Abstract
Background
The role of human papilloma virus (HPV) in oral lichen planus (OLP) is controversial.
Objectives
The primary aim of the current study is to calculate the pooled risk estimates of HPV infection in OLP when compared with healthy controls.
Methods
Bibliographic searches were conducted in three electronic databases. Articles on the association between HPV and OLP were selected from case-control studies or cross-sectional studies, following predefined criteria. Pooled data were analyzed by calculating odds ratios (OR) and 95% confidence interval (CI).
Results
Of the 233 publications identified, 22 case-control studies met the inclusion criteria. Collectively, 835 cases and 734 controls were available for analysis. The summary estimate showed that OLP patients have significantly higher HPV prevalence (OR: 6.83; 95% CI: 4.15–11.27) than healthy controls. In subgroup analyses, the association of HPV and OLP varied significantly by geographic populations. The ORs ranged from 2.43 to 132.04. The correlation of HPV and erosive-atrophic oral lichen planus (EA-OLP) (OR: 9.34) was comparable and well above that of HPV and non-EA-OLP (OR: 4.32). Among HPV genotypes, HPV 16 showed an extremely strong association with OLP (OR: 11.27), and HPV 18 showed a relatively strong one (OR: 6.54).
Conclusion
In conclusion, a significant association was found between HPV and OLP. The strength of the association varied across geographic populations, clinical types of OLP, and HPV genotypes. The results suggest that HPV might play an important causal role in OLP and in its malignant to progression.
Introduction
Oral lichen planus (OLP) is a common chronic autoimmune disorder, which may present epithelial thickening or atrophy with or without ulceration [1]. Clinically, there are six different types: papule, reticular, plaque, atrophic, erosive and bullous. The most common type is reticular. All types of OLP can be pooled in 2 clinical groups: erosive-atrophic forms (EA-OLP), including erosive, atrophic, bullous and mixed EA variants; and non-erosive-atrophic forms (non-EA-OLP), involved papule, reticular, plaque and mixed non-EA variants. EA-OLP is more prone to malignant transformation than non-EA-OLP [2]. The prevalence of HPV in OLP has been reported to range from 0.5 to 2.2%, varying according to geographic location [3].
Although the etiology of OLP is still unknown, it is generally accepted to be a T-cell-mediated inflammatory disease [1]. The reaction of these specific CD8+ T cells is similar to what occurs during a viral infection, in which a virus can act as a cytoplasmic antigen or induce the expression of host cell proteins, resulting in a differing host cell protein profile [4]. In this way, exploring the possibility of viral involvement in the pathogenesis of OLP is improving.
As early as 1987, the association between HPV and OLP was reported [5]. In one study published prior to 1998, 107 OLP samples were studied, and 23% were HPV-positive. The first three most prevalent HPV types were HPV 6, 11, and 16. Then 1929 normal oral mucosa samples were tested for HPV DNA, and 11% were found to be positive [6]. The rate of HPV in OLP was twice as high as in normal cases. On the basis of these studies, the case-control studies of HPV and OLP correlation were increasingly growing.
However, results have been conflicting: some studies seemed to show that HPV did play an important role in OLP [7–10], but others disagreed [11–13]. There have been some reviews of the HPV and OLP, but most of them are qualitative analyses. Very few reviews have included quantitative analysis. There was one meta-analysis of HPV in oral carcinoma and oral potentially malignant disorders (OPMD). OLP was a sub-analysis of OMPD, and the OR of the link between HPV and OLP was 5.12 [14]. The results indicated a strong association, but the articles included were limited, a more comprehensive meta-analysis is needed.
Two English databases (PubMed and Web of Science) and one Chinese database (CNKI) were screened in order to collect more articles. Finally, 22 case-control studies were included to perform this systematic review and meta-analysis. The primary aim of the current paper was to clarify the magnitude of the association between HPV and OLP, and to get a further understanding of it concerning with different geographical populations, different OLP clinical types and HPV genotypes.
Materials and Methods
Data sources
A systematic search was performed in PubMed (www.ncbi.nlm.nih.gov/pubmed), Web of Science (SCI) (http://webofknowledge.com/) and CNKI (http://epub.cnki.net/) to screen relevant literature, until the last search update on September 21, 2015. The search terms were (lichen planus or oral lichen planus or LP or OLP) and (human papillomavirus or HPV). The reference lists of selected papers were searched to identify additional studies.
Data extraction
The screening process was carried out by two investigators (J. Ma and J. Zhang) independently under the same criteria (Fig 1). Disagreements about eligibility were settled by consensus with a third investigator (Y. Zhang). The following details were recorded from each study: first author, publication date, country, clinical type of OLP, number of OLP patients and healthy controls, test methods, and HPV genotypes (Table 1).
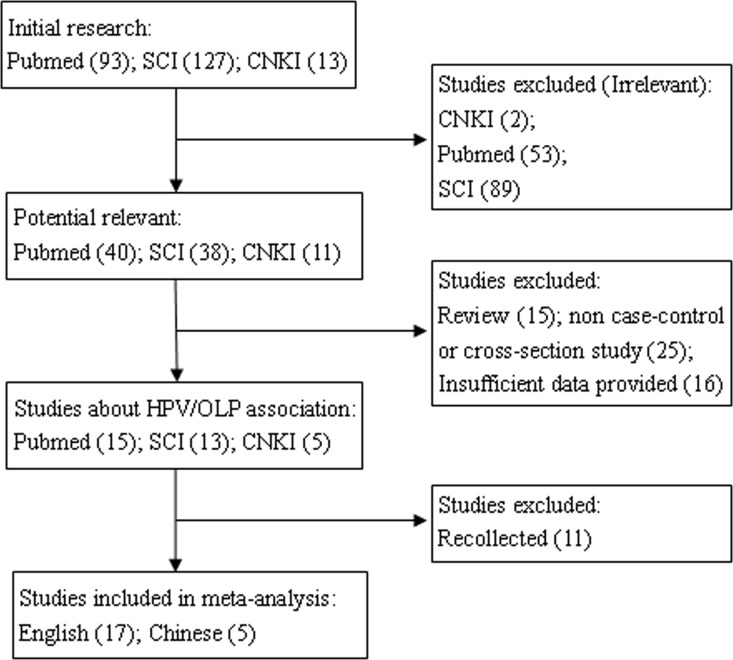
Fig 1. Flow chart of literature searches for this meta-analysis.
Table 1. Characteristics of the 22 included studies in this meta-analysis.
| Reference | Nation | Clinical type | OLP(n/N) | Control(n/N) | Detection method | HPV genotypes |
|---|---|---|---|---|---|---|
| Pol CA et al., 2015 | India | 21/30 | 0/30 | IHC | HPV16 | |
| Arirachakaran P et al., 2013 | Thailand | 1/37 | 0/37 | PCR | NA | |
| Yildirim B et al., 2011 | Turkey | 14/65 | 0/15 | IHC | HPV16 | |
| Debanth S et al., 2009 | India | 6/6 | 3/35 | PCR | HPV16, 18, 31, 33, 35, 39, 45, 51,52, 56, 58, 59,68 | |
| Fehér E et al., 2009 | Hungary | 39/119 | 3/72 | PCR | HPV6.11.16.18.31.33 | |
| Razavi SM et al., 2009 | Iran | 9/29 | 1/14 | PCR | HPV18 | |
| Szarka K et al., 2009 | Hungary | 39/119 | 3/72 | PCR | NA | |
| EA-OLP | 26/61 | 3/72 | ||||
| non-EA-OLP | 13/58 | 3/72 | ||||
| Yu Hong et al., 2007 | China | 33/57 | 3/20 | PCR | HPV16 | |
| Cianfriglia F et al., 2006 | Italy | 3/15 | 2/10 | ISH | NA | |
| Giovannelli L et al., 2006 | Italy | 12/49 | 11/49 | PCR | NA | |
| Campisi G et al., 2004 | Italy | 14/71 | 5/90 | PCR | HPV16.18.31.6 | |
| EA-OLP | 9/44 | 5/90 | ||||
| non-EA-OLP | 5/27 | 5/90 | ||||
| Ma Jian et al., 2003 | China | 26/30 | 5/18 | PCR | NA | |
| OFlatharta C et al., 2003 | Ireland | 10/38 | 0/20 | PCR | HPV16 | |
| Giovannelli L et al., 2002 | Italy | 9/34 | 5/90 | PCR | NA | |
| Li Hui et al., 2000 | China | 9/30 | 3/40 | PCR | NA | |
| Sand L et al., 2000 | Sweden | 6/22 | 0/12 | NA | NA | |
| Qi Yanchun et al., 1999 | China | 9/30 | 3/40 | PCR | NA | |
| Lei Lei et al., 1997 | China | 9/22 | 2/10 | PCR | NA | |
| EA-OLP | 3/4 | 2/10 | ||||
| non-EA-OLP | 6/18 | 2/10 | ||||
| Vespe M et al., 1997 | Germany | 3/7 | 0/33 | PCR | HPV16.18.31 | |
| Boyd AS et al., 1996 | USA | 11/13 | 0/10 | PCR | NA | |
| Cox M et al., 1993 | UK | 3/4 | 3/5 | PCR | HPV16 | |
| Maitland NJ et al., 1987 | UK | 7/8 | 5/12 | PCR | HPV16 |
n: numbers of HPV positive subjects; N: numbers of total subjects; IHC: immunohistochemical staining; PCR: polymerase chain reaction; ISH: in situ hybridization; NA: not available; EA-OLP: erosive-atrophic oral lichen planus; non-EA-OLP: non-erosive-atrophic oral lichen planus.
Selection criteria
Studies included in the meta-analysis had to meet the following criteria: (i) address the relationship between HPV and OLP; (ii) full-text papers; and (iii) original case-control or cross-sectional studies. Studies were excluded if they included any of the following: (i) reviews; (ii) incomplete data; and (iii) republished articles or reused data.
Quality assessment
Results of observational studies are greatly influenced by the design of the research. To guarantee the quality of the meta-analysis, each included paper was carefully assessed using the standard proposed by Chalmers et al. [15], including selection of subjects, comparability between groups and outcome presented. The selected studies were scored on an ordinal star scale from 1 to 9, with higher scores representing higher quality.
Statistical analysis
The meta-analysis was conducted using RevMan5, a copyrighted freeware developed by the Cochrane Collaboration, for preparing and maintaining reviews (http://www.cochrane-net.org/revman). Heterogeneity among different studies was measured by calculating χ2 and I2, if P < 0.05 and I2 > 50% were considered statistically significant. The primary outcome was reported as pooled odds ratios (OR) with 95% confidence intervals (CI), calculated using the random-effects model when heterogeneity existed or the fixed-effects model when no heterogeneity was found. To investigate potential for publication bias, the funnel plot asymmetry of the included studies was evaluated. Subgroup analysis was undertaken for geographic differences, clinical types of OLP, and HPV genotypes.
Sensitivity analysis
Low-quality studies (five stars or fewer) were excluded to investigate potential selection bias. Articles with different methods of HPV detection were analyzed to assess detection bias.
Results
Overall information on the studies included
The process of search strategy is showed in Fig 1. From the 233 articles identified through an initial research, 144 were excluded for lack of relevance to OLP. After further reading, 15 papers were excluded for reviews, 25 papers were excluded without case-control or cross-sectional design, and 16 studies with insufficient data were excluded. Eleven papers were both collected in PubMed and SCI database. In the end, 22 articles met the criteria. The main information of the 22 selected studies is listed in Table 1. The analysis covered 12 countries, 5 of Asia, specifically China [16–20], India [9, 10], Iran [21], Thailand [22], and Turkey [23]; 6 in Europe, specifically Italy [12, 13, 24, 25], Hungary [26, 27], England [5, 11], Germany [8], Ireland [28], and Sweden [29]; 1 in North American, specifically the United States [7].
Results of quality assessment
On the basis of the criteria described previously [15], 20 studies were evaluated as superior quality, with scores of six or more stars, and 2 studies were of low quality, with five stars or fewer (Table 2). Nine articles lacked a definition or diagnosis of OLP [5, 7, 11, 12, 16–20]. Three papers did not clearly explain the selection criteria for the controls [8, 11, 25], and 1 paper used diseased patients as controls [7]. Seventeen papers either did not give information about the sex of subjects or the sexes between cases and controls were incomparable [5, 7, 8, 10–13, 17–21, 23, 24, 26, 28, 29].
Table 2. Assessment of quality and score for 22 studies included.
| Reference | Selection of subjects | Comparability | Outcome | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | Age | Sex | Living area | Race | ||||
| Pol CA et al., 2015 | * | * | * | * | * | * | ** | 8 | ||
| Arirachakaran P et al., 2013 | * | * | * | * | * | * | * | ** | 9 | |
| Yildirim B et al., 2011 | * | * | * | * | * | ** | 7 | |||
| Debanth S et al., 2009 | * | * | * | * | * | * | * | ** | 9 | |
| Fehér E et al., 2009 | * | * | * | * | * | ** | 7 | |||
| Razavi SM et al., 2009 | * | * | * | * | * | ** | 7 | |||
| Szarka K et al., 2009 | * | * | * | * | * | * | * | ** | 9 | |
| Yu Hong et al., 2007 | * | * | * | * | * | * | ** | 8 | ||
| Cianfriglia F et al., 2006 | * | * | * | * | ** | 6 | ||||
| Giovannelli L et al., 2006 | * | * | * | * | * | ** | 7 | |||
| Campisi G et al., 2004 | * | * | * | * | * | ** | 7 | |||
| Ma Jian et al., 2003 | * | * | * | * | ** | 6 | ||||
| OFlatharta C et al., 2003 | * | * | * | * | * | ** | 7 | |||
| Giovannelli L et al., 2002 | * | * | * | * | * | ** | 7 | |||
| Li Hui et al., 2000 | * | * | * | * | ** | 6 | ||||
| Sand L et al., 2000 | * | * | * | * | * | ** | 7 | |||
| Qi Yanchun et al., 1999 | * | * | * | * | ** | 6 | ||||
| Lei Lei et al., 1997 | * | * | * | * | * | ** | 7 | |||
| Vespe M et al., 1997 | * | * | * | * | ** | 6 | ||||
| Boyd AS et al., 1996 | * | * | * | ** | 5 | |||||
| Cox M et al., 1993 | * | * | * | ** | 5 | |||||
| Maitland NJ et al., 1987 | * | * | * | * | ** | 6 | ||||
(1) Was there a specific definition of the diagnosis of this disease in the article?
(2) Were the selection criteria for the patients in the study specifically described?
(3) How representative was the control group with respect to the source population of cases enrolled? Comparability: Were the groups comparable with respect to age, sex, living area or race? The “Outcome” item was scored for double asterisks (**) in cells; others were scored for a single asterisk (*).
Meta-analysis
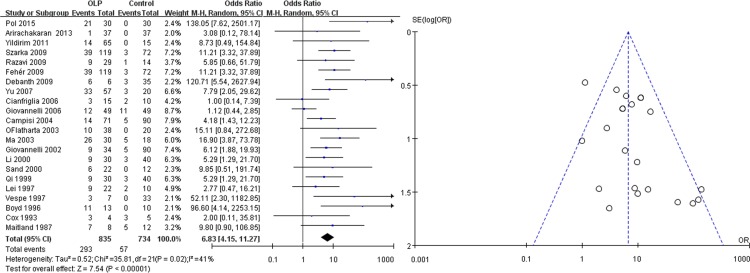
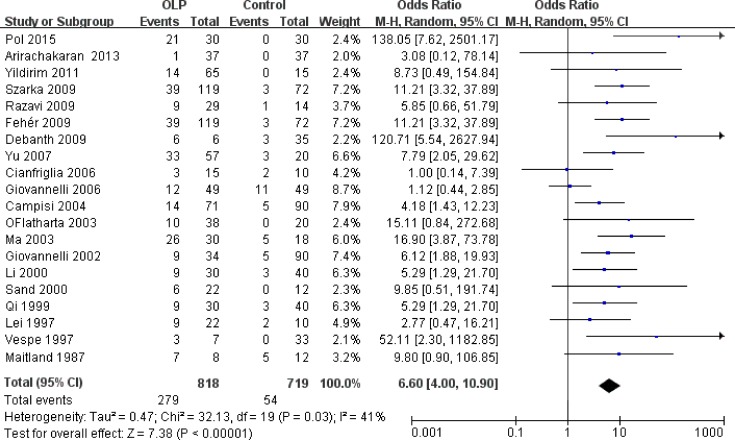
As shown in Fig 2, the studies had a total of 835 patients and 734 controls, of which 293 cases (35.09%) and 57 controls (7.77%) were HPV positive. The χ2 and I2 were 35.81 (P = 0.02) and 41%, respectively, suggesting heterogeneity. The random-effects model was used to analyze the data. The pooled OR was 6.83 (95%CI: 4.15–11.27), and the overall effect Z value was 7.54 (P < 0.00001), which indicated a strong association between HPV and OLP. The visual examination of the symmetry of the funnel plot did not suggest a large publication bias (Fig 2).
Fig 2. Forest and funnel plots of 22 included studies on the association between HPV and OLP.
Subgroup analyses
We further conducted subgroup analyses of all included studies based on geographic population, OLP clinical types and HPV genotypes respectively, to determine the influencing factors that may impact the overall results.
Association of HPV with OLP in terms of geographic population
The 22 articles included 12 countries in Asia, Europe, and North America. The association varied significantly by geographic population (Table 3). In Asia (OR: 9.37), it was strongest in India (OR: 132.04), followed by Turkey (OR: 8.73), China (OR: 6.58), Iran (OR: 5.85), and Thailand (OR: 3.08). There were 11 studies that reported the association of OLP and HPV (OR: 5.15) in Europe, including Germany (OR: 52.11), Ireland (OR: 15.11), Hungary (OR: 11.21), Sweden (OR: 9.85), England (OR: 5.34) and Italian (OR: 2.43). The relationship between OLP and HPV was especially significant in the U.S. study (OR: 96.60).
Table 3. Association of HPV with OLP in terms of geographic population.
| Region | Geographical | Articles | OLP | Control | OR (95% CI) |
|---|---|---|---|---|---|
| population | involved | (n/N) | (n/N) | ||
| Asia | Asia | 10 | 137/336 | 20/259 | 9.37[5.38,16.30] |
| India | 2 | 27/36 | 3/65 | 132.04[14.92,1168.20] | |
| Turkey | 1 | 14/65 | 0/15 | 8.73[0.49,154.84] | |
| China | 5 | 86/169 | 16/128 | 6.58[3.44,12.60] | |
| Iran | 1 | 9/29 | 1/14 | 5.85[0.66,51.79] | |
| Thailand | 1 | 1/37 | 0/37 | 3.08[0.12,78.14] | |
| Europe | Europe | 11 | 145/486 | 37/465 | 5.15[2.56,10.34] |
| Germany | 1 | 3/7 | 0/33 | 52.11[2.30, 1182.85] | |
| Ireland | 1 | 9/29 | 1/14 | 15.11[0.84,272.68] | |
| Hungary | 2 | 78/238 | 6/144 | 11.21[3.32,37.89] | |
| Sweden | 1 | 6/22 | 0/12 | 9.85[0.51,191.74] | |
| England | 2 | 10/12 | 8/17 | 5.34[0.91,31.45] | |
| Italy | 4 | 38/169 | 23/239 | 2.43[1.38,4.27] | |
| North American | America | 1 | 11/13 | 0/10 | 96.60[4.14,2252.15] |
n, positive number of subjects; N, total number of subjects
Association of HPV with OLP in terms of clinical type
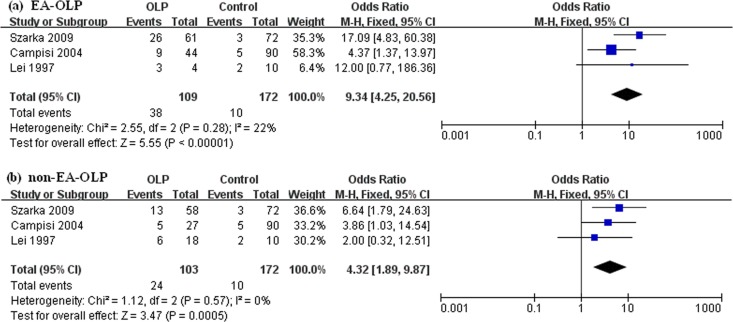
Two types of OLP were involved, EA-OLP and non-EA-OLP. There were only 3 studies mentioned [20, 24, 27], including Hungary (OR: 17.09 vs 6.64), Italy (OR: 4.37 vs 3.86), and China (OR: 12.00 vs 2.00). As shown in Fig 3, the prevalence of HPV differed significantly between the more risky EA-OLP (OR: 9.34; 95%CI: 4.25–20.56) and non-EA-OLP (OR: 4.32; 95%CI: 1.89–9.87).
Fig 3. Forest plots of the association between HPV and OLP in terms of clinical type.
(a) EA-OLP and (b) non-EA-OLP.
Association of HPV with OLP in terms of HPV genotype
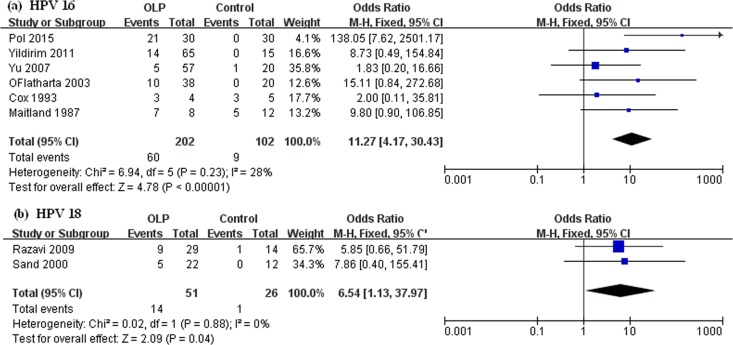
The association of OLP and HPV varied across different HPV genotypes. Among the 22 included articles, HPV16 was the most frequently reported genotype, and HPV18 was the second. ORs of the correlation between HPV16 and OLP from 1.83 to 138.05, the pooled OR of 6 papers was 11.27 (95%CI: 4.17–30.43) (Fig 4A). Only Razavi SM et al. [21] and Sand L et al. [29] reported the association between HPV18 and OLP (pooled OR: 6.54) (Fig 4B).
Fig 4. Forest plots of the association between HPV and OLP in terms of HPV genotypes.
(a) HPV16 and (b) HPV18.
Sensitivity analysis
The exclusion of low-quality studies did not change the summary estimate significantly, OR was 6.60 (Fig 5). Comparing results across detection methods was not considered feasible, given that only 2 studies used IHC, 1 used ISH, and 19 used PCR.
Fig 5. Forest plots of the association between HPV and OLP in high-quality studies.
Discussion
OLP is a chronic inflammatory mucocutaneous disease whose pathogenesis is still the object of much speculation. Different mechanisms by which OLP may develop have been hypothesized in recent years. The association between OLP and viral infections is one of the most controversial positions. The most widely studied viruses in OLP are HPV and hepatitis-C (HCV) [30].
HPVs are small, double-stranded, and circular DNA viruses. There are approximately 40 genotypes known to infect the oral cavity and urogenital tract [31]. The incidence of HPV oral infection has increased in recent decades, and the infection rate seems to be associated with age and gender [32]. Given the U.S. Annual Report on cancer in 2013, the incidence of HPV-positive oropharyngeal (OP) cancers has increased proportionally, while the total incidence of cancer has recently declined [33]. The latest analysis of U.S. cancer registry data showed that the number of HPV-positive OP cancers diagnosed each year may exceed that of invasive cervical cancers by 2020 [32]. In this way, the association of HPV with OLP and oral cancer has received more attention.
This meta-analysis showed a significantly strong association between OLP and HPV. The pooled OR showed that OLP patients have about a 7-fold higher risk of HPV infection than controls, which was consistent with results reported by Syrjänen et al. in 2011 [14]. Besides, we found that the correlation of HPV and OLP vary not only according to geographic populations, but also clinical types of OLP and HPV genotypes. This is the most comprehensive meta-analysis of this issue ever performed.
The current study showed there to be wide variations in the association of OLP and HPV with regard to different geographic populations, which was consistent with results reported by Lodi G et al. [34]. Among the 12 countries included, the ORs of HPV/OLP association ranged from 1.00 to 138.05. Studies performed in Italy (OR: 1.12, 1.00) and the U.K. (OR: 2.00) did not show any significant relationship between HPV and OLP [11–13]. In contrast, patients with OLP in India (OR: 132.04), the United States (OR: 96.60), Germany (OR: 52.11), Ireland (OR: 15.11), and Hungary (OR: 11.21) showed an extremely strong association. In this way, efforts into exploring the correlation between HPV and OLP should focus mainly on epidemiological studies of different populations.
The most common clinical forms of OLP are EA-OLP and non-EA-OLP. In the current meta-analysis, the association between HPV and EA-OLP (OR: 9.34) was comparable and stronger than the association of HPV and non-EA-OLP (OR: 4.32). The risk of progression to malignancy for non-EA-OLPs was approximately 0.5%, but in EA-OLPs it was at least 3.5–4.0% during similar follow-up periods [27]. All this adds up to a hypothesis that differences in HPV prevalence may influence OLP malignant potential. This could shed light on the possibility of a potential prognostic application of HPV detection in OPMD.
Here, the association strength between HPV 16 and OLP was extremely high (OR: 11.27), and HPV types16 has been classified as high-risk (HR) type and reported to be associated with malignancy [35]. Besides, HPV16 has also been reported to be the most common infectious HPV genotype in oral squamous cell carcinoma (OSCC). Its prevalence was 16%. OLP is an OPMD of the oral mucosa with a transformation rate of 0–6.25% [2, 34], so whether HPV 16 has a causal role in OLP malignancy, a long-term follow up is needed to determine.
Recent data from case-control and meta-analytic studies suggested that HPV is a causal factor of the development of several types oropharyngeal and oral cavity squamous carcinomas [36–39]. However, only small proportion of individuals who become infected with HPV will develop OSCC. A recent study showed that patient individual susceptibility to HPV infection, and other biomarkers may be related to OSCC development [40]. Other studies showed that the prognosis, age of onset, and incidence for men and women were different between HPV-positive and HPV-negative oral cancer patients [33]. In this way, determining the HPV infection status of HPV-associated OPMD and OSCC patients may be important to prognosis, treatment, and prevention strategies.
The major limitations of the current meta-analysis are the detection/diagnostic bias and selection bias of the case-control studies that were included. The 22 articles evaluated in this meta-analysis used three kinds of HPV detection methods: PCR, ISH and IHC. PCR is exquisite sensitive and widespread used, it is generally considered as the gold standard in most meta-analysis [14]. However, PCR detection is prone to false positive results because of contamination may occur during sampling, processing and PCR protocols [7]. ISH can be more sensitive in cases in which only a few cells in the sample tissue contain high copy numbers of the virus [41], but its results can influenced by the quality of the sample (e.g. frozen or fixed) [42]. In this way, detection and selection biases limit the usefulness of these results due to possible errors of these kinds. In future work, a uniform research standard should be established to perform a more precise comparison of the results.
Conclusion
In conclusion, this systematic review and meta-analysis showed a significant association between HPV and OLP. All sub-analyses strongly and consistently indicated this association. Because of the lack of prospective cohort studies, it was not possible to take a position on the relationship between HPV infection and oral malignancy. More prospective cohort studies are needed to formally confirm the role of HPV as an etiological agent of OLP, and a uniform research standard should be established to produce get more convincing results.
Supporting Information
(DOC)
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Fund project of China, no. 81273280 and 81272176 (http://www.nsfc.gov.cn/).
References
- 1.Epstein JB, Wan LS, Gorsky M, Zhang L. Oral lichen planus: progress in understanding its malignant potential and the implications for clinical management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96(1):32–7. [DOI] [PubMed] [Google Scholar]
- 2.Gorsky M, Epstein JB. Oral lichen planus: malignant transformation and human papilloma virus: a review of potential clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111(4):461–4. 10.1016/j.tripleo.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 3.McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008; 37(8):447–53. 10.1111/j.1600-0714.2008.00662.x [DOI] [PubMed] [Google Scholar]
- 4.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 5th edition chapter 15. Philadelphia, Pa, USA: WB Saunders; 2004. pp. 345–366. [Google Scholar]
- 5.Maitland NJ, Cox MF, Lynas C, Prime SS, Meanwell CA, Scully C. Detection of human papillomavirus DNA in biopsies of human oral tissue. Br J Cancer. 1987; 56(3):245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrjänen S, Syrjänen K HPV infections of the oral mucosa, Chapter 17 In: Syrjänen K, Syrjänen S, eds. Papillomavirus infections in human pathology. J. Wiley &Sons: New York; 2000. pp. 379–412. [Google Scholar]
- 7.Boyd AS, Annarella M, Rapini RP, Adler-Storthz K, Duvic M. False-positive polymerase chain reaction results for human papillomavirus in lichen planus. Potential laboratory pitfalls of this procedure. J Am Acad Dermatol. 1996; 35(1):42–6. [DOI] [PubMed] [Google Scholar]
- 8.Vesper M, Riethdorf S, Christoph E, Ruthke A, Schmelzle R, Loning T. Detection of human papillomavirus (HVP)-DNA in oral manifestation of lichen planus. Mund Kiefer Gesichtschir. 1997; 1(3):146–9. [DOI] [PubMed] [Google Scholar]
- 9.Debanth S, Singh PA, Mehrotra R, Singh M, Gupta SC, Pandya S, et al. Human papillomavirus infection and premalignant lesions of the oral cavity: A cross-sectional study in Allahabad, North India. Asia-Pac J Clin Onco. 2009; 5(2):111–8. [Google Scholar]
- 10.Pol CA, Ghige SK, Gosavi SR. Role of human papilloma virus-16 in the pathogenesis of oral lichen planus—an immunohistochemical study. Int Dent J. 2015; 65(1):11–4. 10.1111/idj.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox M, Maitland N, Scully C. Human herpes simplex-1 and papillomavirus type 16 homologous DNA sequences in normal, potentially malignant and malignant oral mucosa. Eur J Cancer B Oral Oncol. 1993; 29B(3):215–9. [DOI] [PubMed] [Google Scholar]
- 12.Cianfriglia F, Di Gregorio DA, Cianfriglia C, Marandino F, Perrone DR, Vocaturo A. Incidence of human papillomavirus infection in oral leukoplakia. Indications for a viral aetiology. J Exp Clin Cancer Res. 2006; 25(1):21–8. [PubMed] [Google Scholar]
- 13.Giovannelli L, Campisi G, Colella G, Capra G, Di Liberto C, Caleca MP, et al. Brushing of oral mucosa for diagnosis of HPV infection in patients with potentially malignant and malignant oral lesions. Mol Diagn Ther. 2006;10(1):49–55. [DOI] [PubMed] [Google Scholar]
- 14.Syrjänen S, Lodi G, von Bultzingslowen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011; 17 Suppl 1:58–72. 10.1111/j.1601-0825.2011.01792.x [DOI] [PubMed] [Google Scholar]
- 15.Chalmers TC, Smith HJ, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981; 2(1):31–49. [DOI] [PubMed] [Google Scholar]
- 16.Yu Hong, Liu Zhongyan. Relationship between Human Papillomavirus and Oral Lichen Planus. Chin J Dermato Venerol Integ Trad W Med. 2007; 6(3):149–50. [Google Scholar]
- 17.Ma Jian, Yu Yi, Ma Rui, Ling Disheng. The detection of Human papillomavirus in the oral lichen planus patients. J Clin Stomatol. 2003; 19(2):113–4. [Google Scholar]
- 18.Li Hui, Bai Jingchun, Cao Fenglin, Wang Huanfu. Correlative Study of Human Papilloma Virus and Oral Lichen Planus with Gene Detection. Heilongjiang Med J. 2000; 6:9–10. [Google Scholar]
- 19.Qi Yanchun, Gao Limin, Hu Jianhong, Cao Fenglin. Correlative Study of Human Papilloma Virus and Oral Lichen Planus with Gene Detection. Herbin Med J. 1999; 19(3):5–6. [Google Scholar]
- 20.lei Lei, Li Hui; Sun Yu. Dection of HPV in Oral Lichen Planus. J Clin Stomatol. 1997; 01:5–7. [Google Scholar]
- 21.Razavi SM, Ghalayani P, Salehi MR, Attarzadeh H, Shahmoradi M. Human papilloma virus as a possible factor in the pathogenesis of oral lichen planus. Dent Res J (Isfahan). 2009; 6(2):82–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Arirachakaran P, Chansaengroj J, Lurchachaiwong W, Kanjanabud P, Thongprasom K, Poovorawan Y. Oral lichen planus in thai patients has a low prevalence of human papillomavirus. ISRN Dent. 2013; 2013:362750 10.1155/2013/362750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yildirim B, Senguven B, Demir C. Prevalence of herpes simplex, Epstein Barr and human papilloma viruses in oral lichen planus. Med Oral Patol Oral Cir Bucal. 2011; 16(2):e170–4. [DOI] [PubMed] [Google Scholar]
- 24.Campisi G, Giovannelli L, Arico P, Lama A, Di Liberto C, Ammatuna P, et al. HPV DNA in clinically different variants of oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 98(6):705–11. [DOI] [PubMed] [Google Scholar]
- 25.Giovannelli L, Campisi G, Lama A, Giambalvo O, Osborn J, Margiotta V, et al. Human papillomavirus DNA in oral mucosal lesions. J Infect Dis. 2002; 185(6):833–6. [DOI] [PubMed] [Google Scholar]
- 26.Fehér E, Gáll T, Murvai M, Kis A, Boda R, Sápy T, et al. Investigation of the occurrence of torque tenovirus in malignant and potentially malignant disorders associated with human papillomavirus. J Med Virol. 2009; 81(11):1975–81. 10.1002/jmv.21627 [DOI] [PubMed] [Google Scholar]
- 27.Szarka K, Tar I, Feher E, Gall T, Kis A, Toth ED, et al. Progressive increase of human papillomavirus carriage rates in potentially malignant and malignant oral disorders with increasing malignant potential. Oral Microbiol Immunol. 2009; 24(4):314–8. 10.1111/j.1399-302X.2009.00516.x [DOI] [PubMed] [Google Scholar]
- 28.OFlatharta C, Flint SR, Toner M, Butler D, Mabruk MJ. Investigation into a possible association between oral lichen planus, the human herpesviruses, and the human papillomaviruses. Mol Diagn. 2003; 7(2):73–83. [DOI] [PubMed] [Google Scholar]
- 29.Sand L, Jalouli J, Larsson PA, Hirsch JM. Human papilloma viruses in oral lesions. Anticancer Res. 2000; 20(2B):1183–8. [PubMed] [Google Scholar]
- 30.Payeras MR, Cherubini K, Figueiredo MA, Salum FG. Oral lichen planus: Focus on etiopathogenesis. Arch Oral Biol. 2013; 58(9):1057–69. 10.1016/j.archoralbio.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 31.Marini A, Wagenmann M, Ting E, Hengge UR. Squamous cell cancer and human papillomavirus infection in oral lichen planus: case report and literature review. Dermatol Surg. 2007; 33(6):756–60. [DOI] [PubMed] [Google Scholar]
- 32.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012; 307(7):693–703. 10.1001/jama.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwer AF, Eisenberg MC, Meza R. Age Effects and Temporal Trends in HPV-Related and HPV-Unrelated Oral Cancer in the United States: A Multistage Carcinogenesis Modeling Analysis. Plos One. 2016; 11(3):e151098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100(2):164–78. [DOI] [PubMed] [Google Scholar]
- 35.Zur HH. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2(5):342–50. [DOI] [PubMed] [Google Scholar]
- 36.Syrjanen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010; 21 Suppl 7:i243–5. [DOI] [PubMed] [Google Scholar]
- 37.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010; 2:15 10.1186/1758-3284-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91(6):622–35. [DOI] [PubMed] [Google Scholar]
- 39.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008; 100(6):407–20. 10.1093/jnci/djn025 [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Zhang Y, Liu L, Song X, Li G. Genetic polymorphisms and HPV infection in oral squamous cell carcinomas. Curr Opin Virol. 2015;14:1–6. 10.1016/j.coviro.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 41.Syrjanen SM. Basic concepts and practical applications of recombinant DNA techniques in detection of human papillomavirus (HPV) infection. Review article. APMIS. 1990; 98(2):95–110. [DOI] [PubMed] [Google Scholar]
- 42.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo ML, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007). Ann Oncol. 2008; 19(10):1681–90. 10.1093/annonc/mdn372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.