Abstract
Purpose
The CONSORT extension for patient reported outcomes (PROs) aims to improve reporting, but guidance on the optimal integration with clinical data is lacking. This study examines in detail the reporting of PROs and clinical data from randomized controlled trials (RCTs) in gastro-intestinal cancer to inform design and reporting of combined PRO and clinical data from trials to improve the ‘take home’ message for clinicians to use in practice.
Materials and Methods
The case study was undertaken in gastro-intestinal cancer trials. Well-conducted RCTs reporting PROs with validated instruments were identified and categorized into those combining PRO and clinical data in a single paper, or those separating data into linked primary and supplemental papers. Qualitative methods were developed to examine reporting of the critical interpretation of the trial results (trial exegesis) in the papers in relation of the PRO and clinical outcomes and applied to each publication category. Results were used to inform recommendations for practice.
Results
From 1917 screened abstracts, 49 high quality RCTs were identified reported in 36 combined and 15 linked primary and supplemental papers. In-depth analysis of manuscript text identified three categories for understanding trial exegesis: where authors reported a “detailed”, “general”, or absent PRO rationale and integrated interpretation of clinical and PRO results. A total of 11 (30%) and 6 (16%) combined papers reported “detailed” PRO rationale and integrated interpretation of results although only 2 (14%) and 1 (7%) primary papers achieved the same standard respectively. Supplemental papers provide better information with 11 (73%) and 3 (20%) achieving “detailed” rationale and integrated interpretation of results. Supplemental papers, however, were published a median of 20 months after the primary RCT data in lower impact factor journals (median 16.8 versus 5.2).
Conclusion
It is recommended that single papers, with detailed PRO rationale and integrated PRO and clinical data are published to optimize trial exegesis. Further work to examine whether this improves the use of PRO data to inform practice is needed.
Introduction
The updated Consolidated Standards of Reporting Trials (CONSORT) extension for patient reported outcomes (PROs) aims to facilitate the use of PRO data in health policy and practice through the transparent reporting of PROs in randomized controlled trials (RCTs)[1]. It makes recommendations for reporting of PRO instrument validity, presentation and handling of missing data and reporting of PRO sample size calculations, data analyses and results within the main text and abstract. The statement endorses reporting the rationale/hypotheses for PRO assessment and it highlights the need for integrated reporting of the PROs with the clinical findings of the paper (extensions and elaborations 2a, 2b and P20/21 and 22). These latter recommendations are particularly essential for critical interpretation of the trial, so called exegesis or a “take-home message”, for clinicians to understand and use results in clinical practice.
The CONSORT extension provides illustrations of how to report these issues. For example, elaboration 2a and extension P2b state that authors should “briefly establish the rationale for including PROs and why specific outcomes were selected”, and “report the rationale for the selection of specific patient-reported outcomes”. Furthermore, the guidelines state (in items P20/21 and elaboration 22) that “the clinical significance of PRO results is often not discussed in RCT reports but should be interpreted in relation to other important clinical outcomes such as survival”. Whilst this is helpful, the level of detail required for reporting the PRO data is unclear and this may have a detrimental impact on the overall clinically relevant trial conclusions.
This problem is further compounded by the way that PRO data are published. Some trials publish results in a single paper combined with clinical findings, which may limit full explanation of PRO results in the context of finite manuscript word limits. Publication of clinical and PROs separately is therefore attractive, however, practicing oncologists may be less likely to read the supplemental paper and thus not use PRO data in decision-making. The PRO CONSORT statement does not provide guidance for PRO reporting within these different scenarios. Whether PRO data are published together with clinical outcomes or separately in two articles, there is a need for optimal reporting of clinical and PROs so that they can be used in clinical practice. The aim of this paper, therefore, is to explore current standards and make recommendations for reporting a combined PRO and clinical ‘take home’ message to use in reporting RCTs in oncology.
Materials and Methods
This study was conducted in two parts. Part 1: systematic identification of well-designed and conducted RCTs reporting PROs with validated instruments, categorisation of papers into combined PRO and clinical reports or linked primary and supplemental reports, and assessment of PRO reporting within each RCT (PRO CONSORT extension). Part 2: development of novel methods to examine the ‘take home/trial exegesis’ message of the RCT and application to the papers identified in Part 1.
Part 1(a) Identification of well-designed and conducted RCTs reporting PROs with validated instruments
Systematic review methodology was used to identify RCTs at a low risk of bias reporting PROs with validated instruments in radical treatments of gastro intestinal oncology. Full-text articles were obtained. Gastro-intestinal oncology trials of radical treatment were chosen because the research team were familiar with the clinical and PRO data in this area, and trials at a low risk of bias are examples of best practice.
Electronic searches were performed in MEDLINE, Embase and Cochrane databases using the OVID SP gateway and Cochrane library. Search terms for esophageal, gastric and colorectal cancer were combined, as were terms for chemotherapy, radiotherapy, surgery or combined treatment. Results were restricted with the application of terms for “randomized clinical trials” and “patient reported outcomes”, and limited to articles published between January 2000 and October 2012 (see full search in S1 Appendix). The search output was imported into Reference Manager software and duplicate records removed. References for relevant studies before the year 2000 were obtained from a previous systematic review [2]. Titles and abstracts were screened by two researchers (AGKM and RM). Serial publications for the same trial (e.g. articles reporting short and long term PROs) were included. Excluded were phase II studies, RCTs of endoscopic and non-biomedical interventions, or trials limited to palliative treatment, screening or premalignant conditions. Only English-language publications were considered. Articles were assessed for risk of bias in the trials by three researchers (AGKM, RM, NB) using the Cochrane tool [3]. Studies classified with potential high or unascertainable risk of bias were excluded. Independent data extraction was conducted by at least two reviewers (AGKM, NB, RM, JMB) using a pre-designed and piloted form. Details of the trial were recorded including disease site, treatment intervention, primary and secondary outcomes, number of participants, and main trial results. The systematic review PRISMA checklist is presented in S2 Appendix.
(b) Categorisation of papers into combined PRO and clinical reports or linked primary and supplemental reports
Where included papers indicated the presence of previously published results from the same trial, these additional papers were sought and included in the analysis. These linked papers are hereafter considered in the order by which they were published, with those published first and second defined as “primary” and “supplemental” respectively. Thus, trials were categorized into those reporting PROs and clinical outcomes in a single, combined paper and those reporting results in linked primary and supplemental papers. The journal impact factor (Thomson Reuters, 2012) and the date of publication for each paper were recorded. Descriptive statistics compared journal impact factor for combined and linked primary and supplementary papers and median times between publications were summarized.
(c) Assessment of PRO reporting using the new CONSORT extension
The PRO CONSORT extension was applied to all trials to establish standards of PRO reporting, with the exception of item P6a which was an inclusion criterion (use of a validated PRO measure). Reporting of item 7a (PRO sample size) was recorded as present if a sample size calculation was completed in trials with patient reported primary outcomes, or if it were not applicable (for example, if there were no PROs as primary outcomes). Descriptive statistics are presented to consider PRO CONSORT standards within trials reporting in a single, combined publication or in linked primary and supplementary papers.
Part 2 (a) Development of methods to present the ‘take home/trial exegesis’ message of PRO with clinical data in trials
Methods to report the ‘take home’ messages of clinical and PROs in trials were developed through an in-depth analysis of items 2a and P2b (rationale/hypotheses for PRO measurement), and P20/21 and 22 (limitations and implications for clinical practice, and interpretation of PROs in relation to clinical outcomes) to identify good practice and produce methods to inform a PRO take home message from trials. All papers were read and re-read independently by at least two (AM, RM and JMB) researchers to become immersed in the data and relevant text was independently coded, copied verbatim into an electronic database and analysed for consistency between researchers. Discrepancies in coding were discussed within the study team (AM, RM and JMB). Methods for reporting trial ‘take home’ message were developed and applied iteratively to relevant text. Deviant examples were sought to challenge theories. Primary quotations are provided in accordance to methods of qualitative rigor. It was also noted whether primary papers indicated the future publication of a supplemental PRO paper (defined as “signposting”).
(b) Application of the novel methods to included trials
The methods described above were applied to the included trials and reported data examined by whether trials were published in combined or linked primary and supplemental reports.
Results
Part 1(a) Identification of well-designed and conducted RCTs reporting PROs with validated instruments
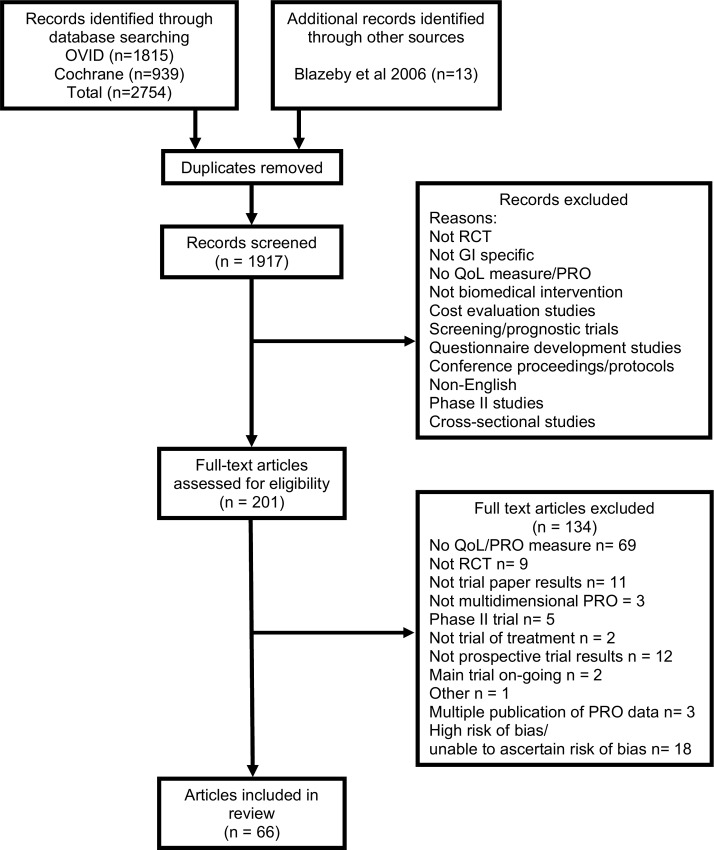
OVID (MEDLINE and Embase) and Cochrane database search yields were 1815 and 939 records. After de-duplication, 1917 abstracts were screened, 1716 excluded, and 201 full text articles further assessed for eligibility, and these were supplemented with 13 studies from a previous systematic review [2]. Sixty-seven articles met the inclusion criteria describing trials at low risk of bias (Fig 1). The 66 included articles reported PROs from 49 RCTs, the majority of which were chemotherapy interventions (22/49, 44.9%) in colorectal disease (36/49, 73.5%, Table 1).
Fig 1. PRISMA diagram of stages of the systematic review.
Table 1. Characteristics of included trials grouped by whether PRO and clinical data were presented in combined PRO and clinical paper or separate primary and supplemental papers.
| Combined clinical and PRO* paper (n = 37) | Separate clinical and PRO papers (n = 15) | |
|---|---|---|
| N (%) | N (%) | |
| Trial type | ||
| • Chemotherapy | 14 (37) | (33) |
| • Radiotherapy | 0 | (14) |
| • Surgery | 12 (32) | (46) |
| • Other‡ | 11 (30) | 7 (46) |
| Disease site | ||
| • Esophagogastric | 22.4) | 0 |
| • Colorectal | 27 (72) | 12 (80) |
| Sample size | ||
| • <100 | 8 (16) | (7) |
| • 100–199 | 4 (10) | (7) |
| • 200–499 | 13 (35) | (33) |
| • 500–999 | 8 (21) | (27) |
| • 1000–1999 | 3 (8) | (27) |
| • 2000–3000 | 1 (3) | 0 |
| Primary outcome | ||
| • Survival | 20 (54) | (46) |
| • Response rate | (8) | 0 |
| • Progression/ recurrence | 6 (16) | (13) |
| • PRO | (16) | 0 |
| • Hospital stay | (5) | 0 |
| • 30 day post-op morbidity | 0 | (7) |
| • Diarrhea | (3) | 0 |
| • Pulmonary infection | (3) | 0 |
| • Unclear | 2 (5) | 0 |
| Journal impact factor | Median (range) | Median (range) |
| • Primary paper | 16.8 (2.8 to 50) | (5.1 to 50) |
| • Supplemental paper | 5.2 (2.1 to 30) | |
| Time between primary and supplemental paper (months) | n/a | 20 (5 to 51) |
‡other biochemical modulators e.g.monoclonal antibody, radioactive yttrium
* PRO: Patient reported outcome
(b) Categorisation of papers into combined PRO and clinical reports or linked primary and supplemental reports
Some 36 (71%) single papers reported combined PRO and clinical results and the remaining 30 were linked primary and supplemental papers. The median journal impact factor for both combined and primary papers was the same (16.8), but supplemental PRO papers were reported in journals with a lower median impact factor (5.2). The mean time between publication of linked primary and supplementary papers was 20 months (range 5 to 51).
(c) Assessment of PRO reporting using the new CONSORT extension
Overall, the reporting of most papers did not meet the new PRO CONSORT standards (S1 Table). There were six (2 combined; 0 primary and 4 supplemental) papers reporting all PRO CONSORT items (Table 2). Primary papers reported the fewest items (median 3, range 2 to 7), typically lower than combined papers (median 6, range 2 to 12) and supplemental papers (median 10, range 4 to 12). The least frequently reported items related to “results” (13a, 15, 16, 17a and 18), and were reported in less than one third of papers. The least frequently reported items related to “results” (13a, 15, 16, 17a and 18), and were reported in less than one third of papers.
Table 2. Analyses of reporting PRO CONSORT extension criteria, grouped by combined PRO and clinical papers, or separate primary and supplemental papers.
| CONSORT PRO item | Combined clinical and PRO paper (n = 36) | Separate clinical and PRO papers (n = 15 pairs) | Total(n = 66) | |||
|---|---|---|---|---|---|---|
| 1° | 2° | |||||
| N (%) | N (%) | N (%) | N (%) | |||
| P1b | The PRO should be identified in the abstract as a primary or secondary outcome. | 28 (78) | 4 (27) | 15 (100) | 47 (71) | |
| 2a/P2b* | The relevant background and rationale for why PROs were assessed in the RCT should be briefly described/ The PROs hypothesis should be stated and relevant domains identified, if applicable. | 22 (61) | 5 (33) | 15 (100) | 42 (64) | |
| P6a** | Evidence of PRO instrument validity and reliability should be provided or cited, if available. | 36 (100) | 15 (100) | 15 (100) | 66 (100) | |
| 7a† | How PRO sample size was determined | 33 (92) | 15 (100) | 15 (100) | 63 (95) | |
| P12a | Statistical approaches for dealing with missing data are explicitly stated | 9 (25) | 1 (7) | 10 (66) | 20 (30) | |
| 13a | The number of PRO outcome data at baseline and at subsequent time points should be made transparent | 6 (17) | 1 (7) | 9 (60) | 16 (24) | |
| 15 | A table showing baseline demographic and clinical characteristics for each group including PRO data | 7 (19) | 0 | 10 (67) | 17 (26) | |
| 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 8 (22) | 0 | 8 (53) | 16 (24) | |
| 17a | For multidimensional PROs, results from each domain and time point specified for analysis. | 9 (25) | 0 | 10 (67) | 19 (29) | |
| 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing prespecified from exploratory including PRO analyses, where relevant | 10 (28) | 3 (20) | 7 (46) | 20 (30) | |
| P20/21‡ | PRO–specific limitations and implications for generalizability and clinical practice | 29 (81) | 3 (20) | 14 (93) | 46 (70) | |
| 22‡ | PRO data should be interpreted in relation to clinical outcomes including survival data, where relevant | 29 (81) | 3 (20) | 14 (93) | 46 (70) | |
Part 2 (a) Development of novel methods to examine the take home message of PRO with clinical data in trials
The new method for understanding and improving combined reporting practice was developed based upon the in-depth analyses, emergent data and iterative discussions with AM and JMB. Sections of verbatim text were coded to items 2a and P2b were classified as providing a 1) detailed rationale/hypothesis—when authors included a specific PRO domain and/or hypothesized effect, 2) general rationale/hypothesis—when authors included non-specific rationale (i.e. “to examine quality of life”) or 3) no rationale provided. Verbatim quotes of PRO rationales are presented in Tables 3 and 4. An example of a detailed rationale included: “…we hypothesized a priori that [intervention] would result in a decrease in the magnitude and rate of decline in HRQL, particularly in physical function and overall well-being”[4], and an illustration of a general rationale included “…to compare…quality of life…between [intervention] and [control]”[5].
Table 3. Reported PRO rationale (items 2a and P2b) and authors’ interpretation of PRO in relation to clinical findings (items P20/21 and 22) in primary reports of trials with separate primary and supplemental papers.
Extracted text was abridged where appropriate, as indicated by a series of periods (…), but otherwise presented verbatim.
| Author [citation] | PRO rationale | Level of detail* | Interpretation of PRO in relation to clinical findings: | Level of detail† | |
|---|---|---|---|---|---|
| Ajani [6] [7] | 1° | “To investigate whether adding [intervention] to [control] could improve patient outcomes (time-to-progression [TTP], overall survival [OS}, quality of life. . .” “Time to 5% definitive deterioration in global health status assed by QLQ-C30 was the primary quality of life parameter. “ | Detailed | “. . . [Intervention] resulted in significantly improved TTP (primary end point), OS, and overall response rate (secondary end points), with global health status (quality of life). . . preserved for a longer time.” | Detailed |
| 2° | “. . .to investigate whether the better efficacy with [intervention] was counterbalanced by. . .the impact. . .on patient QOL” “The primary endpoint of the QOL assessment was. . .global health status” | Detailed | “.. significantly better preservation of QOL for patients treated with [intervention].. as a result of a significantly higher level of efficacy. . . . despite a higher incidence of some toxicities. . .” | General | |
| Au[4] [8] | 1° | “. . .no trials have demonstrated an effect of [intervention] on. . . .quality of life. . .” “The secondary end points were. . .quality of life, assessed by mean changes in scores of physical function and global health status..” | Detailed | “[Intervention] improves overall survival and progression-free survival and preserves quality of life measures. . .” | General |
| 2° | “…. we hypothesized a priori that [intervention] would result in a decrease in the magnitude and rate of decline in HRQL, particularly in physical function and overall well-being.” | Detailed | “Patients who received [intervention] experienced significantly less HRQL deterioration and a longer time before clinically significant deterioration occurred. These results are important, because…although [intervention]. . . results in improved OS, PFS, RR, and DCR…the magnitude of these benefits. . .was not large.” “. . .[intervention] offers clinically important survival and HRQL benefits. . .” | General | |
| de Boer [9] [10] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .to compare the quality of life of patients. . .who underwent [intervention] with patients.. who underwent [control]” | General | “..comparing. . .quality of life..is of great interest because a choice between the..two treatment options proves to be difficult..based on overall survival. However…no lasting differences in the quality of life of patients. . .were found.” | General | |
| Braga[11] [12] | 1° | “To clarify the value of [intervention]. . .quality of life. . .should be considered” | General | “[intervention] resulted in earlier postoperative recovery, better cosmesis and improved quality of life. . .compared to [control].” | General |
| 2° | “The primary endpoint was to compare the impact of [intervention] and [control] on 30-day postoperative morbidity.” “Recovery of social and physical activity was evaluated. . .by a specificd adaptation of the SF-36. . .” | Detailed | “. . .the [intervention] resulted in a reduction of both the overall morbidity rate and the length of hospital stay, and in a faster recovery of physical and social activity.” | Detailed | |
| Chau [13] [14] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .to assess QOL. . . .in patients receiving [intervention]” | General | “[Intervention] was associated with significantly better quality of life. . . Due to the shorter treatment duration, [intervention] had a faster time of QOL recovery. Quality adjusted survival was also in favour of the [intervention]… | General | |
| Hallböök [15] [16] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “We hypothesized that such clear differences in clinical bowel function [with the intervention] would also be reflected in the score of a general quality of life instrument……” | Detailed | “The observed difference in clinical bowel function was not. . . reflected in an improved QOL score. . .” | General | |
| Janson [17] [18] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .with the hypothesis that [intervention] results in an improved HRQL when compared with [control]” | Detailed | “HRQL was better..after [intervention]. At present, several studies indicate that the oncologic results are at least equal after [intervention].” | General | |
| Kabbinavar [19] [20] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “The primary HRQoL endpoint was the time to deterioration in HRQoL measured by the Colorectal Cancer Subscale score” | Detailed | “this prospective HRQoL analysis supports the clinical benefit of [intervention] in improving time to disease progression and prolonging overall survival, without compromising patients’ HRQoL”. | General | |
| King [5] [21] | 1° | “The aim of this study was to compare. . . quality of life. . .in a prospective group of patients undergoing [intervention]” | General | “Patients undergoing [intervention] stay in hospital half as long. . .with no. . .deterioration in quality of life. . .” “. . .clinical improvements resulting from [intervention] did not cause significant deterioration in quality of life. . .” | General |
| 2° | “. . .to compare recovery after [intervention] and [control]. . .using. . .self-report and observer data.” | General | “The earlier discharge in the [intervention] group did not result in any deterioration in quality of life outcomes compared with those in the [control] group” “Despite perioperative optimization of [control], short-term outcomes were better following [intervention]. There was no deterioration in quality of life or increased cost associated with the [intervention].” | General | |
| Kopec [22] [23] | 1° | “A secondary aim was to compare quality of life. . .” | General | No integration of PRO and clinical data | Absent |
| 2° | “We hypothesized that the [intervention] would be associated with higher HRQL and that it would be perceived as more convenient.” …. “The primary end point for this study was the FACT-C total score.” | Detailed | “The efficacy of the two regimens is similar, as demonstrated.. by the survival and disease-free survival analyses. . . This underscores the importance of patient-reported outcomes..” “Both regimens … do not differ in their impact on HRQL.” | General | |
| Marijnen [24] [25] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .we studies the effects of [intervention] on the HRQL and sexual functioning. . .” | Detailed | “The results of this study enable physicians and patients to weigh the beneficial effect of [intervention] on local recurrence against the price to be paid in terms of HRQL and sexual functioning.” | Detailed | |
| Siena [26] [27] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .exploratory analyses were conducted that assessed the association between [trial outcome variables] and HRQoL” | General | “. . . lack of disease progression was associated with … higher HRQoL for [intervention] patients only. . .Lack of disease progression was associated with better symptom control, HRQoL, and OS. | General | |
| Stephens [28] [29] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “. . .the advantages of [intervention] to all patients needs to be balanced against any negative impact on patients’ quality of life”. “. . .the primary quality-of-life aims as “What is the longer-term (2-year) effect of the treatments on (1) sexual function and (2) bowel function?” Secondary outcome measures were “What is the effect of treatment on physical function and general health?” To address these questions, the sexual dysfunction and bowel function scales from the QLQ-CR38 and the physical function and general health scales from the MOS SF-36 were used.” | Detailed | “Therefore our results, together with those of the Dutch trial, provide convincing data on the impact of surgery and PRE on sexual and bowel function.” “The information presented in this article should allow clinicians to discuss with patients an estimate of the benefit of PRE in terms of reduction in LR risk balanced against the detrimental toxicity that is attributable to PRE.” | Detailed | |
| Weeks [30] [31] | 1° | No PRO rationale | Absent | “The detailed quality of life component of this trial suggests that greater benefits in terms of the quality of life and recovery may be possible if fewer procedures are converted.” | General |
| 2° | “The trial was also designed to test the hypothesis that [intervention] is associated with superior QOL outcomes” “the study protocol specified. . .the variability in pain distress item and the global ratings scale” | Detailed | “[Intervention].. results in statistically significant but clinically modest decreases in the duration of postoperative in-hospital analgesia and in length of stay. . . However, these differences do not translate into statistically significant improvements in symptoms or QOL. . .” | General | |
| Wu[32] [33] | 1° | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| 2° | “We hypothesised that patients receiving [the intervention] would have more symptoms and greater fatigue than patients receiving [the control], with treatment arms difference most prominent at the 6-month assessment, and probably continuing up to 1 year after random assignment.” | Detailed | “Although the morbidity rate was higher in [intervention] patients than in [control] patients, our analysis indicates that [intervention] did not adversely influence QOL” | General |
* Detailed rationale/hypothesis: specifying a PRO domain or hypothesized effect; general rationale/hypothesis: any other description; absent: no rationale. See methods for more details
† Interpretation was considered “detailed” where authors discussed the direction of change (e.g. increased/decreased/no change) of a specific PRO domain (e.g. physical function) in relation to the direction of change of a specific clinical outcome (e.g. survival). All other discussions, where present, were considered “partial” interpretations. Where no appropriate text was identified, interpretation was considered “absent”. See methods for more details
Table 4. Reported PRO rationale (items 2a and P2b) and authors’ interpretation of PRO in relation to clinical findings (items P20/21 and 22) in reports of trials with combined clinical and PRO papers.
Extracted text was abridged where appropriate, as indicated by a series of periods (…), but otherwise presented verbatim.
| Author [citation] | PRO rationale | Level of detail* | Interpretation of PRO in relation to clinical findings | Level of detail† |
|---|---|---|---|---|
| Biere [34] | “We compared [intervention] with [control]. . . to assess the rate of pulmonary infection and quality of life associated with [intervention]” | General | “In this trial, [intervention] resulted in a lower incidence of pulmonary infections 2 weeks after surgery and during stay in hospital, a shorter hospital stay, and better short-term quality of life than did [control], with no compromise in the quality of the resected specimen.” “Additionally, [intervention] preserved quality of life better than [control] did. After 6 weeks, the SF 36 questionnaire and global health experience in the EORTC C30 module were better for patients in the [intervention] group than for those in the [control] group. In the oesophageal-specific OES 18 questionnaire, pain and talking were adversely affected in patients in the [control] group as compared with those in the [intervention] group.” | Detailed |
| Bramhall [35] | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| Carmichael [36] | No PRO rationale | Absent | “The safety advantages of [intervention] surprisingly did not lead to demonstrable improvement in quality of life.” | General |
| Cunningham [37] | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| de Gramont [38] | “. . .to compare the two treatments in terms of. . .QoL” | General | “The [intervention] seems beneficial. . ., demonstrating a prolonged progression free survival with acceptable tolerability and maintenance of QoL.” “Median QoL scores were similar for the two arms. . ., despite the increased incidence of [treatment]-related side effects …” | General |
| Doeksen [39] | “The objective. . .was to compare functional and surgical results of [intervention] with [control] and their impact on quality of life.” “The primary end-point was the function. . . assessed at 12 months by the validated COlo-Rectal Functional Outcome (COREFO) questionnaire’s summary score.” | Detailed | “. . .a better functional outcome was found in patients with [intervention] than [control]. These functional differences did not influence health-related and overall quality of life.” | General |
| Douillard [40] | “The QLQ-C30 questionnaire was analysed with the global health status/QoL scale (QL) as the primary endpoint. . .” | Detailed | “[Intervention] was well-tolerated and increased response rate, time to progression, and survival, with a later deterioration in quality of life.” † | General |
| Douillard [41] | No PRO rationale | Absent | “It was surprising that there was no observed difference between the treatment arms in quality of life, despite the clear reduction in toxicity with [intervention].” | General |
| Fein [42] | “. . .to identify optimal [treatment] in terms of quality of life” | General | “There were no differences in operative time, postoperative complications, and mortality.., there were no benefits of [intervention] in terms of quality of life, independent of the resection status. In the third, fourth, and fifth year after surgery quality of life was significantly improved for patients with [intervention]. | General |
| Fields [43] | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| Fuchs [44] | “. . .to compare. . .effect on patient quality of life of these two [treatments]” | General | “This. . . trial provides comparative data on the efficacy, tolerability, and effect on patient quality of life between the [treatments].” | General |
| Furst [45] | “. . .we tested [intervention] with [control] for. . . .quality of life. . .” | General | No integration of PRO and clinical data | Absent |
| Gray [46] | “. . .to assess whether [intervention] could. . . .change quality of life” | General | “No decrease in quality of life was observed which is in accord with the lack of serious toxicity and treatment-related complications.” “[intervention] increases treatment effectiveness when measured by tumor response and time to disease progression and suggests an increase in survival for patients surviving more than 15 months. [Intervention] does not compromise quality of life or add significant toxicity.” | General |
| Guillou [47] | No PRO rationale | Absent | “no differences were recorded between [control] and [intervention]. . . with respect to tumour and nodal status, short term endpoints, and quality of life.” | General |
| Hoksch [48] | “. . .to evaluate the quality of life during the first postoperative year comparing [intervention] and [control]” | General | “In this study of global health status and quality of life, patients operated on with [control procedure] did not reach their preoperative values compared to the patients with the [intervention]. . .” “The clinical advantage manifested 6 months after operation. . . For that reason only patients with a good long-term prognosis might benefit from [intervention]. | Detailed |
| Jayne [49] | No PRO rationale | Absent | “[Intervention]. . . . is as effective as [control] in terms of oncological outcomes and preservation of QoL” | General |
| Kang [50] | No PRO rationale | Absent | “. . .[intervention] is feasible and does not increase short-term oncological risks, which are predicted by CRM positivity and macroscopic quality of TME specimens. . .The results of this trial also suggest that [intervention] results in a better quality of life for up to 3 months. . .” | General |
| Kataria [51] | “To compare the quality of life (QOL) in patients undergoing [intervention] with [control]. . .” “The objective of this study is to assess the QOL following [intervention]. . .” | General | No integration of PRO and clinical data | Absent |
| Kemeny [52] | “We hypothesized that patients in the [intervention] arm would have better physical and social functioning, fewer role limitations due to their emotional health, and better health perceptions than patients in the [control] arm.” | Detailed | “[Intervention] prolonged the median survival. . . was associated with a greater likelihood of objective tumor responses. . ., enhanced time to hepatic progression. . ., and improved physical functioning (QoL measurements).” | Detailed |
| Kohne [53] | No PRO rationale | Absent | No integration of PRO and clinical data | Absent |
| Lal [54] | “No studies have evaluated whether [intervention] is superior. . .in terms of. . .quality of life” | Detailed | “There were no improvements in failure-free survival from continuing [intervention]. . . . However,.. there was no deterioration in QoL. . .” | General |
| Maughan [55] | “Several specific quality-of-life endpoints were predefined in the protocol: palliation of key symptoms, toxic effects, psychological effect, functional status, social functioning, and overall quality of life” | Detailed | “[A] and [B] regimens were similar in terms of survival, quality of life, and response rates. [C] showed similar response rates and overall survival to the [A] regimen and was easier to administer, but resulted in greater toxicity and inferior quality of life.”‡ “Since there was similar overall survival, quality of life became an important outcome measure.” | General |
| Punt [56] | “The primary objective. . .was to examine the treatment effect on the mean global health status score. . .” | Detailed | No integration of PRO and clinical data | Absent |
| Punt [57] | “The primary objective. . .was to examine the treatment effect on the mean global health status score. . .” | Detailed | “[Intervention]. . .results in a small but significant improvement in progression free survival without adding toxicity or worsening QoL. . . .” † | General |
| Rao [58] | No PRO rationale | Absent | “Although there was a trend in favor of [intervention] for progression free survival, and more patients had stable disease, this did not translate in an improved QOL or survival advantage.” | General |
| Ross [59] | “…we report results. . .comparing [intervention] with [control] using. . .QOL. . .as the study’s end points” | General | “The equivalent efficacy of [intervention] was demonstrated, but QOL was superior with [control].” | General |
| Sailer [60] | “Randomised trials. . .have shown functional superiority of [intervention]. . .it was hypothesized that significant differences in bowel function should also be reflected in quality of life” “Sample size analysis was based on. . .global health status. . .” | Detailed | “. . .patients undergoing [intervention] may not only expect better functional results but also an improved quality of life. . .” | General |
| Saini [61] | “. . .to assess QOL of patients undergoing [treatment]” | General | “this study has demonstrated that the [intervention] is associated with less acute toxicity and less impairment of QOL than [control]. Furthermore, this has been achieved without any obvious adverse effect on outcome” | General |
| Saltz [62] | No PRO rationale | Absent | “the [intervention] was associated with higher rates of tumor regression, progression-free survival, and overall survival without compromising the quality of life.” | General |
| Sobrero [63] | No PRO rationale | Absent | “.. Progression free survival was significantly longer in experimental. . ., while the overall survival was similar in both arms. . .; quality of life was similar as well.” † | General |
| Sobrero [64] | No PRO rationale | Absent | “.. [intervention] reduced the risk of progression.., and improved median progression free survival. . ., and response rate.. The QOL assessments also support this benefit. Global health status as well as physical, emotional, and cognitive functioning were significantly better with [intervention].” | Detailed |
| Tebbutt [65] | No PRO rationale | Absent | “the addition of [intervention]. . .has no effect on response rates compared with [control]. In addition, there was no significant effect on overall survival or quality of life,. . .” | General |
| Tol [66] | No PRO rationale | Absent | “the [intervention] resulted in a significant decrease in progression free survival and a poorer quality of life” | General |
| Van Hooft [67] | “We aimed to establish whether [intervention] has better health outcomes than does [control]” “The primary outcome was mean global health status. . . assessed with the QL2 subscale of the European Organisation for Research and Treatment of Cancer quality of life questionnaire.” “This measure was chosen because the outcome of the treatments, such as need for a stoma, incisional hernia, lengthy intensive care, and hospital stay, might affect patients’ quality of life” | Detailed | “. . .[intervention] or [control] did not have any distinct benefits for global health status, mortality, morbidity, other quality of life dimensions, and stoma rates.” | Detailed |
| Vlug [68] | “. . .combining the [intervention] will result in the fastest postoperative recovery.” | Detailed | “Treatment groups had similar morbidity, reoperation and readmission rates, equal in-hospital mortality, comparable levels of quality of life. . .” | General |
| Zachariah [69] | . . . .”if [intervention] was efficacious in reducing treatment-induced diarrhea, better QoL and bowel scores were expected for the [intervention] for all instruments.” | Detailed | “We found that [intervention] did not show a statistically significant reduction in the incidence or severity of diarrhea or change in patient-reported bowel function. . .” | Detailed |
* Detailed rationale/hypothesis: specifying a PRO domain or hypothesized effect; general rationale/hypothesis: any other description; absent: no rationale. See methods for more details
† Interpretation was considered “detailed” where authors discussed the direction of change (e.g. increased/decreased/no change) of a specific PRO domain (e.g. physical function) in relation to the direction of change of a specific clinical outcome (e.g. survival). All other discussions, where present, were considered “partial” interpretations. Where no appropriate text was identified, interpretation was considered “absent”. See methods for more details
Likewise, sections of text coded to the items P20/21 and 22 were developed as 1) detailed interpretations—where authors discussed the hypothesized effect of a specific PRO in relation to the hypothesized effect of a clinical outcome, 2) general interpretations–when authors include non-specific interpretation of PROs in relation to clinical outcomes, or 3) no integrated PRO and clinical interpretation of results in the paper. An example of detailed interpretation of findings includes “… [Intervention] resulted in significantly improved TTP [time to progression] (primary end point), OS [overall survival], and overall response rate (secondary end points), with global health status (quality of life)… preserved for a longer time.”[6]
Part 2, b) Application of the methods to included trials
Of the 36 trials reporting combined papers, there were 11 (30%) papers that provided a PRO rationale/hypothesis, 10 (30%) providing general information and 15 (40%) not providing a PRO rationale (Table 5). The interpretation of PRO data in the context of clinical outcomes were detailed in six (16%), general in 24 (65%) and absent in 7 (17%) papers (Items P20/21 and 22, Table 3). There were seven papers that described both detailed PRO rationale/hypotheses and detailed interpretations of PROs in relation to clinical outcomes [5, 6, 13, 26, 30, 68, 70].
Table 5. Novel methods for assessing CONSORT PRO extension items 2a/P2b and P20/21/22, grouped by combined PRO and clinical papers, or linked primary and supplemental papers (n = 67).
| CONSORT PRO item | Combined clinical and PRO paper (n = 36) | Linked clinical and PRO papers (n = 15 pairs) | |
|---|---|---|---|
| 1° | 2° | ||
| N (%) | N (%) | N (%) | |
| Rationale/hypothesis (2a/P2b)* | |||
| • Detailed | 11 (31) | 2 (14) | 73) |
| • General | 10 (28) | 3 (20) | (27) |
| • Absent | 15 (41) | 10 (66) | 0 |
| Interpretation of findings (P20/21/22)† | |||
| • Detailed | 6 (17) | 1 (7) | (20) |
| • General | 2 (64) | 4 (27) | 80) |
| • Absent | 7 (19) | 10 (66) | 0 |
* Detailed rationale/hypothesis: specifying a PRO domain or hypothesized effect; general rationale/hypothesis: any other description; absent: no rationale. See methods for more details
† Interpretation was considered “detailed” where authors discussed the direction of change (e.g. increased/decreased/no change) of a specific PRO domain (e.g. physical function) in relation to the direction of change of a specific clinical outcome (e.g. survival). All other discussions, where present, were considered “partial” interpretations. Where no appropriate text was identified, interpretation was considered “absent”. See methods for more details
Where PRO and clinical results were published separately (n = 15), most primary papers did not provide any PRO rationale (n = 10, 66%) or text interpreting PROs in relation to clinical findings (n = 10, 66%). One primary paper provided detailed descriptions of both these issues. In comparison, all supplemental papers described PRO rationales and most (14, 93%) contained detailed interpretation of PROs in relation to clinical findings, although only two had detailed descriptions of both of these. Of the 15 primary papers, 66% signposted the presence of the supplemental PRO report.
Conclusions
Reporting PRO rationale linked to clinical hypotheses, and clear reporting of PRO results interpreted appropriately in the context of the clinical outcomes are critical to ensure that oncologists gather a “take-home” message to communicate to patients which encompasses clinical and PROs. This review explored this issue in detail. Patient reported outcome reporting standards were at lowest levels in primary clinical papers (where clinical trial data was reported separately to the supplementary PROs). Whilst supplemental papers provided more detail there was a 5 to 51 month delay in publication in less well cited journals, thus diminishing their impact. Most (71%) trials did report combined results, demonstrating that it is possible to do so. New methods to examine reporting of trial “take home” messages recommend that detailed information about domain specific PRO rational and interpretation with clinical data are supplied within a main trial paper to arm clinicians with relevant outcomes to use in decision-making. Authors need to be allowed space to report these details alongside clinical outcomes to inform the take home message from papers to help clinicians in practice. Where this is not appropriate for scientific reasons, for example, if primary outcome data are available before secondary PROs, then this could be explicitly stated.
Other systematic reviews have shown that PRO reporting standards are poor [71–77] which contributed to the need for the development of the PRO CONSORT extension. Similarly, other papers have recommended clear PRO hypotheses and integration with clinical findings, however, there is no empirical data presented on how to best achieve this [78]. Reviews also have examined how PROs in RCTs influence decision-making and confirm that PRO information is not used in practice [77, 79, 80]. Previous work, however, has not provided solutions to these problems or considered the conceptual reasons why PRO data are not used in practice. Theoretically, failure of PRO data to have an impact on clinical practice may stem from problems that start with the trial design and conduct, compounded by poor reporting and separate PRO and clinical publications. Further research is now needed to investigate whether improved reporting will have the desired effect of informing patient-centred care, clinical decision making and health policy decisions. For example, work could include a study directly exploring oncologists’ views of trial reports following the introduction of new reporting guidelines would be informative. Additionally in-depth research of clinical decision-making in multi-disciplinary teams or of oncology consultations may be undertaken to examine how PRO data are used.
Research needs to be targeted into each of these areas in order to understand how improvements, such as the recently published SPIRIT statement [81] for improving RCT protocols or the CONSORT PRO extension, impact clinical practice. What is clear is that cancer patients want information about PROs and indeed rate such data of similar importance to survival information [82–84]. It is therefore critical that oncologists communicate PRO data in the context of a shared doctor-patient consultation and methods to do so are being established [85–87]. This may have occurred because authors split trial results and deliberately left the PRO methodology and findings to the supplemental report.
This review included a systematic search for studies using PRISMA guidelines [88] and transparent methodology for an in-depth analyses if the textual data, but there were some limitations. Trials of radical treatments of gastrointestinal cancers were selected for analyses because the authors were familiar with the PRO and clinical data in this field. It is conceivable that including trials in other diseases or in the palliative setting may have identified different reporting standards. For example, in the palliative setting, authors may make greater reference to PROs and their integration with clinical outcomes because the main focus of treatment is not to cure disease. Further work is needed to examine this area in detail. In addition, studies with high or unascertainable risk of bias were excluded because lower standards of reporting are associated with bias and likely poor PRO reporting [89], and it is possible that important data were missed. This is considered to be unlikely however, because even the included “high-quality” trials demonstrated significant reporting weaknesses and inclusion of poor quality trials would probably not yield exemplar practice.
In summary, this review presents and evidence based way of implementing the new CONSORT PRO extension items 2a, P2b, P20/21 and 22 based on current literature. It is recommended that RCTs report domain-specific PRO rationale with anticipated treatment effects, and integrate these findings with specific clinical outcomes in a single combined report. It is acknowledged that trials are structured around their primary (often clinical) endpoints, and it is appropriate to prioritise these data at the expense of other outcomes. It seems unnecessary, however, to relegate evaluation of patient experience to reports that may be less likely to influence practice. The adoption of better standards for PRO reporting could facilitate the use of PRO data by oncologists and patients for informed decision making.
Supporting Information
(DOCX)
(PDF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by United Kingdom Medical Research Council Collaboration and innovation for Difficult and Complex randomised controlled Trials In Invasive procedures Hub grant code MR/K025643/1 director (to JMB). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Calvert M, Brundage M, Jacobsen PB, Schunemann HJ, Efficace F. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health and quality of life outcomes. 2013;11:184 10.1186/1477-7525-11-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazeby JM, Avery K, Sprangers M, Pikhart H, Fayers P, Donovan J. Health-related quality of life measurement in randomized clinical trials in surgical oncology. Journal of Clinical Oncology. 2006;24(19):3178–86. [DOI] [PubMed] [Google Scholar]
- 3.The Cochrane C. Cochrane handbook for systematic reviews of interventions2009.
- 4.Au HJ, Karapetis CS, O'Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. Journal of Clinical Oncology. 2009;27(11):1822–8. 10.1200/JCO.2008.19.6048 [DOI] [PubMed] [Google Scholar]
- 5.King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, et al. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. British Journal of Surgery. 2006;93(3):300–8. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. Journal of Clinical Oncology. 2007;25(22):3210–6. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. Journal of Clinical Oncology. 2006;24(31):4991–7. [DOI] [PubMed] [Google Scholar]
- 8.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. New England Journal of Medicine. 2007;357(20):2040–8. [DOI] [PubMed] [Google Scholar]
- 9.de Boer AGEM, van Lanschot JJB, van Sandick JW, Hulscher JBF, Stalmeier PFM, De Haes JCJM, et al. Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. Journal of Clinical Oncology. 2004;22(20):4202–8. [DOI] [PubMed] [Google Scholar]
- 10.Hulscher JBF, van Sandick JW, De Boer AGEM, Wijnhoven BPL, Tijssen JGP, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. New England Journal of Medicine. 2002;347(21):1662–9. [DOI] [PubMed] [Google Scholar]
- 11.Braga M, Frasson M, Zuliani W, Vignali A, Pecorelli N, Di Carlo V. Randomized clinical trial of laparoscopic versus open left colonic resection. British Journal of Surgery. 2010;97(8):1180–6. 10.1002/bjs.7094 [DOI] [PubMed] [Google Scholar]
- 12.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, et al. Laparoscopic versus open colorectal surgery—A randomized trial on short-term outcome. Annals of Surgery. 2002;236(6):759–66. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chau I, Norman AR, Cunningham D, Iveson T, Hill M, Hickish T, et al. Longitudinal quality of life and quality adjusted survival in a randomised controlled trial comparing six months of bolus fluorouracil/leucovorin vs. twelve weeks of protracted venous infusion fluorouracil as adjuvant chemotherapy for colorectal cancer. European Journal of Cancer. 2005;41(11):1551–9. [DOI] [PubMed] [Google Scholar]
- 14.Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Annals of Oncology. 2005;16(4):549–57. [DOI] [PubMed] [Google Scholar]
- 15.Hallbook O, Hass U, Wanstrom A, Sjodahl R. Quality of life measurement after rectal excision for cancer. Comparison between straight and colonic J-pouch anastomosis. ScandJ Gastroenterol. 1997;32(5):490–3. [DOI] [PubMed] [Google Scholar]
- 16.Hallbook O, Pahlman L, Krog M, Wexner SD, Sjodahl R. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Annals of Surgery. 1996;224(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janson M, Lindholm E, Anderberg B, Haglind E. Randomized trial of health-related quality of life after open and laparoscopic surgery for colon cancer. Surgical Endoscopy. 2007;21(5):747–53. [DOI] [PubMed] [Google Scholar]
- 18.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. The lancet oncology. 2005;6:477–84. [DOI] [PubMed] [Google Scholar]
- 19.Kabbinavar FF, Wallace JF, Holmgren E, Yi J, Cella D, Yost KJ, et al. Health-related quality of life impact of bevacizumab when combined with irinotecan, 5-fluorouracil, and leucovorin or 5-fluorouracil and leucovorin for metastatic colorectal cancer. Oncologist. 2008;13(9):1021–9. 10.1634/theoncologist.2008-0003 [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer.[see comment]. New England Journal of Medicine. 2004;350(23):2335–42. [DOI] [PubMed] [Google Scholar]
- 21.King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. International Journal of Colorectal Disease. 2008;23(8):795–800. 10.1007/s00384-008-0478-0 [DOI] [PubMed] [Google Scholar]
- 22.Kopec JA, Yothers G, Ganz PA, Land SR, Cecchini RS, Wieand HS, et al. Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: results from National Surgical Adjuvant Breast and Bowel Project Trial C-06. Journal of Clinical Oncology. 2007;25(4):424–30. [DOI] [PubMed] [Google Scholar]
- 23.Lembersky BC, Wieand HS, Petrelli NJ, O'Connell MJ, Colangelo LH, Smith RE, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. Journal of Clinical Oncology. 2006;24(13):2059–64. [DOI] [PubMed] [Google Scholar]
- 24.Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. Journal of Clinical Oncology. 2005;23(9):1847–58. [DOI] [PubMed] [Google Scholar]
- 25.Kapiteijn E, Marijnen CAM, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New England Journal of Medicine. 2001;345(9):638–46. [DOI] [PubMed] [Google Scholar]
- 26.Siena S, Peeters M, Van CE, Humblet Y, Conte P, Bajetta E, et al. Association of progression-free survival with patient-reported outcomes and survival: results from a randomised phase 3 trial of panitumumab. British Journal of Cancer. 2007;97(11):1469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of Clinical Oncology. 2007;25(13):1658–64. [DOI] [PubMed] [Google Scholar]
- 28.Stephens RJ, Thompson LC, Quirke P, Steele R, Grieve R, Couture J, et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients' quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. Journal of Clinical Oncology. 2010;28(27):4233–9. 10.1200/JCO.2009.26.5264 [DOI] [PubMed] [Google Scholar]
- 29.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial.[see comment]. Lancet. 2009;373(9666):811–20. 10.1016/S0140-6736(09)60484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287(3):321–8. [DOI] [PubMed] [Google Scholar]
- 31.Nelson H, Sargent D, Wieand HS, Fleshman J, Anvari M, Stryker SJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. New England Journal of Medicine. 2004;350(20):2050–9. [DOI] [PubMed] [Google Scholar]
- 32.Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, et al. Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. British Journal of Cancer. 2008;98(1):54–9. 10.1038/sj.bjc.6604097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. The lancet oncology. 2006;7:309–15. [DOI] [PubMed] [Google Scholar]
- 34.Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 35.Bramhall SR, Hallissey MT, Whiting J, Scholefield J, Tierney G, Stuart RC, et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomised trial. British Journal of Cancer. 2002;86(12):1864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A, et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2002;20(17):3617–27. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New England Journal of Medicine. 2008;358(1):36–46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 38.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. Journal of Clinical Oncology. 2000;18(16):2938–47. [DOI] [PubMed] [Google Scholar]
- 39.Doeksen A, Bakx R, Vincent A, van Tets WF, Sprangers MA, Gerhards MF, et al. J-pouch vs side-to-end coloanal anastomosis after preoperative radiotherapy and total mesorectal excision for rectal cancer: a multicentre randomized trial. Colorectal Disease. 2012;14(6):705–13. doi: 10.1111/j.1463-1318.2011.02725.x. 10.1111/j.1463-1318.2011.02725.x [DOI] [PubMed] [Google Scholar]
- 40.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–7. [DOI] [PubMed] [Google Scholar]
- 41.Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2002;20(17):3605–16. [DOI] [PubMed] [Google Scholar]
- 42.Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Annals of Surgery. 2008;247(5):759–65. 10.1097/SLA.0b013e318167748c [DOI] [PubMed] [Google Scholar]
- 43.Fields AL, Keller A, Schwartzberg L, Bernard S, Kardinal C, Cohen A, et al. Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. Journal of Clinical Oncology. 2009;27(12):1941–7. 10.1200/JCO.2008.18.5710 [DOI] [PubMed] [Google Scholar]
- 44.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. Journal of Clinical Oncology. 2003;21(5):807–14. [DOI] [PubMed] [Google Scholar]
- 45.Furst A, Burghofer K, Hutzel L, Jauch KW. Neorectal reservoir is not the functional principle of the colonic J- pouch: the volume of a short colonic J-pouch does not differ from a straight coloanal anastomosis. DisColon Rectum. 2002;45(5):660–7. [DOI] [PubMed] [Google Scholar]
- 46.Gray B, Van HG, Hope M, Burton M, Moroz P, Anderson J, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Annals of Oncology. 2001;12(12):1711–20. [DOI] [PubMed] [Google Scholar]
- 47.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–26. [DOI] [PubMed] [Google Scholar]
- 48.Hoksch B, Ablassmaier B, Zieren J, Muller JM. Quality of life after gastrectomy: Longmire's reconstruction alone compared with additional pouch reconstruction. World J Surg. 2002;26(3):335–41. [DOI] [PubMed] [Google Scholar]
- 49.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. Journal of Clinical Oncology. 2007;25(21):3061–8. [DOI] [PubMed] [Google Scholar]
- 50.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncology. 2010;11(7):637–45. 10.1016/S1470-2045(10)70131-5 [DOI] [PubMed] [Google Scholar]
- 51.Kataria K, Verma GR, Malhotra A, Yadav R. Comparison of quality of life in patients undergoing transhiatal esophagectomy with or without chemotherapy. Saudi Journal of Gastroenterology. 2012;18(3):195–200. doi: 10.4103/1319-3767.96454. 10.4103/1319-3767.96454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). Journal of Clinical Oncology. 2006;24(9):1395–403. [DOI] [PubMed] [Google Scholar]
- 53.Kohne CH, Wils J, Lorenz M, Schoffski P, Voigtmann R, Bokemeyer C, et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. Journal of Clinical Oncology. 2003;21(20):3721–8. [DOI] [PubMed] [Google Scholar]
- 54.Lal R, Dickson J, Cunningham D, Chau I, Norman AR, Ross PJ, et al. A randomized trial comparing defined-duration with continuous irinotecan until disease progression in fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer. Journal of Clinical Oncology. 2004;22(15):3023–31. [DOI] [PubMed] [Google Scholar]
- 55.Maughan TS, James RD, Kerr DJ, Ledermann JA, McArdle C, Seymour MT, et al. Comparison of survival, palliation, and quality of life with three chemotherapy regimens in metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2002;359(9317):1555–63. [DOI] [PubMed] [Google Scholar]
- 56.Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002;360:671–7. [DOI] [PubMed] [Google Scholar]
- 57.Punt CJ, Keizer HJ, Douma J, Skovsgaard T, Schuller J, Muller EW, et al. Trimetrexate as biochemical modulator of 5-fluorouracil/leucovorin in advanced colorectal cancer: final results of a randomised European study. Annals of Oncology. 2002;13(1):81–6. [DOI] [PubMed] [Google Scholar]
- 58.Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. Journal of Clinical Oncology. 2004;22(19):3950–7. [DOI] [PubMed] [Google Scholar]
- 59.Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. Journal of Clinical Oncology. 2002;20(8):1996–2004. [DOI] [PubMed] [Google Scholar]
- 60.Sailer M, Fuchs KH, Fein M, Thiede A. Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. BrJ Surg. 2002;89(9):1108–17. [DOI] [PubMed] [Google Scholar]
- 61.Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. British Journal of Cancer. 2003;88(12):1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2000;343:905–14. [DOI] [PubMed] [Google Scholar]
- 63.Sobrero A, Zaniboni A, Frassineti GL, Aschele C, Guglielmi A, Giuliani R, et al. Schedule specific biochemical modulation of 5-fluorouracil in advanced colorectal cancer: a randomized study. GISCAD, IOR and collaborating centers. Annals of Oncology. 2000;11(11):1413–20. [DOI] [PubMed] [Google Scholar]
- 64.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. Journal of Clinical Oncology. 2008;26(14):2311–9. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 65.Tebbutt NC, Norman A, Cunningham D, Iveson T, Seymour M, Hickish T, et al. A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Annals of Oncology. 2002;13(10):1568–75. [DOI] [PubMed] [Google Scholar]
- 66.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. New England Journal of Medicine. 2009;360(6):563–72. 10.1056/NEJMoa0808268 [DOI] [PubMed] [Google Scholar]
- 67.van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Holzik MF, Grubben MJ, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial.[Erratum appears in Lancet Oncol. 2011 May;12(5):418]. Lancet Oncology. 2011;12(4):344–52. 10.1016/S1470-2045(11)70035-3 [DOI] [PubMed] [Google Scholar]
- 68.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Annals of Surgery. 2011;254(6):868–75. 10.1097/SLA.0b013e31821fd1ce [DOI] [PubMed] [Google Scholar]
- 69.Zachariah B, Gwede CK, James J, Ajani J, Chin LJ, Donath D, et al. Octreotide acetate in prevention of chemoradiation-induced diarrhea in anorectal cancer: randomized RTOG trial 0315. Journal of the National Cancer Institute. 2010;102(8):547–56. 10.1093/jnci/djq063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481).[see comment]. Journal of Clinical Oncology. 2006;24(9):1395–403. [DOI] [PubMed] [Google Scholar]
- 71.Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'Haese S, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: A checklist for evaluating HRQOL outcomes in cancer clinical trials—Does HRQOL evaluation in prostate cancer research inform clinical decision making? Journal of Clinical Oncology. 2003;21(18):3502–11. [DOI] [PubMed] [Google Scholar]
- 72.Lee CW, Chi KN. The standard of reporting of health-related quality of life in clinical cancer trials. Journal of Clinical Epidemiology. 2000;53(5):451–8. [DOI] [PubMed] [Google Scholar]
- 73.Efficace F, Bottomley A, van Andel G. Health related quality of life in prostate carcinoma patients—A systematic review of randomized controlled trials. Cancer. 2003;97(2):377–88. [DOI] [PubMed] [Google Scholar]
- 74.Efficace F, Horneber M, Lejeune S, Van Dam F, Leering S, Rottmann M, et al. Methodological quality of patient-reported outcome research was low in complementary and alternative medicine in oncology. Journal of Clinical Epidemiology. 2006;59(12):1257–65. [DOI] [PubMed] [Google Scholar]
- 75.Kong SX, Gandhi SK. Methodologic assessments of quality of life measures in clinical trials. Annals of Pharmacotherapy. 1997;31(7–8):830–6. [DOI] [PubMed] [Google Scholar]
- 76.Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. European Journal of Cancer. 2008;44(13):1793–8. 10.1016/j.ejca.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 77.Efficace F, Kemmler G, Vignetti M, Mandelli F, Molica S, Holzner B. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials—A systematic review to evaluate the added value in supporting clinical decision making. European Journal of Cancer. 2008;44(11):1497–506. 10.1016/j.ejca.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 78.Lipscomb J, Reeve BB, Clauser SB, Abrams JS, Bruner DW, Burke LB, et al. Patient-reported outcomes assessment in cancer trials: taking stock, moving forward. J Clin Oncol. 2007;25(32):5133–40. . [DOI] [PubMed] [Google Scholar]
- 79.Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Theberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001–2009). Journal of the National Cancer Institute. 2011;103:178–231. 10.1093/jnci/djq508 [DOI] [PubMed] [Google Scholar]
- 80.Ganz PA. Assessing the Quality and Value of Quality-of-Life Measurement in Breast Cancer Clinical Trials. Journal of the National Cancer Institute. 2011;103(3). [DOI] [PubMed] [Google Scholar]
- 81.Chan A- W, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–2. 10.1016/S0140-6736(12)62160-6 [DOI] [PubMed] [Google Scholar]
- 82.Davidson JR, Brundage MD, Feldman-Stewart D. Lung cancer treatment decisions: Patients' desires for participation and information. Psycho-Oncology. 1999;8(6):511–20. [DOI] [PubMed] [Google Scholar]
- 83.Brundage M, Feldman-Stewart D, Leis A, Bezjak A. The importance of quality of life information to a lung cancer (NSCLC) chemotherapy treatment decision—Results of a randomized evaluation. Psycho-Oncology. 2007;16(9):S7-S. [Google Scholar]
- 84.Feldman-Stewart D, Brundage MD, Hayter C, Groome P, Nickel JC, Downes H, et al. What questions do patients with curable prostate cancer want answered? Medical Decision Making. 2000;20(1):7–19. [DOI] [PubMed] [Google Scholar]
- 85.McNair AGK, Brookes ST, Davis CR, Argyropoulos M, Blazeby JM. Communicating the Results of Randomized Clinical Trials: Do Patients Understand Multidimensional Patient-Reported Outcomes? Journal of Clinical Oncology. 2010;28(5):738–43. 10.1200/JCO.2009.23.9111 [DOI] [PubMed] [Google Scholar]
- 86.Brundage M, Feldman-Stewart D, Leis A, Bezjak A, Degner L, Velji K, et al. Communicating quality of life information to cancer patients: A study of six presentation formats. Journal of Clinical Oncology. 2005;23(28):6949–56. [DOI] [PubMed] [Google Scholar]
- 87.Brundage M, Bass B, Jolie R, Foley K. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Quality of Life Research. 2011;20(7):979–85. 10.1007/s11136-011-9848-0 [DOI] [PubMed] [Google Scholar]
- 88.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Plos Medicine. 2009;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. British Medical Journal. 2010;340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.