gPPI) were extracted from the significant clusters for the fear face, neutral face, and neutral object contrasts using EasyROI toolbox (http://www.sbirc.ed.ac.uk/LCL/LCL_M1.html).
RESULTS
Temperament and Behavioral Data
The inhibited children were significantly more inhibited than uninhibited children on parent report, behavioral assessment, and self-report (p < .001; Table 1). The three measures of temperament—parent report, child report, and behavioral ratings—were significantly correlated (Table S1, available online). Parent and child reports of anxiety symptoms were significantly correlated and were also correlated with temperament measures (Table S1, available online). Inhibited children had more anxiety symptoms; however, anxiety levels were below the clinical cut-offs/norms (Table S2, available online). There were no significant group differences in social communication skills, hyperactivity, or depression (Table 1).
Inhibited and uninhibited children showed similar ratings on reaction times and valence ratings overall and by stimulus type, condition, and type x condition (Table S3, available online). There was an interaction of temperament x condition (fear face versus neutral face) on the hit rate, whereby inhibited children were significantly more likely to miss a neutral face cue than a fear face cue (neutral face cue: 8.0% ± 10.1%; fear face cue: 4.1% ± 5.9% missed; p = .04).
Task Analyses
Region of Interest Analyses
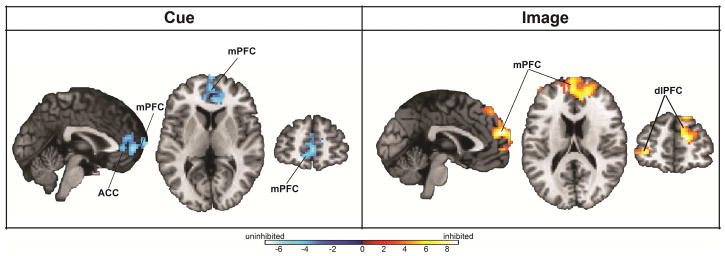
During anticipatory processing, there were significant temperament x condition interactions in two prefrontal cortical regions—the rostral portion of the anterior cingulate cortex (ACC) and the medial prefrontal cortex (mPFC; p < .05, corrected; Figure 2; see Table S4, available online). Post hoc analyses revealed that uninhibited children showed a robust pattern of prefrontal engagement during anticipation of fear faces that was absent in inhibited children (see Figure S1, available online).
Figure 2.
Group differences in brain activation during anticipation and viewing of faces. Note: During anticipation there was an interaction of temperament x condition in the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) (all p < .05, corrected). When viewing faces, there was an interaction of temperament x condition in the mPFC and bilateral dorsolateral prefrontal cortex (dlPFC; p < .05, corrected).
During face viewing, temperament x condition interactions were observed in the mPFC and bilateral dorsolateral PFC (p < .05, corrected; Figure 2; see Table S4, available online). Post hoc analyses showed that inhibited children had heightened prefrontal cortical reactivity to faces (both fear and neutral); in contrast, uninhibited children showed relatively little neural response to viewing faces.
Whole-Brain Analyses
In the whole-brain analyses, significant temperament x condition interactions were found in the mPFC, right dlPFC, frontal pole, and precuneus during face viewing (p < .05, corrected; see Table S4, available online). There were no whole-brain temperament x condition differences during anticipatory processing.
Functional Connectivity Analyses
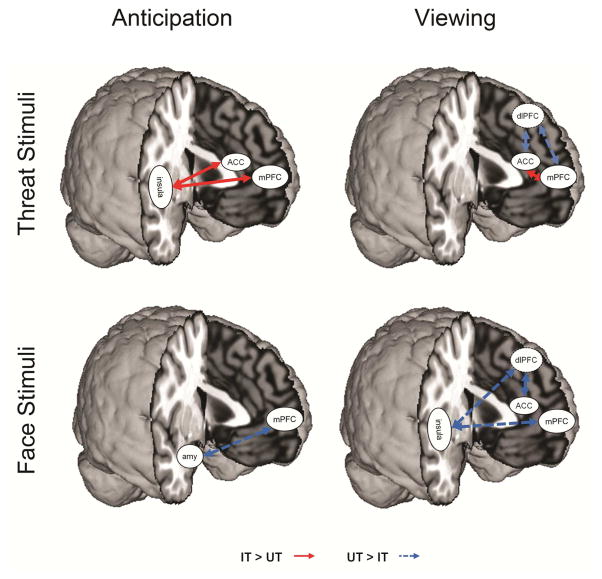
In the follow-up functional connectivity analyses, we examined connectivity with the key regions identified in the task analyses. During anticipation of faces (both fear and neutral), inhibited children had reduced mPFC-amygdala connectivity compared to uninhibited children (both p < .05, Figure 3, see Table S5 and Figure S2, available online). During anticipation of fear faces specifically, inhibited children had increased mPFC-insula and ACC-insula connectivity compared to the uninhibited children.
Figure 3.
Differences in connectivity during threat and face anticipation and viewing. Note: During anticipation of viewing threat, the inhibited group had stronger connectivity between the insula and anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC). When viewing threat, the inhibited group had less connectivity between the dorsolateral prefrontal cortex (dlPFC) and the ACC and mPFC. Connectivity between the ACC and mPFC was increased during threat viewing in the inhibited group. When anticipating faces overall, the inhibited group had less connectivity between the mPFC and amygdala (amy). When viewing faces overall, the inhibited group also had less connectivity among a prefrontal-insular network, including the dlPFC, ACC, mPFC, and insula.
During viewing of faces (fear and neutral), inhibited children had reduced connectivity between dlPFC-ACC and mPFC-insula relative to uninhibited children. During fear face viewing specifically, inhibited children had decreased connectivity between the dlPFC-ACC and dlPFC-mPFC but increased connectivity between the mPFC-ACC (p < .05, Figure 3; see Table S5 and Figure S2, available online). Thus, widespread alterations in connectivity during anticipation and face viewing were observed between prefrontal regions and limbic regions as well as among prefrontal regions.
DISCUSSION
Here, we provide the first report, to our knowledge, of the neural correlates of anticipatory processing and face viewing in young children at high risk for developing anxiety disorders. We identified an altered prefrontal cortex (PFC) response associated with anxiety risk. Uninhibited children demonstrated a proactive pattern, with increased PFC engagement during fear anticipation followed by disengagement during fear face viewing, suggesting that they had successfully prepared to view fear faces during the anticipation period. In contrast, the inhibited children had a similar pattern across all event types during anticipation—they failed to engage PFC regions and instead had heightened PFC activation when viewing all social stimuli, regardless of threat. This pattern suggests that inhibited (high-risk) children were both unable to effectively prepare for threat and showed a delayed PFC response, emerging only in the context of social stimuli. The connectivity findings highlight the medial PFC as a key hub in the circuits underlying alterations in connectivity across all conditions. Thus, findings from this study provide evidence for a neural signature of anxiety risk in children that is characterized by delayed PFC engagement and reduced PFC-limbic connectivity.
The medial PFC region where both activation and connectivity differences were observed is similar to our previous study of inhibited adults;24 however, the pattern of findings in children was unique, suggesting important developmental considerations. In the current study, uninhibited children showed significant prefrontal cortical activation during threat anticipation, whereas in our previous study, uninhibited adults showed relatively little brain activation during anticipation.24 This age difference is consistent with findings in healthy individuals56 and suggests a developmental shift, whereby uninhibited children respond to the anticipation of mild threat by proactive preparation, whereas for young adults, this preparation is either very rapid (and so undetected), or unnecessary. In the current study, inhibited children failed to engage the PFC during threat anticipation, whereas in the previous study, inhibited adults had heightened PFC activation during threat anticipation. Critically, the previous study included inhibited adults with and without anxiety disorders, and higher prefrontal cortex activation predicted lower anxiety symptoms and better coping skills.24 The PFC undergoes protracted development57–59 that parallels the development of cognitive control, and in neuroimaging studies, increased mPFC and dlPFC activity correlates with enhanced cognitive control and emotion regulation.60,61 Thus, across development, differences in prefrontal cortical function likely emerge and the high-risk children who are able to engage the PFC in preparation for threat are likely to be the most resilient to developing anxiety. In this case, therapies that target the PFC may provide a novel approach to preventing and treating anxiety disorders in children.
While increased amygdala activation is a relatively consistent finding in anxious children with anxiety62 and has been shown in anticipation tasks in children and adolescents with anxiety,63–65 in this study inhibited children did not have hyperactive amygdala responses. Importantly, findings may depend on the task used to probe brain function. For example, two previous studies of anticipatory processing in inhibited adolescents and young adults failed to find temperament differences in amygdala function.24,65 However, amygdala hyperactivity was observed in inhibited children using an executive-attention task.26 Studies that include multiple different tasks will be instrumental in elucidating task effects. A second possibility is that amygdala hyperactivity is specific to anxiety62 and is not a neural correlate of anxiety risk. Future studies are needed to examine the neural circuitry in inhibited children with and without anxiety disorders to isolate their unique effects.
These findings should be interpreted in the context of the study limitations. First, we used an extreme discordant phenotypes approach,33 which maximizes the ability to find group differences in initial investigations. One limitation of this approach is that the full range of temperament values was not included; future studies should include children with average temperament scores. Another limitation is that the uninhibited children—while at low risk for developing anxiety—may be at increased risk for developing ADHD66 or other externalizing symptoms.67 While children in this study would never meet criteria for ADHD (given that ADHD symptoms must be present by age 7), they may still be at heightened risk for other disorders. In order to isolate risk for disease, inhibited children with psychiatric disorders were also excluded from the study. One limitation of this approach is that the highest-risk children—those who had an early-onset anxiety disorder—were not represented in this sample. Studies of younger children and longitudinal studies will be necessary to disentangle the unique contributions of inhibited temperament and anxiety disorders to brain function. Finally, the connectivity analyses performed here focused on the observed differences from the task; although the connectivity findings are statistically independent from the task findings, it is important to acknowledge that the interpretations should be limited to this task and that replication in an independent sample is needed.
These findings point to a neural signature of anxiety risk characterized by prefrontal cortex hypoactivity during threat anticipation with a shift to prefrontal cortex hyperactivity during face viewing. High-risk children show specific alterations in engaging prefrontal cortical resources to prepare for an upcoming aversive event and hyper-reactivity to social stimuli. Studies in adults with anxiety disorders have shown alterations in prefrontal cortex function,68 and here we show that alterations in prefrontal cortex activation may be a neural signature of anxiety risk. Following these children longitudinally will help us understand whether these altered PFC responses in the inhibited group increase vulnerability for anxiety disorders. Importantly, these findings highlight the need to focus on the PFC. Specifically, strategies that focus on rapid engagement of the prefrontal cortex during anticipation of threat may be beneficial for these high-risk children and may be a critical component of preventive interventions.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institute of Mental Health (T32-MH018921; F30- MH097344 to J.A.C.; R01-MH068391 to U.R.), the National Institute for General Medical Studies (T32- GM07347 to Vanderbilt Medical Scientist Training Program), the Vanderbilt Institute for Clinical and Translational Research (UL1-TR000445), the Vanderbilt Department of Psychiatry, and the Vanderbilt University Institute of Imaging Science.
The authors would like to thank Suzanne Avery, PhD, and Brittany Matthews, undergraduate research assistant, of Vanderbilt University, and April Seay, MD, of Duke University, for their work in collecting this research sample.
Footnotes
Supplemental material cited in this article is available online.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Blackford served as the statistical expert for this research.
Disclosure: Drs. Clauss, Benningfield, Rao, and Blackford report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64(8):903–913. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 4.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40(9):1086–1093. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CW, Park S, Cornblatt B. Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2006;81(2–3):211–215. doi: 10.1016/j.schres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biederman J, Rosenbaum JF, Bolduc-Murphy EA, et al. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Adolesc Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, Rosenbaum JF, Hirshfeld DR, et al. Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry. 1990;47(1):21–26. doi: 10.1001/archpsyc.1990.01810130023004. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum JF, Biederman J, Hirshfeld DR, et al. Further evidence of an association between behavioral inhibition and anxiety disorders: Results from a family study of children from a non-clinical sample. J Psychiatr Res. 1991;25(1):49–65. doi: 10.1016/0022-3956(91)90015-3. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 14.Hirshfeld-Becker DR, Biederman J, Henin A, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28(3):225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Prog Neurobiol. 2015;127–128:23–45. doi: 10.1016/j.pneurobio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz CE, Wright CI, Shin L, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 18.Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10(1):145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol Psychiatry. 2012;17:1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neurosci. 2013;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc Cogn Affect Neurosci. 2011;6:621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clauss JA, Cowan RL, Blackford JU. Expectation and temperament moderate amygdala and dorsal anterior cingulate cortex responses to fear faces. Cogn Affect Behav Neurosci. 2011;11(1):13–21. doi: 10.3758/s13415-010-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clauss JA, Avery SN, VanDerKlok RM, et al. Neurocircuitry underlying risk and resilience to social anxiety disorder. Depress Anxiety. 2014;31(10):822–833. doi: 10.1002/da.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarcho JM, Fox NA, Pine DS, et al. Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depress Anxiety. 2014;31:53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu X, Taber-Thomas BC, Pérez-Edgar K. Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biol Psychol. 2015 Sep 6; doi: 10.1016/j.biopsycho.2015.08.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarcho JM, Fox NA, Pine DS, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol Psychol. 2013;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamm C, Walker OL, Degnan KA, et al. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev Sci. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aupperle RL, Allard CB, Grimes EM, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- 32.Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 33.Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. Eur J Pharmacol. 2000;410(2–3):107–120. doi: 10.1016/s0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test Manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Bishop G, Spence SH, McDonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Dev. 2003;74(6):1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 38.Dyson MW, Klein DN, Olino TM, Dougherty LR, Durbin CE. Social and non-social behavioral inhibition in preschool-age children: differential associations with parent-reports of temperament and anxiety. Child Psychiatry Hum Dev. 2011;42(4):390–405. doi: 10.1007/s10578-011-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broeren S, Muris P. A psychometric evaluation of the Behavioral Inhibition Questionnaire in a non-clinical sample of Dutch children and adolescents. Child Psychiatry Hum Dev. 2009;41(2):214–229. doi: 10.1007/s10578-009-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59(6):1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 41.Fox NA. Factors contributing to the emergence of anxiety among behaviorally inhibited children: the role of attention. New Dir Child Adolesc Dev. 2010;2010(127):33–49. doi: 10.1002/cd.261. [DOI] [PubMed] [Google Scholar]
- 42.Ballespí S, Jané MC, Riba MD. Reliability and validity of a brief clinician-report scale for screening behavioral inhibition. J Psychopathol Behav Assess. 2013;35:321–334. [Google Scholar]
- 43.Birmaher B, Khetarpal S, Brent D, et al. The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Beidel DC, Turner SM, Morris TL. A new inventory to assess childhood social anxiety and phobia: the Social Phobia and Anxiety Inventory for Children. Psychol Assess. 1995;7:73–9. [Google Scholar]
- 45.Gensthaler A, Möhler E, Resch F, et al. Retrospective assessment of behavioral inhibition in infants and toddlers: development of a parent report questionnaire. Child Psychiatry Hum Dev. 2013;44:152–165. doi: 10.1007/s10578-012-0316-z. [DOI] [PubMed] [Google Scholar]
- 46.Skuse DH. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187(6):568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 47.Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. J Abnorm Child Psychol. 1986;14(1):25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- 48.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 49.Egger HL, Pine DS, Nelson E, et al. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. Int J Methods Psychiatr Res. 2011;20:145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- 51.de Bie HMA, Boersma M, Wattjes MP, et al. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr. 2010;169(9):1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. NeuroImage. 2005;27(3):624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. NeuroImage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gläscher JP, Gitelman D. [Accessed January 17, 2016];Contrast weights in flexible factorial design with multiple groups of subjects. http://www.sbirc.ed.ac.uk/cyril/download/Contrast_Weighting_Glascher_Gitelman_2008.pdf. Published 2008.
- 56.Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 58.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huttenlocher PR. Morphometric study of human cerebral cortex development. In: Johnson MH, Munakata Y, Gilmore RO, editors. Brain Development and Cognition: A Reader. 2. Malden: Blackwell Publishing; 2002. pp. 117–128. [Google Scholar]
- 60.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65(11):1303. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams LE, Oler JA, Fox AS, et al. Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2015;40:1428–1435. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyer AE, Benson B, Choate VR, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26:229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirshfeld-Becker DR, Biederman J, Faraone SV, Violette H, Wrightsman J, Rosenbaum JF. Temperamental correlates of disruptive behavior disorders in young children: preliminary findings. Biol Psychiatry. 2002;51:563–574. doi: 10.1016/s0006-3223(01)01299-9. [DOI] [PubMed] [Google Scholar]
- 67.Degnan KA, Almas AN, Henderson HA, Hane AA, Walker OL, Fox NA. Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Dev Psychol. 2014;50:2311–2323. doi: 10.1037/a0037751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.