Pulmonary arterial hypertension (PAH) is a rare, progressive, and fatal disease that predominantly affects women. Sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling are two major causes for the elevation of pulmonary vascular resistance (PVR) in patients with PAH. Without treatment, the elevated PVR and pulmonary arterial pressure (PAP) increase the afterload to the right ventricle, which can progress to right heart failure and death. Despite significant progress in research, PAH remains a devastating disease with a poor long-term prognosis. The estimated median survival of patients with PAH is only 2.8 years, with 1-, 3-, and 5-year survival rates of 68, 48, and 34%, respectively (1). Current therapies remain insufficient with the absence of effective disease-modifying or preventive interventions. Investigations integrating novel and robust technologies and methods are urgently needed to help shed further insight into the molecular underpinnings of PAH and close these therapeutic gaps.
To date, the exact pathogenic mechanisms responsible for PAH are not fully understood; however, a number of potentially causative mutations in genes primarily related to PAH have been discovered via conventional linkage analysis and, more recently, next-generation sequencing (NGS) technologies. Rare variants in genes related to transforming growth factor β signaling, membrane receptors and ion channels, integral membrane proteins, serine-threonine kinases, and DNA-binding proteins have been reported in familial and sporadic cases of PAH (2–7). Most of these reports reveal gain-of-function or loss-of-function mutations, contributing to the development of PAH or leading to the increased risk of developing PAH. The first reports of genetic contributions to PAH were identified by gene linkage studies in which mutations in the gene encoding bone morphogenetic protein receptor type 2 (BMPR2) were found in approximately 75% of cases of heritable PAH and in ∼20% of patients with idiopathic PAH (IPAH) (5, 6). Later, mutations in the genes encoding activin receptor-like kinase 1 (ALK1, also called ACVRL1) and endoglin (ENG), which are associated with hereditary hemorrhagic telangiectasia, were also reported to be associated with PAH (3, 8, 9). In rare cases, mutations in the genes encoding mothers against decapentaplegic homologs (SMAD), which are also involved in the transforming growth factor β/BMP signaling, have been identified as underlying causative factors in patients with PAH (10, 11). Furthermore, genetic variants in genes encoding canonical transient receptor potential 6 (TRPC6) and Kv1.5 (KCNA5) channels have been demonstrated to be associated with patients with IPAH, using a candidate gene sequencing approach (12, 13). Chida and colleagues screened for mutations in genes related to Notch3 signaling in 41 patients with PAH who had no other identifiable mutations of the genes known to be associated with PAH, and identified two novel missense mutations in NOTCH3 in two patients (14).
Although genome-wide association studies have successfully reported numerous PAH susceptibility variants, in general, the risk alleles account for only a small proportion of estimated heritability (15, 16). Recently, Germain and colleagues first conducted a two-stage genome-wide association study of patients with IPAH and heritable PAH without detectable BMPR2 mutations and identified the CBLN2 locus associated with IPAH and heritable PAH (17). As with many other complex diseases, there are likely rare (<5% of the population) variants that exist in the pathogenesis of PAH that are not captured by genome-wide association studies, but could have a larger effect size while not being causative. With the decreasing cost of NGS technologies, whole-genome sequencing and whole-exome sequencing (WES) have enabled the rapid identification of many rare variants, some with a large effect size, that have facilitated the understanding of both Mendelian and complex traits. WES defines the protein-coding sequences of the human genome and has been a powerful tool for the detection of Mendelian variants. More recently, as the tool becomes cost-effective, there is a growing interest in the detection of potential variants in complex traits as well. In one of the earliest studies to apply WES to PAH, Austin and colleagues identified novel mutations in the gene encoding caveolin 1 (CAV1) in patients with either familial or idiopathic PAH (2). Using WES to study a family in which multiple members had PAH without mutations in BMPR2, ALK1, ENG, SMAD, and CAV1, Ma and colleagues identified association of KCNK3 with familial and idiopathic PAH (7). In another study using WES in PAH, Eyries and colleagues linked recessive variants of a single gene, EIF2AK4 (also called GCN2), to pulmonary veno-occlusive disease development in all 13 families (18). More recently, by analyzing exome sequence data from 12 unrelated patients with IPAH lacking BMPR2 mutations, de Jesus Perez and colleagues identified the DNA topoisomerase II–binding protein 1 (TOPBP1) gene, which is involved in the response to DNA damage and replication stress, as a risk factor for IPAH (4).
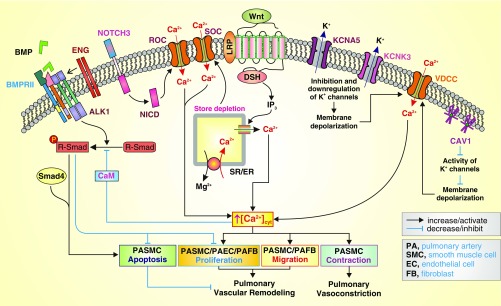
In this issue of the Journal, Hemnes and colleagues (pp. 464–475) have used WES in a small cohort of patients with IPAH that have been well-phenotyped with and without vasodilator responsiveness (19). The authors demonstrated multiple rare genetic variants that converge on cytoskeletal functions and the Wnt signaling pathway in patients with PAH. Importantly, using stringent filtering criteria and comparing it with idiopathic pulmonary fibrosis, the authors highlighted the potential for missed variants of significance. The current article opens up a new realm of possibilities in future PAH research and clinical practice as WES becomes more affordable. With WES, the potential to analyze known mutations (in BMPR2, ALK1, KCNK3, and CAV1, for example), as well as evaluate novel disease-modifying variants in patients with and without a family history of PAH, is now feasible with one approach. Although the authors did not find a variant across all forms of PAH, including vasodilator responsive and nonresponsive PAH, the technique also allows the search for these variants in other tightly phenotyped subgroups of PAH, such as scleroderma-PAH or HIV-associated PAH, in a research and clinical setting. Although no single SNP was significant across all patients with PAH in the current study, the use of pathway-based analyses, composed of several variants across multiple genes, highlighted WNT pathway-modifying genes associated with PAH. This observed molecular pathway was then validated in in vitro studies confirming elevated WNT activity in fibroblasts from patients with PAH. In addition, the authors identified (while low in sample size) a high frequency of genetic variations in smooth muscle cell (SMC) contraction genes and greater overall genetic variation per pathway in patients with vascular-responsive PAH than in patients with nonvascular response PAH; these novel observations strongly suggest genetic predisposition to the development of PAH, as well as the role of WNT pathway and SMC contraction genes in the development of PAH. Given that an increase in cytosolic Ca2+ concentration ([Ca2+]cyt) is one of the major contributors to the development of PAH by inducing pulmonary arterial SMC (PASMC) contraction and pulmonary vasoconstriction, and by stimulating pulmonary vascular cell migration and proliferation, causing concentric pulmonary vascular wall thickening (20), Figure 1 shows a proposed mechanism by which the previously established PAH genes with observed rare variants, including those from the current study, link with the Ca2+ signaling cascade to induce pulmonary vasoconstriction and vascular remodeling.
Figure 1.
Proposed mechanisms by which the genes associated with pulmonary arterial hypertension (PAH; based on emerging genetic sequencing studies) and the Wnt signaling cascade (based on the current study by Hemnes and colleagues) functionally interact with the Ca2+ signaling pathway to cause pulmonary arterial smooth muscle cell (PASMC) contraction and pulmonary vasoconstriction, PASMC proliferation/migration and pulmonary vascular wall thickening, and inhibition of PASMC apoptosis resulting in pulmonary vascular remodeling (19). On the basis of case–control studies, the current work by Hemnes and colleagues has found Wnt pathway and smooth muscle contraction pathway variants to be significantly overrepresented in PAH and in vasodilator-responsive PAH, respectively (19). Activation of Wnt signaling has been shown to induce Ca2+ release from the sarcoplasmic (SR) or endoplasmic (ER) reticulum by inositol 1,4,5-trisphosphate (IP3)-mediated activation of IP3 receptor, a Ca2+ release channel expressed in the ER/SR membrane. The Wnt-mediated Ca2+ mobilization from the SR/ER to the cytosol causes Ca2+ store depletion that subsequently opens the store-operated Ca2+ channels (SOC), formed by Orai1/2 and TRPC6 channel subunits, to induce store-operated Ca2+ entry (SOCE). Mutations in genes encoding KCNK3, a two-pore domain K+ channel, and KCNA5, a voltage-gated K+ channel, may lead to transcriptional down-regulation and/or functional inhibition of KCNK3 and KCNA5, causing membrane depolarization that opens voltage-dependent Ca2+ channels (VDCC) and induces voltage-dependent Ca2+ entry. Furthermore, up-regulated Notch3 and enhanced Notch signaling have been demonstrated to functionally activate receptor-operated Ca2+ channels (ROC) and induce receptor-operated Ca2+ entry (ROCE) via Notch intracellular domain (NICD) or other unknown mechanisms (22). Caveolin-1 (CAV1) has been shown to inhibit various K+ channels, cause membrane depolarization, and induce Ca2+ influx through VDCC. The increased cytosolic Ca2+ concentration ([Ca2+]cyt) causes PASMC contraction, leading to pulmonary vasoconstriction, and stimulates PASMC migration and proliferation, leading to pulmonary vascular remodeling (or concentric pulmonary arterial wall thickening). Bone morphogenetic protein (BMP)/bone morphogenetic protein receptor type 2 (BMPR2)/Smad signaling has been demonstrated to induce PASMC apoptosis and inhibit PASMC and pulmonary arterial endothelial cell (PAEC) proliferation (23, 24). Mutations in the BMPR2 gene identified in patients with idiopathic and heritable PAH result in the inhibition of PASMC apoptosis and enhancement of PASMC/PAEC proliferation, contributing to the development and progression of pulmonary vascular remodeling. Calmodulin (CaM) is a Ca2+-sensitive protein that physically interacts with Smad and induces Ca2+-dependent inhibition of transforming growth factor β/activin signaling (25, 26); this suggests that elevated [Ca2+]cyt and activated CaM can lead to inhibition of BMP/BMPR2/Smad signaling, and ultimately to inhibited PASMC apoptosis and enhanced PASMC proliferation. DSH = dishevelled; ENG = endoglin; LRP = low-density lipoprotein receptor–related protein; Smad = mothers against decapentaplegic homologs.
In concert with previous findings in the genetics of PAH with identification of a number of causative genes, this study will undoubtedly open up new pipelines of research on PAH. However, these enlightening studies using NGS technologies raise several important issues. First, the role of the inherited variants remains difficult to ascertain, despite evidence to support their genetic association with PAH susceptibility. How the causative genes associate with the PAH phenotype, and how several variations may interact with each other, remain unanswered. Clinical outcomes associated with the causative genes (and variants) may also exceed the boundaries of PAH because the same genes associated with PAH may also be associated with other cardiovascular disorders. Another current roadblock is the lack of knowledge in all of the pertinent mechanisms through which these genes contribute to the development and progression of PAH. Further studies testing whether these variants lead to gain or loss of gene expression and activity, as well as their influence on PASMC contraction and pulmonary vascular cell proliferation, migration, and apoptosis, are required to validate their roles in the development and progression of PAH. In addition, although the technological sequencing methods have evolved and become more cost-effective, a major bottleneck remains in the computational and bioinformatics methodology to analyze NGS data. We need improved tools in statistical and bioinformatics methods for reducing the rate of false-positive and false-negative variant calls, calling insertion/deletion, prioritizing candidate causal variants, and predicting and annotating the potential functional effect for disease gene discovery or molecular diagnostics (21). Although these issues appear daunting to tackle, leveraging these emerging genome-editing technologies in combination with the development of animal models that recapitulate the mutations of causative genes should lead to a more comprehensive understanding of the genetic and molecular causes of PAH.
In summary, WES and whole-genome sequencing have revealed a number of rare variants of genes involved in the development and progression of PAH and will continue to be an important tool for future research on PAH genetics. Larger cohorts of samples will be required in genetic studies, and therefore large-scale collaborative efforts involving international and national consortia, as well as physicians, pathophysiologists, geneticists, bioinformaticians, epidemiologists, and statisticians, are necessary to capitalize on these emerging technologies and their ability to dissect the underlying genetic mechanisms of PAH. Ultimately, these efforts and technologies can pave the way for personalized diagnostics and therapeutics in the clinical care for PAH.
Supplementary Material
Footnotes
Supported in part by the grants from the NHLBI of the National Institutes of Health (HL115014, HL066012, and HL125208).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, III, Palomero T, Sumazin P, Kim HR, Talati MH, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaouat A, Coulet F, Favre C, Simonneau G, Weitzenblum E, Soubrier F, Humbert M. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jesus Perez VA, Yuan K, Lyuksyutova MA, Dewey F, Orcholski ME, Shuffle EM, Mathur M, Yancy L, Jr, Rojas V, Li CG, et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:1260–1272. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Trégouët DA, Borczuk A, Rosenzweig EB, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girerd B, Montani D, Coulet F, Sztrymf B, Yaici A, Jaïs X, Tregouet D, Reis A, Drouin-Garraud V, Fraisse A, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med. 2010;181:851–861. doi: 10.1164/rccm.200908-1284OC. [DOI] [PubMed] [Google Scholar]
- 9.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasim MT, Ogo T, Ahmed M, Randall R, Chowdhury HM, Snape KM, Bradshaw TY, Southgate L, Lee GJ, Jackson I, et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat. 2011;32:1385–1389. doi: 10.1002/humu.21605. [DOI] [PubMed] [Google Scholar]
- 11.Shintani M, Yagi H, Nakayama T, Saji T, Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46:331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 12.Remillard CV, Tigno DD, Platoshyn O, Burg ED, Brevnova EE, Conger D, Nicholson A, Rana BK, Channick RN, Rubin LJ, et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007;292:C1837–C1853. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chida A, Shintani M, Matsushita Y, Sato H, Eitoku T, Nakayama T, Furutani Y, Hayama E, Kawamura Y, Inai K, et al. Mutations of NOTCH3 in childhood pulmonary arterial hypertension. Mol Genet Genomic Med. 2014;2:229–239. doi: 10.1002/mgg3.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do R, Kathiresan S, Abecasis GR. Exome sequencing and complex disease: practical aspects of rare variant association studies. Hum Mol Genet. 2012;21:R1–R9. doi: 10.1093/hmg/dds387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day-Williams AG, Zeggini E. The effect of next-generation sequencing technology on complex trait research. Eur J Clin Invest. 2011;41:561–567. doi: 10.1111/j.1365-2362.2010.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germain M, Eyries M, Montani D, Poirier O, Girerd B, Dorfmüller P, Coulet F, Nadaud S, Maugenre S, Guignabert C, et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet. 2013;45:518–521. doi: 10.1038/ng.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, Chelghoum N, Coulet F, Bonnet D, Dorfmüller P, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 19.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, Robbins IM, Blackwell TS, Cogan J, Loyd JE, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca²⁺ signaling. Am J Physiol Heart Circ Physiol. 2012;302:H1546–H1562. doi: 10.1152/ajpheart.00944.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 22.Smith KA, Voiriot G, Tang H, Fraidenburg DR, Song S, Yamamura H, Yamamura A, Guo Q, Wan J, Pohl NM, et al. Notch activation of Ca2+ signaling in the development of hypoxic pulmonary vasoconstriction and pulmonary hypertension. Am J Respir Cell Mol Biol. 2015;53:355–367. doi: 10.1165/rcmb.2014-0235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 24.Fantozzi I, Platoshyn O, Wong AH, Zhang S, Remillard CV, Furtado MR, Petrauskene OV, Yuan JX. Bone morphogenetic protein-2 upregulates expression and function of voltage-gated K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L993–L1004. doi: 10.1152/ajplung.00191.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman CM, Kariapper MS, Mathews LS. Smad proteins physically interact with calmodulin. J Biol Chem. 1998;273:677–680. doi: 10.1074/jbc.273.2.677. [DOI] [PubMed] [Google Scholar]
- 26.Wicks SJ, Lui S, Abdel-Wahab N, Mason RM, Chantry A. Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol Cell Biol. 2000;20:8103–8111. doi: 10.1128/mcb.20.21.8103-8111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.