Abstract
Rationale: Degradation of the endothelial glycocalyx, a glycosaminoglycan (GAG)-rich layer lining the vascular lumen, is associated with the onset of kidney injury in animal models of critical illness. It is unclear if similar pathogenic degradation occurs in critically ill patients.
Objectives: To determine if urinary indices of GAG fragmentation are associated with outcomes in patients with critical illnesses such as septic shock or acute respiratory distress syndrome (ARDS).
Methods: We prospectively collected urine from 30 patients within 24 hours of admission to the Denver Health Medical Intensive Care Unit (ICU) for septic shock. As a nonseptic ICU control, we collected urine from 25 surgical ICU patients admitted for trauma. As a medical ICU validation cohort, we obtained serially collected urine samples from 70 patients with ARDS. We performed mass spectrometry on urine samples to determine GAG (heparan sulfate, chondroitin sulfate, and hyaluronic acid) concentrations as well as patterns of heparan sulfate/chondroitin sulfate disaccharide sulfation. We compared these indices to measurements obtained using dimethylmethylene blue, an inexpensive, colorimetric urinary assay of sulfated GAGs.
Measurements and Main Results: In septic shock, indices of GAG fragmentation correlated with both the development of renal dysfunction over the 72 hours after urine collection and with hospital mortality. This association remained after controlling for severity of illness and was similarly observed using the inexpensive dimethylmethylene blue assay. These predictive findings were corroborated using urine samples previously collected at three consecutive time points from patients with ARDS.
Conclusions: Early indices of urinary GAG fragmentation predict acute kidney injury and in-hospital mortality in patients with septic shock or ARDS.
Clinical trial registered with www.clinicaltrials.gov (NCT01900275).
Keywords: glycocalyx, heparan sulfate, chondroitin sulfate, hyaluronic acid, glycomics
At a Glance Commentary
Scientific Knowledge on the Subject
Degradation of the glomerular endothelial glycocalyx (a glycosaminoglycan [GAG]-rich layer lining the vascular lumen) is associated with the onset of kidney injury in animal models of critical illness. Although glomerular glycocalyx degradation can be detected via urine indices of GAG fragmentation, these indices have yet to be investigated as predictors of patient outcomes in critical illness.
What This Study Adds to the Field
This study, which used state-of-the-art mass spectrometry approaches in three different intensive care unit cohorts, is the first to demonstrate that urine GAG fragmentation predicts the development of acute kidney injury and mortality in critically ill patients. This predictive ability remains when using a simple, low-cost measure of GAG fragmentation, suggesting bedside applicability of our findings.
Since the original descriptions of “putrefaction” by Hippocrates, sepsis has been consistently recognized as a major source of human suffering and mortality (1). Today, sepsis and its associated infections remain the major cause of in-hospital death in the United States (2) and intensive care units (ICUs) worldwide (3, 4). As sepsis mortality increases with the incremental onset of organ failure (5), early identification of at-risk organ systems may enable personalization of therapies that could benefit patients with evolving sepsis.
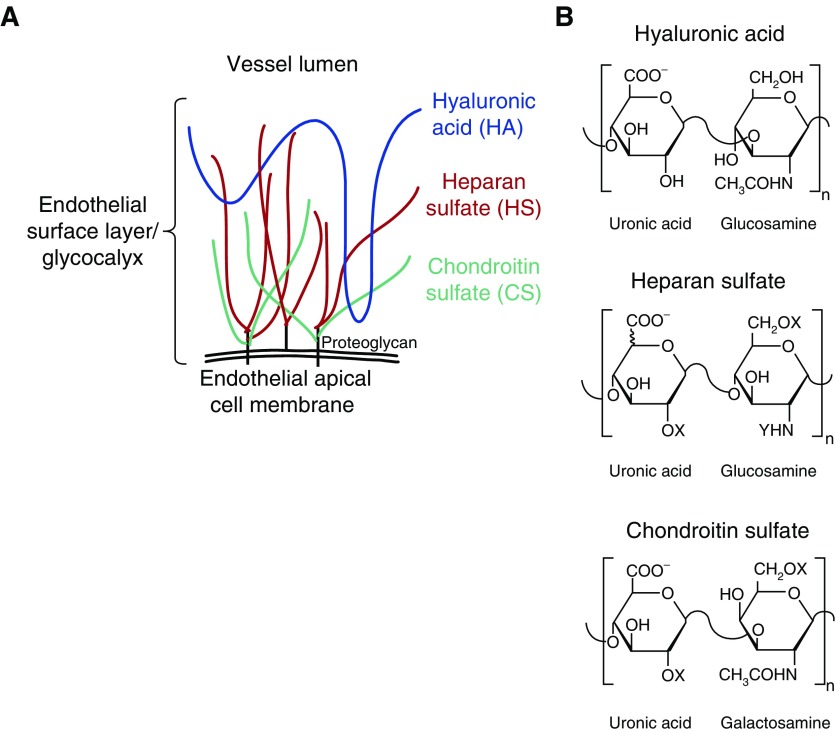
There is increasing appreciation for the importance of the endothelial glycocalyx to lung (6) and kidney (7, 8) homeostasis. The glycocalyx (and its in vivo manifestation, the endothelial surface layer) is a substantial layer of glycosaminoglycans (GAGs) and associated proteoglycans lining the vascular lumen (Figure 1). The glycocalyx contributes to several essential endothelial functions, including (1) maintenance of the endothelial barrier to fluid and protein, (2) the mechanotransduction of shear stress into endothelial nitric oxide production, and (3) the regulation of leukocyte-endothelial adhesion. Accordingly, dysfunction of the endothelial glycocalyx may contribute to the tissue edema, aberrant vascular tone, and inappropriate inflammation characteristic of septic organ injury.
Figure 1.
Endothelial glycocalyx glycosaminoglycans. (A) The endothelial glycocalyx (and its in vivo equivalent, the endothelial surface layer) is composed of glycosaminoglycans including proteoglycan-bound heparan sulfate (HS) and chondroitin sulfate (CS) as well as proteoglycan-independent hyaluronic acid (HA). (B) Glycosaminoglycans are composed of linear polymers of disaccharide repeats. CS and HS may be additionally modified by sulfation (X = SO− or H; Y = SO− or COCH3). n = number of times this disaccharide sequence repeats.
Using a mouse model of polymicrobial sepsis, we recently observed septic induction of renal heparanase, a heparan sulfate (HS)-specific endoglucuronidase (7). HS, the most abundant glycocalyx GAG, is a linear sulfated polysaccharide composed of repeating hexuronic acid: glucosamine disaccharides (Figure 1). Accordingly, renal heparanase activation was associated with fragmentation of glomerular HS, leading to loss of glomerular filtration. This early, pathogenic induction of glomerular heparanase could be detected in mice via urinary assays, including HS degradation activity (7).
We recently developed a high-sensitivity, mass spectrometry–based approach to detect and characterize fragmented GAGs within biologic samples from critically ill patients (9). This high- sensitivity approach allows for not only quantification/characterization of HS fragments but also detection of other glycocalyx GAGs, including chondroitin sulfate (CS, a sulfated linear polysaccharide composed of repeating hexuronic acid:N-acetylgalactosamine disaccharides) and hyaluronic acid (HA, an unsulfated linear polysaccharide of repeating glucuronic acid:N-acetylglucosamine disaccharides, Figure 1). As CS and HS sulfation is a critical determinant of glycosaminoglycan–protein interaction, mass spectrometry characterization of disaccharide sulfation patterns may additionally provide insight into the biologic impact of fragmented GAGs on signal transduction.
Building on these mechanistic animal data (and leveraging our newly developed GAG analytical techniques), we hypothesized that septic shock in humans would be associated with pathologic degradation of the glomerular endothelial glycocalyx and excretion of GAG fragments into the urine. Urinary GAGs would therefore be associated with the development of kidney injury and, ultimately, mortality. We performed a prospective study of patients with septic shock to test this hypothesis. We compared our findings to samples obtained from cohorts of critically ill patients with trauma (9) and patients with acute respiratory distress syndrome (ARDS) (10).
Methods
Prospective Enrollment of Human Subjects
Between July 2013 and November 2014, we prospectively enrolled adult patients admitted to the Denver Health Medical Center (Denver, CO) medical ICU with a diagnosis of septic shock, as defined in the online supplement (ClinicalTrials.gov NCT01900275). Patients were eligible for inclusion if the diagnosis of septic shock was made less than 24 hours before enrollment. We concurrently enrolled adult patients admitted within the previous 24 hours to the Denver Health Medical Center surgical ICU for severe trauma, as defined by an Injury Severity Score greater than 15. A subset of these surgical ICU samples have been previously used to optimize mass spectrometry measures of urine GAGs (9). Exclusion criteria are described in the online supplement.
Urine collection occurred at enrollment, as described in the online supplement. All protocols were approved by the Colorado Multiple Institutions Review Board (COMIRB, approval #13-0425).
The primary outcome was the development of worsening renal function between 24 and 72 hours after enrollment. We defined worsening renal function as the development of new Acute Kidney Injury Network stage 2 criteria: a twofold increase in serum creatinine from admission values (or >0.5 mg/dl absolute change if baseline Cr > 4.0 mg/dl) or decreased urine output (<0.5 ml/kg/h × 12 h) (11). We recorded in-hospital mortality as a secondary outcome. All bloodwork and urine output measurements were recorded as part of standard clinical care.
Validation Cohort of ARDS Urine Samples
We obtained urine samples from a study of urine IL-18 in ARDS (10). These samples had been collected from patients 0, 1, and 3 days after enrollment in the Acute Respiratory Distress Syndrome Network ARMA (Respiratory Management in ALI/ARDS) study (12); the NHLBI provided permission for continued use of these remnant samples. Samples were obtained from 70 patients with ARDS with normal renal function at baseline (serum creatinine ≤ 1.2 mg/dl); 22 patients (cases) later developed acute kidney injury (AKI, as defined by greater than twofold increase in baseline serum creatinine), and 48 patients (control subjects) maintained normal renal function. The Colorado Multiple Institutions Review Board authorized the “exempt” use of these deidentified samples, as previously described (10) and confirmed before current analyses.
Urine Analyses
Urine glycosaminoglycan content was determined using mass spectrometry, as described in the online supplement (9). Urinary heparanase activity, urinary bikunin, and urinary dimethylmethylene blue (DMMB) were measured as described in the online supplement.
Statistical Analyses
Statistical analyses are described in the online supplement. Data are presented as mean ± SE.
Results
Urinary GAGs in Medical and Surgical ICU Patients
Between July 2013 and October 2014, we enrolled 30 medical ICU patients with vasopressor-dependent septic shock. Twenty-five concurrently enrolled surgical ICU patients with severe trauma served as a contemporaneous ICU control group (see Table E1 in the online supplement). As expected, patients with septic shock were more likely than trauma patients to develop new/worsening renal dysfunction in the 72 hours after study enrollment or to die during their hospitalization.
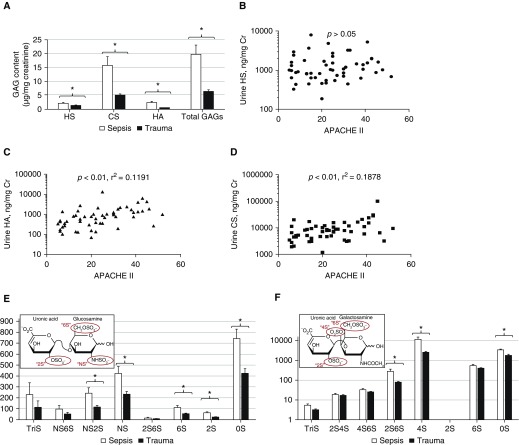
Urine GAGs were significantly elevated in patients with septic shock as compared with their surgical ICU comparators (Figure 2A). Although HS concentrations were independent of severity of illness (Figure 2B), urinary concentrations of HA and CS correlated with Acute Physiology and Chronic Health Evaluation (APACHE) II scores (Figures 2C and 2D). Accordingly, total GAGs correlated with APACHE II (P < 0.01, r2 = 0.2059). Urine HS, HA, CS, and total GAGs were independent (P > 0.3) of serum creatinine or estimated glomerular filtration rate (a clinical index inclusive of age, sex, serum creatinine, and race) at urine collection.
Figure 2.
Urinary glycosaminoglycans (GAGs) in septic shock and trauma. (A) Urine GAGs (including heparan sulfate [HS], chondroitin sulfate [CS], and hyaluronic acid [HA]) were significantly elevated in patients with septic shock (collected within 24 h of shock diagnosis) in comparison to surgical intensive care unit–admitted trauma patients. (B) Urine HS was not associated with severity of illness (Acute Physiology and Chronic Health Evaluation [APACHE] II) in the combined surgical/medical population. In contrast, urine HA (C) and CS (D) were associated with severity of illness. Mass spectrometry measurements revealed that patients with septic shock had distinct patterns of urine HS (E) and CS (F) disaccharide sulfation (inset). *P < 0.05. NS = N-sulfated.
To investigate the association of GAG sulfation (e.g., HS, CS) with the cause of critical illness, we digested isolated GAGs into constituent disaccharides and performed mass spectrometry. As detailed in Figure 2E, urine NS2S, NS, 6S, 2S, and unsulfated heparan disaccharides were significantly elevated in patients with septic shock as compared with trauma patients. Furthermore, urine 2S6S, 4S, and unsulfated chondroitin disaccharides were elevated in septic shock (Figure 2F).
The observed elevation in N-sulfated (e.g., NS, NS6S, NS2S, tri-sulfated) HS fragments suggested the action of heparanase, an HS-specific endoglucuronidase that preferentially releases N-sulfated heparan fragments. Consistent with our previous findings using a mouse model of polymicrobial sepsis (7), patients with septic shock demonstrated elevated urinary HS degradation activity (a marker of heparanase activity, Fig E1A). Urinary HS degradation activity correlated closely with urinary HS, suggesting that heparan fragmentation was a consequence of heparanase activity (Fig E1B). Furthermore, in a manner consistent with urinary HS (Figure 2B), urinary HS degradation activity did not correlate with severity of illness (Fig E1C).
Given the significant septic shock–associated elevation in urine chondroitin 4S disaccharides, we sought to determine if urinary bikunin (a urine proteoglycan enriched in 4S-CS) was elevated in patients with septic shock. Although urinary bikunin and 4S-CS concentrations were correlated (P = 0.03), this association was weak (r2 = 0.08), and there were no differences between patients with septic shock and trauma patients (0.331 ng bikunin/mg creatinine vs. 0.359 ng bikunin/mg creatinine; P = 0.72).
Urinary GAGs Predict the Development/Progression of Renal Dysfunction in Septic Shock
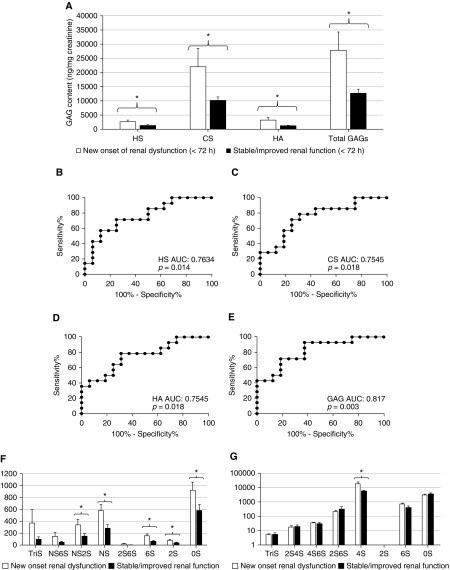
On the basis of our previous animal investigations (7), we hypothesized that urinary GAG fragmentation would be an early event in the development of septic kidney injury in humans. Indeed, urinary concentrations of HS (Figures 3A, 3B, and 3F) and urine HS degradation activity (Table E2) within 24 hours of septic shock onset correlated with later (i.e., within 72 h of enrollment) renal dysfunction, as defined by new onset of Acute Kidney Injury Network 2 criteria.
Figure 3.
Urine glycosaminoglycans (GAGs) predict the development/progression of renal dysfunction in septic shock. (A) In urine collected within 24 hours of the diagnosis of septic shock, GAG concentrations (heparan sulfate [HS], chondroitin sulfate [CS], and hyaluronic acid [HA]) were significantly elevated in patients who developed new Acute Kidney Injury Network 2 criteria (>twofold increase in serum creatinine or <0.5 ml/kg/h urine output) between 24 and 72 hours after urine collection. (B–E) Urine HS (B), CS (C), HA (D), and total GAGs (E) have significant predictive value for the development of renal dysfunction, as demonstrated by receiver operating characteristic curves. Mass spectrometry measurements revealed that patients with septic shock who developed new/progressive renal dysfunction had distinct patterns of urine HS (F) and CS (G) disaccharide sulfation. *P < 0.05. AUC = area under the receiver operating characteristic curve; NS = N-sulfated.
Changes in urinary CS and HA similarly predicted new renal dysfunction (Figures 3A, 3C, 3D, and 3G), with total GAGs demonstrating a high (0.817) area under the receiver operating characteristic (ROC) curve as a kidney injury predictor (Figure 3E). The sensitivities and specificities of various thresholds of urinary glycosaminoglycans in septic shock are detailed in Table E3. Similarly robust predictive characteristics were seen with HS disaccharides, including NS2S, NS, 6S, 2S, and 0S (Figure 3F, Table E2). Of CS disaccharides, only 4S sulfation predicted the onset/progression of renal dysfunction (Figure 3G, Table E2). The predictive ability of urine GAGs remained even after controlling for severity of illness (APACHE II, Table E2).
Due to the small size of our septic shock cohort, we were unable to exclude patients with abnormal serum creatinine at study enrollment. As such, our findings are not direct predictors of de novo AKI but rather the onset or progression of renal dysfunction from baseline function at study enrollment. However, in sensitivity analyses limited to patients with septic shock with creatinine less than 2.0 mg/dl at enrollment, HS (P = 0.05) and total GAGs (P = 0.0287) remained associated with renal dysfunction onset/progression. Additional analyses inclusive of all critically ill patients (both septic shock and trauma) showed strong associations between HS, CS, HA, or total GAGs and renal dysfunction (P < 0.001 for each); these associations remained statistically significant when limited to patients with baseline serum creatinine less than 2.0 mg/dl (data not shown).
In accordance with previous studies of urinary GAGs, we elected to normalize GAG concentrations to urine creatinine, thereby controlling for urine dilution (13, 14). However, absolute (raw) concentrations of urine HS, CS, HA, and total GAGs remained statistically associated with worsening renal dysfunction (P < 0.05), with robust area under the ROC curve measurements (0.8884, 0.8348, 0.8705, and 0.8482, respectively). As with normalized GAGs, absolute concentrations of heparan NS2S, NS, 6S, 2S, and 0S disaccharides remained statistically associated with renal dysfunction. In contrast to normalized CS, absolute concentrations of chondroitin 2S4S, 4S6S, 4S, and 6S disaccharides were associated with later renal dysfunction (P < 0.05).
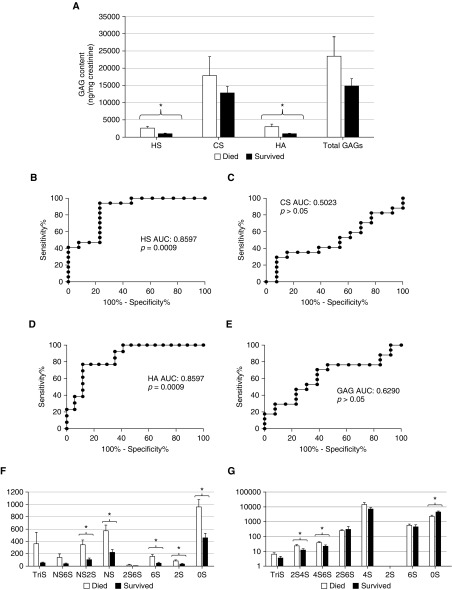
Urinary HS and HA Predict Hospital Mortality in Patients with Septic Shock
Urinary HS and HA (collected within 24 h of diagnosis and normalized to urine creatinine) were significantly elevated in patients with septic shock who later died during their hospitalization (Figures 4A, 4B, and 4D). HS remained an independent predictor of mortality when controlling for severity of illness (Table E4). The sensitivities and specificities of various thresholds of urinary glycosaminoglycans for septic shock mortality are detailed in Table E3 and Fig E2.
Figure 4.
Urine glycosaminoglycans (GAGs) predict hospital mortality in septic shock. (A) In urine collected within 24 hours of the diagnosis of septic shock, urine heparan sulfate (HS) and hyaluronic acid (HA) were significantly elevated in patients who died during their hospitalization. Urine HS (B) and HA (D) have significant predictive value for mortality, as demonstrated by receiver operating characteristic curves. Conversely, urine chondroitin sulfate (CS) (C) and total GAGs (E) did not predict mortality. Mass spectrometry measurements revealed that patients with septic shock who died had distinct patterns of urine HS (F) and CS (G) disaccharide sulfation. *P < 0.05. AUC = area under the receiver operating characteristic curve; NS = N-sulfated.
Similar to renal dysfunction (Figure 3F), NS2S, NS, 6S, 2S, and 0S heparan disaccharides were strongly predictive of hospital mortality (Figure 4F, Table E4). In contrast to kidney dysfunction (Figure 3G), 4S chondroitin disaccharides did not predict mortality, although 2S4S, 4S6S, and 0S did (Figure 4G, Table E4).
Identical associations with hospital mortality were observed when we repeated analyses using absolute concentrations (ng/ml) of HS, CS, HA, total GAGs, and CS/HS disaccharides (data not shown).
Alternative Measures of Urinary GAGs
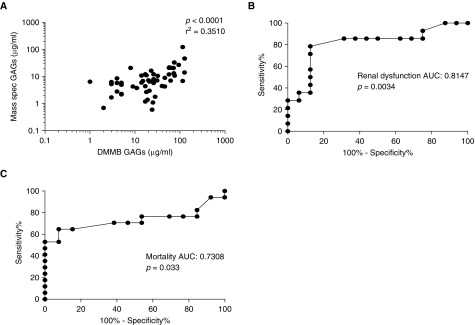
As mass spectrometry is expensive and cannot be easily performed at the bedside, we sought to determine the predictive value of simplified measurements of urinary GAGs. DMMB is a colorimetric assay that detects sulfated GAGs in urine at a fraction of the cost of mass spectrometry (cost per sample in our study: $2 vs. $200). To best approximate a simple, point-of-care assay, DMMB measures were not normalized to urine creatinine. These DMMB measures of sulfated GAGs correlated well with mass spectrometry measures of total GAGs (Figure 5A). Accordingly, urine DMMB (collected within 24 hours of septic shock onset) predicted progressive renal dysfunction (Figure 5B) and hospital mortality (Figure 5C).
Figure 5.
Dimethylmethylene blue (DMMB) colorimetric assay of urinary glycosaminoglycans (GAGs). (A) DMMB, a colorimetric assay that identifies sulfated glycosaminoglycans (GAGs), correlates with urine GAGs as measured by mass spectrometry. Accordingly, DMMB predicted the onset/progression of renal dysfunction (B) and in-hospital mortality (C) in patients with septic shock. AUC = area under the receiver operating characteristic curve.
Urinary GAG Fragmentation in Patients with ARDS with Normal Baseline Renal Function
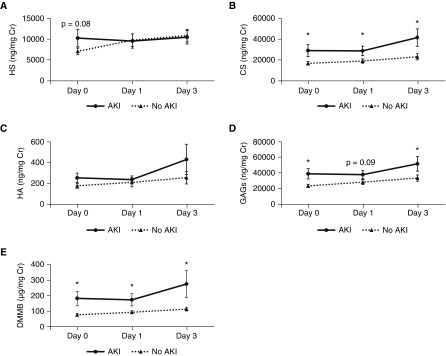
We obtained urine samples collected from patients with ARDS 0, 1, and 3 days after enrollment in the ARDS Network ARMA study (12). As previously described (10), all 70 patients in this cohort had normal renal function at baseline; 22 patients later developed AKI (Table E5). Patients who later developed AKI had significantly elevated urinary total glycosaminoglycans at Days 0 and 3, largely derived from a consistently elevated urinary CS (Figure 6). The sensitivities and specificities of various thresholds of urinary glycosaminoglycans in ARDS are detailed in Table E6. Disaccharide analyses revealed that urine CS was largely 4-O sulfated (Fig E3). Although total HS demonstrated a nonsignificant association with AKI (P = 0.08) at Day 0, N-sulfated and 6-sulfated HS fragments were statistically different between groups with and without AKI (Figure 6, Figure E3).
Figure 6.
Urinary indices of glycosaminoglycan (GAG) degradation and acute kidney injury (AKI) in acute respiratory distress syndrome. Urine was collected from patients with acute respiratory distress syndrome 0, 1, and 3 days after study enrollment and analyzed by mass spectrometry for (A) heparan sulfate (HS), (B) chondroitin sulfate (CS), (C) hyaluronic acid (HA), or (D) total GAGs (reflecting the sum of HS, CS, and HA). In addition, sulfated glycosaminoglycans were measured using the colorimetric dimethylmethylene blue (DMMB) assay (E). At baseline, all patients had normal renal function. A subset of patients later developed AKI; the remainder of patients remained with normal renal function. *P < 0.05 between AKI and no AKI groups.
Absolute Day 0 glycosaminoglycan data revealed similar associations as normalized data, with the exception that absolute HS was significantly elevated in patients who later developed AKI (5,877.42 ± 1,501.63 ng/ml vs. 3,306.44 ± 430.80 ng/ml, P = 0.03). There were no differences between groups in absolute urine glycosaminoglycan levels at Day 1. At Day 3, only absolute CS was significantly different between AKI and non-AKI groups (12,012.98 ± 2,162.19 ng/ml vs. 8,300.30 ± 821.58 ng/ml, P = 0.05).
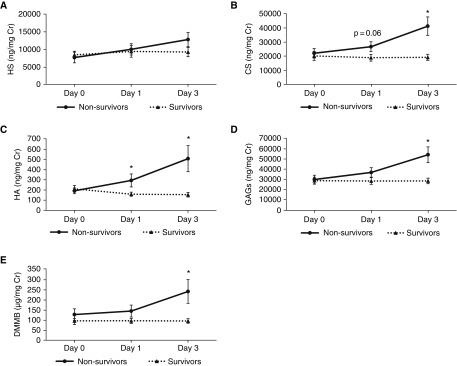
Urinary glycosaminoglycan indices were directly (and temporally) associated with in-hospital mortality in patients with ARDS (Figure 7). At Day 3, N-sulfated and unsulfated HS disaccharides were associated with mortality; nearly every CS sulfation pattern (with the exception of TriS and 2S) was associated with mortality (Figure E4). In contrast to patients with septic shock, the mortality-predictive ability of urinary indices in ARDS was lost when values were not normalized for urine creatinine.
Figure 7.
Urinary indices of glycosaminoglycan (GAG) degradation and hospital mortality in acute respiratory distress syndrome. Urine was collected from patients with acute respiratory distress syndrome 0, 1, and 3 days after study enrollment and analyzed by mass spectrometry for (A) heparan sulfate (HS), (B) chondroitin sulfate (CS), (C) hyaluronic acid (HA), or (D) total GAGs (reflecting the sum of HS, CS, and HA). In addition, sulfated glycosaminoglycans were measured using the colorimetric dimethylmethylene blue (DMMB) assay (E). *P < 0.05 between nonsurvivors and survivors.
Urinary GAGs and Pulmonary versus Nonpulmonary Etiologies of Illness
As we have previously observed distinct plasma GAG fragmentation patterns in pulmonary and nonpulmonary sepsis (15), we measured the correlation of urinary GAG fragments with the infectious site causative of septic shock. Urinary GAG fragments in patients with septic shock did not correlate with source of infection (pulmonary vs. nonpulmonary, P > 0.3 for all GAGs studied). In patients with ARDS, there was a nonsignificant elevation of urine HS (Day 0) in patients with nonpulmonary sepsis–induced ARDS as opposed to direct (pneumonia) injury (10,123.62 ± 1,980.71 ng/mg Cr vs. 6,979.37 ± 784.75 ng/mg Cr, P = 0.08).
Discussion
In this study, we found that septic shock was associated with an early increase in urinary GAGs, predictive of ongoing/progressive renal dysfunction in the ensuing 72 hours. The ability of urinary GAGs to predict renal dysfunction persisted even after controlling for severity of illness. Accordingly, urine HS and HA were associated with septic shock mortality, with HS remaining predictive of mortality after controlling for severity of illness. Comparable urinary GAG fragmentation was noted in patients with ARDS with normal renal function at baseline who later developed AKI. These mass spectrometry–based measures could be largely replicated using an inexpensive, rapidly performed colorimetric assay of sulfated glycosaminoglycans. As such, urinary glycosaminoglycans are promising biomarkers, with both diagnostic and prognostic implications in critical illness.
Our observation (Fig E1) that urine HS correlated strongly with urine HS degradation activity in septic shock is consistent with septic induction of glomerular heparanase, as predicted by animal models of septic kidney injury (7). These findings are compatible with a renal source of HS degradation (e.g., within the glomerular glycocalyx) and may not simply reflect circulating plasma HS (i.e., released from extrarenal injury). This potential renal selectivity of urine HS fragmentation is supported by the absence of association between urine HS and the etiology of septic shock (pulmonary vs. nonpulmonary), contrasting the significant associations we have previously observed between plasma HS and infection source during sepsis (15). Of note, although our previous preclinical studies suggested a glomerular source of heparanase induction and HS fragmentation during septic kidney injury (7), we cannot exclude alternative sources of glycosaminoglycan fragmentation (e.g., tubules) in human sepsis.
Interestingly, in patients with ARDS, baseline levels of urine HS were significantly elevated in comparison to our septic shock cohort (which was characterized by a low prevalence of ARDS). Although we cannot exclude a “batch effect” of short-term (i.e., septic shock/trauma) versus prolonged (ARDS) sample storage, we speculate that in ARDS, elevated levels of circulating HS fragments (reflecting pulmonary induction of heparanase [6]) may “spill” into the urine, obscuring the correlation of urinary HS with glomerular pathophysiology. Indeed, the presence of glycosaminoglycan “spilling” is supported by a strong trend of increased urine HS in patients with ARDS with nonpulmonary sepsis, a pattern observed in the plasma of such patients (15). This increased level of urinary GAGs in patients with ARDS prevents determination of a single “cutoff” that consistently diagnoses impending kidney injury or mortality in both patients with septic shock and patients with ARDS. Determination of a broadly applicable diagnostic threshold for urinary GAGs will require additional multicenter studies of heterogeneous critically ill patient populations.
Although activation of glomerular heparanase can explain increased urine HS, this enzyme cannot directly account for the observed increases in HA and CS. Indeed, HA and CS were more closely associated with outcomes than HS in the ARDS cohort. These unexpected findings suggest the activation of additional GAG-degrading renal enzymes in septic shock and ARDS. Elevated urinary CS has been previously observed in children with meningococcal sepsis (16), with roughly equivalent elevations of 4S- and 6S-CS, contrasting the 4S-predominance seen in our patients. Interestingly, 4S-CS is commonly found within bikunin, a proteoglycan constituent of the urinary trypsin inhibitor IαI (17). However, bikunin-associated CS is typically characterized by a 1:2 ratio of 4S:0S disaccharides (17), inconsistent with the pattern observed in our patients with septic shock or ARDS. Accordingly, we found little association between urine CS and bikunin in patients with septic shock. The elevations of urine CS in septic shock contrast the relative stability of plasma CS in our previous study of sepsis-associated respiratory failure (15), again suggesting that urine GAG fragments primarily reflect localized renal degradation.
HA has been implicated in several renal diseases, including renal ischemia-reperfusion injury (18–20). We observed that patients with septic shock had a significant increase in urine HA, which was predictive of renal dysfunction and (on unadjusted analyses) in-hospital mortality. Similarly, urine HA predicted mortality in patients with ARDS. Recent reports have highlighted the utility of urine HA as an early biomarker of kidney injury after intraoperative cardiopulmonary bypass (13). We previously found no association between plasma HA and nonpulmonary sepsis in patients with respiratory failure (15), further supporting the conclusion that urinary HA fragments reflect renal HA degradation (and not systemic HA degradation) during septic shock.
The potential value of urine GAGs as a predictive biomarker may be limited by concerns about generalizability, given the high mortality in the septic shock and ARDS cohorts. However, we are reassured that the prognostic utility of urine GAGs remains even when expanding our prospectively enrolled cohort to include the less severely ill trauma population. Furthermore, the association of urine GAGs with renal dysfunction (and urine HS with mortality) persisted when controlling for severity of illness in septic shock. Although APACHE II contains indices of renal dysfunction, this potential collinearity would be expected to decrease the predictive ability of urinary GAGs. On the contrary, urine GAGs meet or exceed previously reported plasma or urine biomarkers of kidney injury (with areas under ROC curves ranging from 0.7–0.9), at much lower cost (21, 22). As individualized severity of illness data were not available for the validation cohort of patients with ARDS, we were unable to similarly control for APACHE II in the analysis of ARDS urine samples. However, these samples were collected from a parent study in which baseline (Day 0) severity of illness scores were identical between the ARDS group that developed AKI and that which maintained normal renal function (10).
The clinical relevance of our findings is supported by the ability to largely replicate the predictive capabilities of our mass spectrometry findings by using DMMB, an inexpensive GAG assay that can be directly performed on urine. Although mass spectrometry affords highly sensitive analyses of GAG concentrations and patterns of disaccharide sulfation, such analyses are time intensive and expensive, costing approximately $200 per sample in our study (not including the costs of mass spectrometry equipment purchase and maintenance and data analysis). In contrast, the DMMB assay, which detects highly sulfated GAGs, including HS and CS, can be easily performed in minutes, at low cost ($2 per sample, not including the relatively inexpensive and commonly available colorimetric plate reader). The generalizability of this DMMB assay should be prospectively tested in a future multicenter study inclusive of lower-acuity sepsis cohorts and/or other systemic illnesses associated with kidney dysfunction (e.g., severe acute pancreatitis).
In summary, our study demonstrates an association between urinary GAG concentrations (measured early in the course of critical illness) and the later onset/progression of renal dysfunction and hospital mortality. These associations, derived from highly sophisticated mass spectrometry approaches, can be replicated with a widely applicable, inexpensive colorimetric assay, demonstrating practical relevance of urinary GAGs as predictive markers in septic shock and ARDS.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Tuder Laboratory (University of Colorado) for their technical advice and the clinical staff of the Denver Health medical ICU and surgical ICU for their care of the enrolled patients. They thank the patients (and their families) of the Denver Health Medical Center and ARMA clinical sites for their participation in these clinical studies.
Footnotes
Supported by NHLBI grant R01 HL125371 (R.J.L. and E.P.S.) and National Institutes of Health/National Center for Advancing Translational Sciences Colorado grant CTSI UL1 TR000154 (E.P.S.).
Author Contributions: E.P.S. designed the study, performed statistical analyses, interpreted the data, and wrote the manuscript. K.H.O., L.A.A., T.D.H., and C.C.B. participated in study design and enrolled study subjects. X.S., L.L., X.L., Y. Yang, M.A.S., Y. Yu, Y.C., and F.Z. designed and performed analyses of urine glycosaminoglycan content. C.L.E. provided patient samples and assisted in study design. I.S.D. participated in study design, performed statistical analyses, interpreted the data, and assisted in manuscript preparation. R.J.L. supervised all analyses of urine glycosaminoglycan content, assisted in study design, interpreted the data, and assisted in manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201511-2281OC on February 29, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Baron RM, Baron MJ, Perrella MA. Pathobiology of sepsis: are we still asking the same questions? Am J Respir Cell Mol Biol. 2006;34:129–134. doi: 10.1165/rcmb.F308. [DOI] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. ICON investigators. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1:e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Li L, Overdier KH, Ammons LA, Douglas IS, Burlew CC, Zhang F, Schmidt EP, Chi L, Linhardt RJ. Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2015;87:6220–6227. doi: 10.1021/acs.analchem.5b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 13.Zarbock A, Meersch M, Van Aken H, Görlich D, Singbartl K. Urinary hyaluronic acid as an early predictor of acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64:737–738. doi: 10.1016/j.jacc.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJ. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289:8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oragui EE, Nadel S, Kyd P, Levin M. Increased excretion of urinary glycosaminoglycans in meningococcal septicemia and their relationship to proteinuria. Crit Care Med. 2000;28:3002–3008. doi: 10.1097/00003246-200008000-00054. [DOI] [PubMed] [Google Scholar]
- 17.Ly M, Leach FE, III, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7:827–833. doi: 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Declèves A-E, Caron N, Voisin V, Legrand A, Bouby N, Kultti A, Tammi MI, Flamion B. Synthesis and fragmentation of hyaluronan in renal ischaemia. Nephrol Dial Transplant. 2012;27:3771–3781. doi: 10.1093/ndt/gfs098. [DOI] [PubMed] [Google Scholar]
- 19.Göransson V, Johnsson C, Jacobson A, Heldin P, Hällgren R, Hansell P. Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol Dial Transplant. 2004;19:823–830. doi: 10.1093/ndt/gfh003. [DOI] [PubMed] [Google Scholar]
- 20.Wüthrich RP. The proinflammatory role of hyaluronan-CD44 interactions in renal injury. Nephrol Dial Transplant. 1999;14:2554–2556. doi: 10.1093/ndt/14.11.2554. [DOI] [PubMed] [Google Scholar]
- 21.Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223. doi: 10.1186/s13054-015-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prucha M, Bellingan G, Zazula R. Sepsis biomarkers. Clin Chim Acta. 2015;440:97–103. doi: 10.1016/j.cca.2014.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.