To the Editor:
Obstructive sleep apnea (OSA) is both a risk factor for type 2 diabetes mellitus (T2DM) (1) and an exceptionally frequent comorbidity with an adverse effect on glycemic control (2–4). To date, it remains unclear whether continuous positive airway pressure (CPAP) treatment of OSA can improve glycemic control. Previous studies examining the effect of CPAP treatment on measures of glucose metabolism in individuals with T2DM have yielded conflicting results (5). In two randomized clinical trials, there was no effect of CPAP on hemoglobin A1c (HbA1c) when compared with either sham CPAP (6) or usual care (7). One potential reason for the failure of OSA treatment to improve chronic glycemic control is insufficient CPAP use. Notably, the mean nightly CPAP use in these two randomized controlled trials was 3.6 and 4.3 hours per night (6, 7). We recently reported a significant association between OSA during REM sleep and glycemic control in T2DM (4). Because REM sleep predominates in the early morning hours before typical awakening, the cardiometabolic benefits of CPAP therapy may not be achieved with the typical CPAP use of 3–4 hours per night (4, 8).
To obtain definite evidence regarding the clinical efficacy of CPAP treatment to improve glucose control in T2DM, we designed a proof-of-concept study to test the hypothesis that 1 week of full-night CPAP treatment of OSA in the laboratory, as compared with sham CPAP (placebo), results in improvement in glycemic control as assessed by mean plasma glucose levels from 24-hour blood sampling. Preliminary data from this study have been previously reported in the form of an abstract (9). This study is registered at ClinicialTrials.gov (NCT01136785).
Methods
Adults with T2DM and OSA were recruited at the University of Chicago between November 2009 and September 2014. Subjects were eligible if they were newly diagnosed and as-yet untreated for T2DM or were taking a stable oral antidiabetic regimen for at least the past 3 months. Exclusion criteria were use of insulin, unstable medical conditions, being a shift worker, and current or past treatment with positive airway pressure or supplemental oxygen for OSA. The protocol was approved by the Institutional Review Board, and all participants gave informed consent.
Nightly bedtime duration in the laboratory was 8 hours, but bedtimes were individually tailored to habitual bedtimes derived from home actigraphy. In-laboratory baseline assessments were conducted over the course of 2 days under strictly controlled dietary conditions and nightly polysomnography. In the afternoon of the second day, blood sampling at 15- to 30-minute intervals for 24 hours was initiated to assess glucose and insulin levels. During the sampling periods, subjects were provided with identical meals served at 09:00 h, 14:00 h, and 19:00 h. Subjects were required to consume each meal within 20 minutes. No other caloric intake was allowed. Subjects were instructed to take their medications at the habitual time. During bedtime hours, the intravenous line was extended to allow blood drawing from an adjacent room.
After baseline assessments, subjects were assigned in a 2:1 ratio to 1 week of either active CPAP or sham CPAP treatment. Before starting treatment, all subjects underwent an overnight in-laboratory CPAP titration (10). The subjects who were assigned to sham CPAP underwent a sham titration night, using a customized CPAP device (11). During the 1-week treatment period, CPAP or sham CPAP adherence for a full 8 hours on each night was achieved by continuous supervision. During the first 5 days of treatment, subjects were allowed to leave the laboratory in the morning but had to return by approximately 20:00 h. In the evening of the fifth day, the subjects were admitted for 2 days to repeat all baseline assessments under the same laboratory conditions.
The primary endpoint was the change in mean 24-hour plasma glucose level after 1 week of active CPAP versus sham CPAP therapy for 8 hours per night. After verifying the distribution of the outcome measure, group differences were tested using two-sided unpaired Student’s t tests with Welch unequal variances. Data are reported as mean ± SEM.
Results
In a 2:1 ratio, 22 eligible subjects who had completed baseline assessments underwent 1 week of in-laboratory active CPAP or sham CPAP therapy and completed the post-treatment assessments. Two subjects assigned to the CPAP group and one subject assigned to the sham group were excluded for protocol violations. In two additional subjects, the sham CPAP device was found to have delivered therapeutic CPAP pressure that effectively treated OSA on each of the 7 treatment nights. Data from these two subjects were, therefore, included in the CPAP-treated group. In a per-treatment analysis, 13 subjects were analyzed in the treated group and six subjects were analyzed in the untreated group. Baseline characteristics of the participants who were treated (n = 13) versus untreated (n = 6) are described in Table 1. Sleep characteristics before and after 1 week of treatment are reported in Table 2. At baseline, there were no differences in polysomnography parameters between the two groups. CPAP treatment was effective every night in the 13 subjects in the CPAP group (i.e., apnea–hypopnea index <5 events/h). In contrast, there was no significant change in the severity of OSA in the six subjects treated with sham CPAP. There was no difference in CPAP use over 1 week of treatment in the two groups (active CPAP, 7.92 ± 0.08 h/night; sham CPAP, 7.87 ± 0.11 h/night; P = 0.32). Weight did not change from baseline to end of treatment period in either group.
Table 1.
Baseline Characteristics in 13 Treated and 6 Untreated Subjects
| Baseline Characteristics | Treated | Untreated | P Value |
|---|---|---|---|
| Age, yr | 56.2 ± 3.2 | 52.3 ± 1.3 | 0.27 |
| Male | 7 (53.8) | 2 (33.3) | 0.63 |
| Ethnicity | 0.63 | ||
| African-American | 6 (46) | 4 (67) | |
| White | 4 (31) | 2 (33) | |
| Hispanic | 2 (15) | 0 (0) | |
| Pacific Islander | 1 (8) | 0 (0) | |
| BMI, kg/m2 | 36.8 ± 2.5 | 40.1 ± 1.9 | 0.41 |
| Diabetes diagnosis, yr | 2.5 ± 1.2 | 7.3 ± 2.3 | 0.09* |
| HbA1c, % | 7.3 ± 0.4 | 7.0 ± 0.3 | 0.63 |
| Poorly controlled diabetes† | 7 (54) | 2 (33) | 0.63 |
| Oral antidiabetic medications, n | 0.42 | ||
| No medication | 6 | 2 | |
| Metformin alone | 3 | 2 | |
| Combination‡ | 4 | 2 | |
| Hypertension | 4 (31) | 3 (50) | 0.62 |
| Dyslipidemia | 4 (31) | 3 (50) | 0.62 |
| OSA severity | 0.33 | ||
| Mild to moderate | 6 (46) | 1 (17) | |
| Severe | 7 (54) | 5 (83) | |
| Sleep duration at home measured by actigraphy, h | 6.4 ± 0.3 | 6.2 ± 0.6 | 0.69 |
Definition of abbreviations: BMI = body mass index; HbA1c = hemoglobin A1c; OSA = obstructive sleep apnea.
Data are given as mean ± SEM or n (%).
P value using Mann-Whitney nonparametric test.
Poorly controlled diabetes defined as HbA1c ≥7.0%.
Includes metformin plus either a sulfonylurea or a thiazolidinedione.
Table 2.
Sleep/Metabolic Measures at Baseline and after Intervention in 13 Treated and 6 Untreated Subjects
| Sleep/Metabolic Measures | Baseline |
Change from Baseline |
|||
|---|---|---|---|---|---|
| Treated | Untreated | Treated | Untreated | P Value* | |
| Polysomnography† | |||||
| Total sleep time, min | 367.2 ± 12.9 | 386.6 ± 16.1 | 13.4 ± 11.2 | 22.5 ± 21.4 | 0.68 |
| Sleep efficiency, % | 76.6 ± 2.7 | 83.3 ± 1.6 | 2.4 ± 2.4 | 2.7 ± 2.5 | 0.95 |
| Rapid eye movement sleep, min | 67.1 ± 8.7 | 64.5 ± 10.4 | 25.0 ± 6.6 | 19.6 ± 7.8 | 0.63 |
| N1 sleep, min | 54.1 ± 7.8 | 68.1 ± 20.0 | −25.5 ± 5.5 | 9.4 ± 10.3 | 0.04 |
| N2 sleep, min | 226.1 ± 10.3 | 236.0 ± 9.5 | 6.4 ± 8.2 | −9.6 ± 15.2 | 0.32 |
| Slow wave sleep, min | 19.9 ± 8.1 | 17.9 ± 10.3 | 7.6 ± 2.8 | 3.1 ± 3.6 | 0.37 |
| Apnea–hypopnea index, per hour of sleep | 39.7 ± 8.0 | 52.3 ± 14.2 | −35.9 ± 7.4 | 0.2 ± 4.6 | 0.006 |
| 3% oxygen desaturation index, per hour of sleep | 24.4 ± 6.1 | 37.4 ± 8.1 | −21.7 ± 5.6 | 1.0 ± 7.5 | 0.03 |
| Sleep time at <90% oxygen saturation, min | 63.9 ± 20.5 | 90.9 ± 22.4 | −58.9 ± 19.6 | −13.8 ± 18.4 | 0.17 |
| Arousal index, per hour of sleep | 49.5 ± 7.6 | 57.5 ± 14.9 | −24.9 ± 6.1 | −0.5 ± 3.6 | 0.02 |
| Glucose metabolism from 24-h blood sampling†‡ | |||||
| 24-h glucose, mg/dl | 152.1 ± 8.8 | 138.4 ± 6.5 | −13.7 ± 3.6 | −2.9 ± 1.4 | 0.01 |
| Overnight glucose, mg/dl | 135.7 ± 8.7 | 110.6 ± 6.3 | −12.6 ± 4.2 | −1.5 ± 2.5 | 0.04 |
| Daytime glucose, mg/dl | 163.1 ± 10.3 | 135.2 ± 9.6 | −13.6 ± 4.1 | −4.9 ± 2.5 | 0.22 |
Data are given as mean ± SEM.
P values by two-sided paired t test for the change from baseline between the treated and untreated groups.
There were no statistically significant differences between all baseline variables among the two groups.
Overnight glucose includes values from 23:00 until 09:00 h. Daytime glucose includes values from 09:00 until 23:00 h.
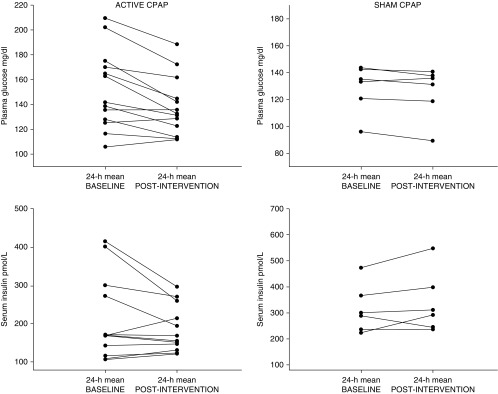
The 24-hour mean plasma glucose decreased significantly more after 1 week of active versus sham CPAP treatment (−13.7 ± 3.6 mg/dl vs. −2.9 ± 1.4 mg/dl; P = 0.013). This decrease in mean plasma glucose was associated with a trend for lower 24-hour mean insulin levels (−25.8 ± 16.5 pmol/L vs. 28.4 ± 21.6 pmol/L; P = 0.071). Improvement in glucose levels was most prominent during the overnight period, resulting in lower morning fasting glucose levels (Table 2). Individual changes in mean 24-hour plasma glucose and serum insulin levels for all participants are illustrated in Figure 1. Importantly, the beneficial effect of CPAP was of larger magnitude in participants with poor glycemic control at baseline.
Figure 1.
Individual changes in mean 24-hour glucose and serum insulin levels. CPAP = continuous positive airway pressure.
Discussion
To the best of our knowledge, this is the first rigorous physiologic proof-of-concept study of CPAP treatment of OSA in T2DM that has positive findings with improvement in glycemic control without an increase in serum insulin levels, suggesting that elimination of OSA decreases insulin resistance.
Our rigorous methodology of 24-hour blood sampling, adherence to standardized meals during 24-hour blood sampling, and ensuring CPAP adherence addresses important limitations faced by several prior studies, such as solely relying on fasting measures of glucose and insulin or HbA1c or being limited by low CPAP adherence. Our findings, however, are in line with a recent randomized controlled trial that reported a significant reduction in HbA1c of 0.4% after 6 months of CPAP therapy in patients with suboptimally controlled T2DM at baseline (mean HbA1c, 7.6%). Of note, in this study, the mean adherence to CPAP was 5.2 hours per night (12). In our study, the degree of improvement in plasma glucose after 1 week of effective CPAP therapy is also equivalent to a drop of approximately 0.4–0.5% of HbA1c, an effect size similar to that achieved by oral pharmacologic agents (13). Moreover, because a drop of 1% in HbA1c levels is associated with clinically significant reduction in major macro- and microvascular complications in T2DM, a 0.4% drop in HbA1c achieved by CPAP therapy has clinical relevance in the overall cardiometabolic health of patients with T2DM and comorbid OSA (13).
One limitation of our study is that two subjects assigned to sham CPAP received defective sham devices that delivered therapeutic pressure. However, as polysomnography data were analyzed for every night in the laboratory, there was no ambiguity whatsoever regarding participants receiving active versus inactive treatment. Therefore, we believe a per-treatment analysis for this physiologic proof-of-concept study is appropriate. We performed additional analysis excluding these two participants (n = 11 receiving active CPAP vs. n = 6 receiving sham CPAP), and the change in 24-hour mean plasma glucose remained larger in the active CPAP group (−12.8 ± 3.5 mg/dl vs. −2.9 ± 4.7 mg/dl; P = 0.045).
In conclusion, CPAP treatment of OSA across 7 entire nights results in a clinically significant improvement in glycemic control in T2DM.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Chuanhong Liao, M.S., and Theodore Karrison, Ph.D., from the University of Chicago Biostatistics Laboratory for their assistance with the statistical analysis. They are grateful to Dr. Esra Tasali, Dr. Suma Dronavalli, Dr. Carol Touma, Dr. Renee Aronsohn, and Dr. Sushmita Pamidi for providing physician supervision during the study. They also thank Dr. Esra Tasali for her contributions in the initial design of the study and interim analysis. This study would have not been possible without the efforts of Nina Massad and Matthew Lagen with study coordination. The authors particularly thank the subjects for participating in this study and the nursing staff of the University of Chicago Clinical Resource Center for their expert assistance.
Footnotes
This work was supported by an investigator-initiated grant from Philips/Respironics (E.V.C.). Other sources of funding included the Diabetes Research and Training Center at the University of Chicago and National Institute of Health grants P01 AG11412 and CTSA UL1 RR024999. B.M. is partly supported by grant R01 HL119161. The funders had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Contributions: E.V.C. designed the study; B.M., D.G., G.B., V.A., F.D., and H.W. collected data; B.M., D.G., G.B., F.D., and E.V.C. performed all analyses; B.M., D.G., G.B., F.D., and E.V.C. participated in data management, analyses, interpretation, and manuscript preparation; E.V.C. obtained study funding; B.M. and E.V.C. oversaw all analyses and take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript; and all authors approved this manuscript in its final form.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes: a historical cohort study. Am J Respir Crit Care Med. 2014;190:218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 2.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37:355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 6.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw JE, Punjabi NM, Naughton MT, Willes L, Bergenstal RM, Cistulli PA, Fulcher GR, Richards GN, Zimmet PZ. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016;194:486–492. doi: 10.1164/rccm.201511-2260OC. [DOI] [PubMed] [Google Scholar]
- 8.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, Peppard PE. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, Van Cauter E. Impact of fully compliant CPAP treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof of concept clinical trial. Am J Respir Crit Care Med. 2015;191:A6417. doi: 10.1164/rccm.201602-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodway GW, Weaver TE, Mancini C, Cater J, Maislin G, Staley B, Ferguson KA, George CF, Schulman DA, Greenberg H, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260–266. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Cerón E, Barquiel B, Bezos AM, Casitas R, Galera R, García-Benito C, Hernanz A, Alonso-Fernández A, Garcia-Rio F.Effect of CPAP on glycemic control in patients with obstructive sleep apnea and type 2 diabetes: a randomized clinical trial Am J Respir Crit Care Med[online ahead of print] 24 Feb 2016; DOI: 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes: 2016. Diabetes Care. 2016;39:S1–S112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.