Abstract
Objectives
Prior studies have shown an anticancer effect of statins in patients with certain malignancies. However, it is unclear whether statins have a mortality benefit in lung cancer. We compared survival of patients with stage IV non-small cell lung cancer (NSCLC) receiving vs. not receiving statins prior to diagnosis.
Methods
Using data from the Surveillance, Epidemiology and End Results registry linked to Medicare claims, we identified 5,118 patients >65 years of age diagnosed with stage IV NSCLC between 2007 and 2009. We used propensity score methods to assess the association of statin use with overall and lung cancer-specific survival while controlling for measured confounders.
Results
Overall, 27% of patients were on statins at time of lung cancer diagnosis. Median survival in the statin group was 7 months, compared to 4 months in patients not treated with statins (p<0.001). Propensity score analyses found that statin use was associated with improvement in overall (hazard ratio [HR]: 0.76, 95% confidence interval [CI]: 0.73–0.79) and lung cancer-specific survival (HR: 0.77, 95% CI: 0.73–0.81), after controlling for baseline patient characteristics, cancer characteristics, staging work-up and chemotherapy use.
Conclusions
Statin use is associated with improved survival among patients with stage IV NSCLC suggesting a potential anticancer effect. Further research should evaluate plausible biological mechanisms as well as test the effect of statins in prospective clinical trials.
Keywords: non-small cell lung cancer, statins, survival analysis, outcomes
1. Introduction
Patients with advanced-stage lung cancer have very poor survival with a median survival of approximately 4 months [1]. Most research has traditionally evaluated cytotoxic chemotherapy for lung cancer treatment; however, there are increasing numbers of studies investigating the repositioning of medications primarily used for non-cancer purposes as anticancer agents. Among these medications are the statins, a class of widely-used lipid lowering agents that work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Some recent data suggest that statins may improve mortality in lung cancer patients [2, 3], although others do not seem to show a benefit [4]. However, prior observational studies [2, 3] included large number of patients with early stage lung cancer; thus, it is possible that the survival benefit observed was due to statins’ effect on cardiovascular risk reduction since many early stage lung cancer patients experience relatively good long-term survival. In addition, staging work-up and treatment were not assessed in these studies, and it is unknown whether there is a benefit to using statins in advanced lung cancer patients who are not treated with chemotherapy and who have very short median survival. Lastly, the study [4] showing no effect of simvastatin on survival in advanced lung cancer patients was a small phase II study and may not have had enough power to find a survival benefit.
In this study, we used a nationally representative, population-based cancer data source in the United States to determine the effect of statins use on survival outcomes among patients with stage IV non-small cell lung cancer (NSCLC).
2. Methods
2.1. Data sources
The study was conducting using the Surveillance, Epidemiology and End Results (SEER) registry (2007–2009) linked to Medicare claims [5]. We selected patients >65 years with histologically-confirmed stage IV NSCLC. We excluded individuals in health care maintenance organizations or those without Part B Medicare insurance due to lack of complete claims and those without Part D coverage for whom we could not ascertain outpatient medications [6]. We excluded patients living in a nursing home at time of diagnosis as they likely had limited functional status.
2.2. Comorbidities and Medications
We obtained data about sociodemographics information from SEER and Medicare databases. We used the Deyo adaptation of Charlson’s index to assess the burden of comorbidities [7–9] and data about use of home health services (restricted to homebound patients), including receipt of home physical therapy, occupational therapy, home health aide or social services, as a proxy for poor performance status [10]. Statin and other lipid-lowering medication use was ascertained from Medicare Part D claims. Following an intention to treat analysis, patients were classified as using specific drugs if there was a pharmacy claim submitted within 6 months prior to cancer diagnosis.
2.3. Cancer-related Factors and Treatment
Data regarding tumor location and histology were obtained from SEER; histologic subtypes were classified using International Classification of Diseases for Oncology [11]. Using Medicare claims, we ascertained use of diagnosis and staging procedures (such as Positron Emission Tomography [PET] scan, mediastinoscopy and fine needle biopsy) and classified patients as treated with chemotherapy if they received treatment within 4 months of cancer diagnosis [12].
2.4. Study outcome
The study outcomes were overall (primary) and lung cancer-specific (secondary) survival determined from Medicare and SEER data, respectively. Survival times were calculated as the period from the date of diagnosis to the date of death; subjects alive as of December 15, 2011 were censored.
2.5. Statistical Analysis
Baseline characteristics were compared using the t-test, chi-square test or Wilcoxon test. Unadjusted Kaplan-Meier curves were plotted for patients treated with or without statins and compared using the log-rank test. We used propensity score methods to control for potential allocation bias [13] since differences in patient characteristics and comorbidities may have influenced statin prescribing. The propensity score represents the probability that a patient will receive a statin based on their known baseline (pre-cancer diagnosis) characteristics. We calculated propensity scores using a logistic model that included patients’ sociodemographics (age, gender, race/ethnicity, marital status, and income quartile), comorbidities (hypertension, hyperlipidemia, diabetes, congestive heart failure, cerebrovascular disease, peripheral vascular disease, history of myocardial infarction), Charlson comorbidity score, and performance status and used regression analysis to evaluate whether covariates were balanced across treatment groups after adjusting for propensity scores.
Cox regression was used to compare overall and lung cancer-specific survival of patients receiving and not receiving statins while adjusting for propensity scores as well as use of PET scan, mediastinoscopy, fine needle biopsy, tumor characteristics and use of oral and systemic chemotherapy. Adjusted analyses were performed using inverse probability weighting, fitting a stratified Cox model according to propensity score quintiles, and matching patients by propensity scores [14]. We conducted secondary stratified analyses by receipt of chemotherapy or PET scan use. Additionally, to assess if the survival benefit was specific to statins, we tested the effect of statins compared to other lipid-lowering medications. Analyses were performed with SAS 9.3 (SAS, Cary, NC) using two tailed p-values. Our study was deemed exempt following Institutional Review Board evaluation at Icahn School of Medicine at Mount Sinai.
3. Results
We identified 5,118 patients over 65 years with stage IV NSCLC. Overall, 1404 (27%) patients were treated with a statin at the time of lung cancer diagnosis. Statin-treated patients were younger (p=0.04), more likely to be female (p<0.01), married (p<0.01), have more comorbidities (p<0.01), and as expected, more likely to have hypertension, diabetes, history of myocardial infarction, congestive heart failure, peripheral vascular disease, or cerebrovascular disease (p<0.01 for all comparisons). Other baseline characteristics were not significantly different between the two groups and all covariates were well-balanced after adjustment for propensity scores (Table 1). Those in the statin group were more likely to have had a PET scan, mediastinoscopy and fine needle biopsy and were also more likely to have been treated with chemotherapy (p<0.01 for all comparisons; Table 2).
Table 1.
Characteristics of Stage IV Non-small Cell Lung Cancer Patients in the SEER-Medicare Database, 2007–2009
| Characteristic | Statin (N=1404) |
No Statin (N=3714) |
P-value | Adjusted P-value* |
|---|---|---|---|---|
| Age, years, mean±SD | 75.3±6.2 | 75.7±6.8 | 0.04 | 0.98 |
| Male, N (%) | 662 (48.3) | 1901 (52.6) | <0.01 | 0.97 |
| Married, N (%) | 694 (50.6) | 1611 (44.6) | <0.01 | 0.96 |
| Race/Ethnicity, N (%) | 0.75 | 0.99 | ||
| White | 1049 (76.5) | 2733 (75.6) | ||
| Black | 135 (9.9) | 377 (10.4) | ||
| Hispanic | 70 (5.1) | 207 (5.7) | ||
| Other | 117 (8.5) | 307 (8.5) | ||
| Income, N (%) | 0.28 | 0.99 | ||
| First quartile | 425 (31.0) | 1122 (31.1) | ||
| Second quartile | 333 (24.3) | 951 (26.3) | ||
| Third quartile | 318 (23.2) | 759 (21.1) | ||
| Fourth quartile | 295 (21.5) | 780 (21.6) | ||
| Comorbidity Score, N (%) | <0.01 | 0.48 | ||
| <1 | 527 (37.5) | 1607 (43.3) | ||
| 1–2 | 265 (18.9) | 886 (23.9) | ||
| >2 | 612 (43.6) | 1221 (32.9) | ||
| Hypertension, N (%) | 1113 (79.3) | 2396 (64.5) | <0.01 | 0.94 |
| Diabetes (without complications), N (%) | 484 (34.5) | 845 (22.8) | <0.01 | 0.82 |
| Diabetes (with complications), N (%) | 110 (7.8) | 180 (4.9) | <0.01 | 0.9 |
| History of myocardial infarction, N (%) | 89 (6.3) | 109 (2.9) | <0.01 | 0.71 |
| Peripheral vascular disease, N (%) | 208 (14.8) | 363 (9.8) | <0.01 | 0.87 |
| Congestive heart failure, N (%) | 229 (16.3) | 480 (12.9) | <0.01 | 0.94 |
| Cerebrovascular disease, N (%) | 146 (10.4) | 244 (6.6) | <0.01 | 0.88 |
SD denotes standard deviation
P-values for analysis adjusting for propensity scores.
Table 2.
Lung Cancer and Treatment Characteristics of Study Subjects
| Characteristic | Statin (N=1404) |
No Statin (N=3714) |
P-value |
|---|---|---|---|
| Tumor Histology, N (%) | 0.10 | ||
| Adenocarcinoma | 811 (59.2) | 2159 (59.7) | |
| Squamous cell | 419 (30.6) | 1010 (28.0) | |
| Large cell | 49 (3.6) | 144 (4.0) | |
| Other | 92 (6.7) | 301 (8.3) | |
| Tumor Site, N (%) | 0.10 | ||
| Upper lobe | 628 (45.8) | 1642 (45.4) | |
| Middle lobe | 66 (4.8) | 124 (3.4) | |
| Lower lobe | 357 (26.0) | 944 (26.1) | |
| Other | 320 (23.3) | 904 (25.0) | |
| Mediastinoscopy, N (%) | 41 (3.0) | 60 (1.7) | <0.01 |
| PET Scan, N (%) | 589 (43.6) | 1140 (32.0) | <0.01 |
| Bone Scan, N (%) | 230 (17.0) | 622 (17.5) | 0.68 |
| Fine Needle Aspiration, N (%) | 428 (31.2) | 834 (23.1) | <0.01 |
| Systemic chemotherapy, N (%) | 733 (53.5) | 1487 (41.2) | <0.01 |
| Oral chemotherapy, N (%) | 262 (18.7) | 516 (13.9) | <0.01 |
PET denotes Positron Emission Tomography
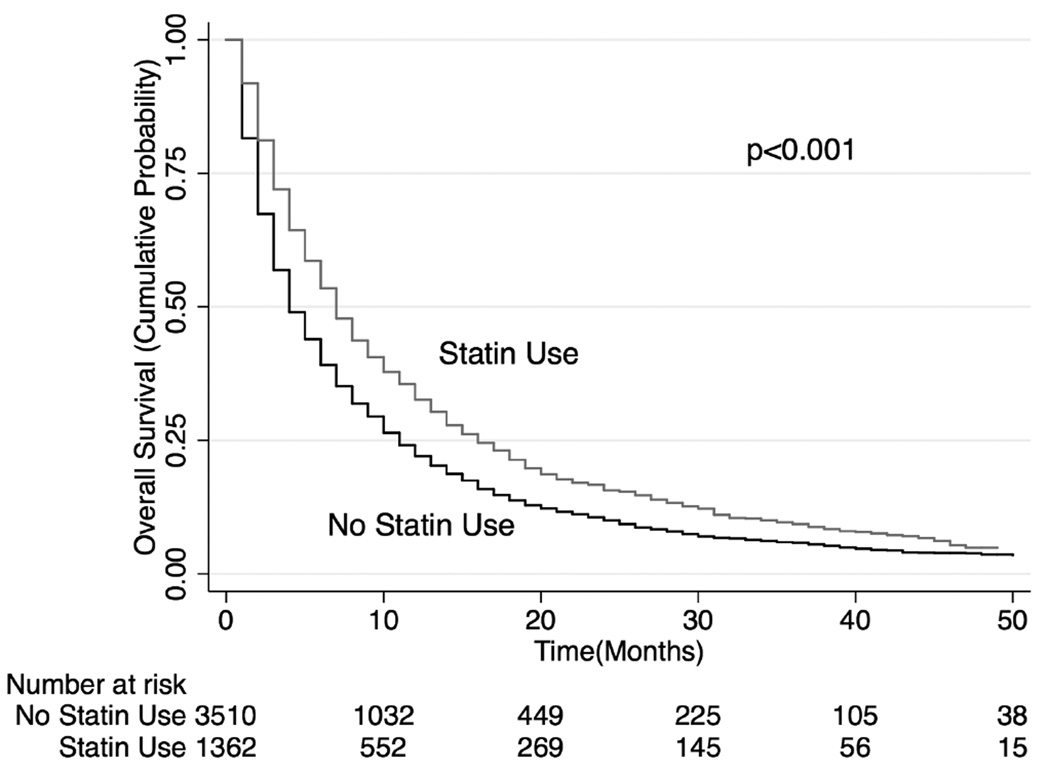
Unadjusted median overall survival (OS) for those in the statin group was 7 months (interquartile range [IQR]: 13 months) compared to 4 months (IQR: 9 months) among those not treated with statin (p<0.001, Figure 1). Inverse probability weighting analysis using Cox regression (and adjusted for staging work-up, cancer characteristics, and oral and systemic chemotherapy use) showed that statin use associated with significantly better overall survival (hazard ratio [HR]: 0.77, 95% confidence interval [CI]: 0.74 to 0.81) and lung cancer-specific survival (HR: 0.78, 95% CI: 0.74–0.82; Table 3). The survival advantage with statins persisted when the analyses were repeated using stratification (HR: 0.75, 95% CI: 0.70–0.80 for OS; HR: 0.74, 95% CI 0.68–0.81 for lung cancer-specific survival) or matching (HR: 0.77, 95% CI: 0.72–0.83 for OS; HR 0.75, 95% CI 0.68–0.82 for lung cancer-specific survival) of study patients by propensity scores.
Figure 1. Kaplan-Meier Overall Survival Curves for all patients in cohort.
Patients in the statin group have better overall survival compared to those in the non-statin group.
Table 3.
Propensity Score Analysis: Comparison of Overall and Lung Cancer-Specific Survival of Patients Treated with and without Statins
| Model | Overall Survival Hazard Ratio (95% CI*) |
Lung Cancer Survival Hazard Ratio (95% CI*) |
|---|---|---|
| Primary Analysis: Entire Cohort | ||
| Inverse Probability Weighted | 0.77 (0.74–0.81) | 0.78 (0.74–0.82) |
| Stratified by Propensity Score Quintiles | 0.75 (0.70–0.80) | 0.74 (0.68–0.81) |
| Matched Analysis (N=4556) | 0.77 (0.72–0.83) | 0.75 (0.68–0.82) |
| Secondary Analyses | ||
| Patients who received systemic chemotherapy | ||
| Inverse Probability Weighted | 0.86 (0.81–0.91) | 0.81 (0.75–0.87) |
| Stratified by Propensity Score Quintiles | 0.83 (0.75–0.92) | 0.78 (0.68–0.89) |
| Matched Analysis (N=2052) | 0.86 (0.78–0.95) | 0.79 (0.70–0.90) |
| Patients who received oral chemotherapy | ||
| Inverse Probability Weighted | 0.87 (0.75–1.03) | 0.94 (0.77–1.15) |
| Stratified by Propensity Score Quintiles | 0.85 (0.66–1.11) | 0.96 (0.69–1.33) |
| Matched Analysis (N=304) | 0.90 (0.69–1.17) | 0.98 (0.70–1.36) |
| Patients who did not receive systemic or oral chemotherapy | ||
| Inverse Probability Weighted | 0.74 (0.70–0.79) | 0.78 (0.73–0.84) |
| Stratified by Propensity Score Quintiles | 0.71 (0.64–0.79) | 0.72 (0.64–0.82) |
| Matched Analysis (N=2220) | 0.70 (0.63–0.78) | 0.71 (0.63–0.81) |
| Patients who underwent a PET scan | ||
| Inverse Probability Weighted | 0.81 (0.75–0.87) | 0.79 (0.72–0.87) |
| Stratified by Propensity Score Quintiles | 0.78 (0.70–0.88) | 0.77 (0.66–0.89) |
| Matched Analysis (N=1625) | 0.82 (0.73–0.91) | 0.78 (0.68–0.91) |
| Patients who did not undergo a PET scan | ||
| Inverse Probability Weighted | 0.77 (0.74–0.81) | 0.79 (0.74–0.84) |
| Stratified by Propensity Score Quintiles | 0.74 (0.68–0.81) | 0.74 (0.67–0.83) |
| Matched Analysis (N=2931) | 0.75 (0.69–0.83) | 0.74 (0.66–0.83) |
| Low potency (vs. high potency) statin | 0.92 (0.88–0.96) | 0.91 (0.87–0.96) |
CI denotes confidence interval. The hazard ratio represents the risk of death of a patient treated with a statin compared with a patient who did not receive a statin. All models were also adjusted for tumor histology, tumor site, receipt of PET scan, mediastinoscopy, fine needle aspiration, and use of systemic and oral chemotherapy.
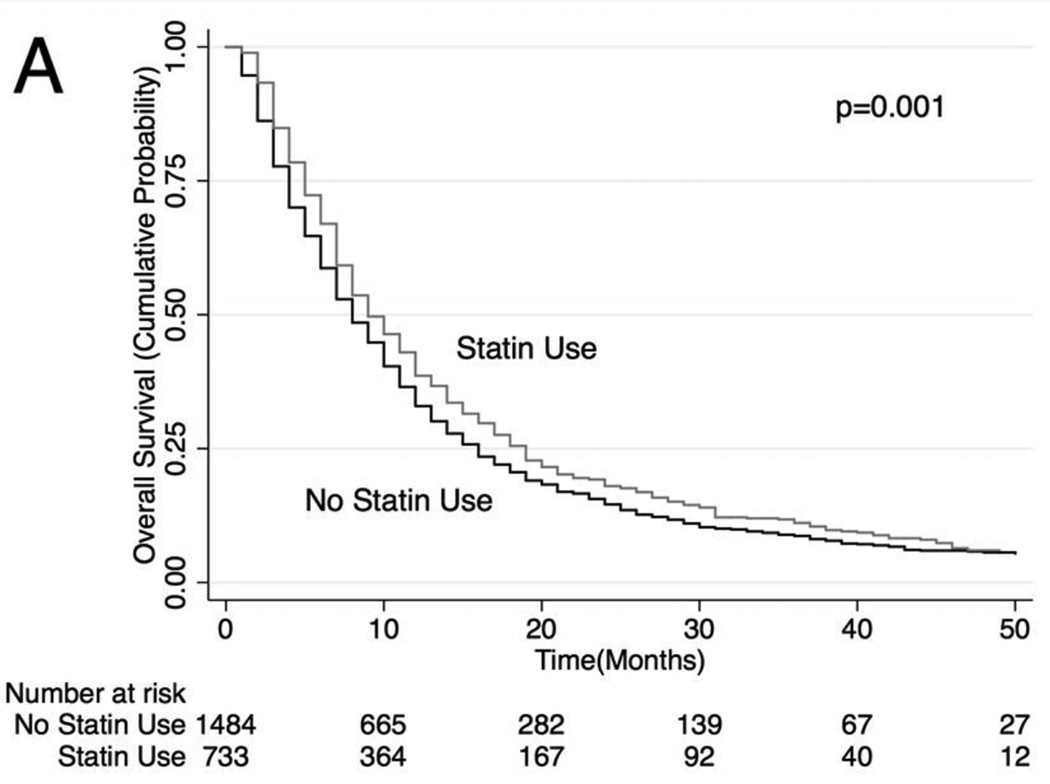
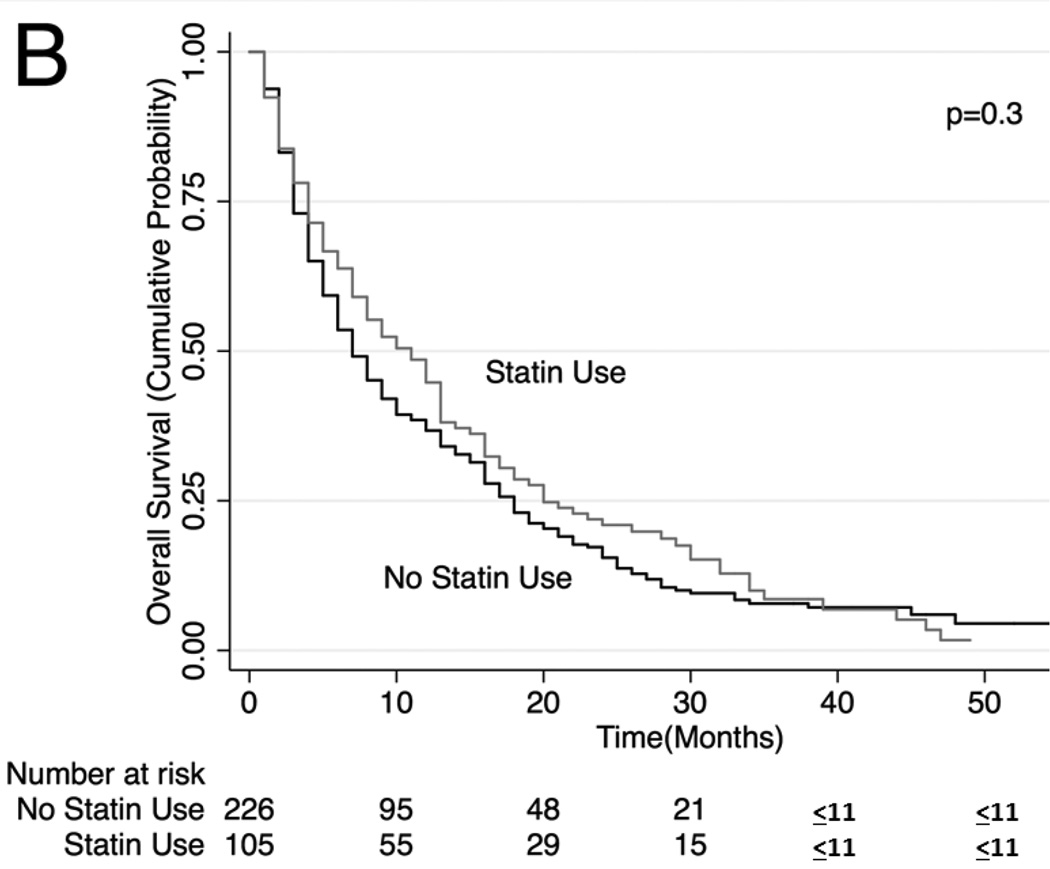
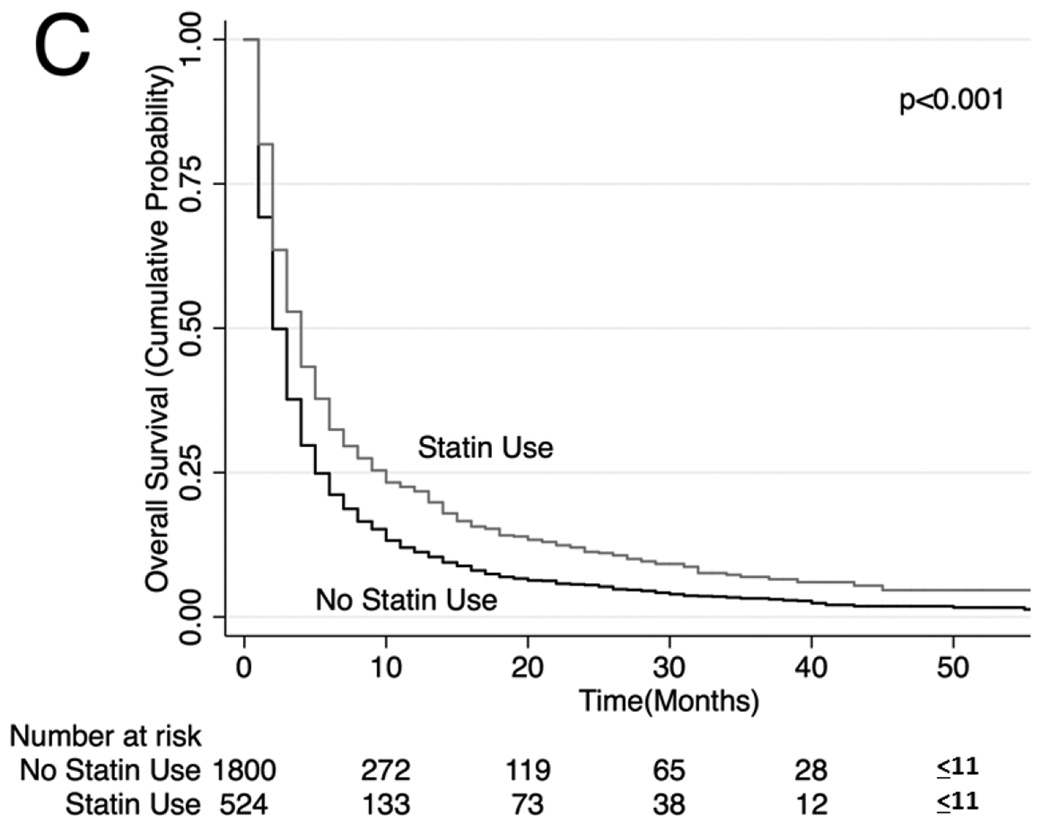
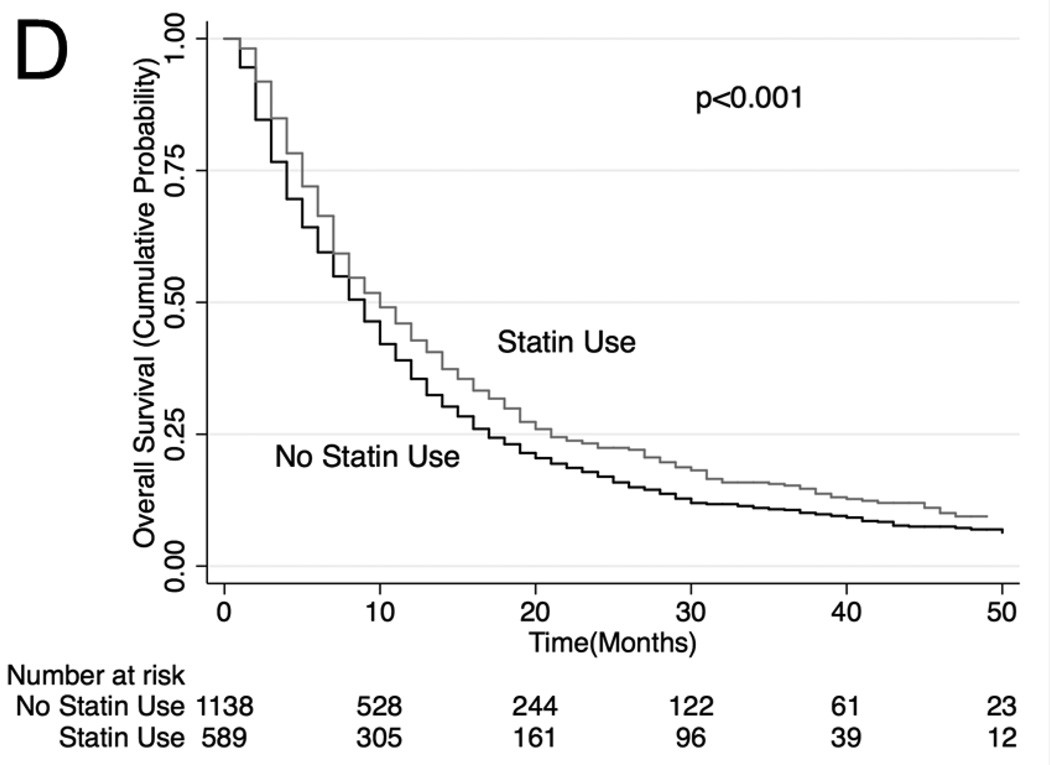
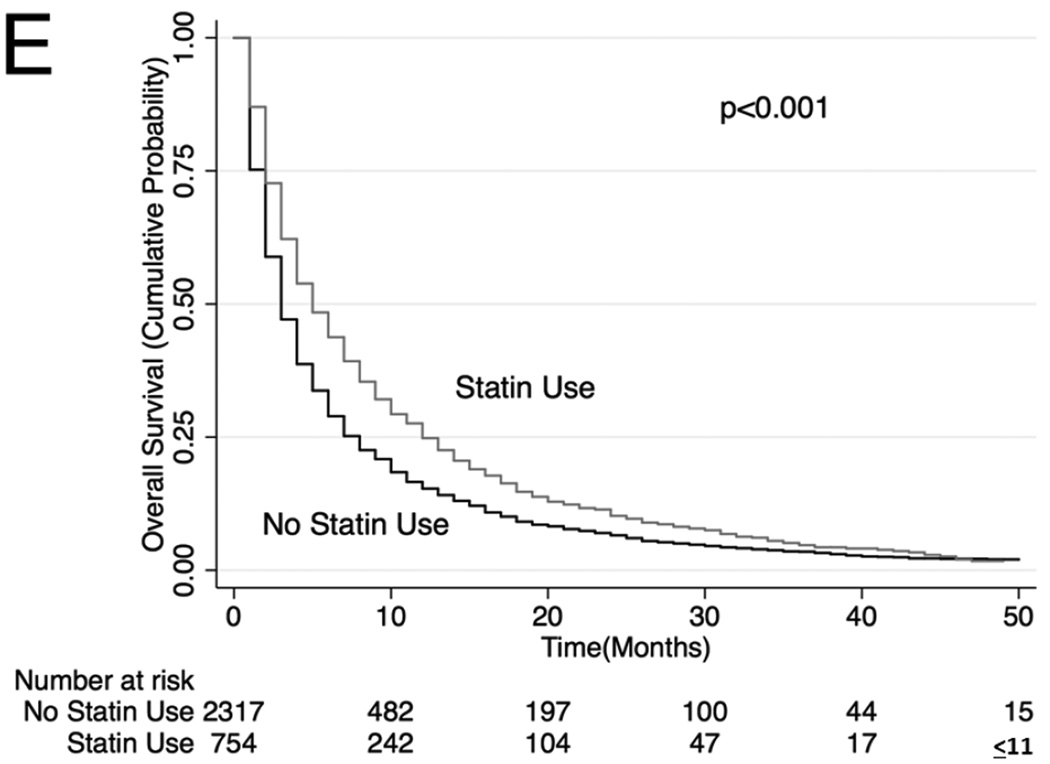
Unadjusted (Figure 2) and adjusted (Table 3) secondary analysis stratified by receipt of systemic chemotherapy (HR: 0.86, 95% CI: 0.81–0.91 for OS and HR 0.81, 95% CI 0.75–0.87 for lung cancer-specific survival among those who received chemotherapy) showed survival benefit with statins. Stratification by those who did not receive systemic or oral chemotherapy also showed a survival benefit with statin use (HR: 0.74, 95% CI: 0.70–0.79 for OS and HR 0.78, 95% CI 0.73–0.84 for lung cancer-specific survival), although stratification by receipt of oral chemotherapy (tyrosine kinase inhibitor) did not show a statistically significant survival benefit with statin use (HR: 0.87, 95% CI: 0.75–1.03 for OS and HR 0.94, 95% CI 0.77–1.15 for lung cancer-specific survival among those who received oral chemotherapy). Lastly, analyses stratifying by PET scan use showed a significant survival advantage conferred by statin use (HR: 0.81. 95% CI: 0.75–0.87; HR 0.79, 95% CI 0.72–0.87 for lung cancer-specific survival among those who underwent PET scan and HR: 0.77, 95% CI: 0.74–0.81 for OS and HR: 0.79, 95% CI: 0.74–0.84 for lung cancer-specific survival among those who did not undergo PET scan). The survival benefit of statins remained significant when secondary analyses were repeated using stratification or matching of patients by propensity scores (Table 3). Analyses of specific types of statins showed that lower potency statins (fluvastatin, lovastatin, pravastatin and simvastatin) conferred a slightly better overall and lung-cancer-specific survival compared to higher potency statins (atorvastatin and rosuvastatin; HR: 0.92, 95% CI: 0.88–0.96 and HR: 0.91, 95% CI: 0.87–0.96, respectively).
Figure 2. Stratified Kaplan-Meier Overall Survival Curves.
Stratified by (A) patients who received systemic chemotherapy, (B) patients who received oral chemotherapy (C) patients who did not receive systemic or oral chemotherapy, (D) patients who received PET scan, (E) patients who did not receive PET scan
4. Discussion
Previous studies have reported that statins are associated with improved survival in some but not other cancers [3, 15, 16]. However, there is limited data regarding the potential effectiveness of statins among patients with lung cancer. Using population-based data, we found that among stage IV NSCLC patients, statin use was associated with significantly better overall and lung cancer-specific survival, even when compared to other lipid-lowering agents. Our results contribute further evidence supporting the potential anti-cancer effects of statins.
This is the first study to investigate statin use specifically amongst a large cohort of patients with advanced NSCLC. A meta-analysis of statins on cancer incidence and mortality did not show a reduction of lung cancer deaths; however these results were limited by the low number of lung cancer cases included in the studies [17]. Another phase 2 study in patients with advanced lung cancer did not find a survival benefit of statins [4]. Our findings of improved overall and lung cancer specific survival are consistent with a large epidemiologic study of statin use among Danish patients showing reduced cancer mortality in lung cancer patients of all stages [3]. In a recent study using a cohort from the United Kingdom, Cardwell et al. found similar improvements in lung cancer and overall survival when they investigated statin use prior to lung cancer diagnosis [2]. However, they also included all stages of NSCLC and small cell lung cancer cases and did not control for stage or use of chemotherapy. We restricted our cases to stage IV NSCLC to allow for a more homogenous group of patients with similar survival.
The mechanisms through which statins may contribute to improving lung cancer survival are unknown. Patients with lung cancer, due to a shared risk factor of smoking, have a large burden of cardiovascular disease. These patients are at increased risk for premature death due to cardiovascular events and treatment with a statin may mitigate this risk. However, given the relatively short life expectancy and the extremely high rate of lung cancer deaths among these patients, our findings should not be explained by a positive impact of statins on cardiovascular events.
A mechanism by which statins may confer survival benefit is through lowering circulating cholesterol levels. Statins inhibit cholesterol synthesis within cells through their effect on the HMG-CoA reductase, the rate-limiting enzyme in the mevalonate and cholesterol-synthesis pathway [18, 19]. Many cholesterol metabolites are involved in cell proliferation, membrane integrity, cell signaling, protein synthesis, and cell-cycle progression. Disruption of these pathways has been hypothesized to inhibit cancer growth and metastasis [18]. Recent findings suggest that statins may also inhibit the nuclear localization of the YAP and TAZ proto-oncogenes via inhibition of the mevalonate pathway [20]. Inhibition of the mevalonate pathway inhibits geranylgeranyl pyrophosphate which is required for the Rho guanine phosphate transferases (GTPases) that activate YAP and TAZ. In vitro studies have also shown that simvastatin can induce cell cycle arrest or possibly apoptosis in human lung cancer cells [21, 22]. Simvastatin downregulates cyclin D1 and cyclin-dependent kinase (CDK) expression and appears to decrease matrix metallopeptidase-9 (MMP-9) levels, possibly by inhibiting the activation of NF-κB.[22] MMP-9 is thought to be important for increasing metastatic potential in cancer cells due to its role in extracellular matrix remodeling and angiogenesis [23, 24]. Statins have also been shown to act downstream of ATP citrate lyase in the cholesterol synthesis pathway and can inhibit NSCLC growth in in vivo mouse models by inhibiting this enzyme [25]. Lastly, in vitro studies show that simvastatin may reverse resistance to tyrosine kinase inhibitors for NSCLC cell lines, which have a T790M mutation of the epidermal growth factor receptor [26]. These findings suggest that multiple mechanisms may be responsible for the improved survival benefit seen in NSCLC patients taking statins [27, 28].
Several strengths and limitations of this study should be noted. First, due to limitations of the database, we are unable to assess smoking history and could not adjust for this variable in the propensity matching. In addition, due to the observational nature of the study, treatment with statins was not randomized. Use of statin therapy may be a reflection of more comprehensive health care or increased health awareness. To address this limitation, we adjusted our analyses for potential confounders such as sociodemographics and comorbidities to create a comparison groups that would have had similar likelihoods of receiving statins. Moreover, statin use prior to NSCLC diagnosis was probably not related to cancer characteristics or other cancer-related prognostic factors. NSCLC patients who were treated with statins were more likely to receive chemotherapy and undergo PET scans; however, the survival benefit of statins was consistent even after stratifying by these factors. We cannot rule out the possibility that residual confounding could explain a small increase in survival. Our study was limited to patients above 65 years of age and consequently we were unable to assess the effect of statins in younger patients.
Use of medications was determined using pharmacy claims, and therefore we have no information on adherence to therapy. Despite this, pharmacy claims data have been shown to have high concordance with pill counts [29]. We used a conservative estimate of medication treatment since some patients receive a three-month supply of their chronic medications. Furthermore, lack of adherence would have biased our results towards the null. Finally, we were not able to assess whether there was a dose-dependent effect of statins on survival, although analysis of specific statins compared to other statins did show a slight survival benefit of lower potency statins compared to higher potency statins. Consistent with an intention-to-treat analysis, we did not include statin use after lung cancer diagnosis since ongoing statin use after cancer diagnosis may suggest a healthier population. In this approach, the assumption is that pre-diagnosis use is correlated with later exposure, although it may not be a perfect marker. The alternative approach, using post diagnosis drug exposure is substantially biased for two main reasons. First, post diagnosis use may be determined by cancer-related factors, such as extent of metastasis, weight loss, poor (or good) prognosis, and thus may lead to confounding by indication. Second, survival bias (i.e., those who survive longer will be exposed to statins for longer periods) will lead to overestimating the beneficial effect of statins. Our approach would tend to bias the results to the null so that our findings are conservative.
In summary, these data suggest that among patients with stage IV non-small cell lung cancer, statin use was associated with improved survival. This effect is consistent with the survival benefit of statins observed in other cancer types. Further prospective studies evaluating the use of statins in conjunction with chemotherapy for stage IV lung cancer can help determine if statins are an effective class of medications for advanced lung cancer treatment and may better elucidate cancer-specific mechanisms of action for statins.
Highlights.
In our sample, 27% of patients were on statins at time of NSCLC diagnosis
Stage IV NSCLC patients on statins prior to cancer diagnosis have improved survival
These findings suggest a potential anticancer effect of statins
Acknowledgments
Funding: This study was supported by the National Institutes of Health (1K07CA166462 to JJL and 1K07CA180782 to KS). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Wisnivesky is a member of the research board of EHE International, has received consulting honorarium from Merck Pharmaceutical, BMS, and Quintiles, and a research grant from Aventis.
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services, and the Office of Strategic Planning, Health Care Finance Administration; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the SEER-Medicare Database. The interpretation and reporting of these data are the sole responsibilities of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All other authors are without any conflicts of interest.
References
- 1.Cetin K, Ettinger DS, Hei YJ, O'Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. 2011;3:139–148. doi: 10.2147/CLEP.S17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardwell CR, Mc Menamin U, Hughes CM, Murray LJ. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2015;24(5):833–841. doi: 10.1158/1055-9965.EPI-15-0052. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 4.Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, Kim HT, Lee JS. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(6):1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 5.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 6.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44(10):921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 8.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of epidemiology. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 10.Wisnivesky JP, Smith CB, Packer S, Strauss GM, Lurslurchachai L, Federman A, Halm EA. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II–IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz AG, editor. International Classification of Diseases for Oncology: ICD-O. 3rd. World Health Organization; 2000. [Google Scholar]
- 12.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 13.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 Supl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 14.Wei L, Lin D, Weissfeld L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 15.Ng K, Ogino S, Meyerhardt JA, Chan JA, Chan AT, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Benson AB, 3rd, et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst. 2011;103(20):1540–1551. doi: 10.1093/jnci/djr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon CY, Pandol SJ, Wu B, Cook-Wiens G, Gottlieb RA, Merz NB, Goodman MT. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One. 2015;10(4):e0121783. doi: 10.1371/journal.pone.0121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. Jama. 2006;295(1):74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 18.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(1):10–19. [PubMed] [Google Scholar]
- 19.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16(4):508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 20.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 21.Liang YW, Chang CC, Hung CM, Chen TY, Huang TY, Hsu YC. Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC) Int J Mol Sci. 2013;14(3):5806–5816. doi: 10.3390/ijms14035806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Pan Y, Ma H, Li W. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol Res. 2013;20(8):351–357. doi: 10.3727/096504013X13657689382897. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 25.Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P, Sukhatme VP. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227(4):1709–1720. doi: 10.1002/jcp.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang KE, Kwon SJ, Kim YS, Park DS, Kim BR, Yoon KH, Jeong ET, Kim HR. Effect of simvastatin on the resistance to EGFR tyrosine kinase inhibitors in a non-small cell lung cancer with the T790M mutation of EGFR. Exp Cell Res. 2014;323(2):288–296. doi: 10.1016/j.yexcr.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Altwairgi AK. Statins are potential anticancerous agents (review) Oncol Rep. 2015;33(3):1019–1039. doi: 10.3892/or.2015.3741. [DOI] [PubMed] [Google Scholar]
- 28.Young RP, Hopkins RJ. The mevalonate pathway and innate immune hyper-responsiveness in the pathogenesis of COPD and lung cancer: potential for chemoprevention. Curr Mol Pharmacol. 2016 doi: 10.2174/1874467209666160112130016. [DOI] [PubMed] [Google Scholar]
- 29.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]