Abstract
Rationale: Pulmonary complications (PCs) cause significant morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HCT). Shifts in gut microbiota have been linked to HCT outcomes; however, their effect on PCs is unknown.
Objectives: To investigate whether changes in gut microbiota are associated with PCs after HCT.
Methods: A single-center observational study was performed on 94 patients who underwent HCT from 2009 to 2011 and who were previously enrolled in a protocol for 16S ribosomal RNA sequencing of fecal microbiota. The primary endpoint, PC, was defined by new abnormal parenchymal findings on chest imaging in the setting of respiratory signs and/or symptoms. Outcomes were collected up to 40 months after transplant. Clinical and microbiota risk factors for PCs and mortality were evaluated using survival analysis.
Measurements and Main Results: One hundred twelve PCs occurred in 66 (70.2%) subjects. A high comorbidity index (hazard ratio [HR], 2.30; 95% confidence interval [CI], 1.30–4.00; P = 0.004), fluoroquinolones (HR, 2.29, 95% CI, 1.32–3.98; P = 0.003), low baseline diversity (HR, 2.63; 95% CI, 1.22–5.32; P = 0.015), and γ-proteobacteria domination of fecal microbiota (HR, 2.64; 95% CI, 1.10–5.65; P = 0.031), which included common respiratory pathogens, predicted PCs. In separate analyses, low baseline diversity was associated with PCs that occurred preengraftment (HR, 6.30; 95% CI, 1.42–31.80; P = 0.016), whereas γ-proteobacteria domination predicted PCs postengraftment (HR, 3.68; 95% CI, 1.49–8.21; P = 0.006) and overall mortality (HR, 3.52; 95% CI, 1.28–9.21; P = 0.016). Postengraftment PCs were also independent predictors of death (HR, 2.50; 95% CI, 1.25–5.22; P = 0.009).
Conclusions: This is the first study to demonstrate prospective changes in gut microbiota associated with PCs after HCT. Postengraftment PCs and γ-proteobacteria domination were predictive of mortality. This suggests an adverse relationship between the graft and lung, which is perhaps mediated by bacterial composition in the gut. Further study is warranted.
Keywords: stem cell transplantation, microbiota, pulmonary disease
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary complications are associated with significant morbidity and mortality after allogeneic hematopoietic cell transplantation. It is possible that gut microbiota, which have been associated with adverse transplant outcomes, contribute to lung injury in this setting. In the absence of a clear understanding of infectious and inflammatory disease mechanisms in this population, tools for prediction, diagnosis, and treatment of transplantation-related pulmonary complications are limited.
What This Study Adds to the Field
This is the first study to use high-throughput sequencing of the 16S rRNA gene to identify gut microbiota composition as a risk factor for pulmonary complications and mortality after allogeneic hematopoietic cell transplantation. As transplantation continues to expand as a treatment modality for diverse hematologic diseases, the identification of microbial mechanisms of lung injury is greatly needed to develop predictive risk tools and inform novel mechanistic hypotheses.
Allogeneic hematopoietic stem cell transplantation (HCT) is a potentially curative therapy for many blood disorders and malignancies. Advances in transplant management have greatly improved outcomes (1). However, pulmonary complications (PCs) are associated with high morbidity and mortality. Although PCs have been reported in up to 70% of HCT recipients (2–4), the epidemiology is poorly defined in this heterogeneous population.
Lung injury after HCT can occur early or late, present as acute or chronic, and involve one or more anatomic compartments of the lower respiratory tract. Risk factors for infectious complications include impairment of host defenses through myeloablative conditioning, T-cell depletion, and the development of graft versus host disease (GVHD) (5). Risk factors for parenchymal inflammation, such as idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage, and organizing pneumonia (OP), are less clear. Host susceptibility to PCs outside of typical engraftment phases may further increase in the setting of critical illness, augmented immunosuppression, and recurrent nosocomial exposures.
Chest imaging is quickly obtained upon clinical suspicion of a respiratory process in transplant patients. Computed tomography (CT) is often preferred because of the low sensitivity of chest X-rays in this population (6, 7). Although nonspecific, abnormal parenchymal patterns such as airspace consolidation, ground glass opacities, nodules, and reticular changes (8) may help inform the differential diagnosis and guide the next step in clinical decision-making (9). However, the presence of any pulmonary infiltrate, whether focal, multifocal, or diffuse, will commonly trigger broad antimicrobial treatment. Untargeted therapy can lead to additional complications, including antibiotic resistance, and may further confound the diagnostic evaluation when respiratory symptoms persist, progress, or recur.
Recently, associations between features of gut microbiota and bloodstream infection (10), Clostridium difficile infection (11), GVHD (12), and mortality (13) were described in allogeneic HCT recipients. Relationships between dysbiosis of gastrointestinal microbiota and allergic airway diseases (14–16), cystic fibrosis (17), and infection-mediated lung inflammation in animal models (18–21) have been suggested. The effects of diet (22) and probiotics (23, 24) on the prevention of pneumonia are also under investigation. The influence of gut microbiota on the development of PCs in HCT recipients, whose gut flora changes markedly upon hospitalization for HCT (10), has not been explored. Because of the likelihood of parallel mucosal changes in the gastrointestinal and respiratory tracts during transplant-related treatments, it is possible that gut–lung cross-talk contributes to PCs after HCT.
The objective of this study was to assess clinical and gut microbiota risk factors for post-transplant PCs, as defined by radiographic parenchymal abnormalities in the setting of respiratory signs and/or symptoms, and associated mortality at a high-volume academic transplantation center. Identification of gut microbiota features and transplant characteristics that may place allogeneic HCT recipients at increased risk for PCs will inform future investigations, with the ultimate goal to advance diagnostic, therapeutic, and prevention practices in this compromised population. Aspects of this research have previously been presented in abstract form (25, 26).
Methods
Study Population and Fecal Sampling Protocol
Chart review was performed on an established cohort of 94 patients who underwent allogeneic HCT from September 4, 2009, to August 4, 2011, and who were enrolled in a protocol for 16S ribosomal RNA (rRNA) sequencing of stool microbiota during hospitalization. Informed consent, patient enrollment, and sequencing methods have been described (10, 13); a brief summary is available in the online supplement.
Data Sources and Definitions
Clinical data
Results of chest imaging and clinical data were collected up to 40 months after transplant. The primary endpoint, PC, was defined as any new pulmonary infiltrate on chest CT (or X-ray if unable to obtain CT) in the setting of respiratory complaints or abnormal vital signs. Two study physicians independently identified each PC, with adjudication by a third in complex cases, as further described in the online supplement. Specific abnormalities identified on pre-transplant imaging were excluded. PCs did not include imaging findings suggestive of cardiogenic pulmonary edema, atelectasis, or recurrent malignancy unless there was concern for a superimposed process. PCs were defined as “preengraftment” if they occurred before recovery of an absolute neutrophil count ≥500 cells/μl for 3 consecutive days and “postengraftment” after the engraftment date (10). PCs were categorized as primarily “infectious” or “inflammatory/other” based on clinical impression in the medical record. PCs were excluded if they developed after a second HCT during the study period.
The endpoint of mortality was defined as the percentage of patients who died because of any cause, and was considered transplant-related in subjects who had not experienced disease relapse or progression (27).
Potential clinical predictors included demographic characteristics, underlying disease, allogeneic stem cell source, donor status, conditioning regimen intensity (28), disease risk score (based on the 2014 American Society for Blood and Marrow Transplantation Request for Information Disease Classifications schema) (29), the HCT comorbidity index (HCTCI; a weighted score of pre-transplantation comorbidity) (30), and antibiotic use during the transplantation hospitalization. The examined pulmonary risk factors included history of smoking, chronic lung disease, abnormal baseline pulmonary function (impaired diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin, obstructive ventilatory defect, restrictive ventilatory defect, and/or oxygen desaturation, defined according to American Thoracic Society/European Respiratory Society guidelines [31]), and the use of a fixed-dose busulfan-containing conditioning regimen.
Microbiota data
Phylogenetic classification for each of the 16S sequences was achieved using a naïve Bayesian classification approach and the Greengenes reference database (13, 32) for this analysis. Based on previous findings in this population, in whom intestinal composition frequently shifted from a diverse microbiota to one largely occupied by a single taxon soon after transplantation (10), we established two microbiota “states” as predictors. A state of low diversity, estimated by calculating the Shannon diversity index (SDI) (33), was defined by a SDI ≤1.5 (10, 34). A state of taxon domination was defined as 30% or greater relative abundance of the most dominant taxon identified in a stool specimen collected until Transplantation Day 35, hospital discharge, or death (10). Using this threshold, domination states by bacteria from the taxa proteobacteria (phylum), Enterococcus (genus), and Streptococcus (genus) were included based on previous findings as the most abundant and clinically relevant taxa in this allogeneic HCT population (10).
The microbiota of these patients were further described by grouping sequences into operational taxonomic units of 97% similarity and calculating α- and β-diversity measures. The SDI was calculated as a measure of α-diversity, and Bray-Curtis dissimilarity distances were calculated and plotted by principal components analysis as a measure of β-diversity.
Statistical Analysis
Fisher’s exact test was used for comparison of characteristics of HCT subjects by PC status. Kaplan-Meier analysis and Cox proportional hazards modeling were used to identify risk factors for PCs and adjusted for potential confounders. Dynamic predictors occurring after HCT, such as acute GVHD, bloodstream infection, disease relapse, antibiotic regimens, and microbiota states, were coded as time-dependent variables (assessed from HCT infusion, Day 0, forward) consistent with evaluation of the primary endpoint. Covariates associated with PCs at a significance level of P < 0.2 on univariate analysis were included in the multivariate model.
Similar calculations were performed using mortality as the outcome of interest. The impact of pre- and postengraftment PCs on mortality was also examined by incorporating an interaction term for PCs and engraftment, both coded as time-dependent predictors. All analyses were performed using the machine learning package R, version 3.2 (R, Vienna, Austria).
Results
Cohort Characteristics
Clinical
Of 94 subjects, 66 (70.2%) had at least one post-transplant PC, with a median time of onset 50 days after HCT. Table 1 shows baseline transplant and pulmonary characteristics of participants by PC. Compared with subjects without PCs, the PC group had significantly lower abnormal baseline pulmonary function tests (PFTs) (P = 0.021), a higher disease risk profile (P = 0.032), a higher HCT cardiac index score (P = 0.013), previous fixed-dose busulfan (P = 0.028), and fluoroquinolone (P = 0.014) or azithromycin (P = 0.003) use during hospitalization. Pretransplant characteristics of this cohort were similar to the overall allogeneic HCT case mix at our institution during the same period, as reported (13).
Table 1.
Baseline Transplant and Pulmonary Characteristics of Hematopoietic Stem Cell Transplantation Patients with and without Pulmonary Complications
| Variable | At Least 1 PC (n = 66; 70.2%) | No PC (n = 28; 29.8%) |
|---|---|---|
| Pretransplant characteristic | ||
| Age, yr | ||
| ≤29 | 6 (9.1) | 1 (3.6) |
| 30–39 | 10 (13.8) | 3 (10.7) |
| 40–49 | 10 (15.2) | 9 (32.1) |
| 50–59 | 19 (28.8) | 9 (32.1) |
| ≥60 | 21 (31.8) | 6 (21.4) |
| Sex, male | 37 (56.1) | 16 (57.1) |
| Race | ||
| White | 52 (78.8) | 23 (82.1) |
| Black | 3 (4.5) | 3(10.7) |
| Asian/Southeast Asian | 9 (13.6) | 1 (3.6) |
| Disease | ||
| Leukemia | 30 (45.5) | 14 (50.0) |
| Lymphoma | 17 (25.8) | 10 (35.7) |
| Multiple myeloma | 7 (10.6) | 1 (3.6) |
| Myelodysplastic syndrome | 9 (13.6) | 3 (10.7) |
| Donor | ||
| Matched-related | 20 (30.3) | 11 (39.3) |
| Matched-unrelated | 20 (30.3) | 8 (28.6) |
| Mismatched-unrelated | 10 (15.2) | 2 (7.1) |
| Umbilical cord | 16 (24.2) | 7 (25.0) |
| Transplant type | ||
| T cell–depleted peripheral blood | 29 (43.9) | 13 (46.4) |
| Unmodified peripheral blood | 21 (31.8) | 8 (28.6) |
| Double umbilical cord | 16 (24.2) | 7 (25.0) |
| Conditioning intensity | ||
| Non-myeloablative | 12 (18.2) | 6 (21.4) |
| Reduced intensity | 21 (31.8) | 9 (32.1) |
| Myeloablative | 33 (50.0) | 13 (46.4) |
| Disease risk | ||
| Low | 14 (21.2) | 13 (46.4) |
| Intermediate | 16 (24.2) | 7 (25.0) |
| High | 36 (54.5) | 8 (28.6) |
| HCT comorbidity index | ||
| ≤1 | 19 (28.8) | 15 (53.6) |
| 2–3 | 22 (33.3) | 10 (35.7) |
| ≥4 | 25 (37.9) | 3 (10.7) |
| Antibiotics administered* | ||
| Vancomycin† | 61 (92.4) | 28 (100.0) |
| β-Lactam† | 61 (92.4) | 25 (89.3) |
| Fluoroquinolone | 51 (77.3) | 14 (50.0) |
| Flagyl | 36 (54.5) | 12 (42.9) |
| Azithromycin | 39 (59.1) | 7 (25.5) |
| Pretransplant pulmonary history | ||
| Smoking (former/current) | 33 (50.0) | 14 (50.0) |
| ≥20 pack-years‡ | 11 (33.3) | 4 (28.6) |
| Previous or chronic lung disease§ | 11 (16.7) | 3 (10.7) |
| Fungal risk factors|| | 43 (74.1) | 19 (86.4) |
| Abnormal chest imaging¶ | 37 (56.1) | 13 (46.4) |
| Abnormal PFT** | 44 (66.7) | 11 (39.3) |
| Impaired DlCO [Hb] | 37 (84.1) | 9 (81.8) |
| OVD | 5 (11.4) | 1 (9.1) |
| RVD | 8 (18.2) | 4 (36.4) |
| Busulfan-containing regimen | 25 (37.9) | 4 (14.3) |
Definition of abbreviations: CT = computed tomography; DlCO [Hb] = diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin; HCT = hematopoietic stem cell transplantation; OVD = obstructive ventilatory defect; PC = pulmonary complication; PFT = pulmonary function test; RVD = restrictive ventilatory defect.
Data are shown as n (%). P values are two-sided and based on Fisher’s exact test; significant values in bold if P < 0.05.
Antibiotics were assessed only during in-patient hospitalization for allogeneic HCT; variables are not mutually exclusive and do not sum to 100%.
Intravenous vancomycin only; β-lactams include cephalosporins, β-lactam–β-lactamase combinations, and carbapenems.
Two former smokers without pack-year data; percent of smokers shown in parentheses.
Asthma, chronic obstructive pulmonary disease, interstitial lung disease, or previous pneumonia.
HCT provider assessment of fungal risk pre-transplant (e.g., history of extensive neutropenia, prolonged steroid use, presumed or confirmed fungal pneumonia); 14 with missing data.
Abnormal parenchymal finding (nodule, consolidation, ground glass, interstitial changes) on baseline chest CT, or infiltrate on chest X-ray if CT is unavailable.
As per American Thoracic Society/European Respiratory Society guidelines (31); seven with missing lung volumes; percent of abnormal PFTs shown in parentheses do not add up to 100% because of the presence of multiple abnormalities per PFT.
Microbiota
As previously stated by Taur and colleagues (10), 439 fecal specimens were collected from 94 subjects (3–8 per patient) during the transplant hospitalization, yielding 1,838,205 high-quality 16S rRNA-encoding sequences (mean 4,187; range 852–9,862 per specimen). In this study, 71.3% (67 of 94) of subjects developed at least one state of low diversity, and 67.0% (63 of 94) developed at least one taxon-dominated state: 38 (40.4%) Enterococcus-dominated, 29 (30.9%) Streptococcus-dominated, and 11 (11.7%) proteobacteria-dominated (all sequences within this phylum belonged to the class γ-proteobacteria, heretofore referred to as a “γ-proteobacteria” domination state). Table 2 describes these microbiota states in subjects according to their PC status, and Table E1 in the online supplement shows microbiota features by mortality status. There were no significant differences between subjects with low diversity at baseline, which was defined as the specimen meeting the defined criteria closest to Day 0 (mean Day −3; range −8 to −1), at engraftment, or by domination states. Ten of 11 subjects who developed γ-proteobacteria domination also developed PCs; however, not all states preceded the PC. There was a trend toward a significant difference in γ-proteobacteria domination in subjects who died compared with those who were alive at study end (9 vs. 2; P = 0.051).
Table 2.
Gut Microbiota States during Transplant Hospitalization and Clinical Outcomes
| Microbiota State | At least 1 PC (n = 66; 70.2%) | No PC (n = 28; 29.8%) |
|---|---|---|
| Taxon domination* | ||
| γ-Proteobacteria | 10 (15.2) | 1 (3.6) |
| Enterococcus | 30 (45.5) | 8 (28.6) |
| Streptococcus | 18 (27.3) | 11 (39.3) |
| Low diversity† | ||
| Baseline‡ | 12 (18.2) | 3 (10.7) |
| Engraftment§ | 33 (53.2) | 11 (42.3) |
Definition of abbreviation: PC = pulmonary complication.
Data are shown as n (%). Using two-sided P values based on Fisher’s exact test with a significance threshold of P < 0.05, there were no statistically significant differences in the absence of time-dependent analysis.
Defined as relative abundance greater than 30% and the most dominant taxon in a stool specimen collected during initial transplantation hospitalization (until Day 35 or death) (10). Seven of 94 patients had a domination state at the time of stem cell infusion (Day 0).
‡Baseline defined as the pretransplant specimen collected most proximal to Day 0 (mean Day −3, range −8 to −1).
§Two individuals did not engraft.
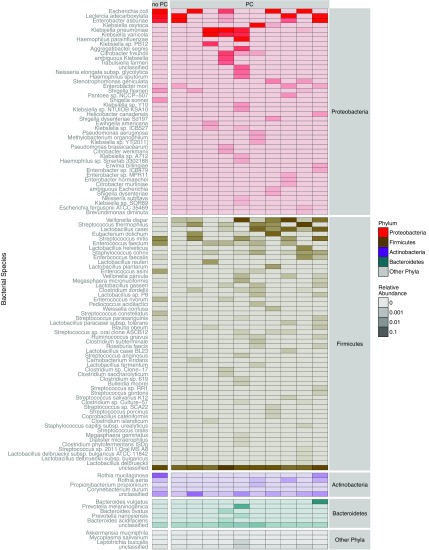
Microbiota domination states were each largely defined by single species, including Enterococcus faecium in 34 of 38 subjects (89.5%; mean relative abundance, 76.4% per specimen; range, 32.0–99.9%) and Streptococcus thermophilus in 26 of 29 (89.7%; mean relative abundance, 73.2%; range, 24.0–99.9%). The species driving γ-proteobacteria domination states were Escherichia coli in 4 of 11 subjects (36.4%; mean relative abundance, 75.8%; range, 56.9–94.8%), Klebsiella spp. in 3 (27.3%; relative abundance of K. oxytoca 89.9%, K. variicola 59.3%, and K. pneumoniae 40.25%), Enterobacter asburiae in 2 (18.2%; mean relative abundance 50.7%; range, 39.3–62.1%), and Leclercia adecarboxylata (relative abundance 47.5%), and Haemophilus parainfluenzae (relative abundance 31.9%) in one subject each. Figure 1 is a heat map that shows the relative abundance of all species identified in 11 subjects with specimens meeting criteria for γ-proteobacteria domination. Figure E1 is a heat map depicting the maximum relative abundance of all γ-proteobacteria species identified in all study specimens by PC status. Plots of α-diversity by PC status and β-diversity by domination state and PC status are shown in Figures E2 and E3, respectively.
Figure 1.
Heat map of bacterial species identified in subjects with γ-proteobacteria domination of fecal microbiota during the transplant hospitalization. The heat map shows the log-transformed relative abundance of all species identified in subjects with fecal specimens meeting criteria for γ-proteobacteria domination (i.e., the first sample with at least 30% relative abundance of γ-proteobacteria and the most abundant taxon identified) between hematopoietic stem cell transplantation infusion and Day 35 or hospital discharge. Based on this definition, each specimen was collected at a different time point (median Day 14 in subjects with pulmonary complications [PCs]; range Days 4–31; and Day 0 in the subject without PCs). Each column represents one patient, stratified by PC status. Each row represents bacterial taxa present in the γ-proteobacteria dominant specimen resolved to the species level, which are clustered by phylum, the highest taxonomic rank to which the species belongs, according to the color legend at the right of the plot. The most abundant species within each sample are from the phylum proteobacteria, by definition, as shown at the top of the heat map. In all subjects, 16S sequences were dominated by only one or two γ-proteobacteria species, including common respiratory pathogens such as Klebsiella pneumoniae, K. oxytoca, and Escherichia coli.
Pulmonary Complications, Mortality, and Other Clinical Outcomes
There were 112 total PC events in 66 subjects during the study period, including 2 PCs in 31 subjects and 3 PCs in 15. Ninety-four of 112 (83.8%) PCs were first diagnosed by chest CT, and 71.4% (80 of 112) of radiographic findings were described as multifocal (35) or diffuse (20). Thirty-three PCs (29.2%) occurred during the first 30 days, and 86 (76.8%) occurred by the end of the first post-transplantation year. PCs developed preengraftment in 19 subjects (28.8%), postengraftment in 56 (84.9%), and during both intervals in 9 patients (13.6%). Forty-eight subjects (51.1%) died during the study, and one-half of the deaths were considered transplant-related. Median time to death was 218 days. Subjects who died were more likely to have abnormal baseline PFTs (P = 0.006) and exposure to azithromycin (P = 0.026) compared with those who were alive at the end of the study period. Figure 2 shows a timeline of PCs and mortality events across all subjects, and highlights subjects who developed fecal domination by Enterococcus and/or γ-proteobacteria during that transplant hospitalization.
Figure 2.
Time to post-transplant pulmonary complications and survival in all subjects. Time to development of pulmonary complications (PCs) after hematopoietic stem cell transplantation (HCT) during the study period, or until death or loss to clinical follow-up is depicted. Each row represents a subject, and the occurrence of a microbiota domination state by Enterococcus or γ-proteobacteria during transplant hospitalization is indicated at the left of the plot (note that time to domination is not indicated). Most of the subjects who died during study follow-up experienced at least one PC before death, and many PCs directly preceded the death event. Median time to first PC was 50 days, and median time to death was 218 days after HCT. Median follow-up was 3.3 years for subjects who remained alive at the end of the study period. There were 14 subjects with multiple domination events during the transplant hospitalization, including 10 in the PC group. Four of these subjects with PCs developed more than one domination event before the occurrence of the PC. For the time-dependent analysis, the specimen defined as having a domination state most proximal to the PC endpoint was analyzed, including the γ-proteobacteria dominant specimen in three subjects and the Enterococcus-dominant specimen in one patient. In the four subjects with multiple domination events and without PCs, the first specimen collected after time 0 was used in the time-dependent analysis, including γ-proteobacteria domination in one subject, Enterococcus domination in one patient, and Streptococcus domination in two subjects (not shown). Enterococcus domination (green square); γ-proteobacteria domination (red square); engraftment (light green diamond); PC (purple circle); dead (closed circle); and alive (open circle).
Table 3 shows results of diagnostic tests that led to a working PC diagnosis. Although most of the events (97; 86.6%) were considered primarily infectious, only 39 (34.8%) of diagnoses had supportive microbiologic or histopathologic data that identified a causative agent. The remainder (58; 59.8%) was attributed to nonspecific infectious processes, such as community-acquired or health care–acquired pneumonia. There were 15 noninfectious primary diagnoses, including 7 (46.6%) within the IPS spectrum (36) and 4 (26.7%) with biopsy-proven interstitial lung disease. In several cases, two processes were treated concomitantly, such as bacterial pneumonia and engraftment syndrome, or viral pneumonitis and alveolar hemorrhage. Of nine subjects who underwent autopsy, seven (77.8%) had histopathologic evidence of lung injuries deemed contributory to death, including bronchopneumonia, acute lung injury, diffuse alveolar damage, and OP.
Table 3.
Infectious and Noninfectious Etiologies of Primary Pulmonary Complication Diagnoses
| Pulmonary Complication (n = 112) | Specimen/Evaluation |
|---|---|
| Infectious* (n = 97) | |
| Bacterial pneumonia (n = 20) | |
| Legionella pneumophilia (n = 2) | Lung tissue FNA/culture† |
| Urine/serotype 1 urine antigen | |
| Legionella micdadei (n = 1) | Pleural fluid/culture† |
| Pseudomonas aeruginosa (n = 4) | BAL/culture |
| Sputum/culture | |
| TA/culture | |
| Blood/culture | |
| Klebsiella pneumoniae (n = 4) | Sputum/culture (n = 2) |
| TA/culture | |
| Blood/culture | |
| Klebsiella oxytoca (n = 3) | Sputum/culture |
| Blood /culture (n = 2) | |
| Escherichia coli (n = 2) | Blood/culture |
| Moraxella catarrhalis (n = 2) | Sputum/culture |
| BAL/culture | |
| Stenotrophomonas maltophilia (n = 1) | Blood/culture |
| Vancomycin-resistant Enterococcus faecium (n = 1) | Blood/culture |
| Endobronchial tissue biopsy/pathology | |
| Viral pneumonia (n = 16) | |
| Human herpes virus 6 (n = 2)‡ | BAL/PCR |
| Cytomegalovirus (n = 2)‡ | BAL /culture |
| Blood/viral antigen, culture | |
| Adenovirus (n = 1)‡ | Blood/PCR |
| Respiratory syncytial virus (n = 5) | NP swab/PCR |
| Parainfluenza (n = 3) | NP swab/PCR |
| Influenza (n = 2) | BAL/PCR (influenza B) |
| NP swab/PCR (influenza A) | |
| Metapneumovirus (n = 1) | NP swab/PCR |
| Fungal pneumonia (n = 3) | |
| Pneumocystis jirovecii (n = 1) | BAL/PCR, cytology |
| Aspergillus spp (n = 1) | BAL/Aspergillus galactomannan antigen |
| Rhizopus (n = 1) | Lung tissue (transbronchial biopsy)/culture |
| Unspecified (n = 58)§ | N/A |
| Inflammatory/other (n = 15)|| | |
| Diffuse alveolar hemorrhage* (n = 2) | BAL |
| Engraftment syndrome (n = 2) | N/A |
| Idiopathic pneumonia syndrome (n = 3) | N/A |
| Organizing pneumonia (n = 3) | Lung tissue: transbronchial, CT-guided and surgical biopsies)/pathology (n = 3) |
| Desquamative interstitial pneumonitis (n = 1) | Lung tissue (surgical biopsy) |
| Drug toxicity (n = 3) | N/A |
| Transfusion reaction (IVIG) (n = 1) | N/A |
Definition of abbreviations: BAL = bronchoalveolar lavage; CT = computed tomography; FNA = fine needle aspirate; IVIG = intravenous immunoglobulin; N/A = not applicable (negative workup); NP = nasopharyngeal; PCR = polymerase chain reaction; TA = tracheal aspirate.
Infectious diagnoses on the basis of diagnostic evaluation of respiratory or peripheral specimens in the appropriate clinical context; other positive results of microbiological testing (e.g., isolation of Staphylococcus aureus [n = 2], rhinovirus [n = 1], and atypical mycobacteria [n = 3] in BAL or sputum specimens were not considered to be likely causes of pulmonary complications [PCs]). Furthermore, two cases of vancomycin-resistant Enterococcus were identified in clinical specimens; however, only one was considered a likely cause of PC (positive blood culture with chest imaging suggestive of septic emboli); the other was derived from pathological examination of an endobronchial lesion biopsied during bronchoscopy; further information about these cases is available in the online supplement.
Culture performed at the New York City Department of Health after negative diagnostic workup at Memorial Sloan Kettering Cancer Center (both BAL and urine antigen were negative for L. pneumophilia in the case of L. micdadei).
Significantly elevated viral titres in lung specimens compared with blood, and/or blood with disseminated disease and supportive clinical and radiographic data suggestive of viral pneumonitis.
Includes community-acquired pneumonia, health care–acquired pneumonia, aspiration pneumonia, and nonspecific fungal pneumonia (e.g., elevated β-d-glucans with nodular opacities on CT).
One case of alveolar hemorrhage in setting of cytomegalovirus pneumonitis; two cases of idiopathic pneumonia syndrome treated with etanercept after negative infectious workup; drug toxicity was presumed to be due to radiation, sirolimus, and busulfan toxicity, respectively.
Other post-transplant complications included disease relapse and/or progression in 21.3% (24), bloodstream infection in 37.2% (36), and acute GVHD in 38.6% (34 of 88 evaluated). Differences between PC groups were not statistically significant.
Risk Factors for Pulmonary Complications
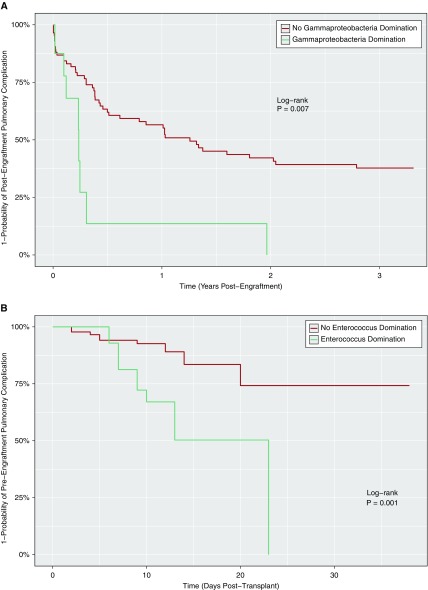
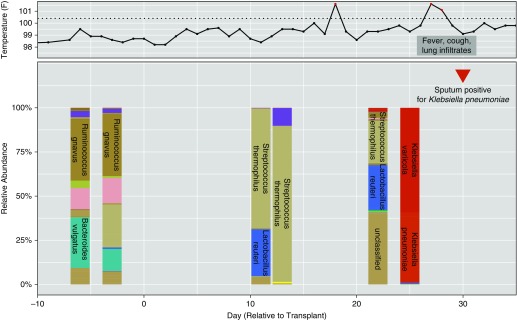
In the Cox hazards model, associations were found between the occurrence of PCs and high HCTCI, fluoroquinolone use, low baseline diversity, and γ-proteobacteria domination of fecal microbiota (Table 4). γ-Proteobacteria domination was a strong, independent predictor of PCs; subjects with this microbiota composition were two to three times more likely to develop a PC, independent of other factors. Low baseline diversity was also an independent predictor, although only in the multivariate model after adjusting for antibiotics during the transplant hospitalization. In separate pre- and postengraftment analyses, low baseline diversity was solely predictive of PCs occurring preengraftment (hazard ratio [HR], 6.30; 95% confidence interval [CI] 1.42–31.80; P = 0.016) (see Table E2). The relationship between Enterococcus-domination and preengraftment PCs (HR, 5.72; 95% CI, 2.12–15.45; P = 0.001) was attenuated after adjusting for antibiotics (HR, 1.38; 95% CI, 0.38–4.95; P = 0.625), whereas γ-proteobacteria domination remained a strong predictor of postengraftment PCs in the fully adjusted model (HR, 3.68; 95% CI, 1.49–8.21; P = 0.006) (see Table E3). Relationships between microbiota domination states and PCs using Kaplan-Meier analysis are shown in Figure 3, including associations between γ-proteobacteria domination and postengraftment PCs (log-rank P = 0.006) (Figure 3A), as well as Enterococcus-domination and preengraftment PCs (log-rank P ≤ 0.001) (Figure 3B). Figure 4 presents a timeline for a case of pneumonia caused by K. pneumoniae, a known respiratory pathogen from the γ-proteobacteria class, which developed after the transition to fecal domination by K. pneumoniae in serial stool samples, raising the possibility of translocation from the gut to the lung.
Table 4.
Predictors of Pulmonary Complications after Allogeneic Hematopoietic Stem Cell Transplantation
| Clinical Predictor | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age, ≥60 yr | 1.22 (0.71–2.05) | 0.462 | ||
| Sex, female | 1.24 (0.75–2.02) | 0.398 | ||
| Race, white vs. other | 0.74 (0.42–1.43) | 0.355 | ||
| Disease, leukemia vs. other | 1.13 (0.69–1.85) | 0.622 | ||
| Donor stem cell type, matched related vs. other | 0.68 (0.41–1.13) | 0.136 | 0.63 (0.35–1.11) | 0.110 |
| Double umbilical cord | 1.43 (0.79–2.48) | 0.230 | ||
| T-cell depletion | 0.89 (0.54–1.46) | 0.653 | ||
| Conditioning intensity, myeloablative vs. other | 1.20 (0.73–1.97) | 0.464 | ||
| Busulfan | 1.59 (0.94–2.63) | 0.082 | 1.72 (0.96–3.04) | 0.068 |
| HCT comorbidity index, ≥4 vs. other* | 2.40 (1.41–3.98) | 0.001 | 2.30 (1.30–4.00) | 0.004 |
| Disease risk, high vs. other† | 1.43 (0.87–2.36) | 0.082 | 1.72 (0.96–3.04) | 0.473 |
| Abnormal baseline PFTs | 1.93(1.12–3.32) | 0.012 | 1.73 (0.99–3.14) | 0.056 |
| Acute GVHD, Day 0 to 100‡ | 1.44 (0.66–2.94) | 0.344 | ||
| Bloodstream infection‡ | 1.35 (0.77–2.29) | 0.286 | ||
| Shannon diversity index <1.5 at baseline§ | 1.69 (0.83–3.11) | 0.138 | 2.63 (1.22–5.32) | 0.015 |
| γ-Proteobacteria domination‡ | 2.30 (1.00–4.66) | 0.051 | 2.64 (1.10–5.65) | 0.031 |
| Enterococcus domination‡ | 1.33 (0.77–2.24) | 0.303 | ||
| Streptococcus domination‡ | 0.83 (0.43–1.48) | 0.535 | ||
| Vancomycin‡|| | 0.75 (0.35–1.97) | 0.532 | ||
| β-Lactam‡¶ | 1.12 (0.582.39) | 0.752 | ||
| Fluoroquinolone‡ | 1.99 (1.20–3.32) | 0.008 | 2.29 (1.32–3.98) | 0.003 |
| Metronidazole‡ | 1.19 (0.69–2.00) | 0.515 | ||
| Azithromycin‡ | 1.42 (0.632.86) | 0.374 | ||
Definition of abbreviations: CI = confidence interval; HCT = hematopoietic stem cell transplantation; PFT = pulmonary function test; GVHD = graft-versus-host disease.
Bold values indicate statistically significant P value <0.05.
HCT comorbidity index groups: low (0–1), intermediate (2–3) or high (≥4) (30).
Disease risk: American Society for Blood and Marrow Transplantation Request for Information Classification as low, intermediate, or high (29).
Time-dependent variables.
Shannon diversity index takes into account number of species and relative abundance (33).
Vancomycin includes intravenous mode of administration only.
β-Lactams include cephalosporins, β-lactam–β-lactamase combinations, and carbapenems.
Figure 3.
(A) Association between γ-proteobacteria domination of gut microbiota and the development of a pulmonary complication (PC) after engraftment. The relationship between γ-proteobacteria domination of fecal microbiota and the development of a postengraftment PC on Kaplan-Meier analysis is shown. Log-rank P values are significant if P < 0.05. (B) Association between Enterococcus domination of gut microbiota and the development of a PC before engraftment. The relationship between the Enterococcus domination state and the development of a preengraftment PC on Kaplan-Meier analysis is shown. Log-rank P values are significant if P < 0.05.
Figure 4.
Shift to dominant Klebsiella pneumoniae in the gastrointestinal tract precedes clinical diagnosis of culture-proven K. pneumoniae pneumonia. Serial changes in gastrointestinal microbiota in a patient who received a double umbilical cord transplant for non-Hodgkin’s lymphoma. On Transplantation Day 29, there was a shift in the composition of stool microbiota to K. pneumoniae gut domination. The patient soon thereafter developed a fever, cough and infiltrates on chest X-ray, and was empirically started on broad-spectrum antibiotics. Sputum culture was significant for K. pneumoniae, with interval chest CT showing multifocal pneumonia. Although it is possible that there was colonization of the respiratory tract that preceded gut domination, because the temporality of the clinical presentation and clinical findings, it is plausible that K. pneumoniae translocated from the gastrointestinal tract to the lungs. Expansion of this taxon in stool specimens likely began several days before observation in the dominant specimen, which cannot be appreciated in this figure (only taxa with relative abundance >30% are depicted). Each stacked bar represents the microbial composition of a single fecal specimen based on taxonomic classification to species level, with percent relative abundance of 16S sequences to 100% shown on the y-axis. Colors denote taxa from distinct phyla, with gradations in the color representing abundant species within that phylum. Sequences from the phylum proteobacteria are red (all are from the class γ-proteobacteria). Species are labeled within the bars representing taxon abundance when there was at least 30% relative abundance, as with K. pneumoniae and K. variicola in the stool specimen preceding the clinical diagnosis of pneumonia. The clinical microbiology result of the sputum culture is shown under the red triangle following the last stool specimen on the timeline. The solid line at the top of the plot depicts the fever trend during transplantation hospitalization, with a red peak indicating temperature higher than 100.4°F (dotted line), concurrent with respiratory signs and symptoms.
Risk Factors for Mortality
In the Cox hazards model, a strong association was found between overall mortality and PCs occurring postengraftment, but not preengraftment (Table 5), which remained after evaluating for a potential interaction between PCs and engraftment. Similarly, Kaplan-Meier analysis demonstrated significant associations between mortality and PCs occurring postengraftment (log-rank P ≤ 0.001), but not preengraftment (log-rank P = 0.131). Other independent predictors of death in the multivariate model included disease relapse, azithromycin use, and γ-proteobacteria domination. Relationships between transplant-related mortality and γ-proteobacteria domination (HR, 4.49; 95% CI, 1.54–11.08; P = 0.008) and with postengraftment PCs (HR, 4.77; 95% CI, 2.23–10.98 ; P ≤ 0.001) were attenuated after adjusting for antibiotics in the full model.
Table 5.
Predictors of Overall Mortality after Allogeneic Hematopoietic Stem Cell Transplantation
| Clinical Predictor | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age, ≥60 yr | 1.25 (0.67––2.23) | 0.465 | ||
| Sex, female | 1.28 (0.73–2.25) | 0.388 | ||
| Race, white vs. other | 0.80 (0.42–1.68) | 0.542 | ||
| Disease, leukemia vs. other | 1.09 (0.62–1.91) | 0.772 | ||
| Donor stem cell type, matched related vs. other | 0.66 (0.37–1.18) | 0.155 | 0.69 (0.34–1.40) | 0.300 |
| Double umbilical cord | 1.41 (0.71–2.61) | 0.311 | ||
| T-cell depletion | 0.94 (0.53–1.64) | 0.816 | ||
| Conditioning intensity, myeloablative vs. other | 1.04 (0.59–1.83) | 0.891 | ||
| HCT comorbidity index, ≥4 vs. other* | 2.24 (1.25–3.95) | 0.007 | 0.91 (0.47–1.76) | 0.785 |
| Disease risk, high vs. other† | 1.07 (0.61–1.88) | 0.815 | ||
| Abnormal baseline PFTs | 2.45 (1.34–4.77) | 0.003 | 1.79 (0.90–3.72) | 0.097 |
| Preengraftment PC‡ | 1.74 (0.88–3.22) | 0.107 | 1.19 (0.54–2.53) | 0.652 |
| Postengraftment PC‡ | 4.29 (2.34–8.28) | <0.001 | 2.50 (1.25–5.22) | 0.009 |
| Acute GVHD‡ | 1.27 (0.68–2.32) | 0.443 | ||
| Bloodstream infection‡ | 1.69 (0.95–2.97) | 0.071 | 1.62 (0.86–3.06) | 0.132 |
| Relapse or progression‡ | 2.92 (1.60–5.20) | 0.001 | 2.68 (1.29–5.48) | 0.008 |
| Shannon diversity index <1.5 at baseline§ | 0.96 (0.40–1.96) | 0.907 | ||
| γ-Proteobacteria domination‡ | 4.34 (1.98–8.60) | 0.001 | 3.52 (1.28–9.21) | 0.016 |
| Enterococcus domination‡ | 1.44 (0.82–2.53) | 0.207 | ||
| Streptococcus domination‡ | 1.35 (0.74–2.39) | 0.323 | ||
| Vancomycin‡|| | 1.67 (0.57–8.10) | 0.393 | ||
| β-Lactam‡¶ | 2.42 (0.82–11.74) | 0.119 | 1.67 (0.51–8.53) | 0.435 |
| Fluoroquinolone‡ | 1.90 (1.05–3.65) | 0.035 | 1.12 (0.53–2.43) | 0.767 |
| Metronidazole‡ | 1.88 (1.06–3.37) | 0.029 | 1.44 (0.73–2.87) | 0.290 |
| Azithromycin‡ | 3.53 (1.99–6.43) | <0.001 | 2.84 (1.49–5.51) | 0.001 |
Definition of abbreviations: CI = confidence interval; HCT = hematopoietic stem cell transplantation; PC = pulmonary complication; PFT = pulmonary function test; GVHD = graft-versus-host disease.
Bold values indicate statistically significant P value <0.05.
HCT comorbidity index groups: low (0–1), intermediate (2–3), or high (≥4) (30).
Disease risk: American Society for Blood and Marrow Transplantation Request for Information Classification as low, intermediate, or high (29).
Time-dependent variables.
Shannon diversity index takes into account number of species and relative abundance (33).
Vancomycin includes intravenous mode of administration only.
β-Lactams include cephalosporins, β-lactam–β-lactamase combinations, and carbapenems.
Discussion
We examined clinical and microbiota predictors of PCs and mortality in a cohort of 94 allogeneic HCT recipients, in whom serial changes in gut flora had been previously evaluated using high-throughput sequencing methods. The composition of gut microbiota, including low baseline diversity and γ-proteobacteria domination, during the early transplant period was associated with the development of PCs. γ-Proteobacteria domination and postengraftment PCs were also independent predictors of death after transplant. To our knowledge, this is the first study to demonstrate a relationship between gut microbiota and lung injury, which may, in turn, increase the risk of death in this population. These findings present an opportunity for further investigation into potential biomarkers and mechanisms of poorly understood pulmonary processes.
From pretransplant conditioning through early engraftment, the gastrointestinal tract bears the brunt of damage to immune and mucosal defenses, leading to systemic translocation of bacteria and subsequent infection (10, 37). By identifying features of fecal microbiota that may predict PCs, we were able to extend the impactful scope of these treatment-related shifts in gastrointestinal flora on clinically relevant endpoints. Early gut domination by γ-proteobacteria, a class of bacteria that includes respiratory pathogens such as K. pneumoniae and K. oxytoca, was associated with the development of postengraftment PCs, which, in turn, more than doubled the risk of mortality. Interestingly, the identification of abundant K. variicola, a recently named and virulent phylogroup of K. pneumoniae (38), in several specimens from subjects with PCs may highlight missed opportunities for isolating potential pathogens in clinical specimens using traditional diagnostic methods. The same may be true for E. asburiae and L. adecarboxylata, formerly known as E. adecarboxylata, which have also been isolated from clinical specimens in patients with pneumonia and bacteremia (39,40).
These findings may suggest direct translocation of bacteria to the lungs during early transplant (as seen in Figure 4) or indirect lung injury caused by microbiota-stimulation of a systemic or local inflammatory response as possible mechanisms; alternatively, associations might reflect overall microbiota status or antimicrobial use. The latter may best explain the attenuation of the relationship between gut domination by bacteria belonging to the genus Enterococcus (where most of the sequences were identified as vancomycin-resistant E. faecium [10]) and preengraftment PCs, because antibiotic use for prophylaxis and/or empiric treatment is heaviest at this time and may foster its aberrant colonization (10). However, although E. faecium is infrequently considered a true lung pathogen in the clinical setting, perhaps because of its diminished virulence compared with K. pneumoniae (41), infection and direct lung injury may occur, especially in the compromised host (42). Conversely, the emergence of low diversity as an independent predictor of PCs only once antibiotics were included in the model is consistent with previous findings in this population (13) and may have biological relevance. Although diversity and domination states did not always occur in close proximity to the PC (an unavoidable limitation to our study design), changes in gut microbiota composition early during HCT might have affected long-term pulmonary health, as shown in association with other post-transplant outcomes (43).
We also observed a correlation between specific antibiotics and PCs, which is difficult to interpret and likely confounded by the context in which antibiotics were administered. For example, subjects who were considered to be at higher risk for pneumonia might have received early empiric coverage of typical and atypical bacteria with fluoroquinolones and/or azithromycin before chest imaging or further diagnostic workup. It is also possible that the relationship between antibiotics and PCs was confounded by their associations with other microbiota taxa or characteristics that were not examined in this study.
Disordered respiratory microbiota, often dominated by γ-proteobacteria, have been associated with several chronic lung diseases (44–50) and complications of lung transplantation (51, 52). The origin of some lung communities has been speculated to derive from nonpulmonary sources, including the oral cavity, upper respiratory tract, and gastrointestinal system. In the absence of lung colonization or infection, several studies have shown that gut dysbiosis triggers inflammation in asthma and allergic airways diseases (14–16), cystic fibrosis (17), and infection-mediated lung inflammation (18–21). Intestinal permeability may be implicated in the translocation of bacteria and/or microparticles, such as lipopolysaccharide, on the pathway to acute lung injury and sepsis-associated acute respiratory distress syndrome (53–55). Because of the emerging recognition of shared mucosal immune responses in these parallel systems, it is possible that changes in gut microbiota associated with treatment-induced mucosal damage elicit injurious lung inflammation, which may persist or recur after engraftment. Further investigation into these relationships will be important to inform novel mechanistic hypotheses and develop predictive risk tools.
Finally, we observed expected clinical risk factors to predict PCs and mortality, such as underlying disease relapse and/or progression and having a high HCTCI score. The latter is a validated summary measurement of multisystem pretransplant comorbidities, including moderate or severe pulmonary impairment, which has been shown to predict mortality (30, 56). PCs have also been associated with HCT-related mortality throughout the literature (57–60), albeit using varied definitions and study designs. Although it is difficult to determine whether PCs proximal to death were causal in our study, their contribution is likely underestimated based on autopsy studies (59, 61). The strength of association between mortality and postengraftment PCs was also interesting. In a study of HCT recipients with bloodstream infections, postengraftment events were associated with worse outcomes, including death (62). “Late onset” noninfectious PCs, which may be subjected to diagnostic inaccuracy (63), have been associated with acute and chronic GVHD (35, 64, 65) and mortality (66). Many subjects in our study had multiple PCs, suggesting susceptibility to recurrent lung injury, incomplete resolution, and/or misdiagnosis and treatment of a previous PC. Most postengraftment PCs were empirically treated as infectious, but they may represent an infection-mediated alloimmune or inflammatory mechanism (67, 68). However, it is reassuring that a preengraftment PC may not be a harbinger of death, as supported by survival rates of HCT recipients in the intensive care unit with acute respiratory distress that developed prior to engraftment who are comparable to noncancer patients in that setting (69).
There are several limitations to this study. First, we used a broad clinical–radiographic definition of post-transplant PCs to maximize capture of poorly defined events. We used commonly accepted abnormal radiographic patterns (8, 9) in conjunction with clinical information, similar to the approach taken by Garcia and colleagues, to identify a “pneumonia syndrome” in patients with acute leukemia (70), and our PC yield compared well with reported estimates (3). However, the inclusion of all PCs into one primary endpoint, regardless of severity, distribution or diagnosis, could inflate or dilute the impact of PCs on mortality. Prospective characterization of clinical and radiographic features of PCs will improve insight into which processes portend the greatest risk of death.
Second, microbiota data were collected solely during transplantation admission, and therefore, they were not contemporaneous with later clinical events. Although PC data were identified retrospectively, the microbiota protocol was prospective in nature. We thus used time-dependent variables to address this issue. Third, the small sample size limited the power to analyze specific measures of baseline pulmonary dysfunction (e.g., impaired diffusing capacity in predicting PCs), and overlapping variables within PFT and HCTCI assessments may have contributed. Lastly, although this cohort was representative of the HCT population at our center (10, 13), it might not reflect patient characteristics or clinical practice elsewhere.
In conclusion, gut microbiota composition, including low diversity and domination by γ-proteobacteria, during the early HCT process is important in determining post-transplant trajectory, including the occurrence of PCs and/or death. The interaction between PCs and engraftment suggests that the graft–lung relationship may play an important role, which may be mediated either directly or indirectly by gut microbiota composition. Further study is warranted to delineate mechanisms, identify risk factors, and design interventions that could improve outcomes for at-risk patients.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Augustine Choi and Dr. Ronald Collman for their input on the manuscript.
Footnotes
Supported by National Institutes of Health awards 5 UL1 TR000457-09 (Weill Cornell Clinical and Translational Science Center: B.H.) and 1 K23 A1-095398-01 (Y.T.); Tow Foundation/Lucille Castori Center for Microbes, Inflammation and Cancer (E.G.P.); and National Cancer Institute P30 CAoo8748 (MSKCC Cancer Center Support Grant/Core Grant).
Author Contributions: Had full access to all of the data and takes responsibility for data integrity and analysis accuracy: B.H. Contributed to data collection: B.H., Y.T., S.M.M., E.R.L., A.I.G., J.N.B., and S.A.G. Contributed to the design of the study and data analysis: B.H., Y.T., S.A.G., and E.G.P. Contributed to the writing of the manuscript: B.H., D.E.S., Y.T., and E.G.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201507-1491OC on February 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996;109:1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- 3.Diab KJ, Yu Z, Wood KL, Shmalo JA, Sheski FD, Farber MO, Wilkes DS, Nelson RP., Jr Comparison of pulmonary complications after nonmyeloablative and conventional allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2012;18:1827–1834. doi: 10.1016/j.bbmt.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Peters SG, Afessa B. Acute lung injury after hematopoietic stem cell transplantation. Clin Chest Med. 2005;26:561–569, vi. doi: 10.1016/j.ccm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 6.Cereser L, Zuiani C, Graziani G, Girometti R, Como G, Zaja F, Bazzocchi M. Impact of clinical data on chest radiography sensitivity in detecting pulmonary abnormalities in immunocompromised patients with suspected pneumonia. Radiol Med. 2010;115:205–214. doi: 10.1007/s11547-009-0433-3. [DOI] [PubMed] [Google Scholar]
- 7.Heussel CP, Kauczor HU, Heussel GE, Fischer B, Begrich M, Mildenberger P, Thelen M. Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: use of high-resolution computed tomography. J Clin Oncol. 1999;17:796–805. doi: 10.1200/JCO.1999.17.3.796. [DOI] [PubMed] [Google Scholar]
- 8.Shah RM, Miller W., Jr Pulmonary complications of transplantation: radiographic considerations. Clin Chest Med. 2005;26:545–560, v. doi: 10.1016/j.ccm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Shorr AF, Susla GM, O’Grady NP. Pulmonary infiltrates in the non-HIV-infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes. Chest. 2004;125:260–271. doi: 10.1378/chest.125.1.260. [DOI] [PubMed] [Google Scholar]
- 10.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinnebrew MA, Lee YJ, Jenq RR, Lipuma L, Littmann ER, Gobourne A, No D, van den Brink M, Pamer EG, Taur Y. Early Clostridium difficile infection during allogeneic hematopoietic stem cell transplantation. PLoS One. 2014;9:e90158. doi: 10.1371/journal.pone.0090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 15.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen and interleukin-13. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures MBio 20123pii:e00251-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesteman AS, Perrin-Guyomard A, Laurentie M, Sanders P, Toutain PL, Bousquet-Mélou A. Emergence of resistant Klebsiella pneumoniae in the intestinal tract during successful treatment of Klebsiella pneumoniae lung infection in rats. Antimicrob Agents Chemother. 2010;54:2960–2964. doi: 10.1128/AAC.01612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, Redpath SA, Perona-Wright G, Blanchet MR, Mohn WW, et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015;135:100–109. doi: 10.1016/j.jaci.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Winglee K, Eloe-Fadrosh E, Gupta S, Guo H, Fraser C, Bishai W. Aerosol Mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLoS One. 2014;9:e97048. doi: 10.1371/journal.pone.0097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 23.Forsythe P. Probiotics and lung immune responses. Ann Am Thorac Soc. 2013:S33–S37. doi: 10.1513/AnnalsATS.201306-156MG. [DOI] [PubMed] [Google Scholar]
- 24.Esposito S, Rigante D, Principi N. Do children’s upper respiratory tract infections benefit from probiotics? BMC Infect Dis. 2014;14:194. doi: 10.1186/1471-2334-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris B, Morjaria S, Littmann E, Giralt S, Taur Y, Pamer E.Pulmonary complications following allogeneic hematopoietic stem cell transplantation at a high-volume, academic transplant center [abstract]. Presented at the American College of Chest Physicians Conference, Austin, Texas, October 29, 2014 [Google Scholar]
- 26.Morjaria S, Littmann E, Geyer A, Giralt S, Taur Y, Pamer E, Harris B.Infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation at a high-volume academic transplant center [abstract]. Presented at the Infectious Disease Society of America Conference, Philadelphia, PA, October 10, 2014 [Google Scholar]
- 27.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, Gupta V, Rizzieri DA, George B, Keating A, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47:810–816. doi: 10.1038/bmt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2010;2010:237–247. doi: 10.1182/asheducation-2010.1.237. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrino R, Viegi G, Brurasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustrafsson P, Hankinson J, et al. Interpretive strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 32.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magurran AE. Biological diversity. Curr Biol. 2005;15:R116–R118. doi: 10.1016/j.cub.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald GM.Biogeography: space, time, and life. New York: John Wiley & Sons, Inc., 2003 [Google Scholar]
- 35.Bolaños-Meade J, Ioffe O, Hey JC, Vogelsang GB, Akpek G. Lymphocytic pneumonitis as the manifestation of acute graft-versus-host disease of the lung. Am J Hematol. 2005;79:132–135. doi: 10.1002/ajh.20315. [DOI] [PubMed] [Google Scholar]
- 36.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Velden WJ, Herbers AH, Feuth T, Schaap NP, Donnelly JP, Blijlevens NM.Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation PLoS One 20105e1515610.1371/journal.pone.0015156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maatallah M, Vading M, Kabir MH, Bakhrouf A, Kalin M, Nauclér P, Brisse S, Giske CG. Klebsiella variicola is a frequent cause of bloodstream infection in the Stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One. 2014;9:e113539. doi: 10.1371/journal.pone.0113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temesgen Z, Toal DR, Cockerill FR., III Leclercia adecarboxylata infections: case report and review. Clin Infect Dis. 1997;25:79–81. doi: 10.1086/514514. [DOI] [PubMed] [Google Scholar]
- 40.Cha SW, Heo JN, Park C, Choi YW, Jeon SC. Enterobacter Asburiae pneumonia with cavitation. J Korean Soc Radiol. 2013;68:217. [Google Scholar]
- 41.Caballero S, Carter R, Ke X, Sušac B, Leiner IM, Kim GJ, Miller L, Ling L, Manova K, Pamer EG.Distinct but spatially overlapping intestinal niches for vancomycin-resistant Enterococcus faecium and Carbapenem-resistant Klebsiella pneumoniae PLoS Pathog 201511e1005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savini V, Gherardi G, Astolfi D, Polilli E, Dicuonzo G, D’Amario C, Fazii P, D’Antonio D. Insights into airway infections by enterococci: a review. Recent Pat Antiinfect Drug Discov. 2012;7:36–44. doi: 10.2174/157489112799829774. [DOI] [PubMed] [Google Scholar]
- 43.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e1–3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z, Chen H, Berger KI, Goldring RM, Rom WN, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1:19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, Ennis M, Boucher RC, Wolfgang MC, Elborn JS. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–1126. doi: 10.1164/rccm.201210-1937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Twomey KB, Alston M, An SQ, O’Connell OJ, McCarthy Y, Swarbreck D, Febrer M, Dow JM, Plant BJ, Ryan RP. Microbiota and metabolite profiling reveal specific alterations in bacterial community structure and environment in the cystic fibrosis airway during exacerbation. PLoS One. 2013;8:e82432. doi: 10.1371/journal.pone.0082432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garzoni C, Brugger SD, Qi W, Wasmer S, Cusini A, Dumont P, Gorgievski-Hrisoho M, Mühlemann K, von Garnier C, Hilty M. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax. 2013;68:1150–1156. doi: 10.1136/thoraxjnl-2012-202917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molyneaux PL, Cox MJ, Willis-Owen SAG, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, Bushman FD, Collman RG. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186:536–545. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One. 2014;9:e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McVey M, Tabuchi A, Kuebler WM.Microparticles and acute lung injury Am J Physiol Lung Cell Mol Physiol 2012303L364–L381.10.1152/ajplung.00354.2011 [DOI] [PubMed] [Google Scholar]
- 54.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 55.Yao YM, Sheng ZY, Tian HM, Yu Y, Wang YP, Yang HM, Guo ZR, Gao WY. The association of circulating endotoxaemia with the development of multiple organ failure in burned patients. Burns. 1995;21:255–258. doi: 10.1016/0305-4179(95)93867-j. [DOI] [PubMed] [Google Scholar]
- 56.Bayraktar UD, Shpall EJ, Liu P, Ciurea SO, Rondon G, de Lima M, Cardenas-Turanzas M, Price KJ, Champlin RE, Nates JL. Hematopoietic cell transplantation-specific comorbidity index predicts inpatient mortality and survival in patients who received allogeneic transplantation admitted to the intensive care unit. J Clin Oncol. 2013;31:4207–4214. doi: 10.1200/JCO.2013.50.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson PA, Lim A, Panek-Hudson Y, Tacey M, Hijazi R, Ng AP, Szer J, Ritchie D, Bajel A. Screening with spirometry is a useful predictor of later development of noninfectious pulmonary syndromes in patients undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:781–786. doi: 10.1016/j.bbmt.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Rano A, Agusti C, Natividada B, Rovira M, Angrill J, Pumarola T, Torres A. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2002;122:253–261. doi: 10.1378/chest.122.1.253. [DOI] [PubMed] [Google Scholar]
- 59.Roychowdhury M, Pambuccian SE, Aslan DL, Jessurun J, Rose AG, Manivel JC, Gulbahce HE. Pulmonary complications after bone marrow transplantation: an autopsy study from a large transplantation center. Arch Pathol Lab Med. 2005;129:366–371. doi: 10.5858/2005-129-366-PCABMT. [DOI] [PubMed] [Google Scholar]
- 60.Nakasone H, Onizuka M, Suzuki N, Fujii N, Taniguchi S, Kakihana K, Ogawa H, Miyamura K, Eto T, Sakamaki H, et al. Pre-transplant risk factors for cryptogenic organizing pneumonia/bronchiolitis obliterans organizing pneumonia after hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:1317–1323. doi: 10.1038/bmt.2013.116. [DOI] [PubMed] [Google Scholar]
- 61.Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow MR, Aubry MC, Afessa B. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 2005;128:1385–1392. doi: 10.1378/chest.128.3.1385. [DOI] [PubMed] [Google Scholar]
- 62.Gudiol C, Garcia-Vidal C, Arnan M, Sánchez-Ortega I, Patiño B, Duarte R, Carratalà J. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49:824–830. doi: 10.1038/bmt.2014.37. [DOI] [PubMed] [Google Scholar]
- 63.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Huisman C, van der Straaten HM, Canninga-van Dijk MR, Fijnheer R, Verdonck LF. Pulmonary complications after T-cell-depleted allogeneic stem cell transplantation: low incidence and strong association with acute graft-versus-host disease. Bone Marrow Transplant. 2006;38:561–566. doi: 10.1038/sj.bmt.1705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmas A, Tefferi A, Myers JL, Scott JP, Swensen SJ, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol. 1998;100:680–687. doi: 10.1046/j.1365-2141.1998.00617.x. [DOI] [PubMed] [Google Scholar]
- 66.Solh M, Arat M, Cao Q, Majhail NS, Weisdorf D. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation. 2011;91:798–803. doi: 10.1097/TP.0b013e31820c85fa. [DOI] [PubMed] [Google Scholar]
- 67.Cooke KR, Yanik G. Acute lung injury after allogeneic stem cell transplantation: is the lung a target of acute graft-versus-host disease? Bone Marrow Transplant. 2004;34:753–765. doi: 10.1038/sj.bmt.1704629. [DOI] [PubMed] [Google Scholar]
- 68.Versluys AB, Rossen JWA, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pène F, Aubron C, Azoulay E, Blot F, Thiéry G, Raynard B, Schlemmer B, Nitenberg G, Buzyn A, Arnaud P, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: a reappraisal of indications for organ failure supports. J Clin Oncol. 2006;24:643–649. doi: 10.1200/JCO.2005.03.9073. [DOI] [PubMed] [Google Scholar]
- 70.Garcia JB, Lei X, Wierda W, Cortes JE, Dickey BF, Evans SE, Ost DE. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Ann Am Thorac Soc. 2013;10:432–440. doi: 10.1513/AnnalsATS.201304-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.