Abstract
Introduction
The strength of evidence underpinning care and treatment recommendations in traumatic brain injury (TBI) is low. Comparative effectiveness research (CER) has been proposed as a framework to provide evidence for optimal care for TBI patients. The first step in CER is to map the existing variation. The aim of current study is to quantify variation in general structural and process characteristics among centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study.
Methods
We designed a set of 11 provider profiling questionnaires with 321 questions about various aspects of TBI care, chosen based on literature and expert opinion. After pilot testing, questionnaires were disseminated to 71 centers from 20 countries participating in the CENTER-TBI study. Reliability of questionnaires was estimated by calculating a concordance rate among 5% duplicate questions.
Results
All 71 centers completed the questionnaires. Median concordance rate among duplicate questions was 0.85. The majority of centers were academic hospitals (n = 65, 92%), designated as a level I trauma center (n = 48, 68%) and situated in an urban location (n = 70, 99%). The availability of facilities for neuro-trauma care varied across centers; e.g. 40 (57%) had a dedicated neuro-intensive care unit (ICU), 36 (51%) had an in-hospital rehabilitation unit and the organization of the ICU was closed in 64% (n = 45) of the centers. In addition, we found wide variation in processes of care, such as the ICU admission policy and intracranial pressure monitoring policy among centers.
Conclusion
Even among high-volume, specialized neurotrauma centers there is substantial variation in structures and processes of TBI care. This variation provides an opportunity to study effectiveness of specific aspects of TBI care and to identify best practices with CER approaches.
Introduction
Traumatic Brain Injury (TBI) is an important threat to public health with a crude incidence rate of up to 849 per 100,000 people in European countries [1, 2]. TBI is emerging as one of the leading causes of death and disability worldwide resulting in huge personal suffering and far-reaching socioeconomic consequences [3, 4].
Different perspectives on various aspects of care exist, and the evidence underpinning guideline recommendations for treatment of patients with TBI is weak [3, 5]. There is growing realization that randomized clinical trials alone will not be able to provide the evidence base that is needed to address these knowledge gaps [6]. Comparative effectiveness research (CER) has been proposed as a good complementary approach to strengthen the evidence base. CER has been defined as “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care” [7]. CER exploits between-center differences in patient management by comparing centers that perform a certain intervention routinely to others that do not. This approach is expected to be particularly suitable for TBI since large between-center differences in both patient management and outcomes have been previously reported [8, 9].
The Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study is a large-scale observational multicenter study focusing on characterization and CER in TBI. The first step for CER is to provide an overview of variation in structures and processes of care in the participating centers (‘provider profiling’). Such an overview can be used to identify areas where large between-center variation exists, to guide future CER analyses. But it can also directly be used for CER. For example, treatment effectiveness of a certain intervention can be studied by comparing outcome in patients from centers that routinely perform the intervention to outcome in patients from centers that do not routinely perform the intervention. Therefore, the objective of the current study is to quantify variation in general structure and process characteristics among centers participating in the CENTER-TBI study and to identify topics for CER.
Material and Methods
CENTER-TBI study
CENTER-TBI is a prospective longitudinal observational study conducted in 72 centers from 20 countries across Europe and Israel [3]. One of the global aims is to “identify the most effective clinical care and provide high-quality evidence in support of treatment recommendations and guidelines” [3]. This will be pursued by CER approaches. For more information, see also www.center-tbi.eu. Before the patient inclusion started, a detailed inventory of center characteristics was performed by distributing a set of questionnaires on structures and process of TBI care: The Provider Profiling (PP) questionnaires (S1 File). This set of questionnaires was distributed among 71 centers, since two CENTER-TBI centers represented different departments from the same hospital with similar structures and processes.
Development process of the Provider Profiling Questionnaires
The PP questionnaires went through a comprehensive developing process to warrant completeness and relevance of topics and face validity of questions. The neurotrauma evidencemap (http://neurotrauma.evidencemap.org/) was searched for gaps and inconsistencies in knowledge of optimal treatment and organization of TBI care, and used to define topics of interest. We included topics relevant for CER as well as topics relevant for descriptive analyses. Initial questions were formulated based on literature and suggestions from experts in the field. Available surveys and questionnaires in the field of TBI or critical care [10, 11] were searched for and used for the (re)formulation of (additional) questions.
Questions related either to structures or processes of general or TBI-specific care. Structure refers to the conditions under which patient care is provided (e.g. the number of beds, trauma center designation, hospital facilities), and process refers to activities that constitute patient care (e.g. general hospital or department policies) [12]. Structural information could be extracted from hospital databases, annual reports and local registries. Process information refers to general policies rather than individual treatment preferences of responsible physicians. General policy was defined as ‘the way the large majority of patients (>75%) with a certain indication would be treated’, recognizing that there might be exceptions. We included open questions and multiple-choice questions. All questions were presented with text boxes that contained definitions and a short explanation about the interpretation and completion of the question. The definitions used in this paper are summarized in the Supplemental material (S2 File).
Experts in the field provided feedback on the initial formulated questions and proposed new questions and topics in three subsequent phases. Consulted experts included neurosurgeons, (neuro)intensivists, neurologists, emergency department (ED) physicians, rehabilitation physicians, medical ethicists, health care economists and epidemiologists. Some of the consulted experts had previous experience with the design and conduct of surveys in the field of TBI or critical care. In a first phase, a small group of involved experts discussed the questionnaires during an email conversation and a group discussion. In a second phase, an international expert panel, consisting of 25 experts from 9 countries, was consulted per email. These experts provided feedback on one or more of the questionnaires. Decisions on proposed content and formulation were then made during a group discussion with a small group of involved experts. These draft PP questionnaires were then pilot-tested in 16 of the participating CENTER-TBI centers. Each center completed two or three questionnaires, such that each questionnaire was pilot-tested at least three times. All answers were checked for unexpected or missing values and ambiguous questions were subsequently reformulated or deleted. Pilot-testers additionally completed a form in which they were asked to provide feedback, which was incorporated accordingly. All these processes resulted in a final set of eleven questionnaires related to different phases of TBI care (see Table 1). In total, there were 321 questions included in the PP.
Table 1. Characteristics of the Provider Profiling questionnaires.
| Questionnaire | No. of questions | Topics |
|---|---|---|
| 1.General | 41 | Structural characteristics of the hospital, catchment area, volume, facilities, staffing characteristics, payment, equipment, costs |
| 2.Medical ethics | 17 | Department of medical ethics, IRB approval, informed consent procedures |
| 3. Prehospital trauma care | 28 | First aid initiatives, dispatch systems, emergency services, hospital reception and initial treatment |
| 4. Emergency department | 50 | Structural characteristics of the ED, imaging, guidelines, ED overcrowding, treatment, admission policy, discharge policy, withdrawal of life support |
| 5. Admission | 22 | Structural characteristics of the ward, admission policy, guidelines, observations, treatment policy, step down beds, discharge policy |
| 6. Structural and organizational aspects of the ICU | 27 | Structural characteristics of the ICU(s), staffing characteristics, admission policy, ICU decision making |
| 7. Treatment at the ICU | 70 | Protocol use, ICP- and CPP monitoring, sedation, non-surgical treatment of severe TBI patients, seizure prophylaxis, treatment of fever, DVT prophylaxis, mechanical ventilation |
| 8. Ethical aspects of the ICU | 20 | Withdrawal of life support, age and ICU admission |
| 9. Neurosurgery | 21 | Volume, staffing characteristics, decision making, protocols, surgical management of mass lesions |
| 10. Rehabilitation | 14 | In-hospital rehabilitation facilities, referral to post-acute care |
| 11. Country | 11 | Health care policy, dispatch systems, insurance |
Note. The provider profiling questionnaires consist of 11 separate questionnaires. Table shows number of questions and topics for each of the questionnaires.
Abbreviations. IRB = institutional review board, ED = emergency department, ICU = intensive care unit, ICP = intracranial pressure, CPP = cerebral perfusion pressure, TBI = traumatic brain injury, DVT = deep venous thrombosis prophylaxis
Distribution of the questionnaires
During presentations and workshops at two consecutive CENTER-TBI investigators meetings, information on the PP questionnaires was provided. Local investigators, as the senior persons supervising the CENTER-TBI study in the centers, were extensively informed in person and per email about the aim of the study and we emphasized the confidentiality of their responses. Additionally, to achieve unequivocal responses, we instructed them on how to respond to the process questions. We emphasized that we were asking for general policies, rather than individual treatment preferences and stimulated discussions with colleagues to identify the general policy of their department/center. Questionnaires were completed using a web-based system (Quesgen Systems Inc.) An instruction video was made available and any questions from local investigators were answered per email.
The local investigators in each center were responsible for the completion process in their center. Staff members with the appropriate expertise and knowledge needed to complete one or more questions or questionnaires. The local investigators were responsible for monitoring progress and checking face validity of all answers. The first author (MC) reminded local investigators regularly and answered any questions by email.
We aimed to receive completed questionnaires before centers started recruiting patients. As CENTER-TBI had a phased start of the inclusion period, PP questionnaires were completed between December 2014 and April 2016.
Questionnaire completion and data cleaning
A questionnaire was considered completed by a center if > 90% of the questions had been answered. Data from participating centers were included in the current paper if the center had completed the first PP questionnaire (‘general’), since the first questionnaire provides the general structure information necessary for provider profiles. The first author (MC) screened the completed questionnaires for missing values and contacted local investigators if any missings were present. They were asked to complete the missing data if possible or provide a reason for missingness. Data were further screened for outliers and local investigators were contacted to confirm values that were considered out of range.
Statistical analyses
To estimate reliability of the questionnaires, we included 17 (5%) duplicate questions, including all question formats. We equally included structure and process questions in the duplicate questions. Concordance rates were estimated by calculating the percentage of overlap between duplicate questions, and presented as mean, median and range. For open questions (e.g. what is the number of intensivist in your center), a maximum difference of 10% was considered concordant. For all hospital characteristics in this paper, frequencies and percentages were presented for categorical variables and medians and interquartile ranges (IQR) were presented for continuous variables. For a more in-depth understanding of the variation among centers, we checked whether there were differences between relatively high- and middle-income countries versus relatively lower-income countries, and also if there were differences between countries from different geographic locations (North and West Europe versus South and East Europe and Israel). We used the Chi-square test, and if appropriate, Fisher’s exact test to examine whether differences between groups were statistically significant (p < .05). The designation into relatively lower-income countries was based on a 2007 report by the European Commission [13]. Bosnia Herzegovina, Bulgaria, Hungary, Latvia, Lithuania, Romania and Serbia were subsequently classified as relatively lower-income countries. The subdivision into geographic location was based on the classification by the United Nations. Austria, Belgium, Denmark, Finland, France, Germany, Lithuania, the Netherlands, Norway, Sweden and the United Kingdom (UK) were subsequently classified as countries from West and North Europe, while all other countries were classified as countries from South and East Europe and Israel. Analyses were performed using the Statistical Package for Social Sciences (SPSS) version 21.
Results
Completion process
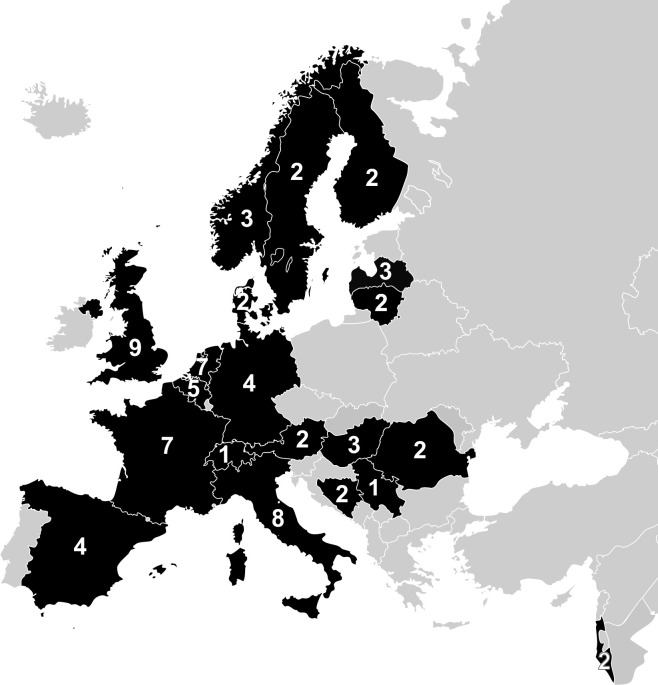
All 71 eligible centers completed the provider profiling questionnaire about general structural and process information. Questionnaires were completed by multiple persons per center, including neurologists, neurosurgeons, trauma surgeons, intensivists, research nurses and administrative staff members. The 71 centers were from 20 European countries (see Fig 1). Each country had 1 to 9 participating centers (median = 2.5). The United Kingdom (UK) had most centers participating (n = 9), while Serbia and Switzerland both had one participating center. Thirteen of the included centers were from relatively lower-income countries and 25 centers were from countries in South and East Europe (including Israel).
Fig 1. Centers and countries included in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study Note.
Reprinted and updated from Maas et al. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury: a prospective longitudinal observational study. Neurosurgery, 76:67–80, under a CC BY licence, with permission from professor A.I. Maas.
Reliability of the questionnaires
The median concordance rate between duplicate questions was 0.85 (mean: 0.81; range 0.44–0.97), meaning that 85% of the responses were similar. Concordance rates were lowest for questions about treatment policy (e.g. on what indications would you admit a patient with mild TBI to the ward) and for open questions (e.g. what is the number of intensivists working at your center). Most multiple-choice questions about structure had concordance rates above 0.90.
General structural characteristics
The participating centers were predominately academic centers (n = 65, 92%), designated as a level I or II trauma center (n = 54, n = 74%) and situated in an urban location (n = 70, 99%, see Table 2). The majority of participants indicated that they had access to a helicopter platform (n = 57, 80%) and an acute trauma team (n = 63, 89%). Around half of the centers (n = 40, 57%) had a dedicated neuro ICU. Centers from relatively high- and middle-income countries more often indicated that they have a dedicated neuro ICU (n = 35, 61%) than centers from relatively lower-income countries (n = 5, 39%, p = .13, S1 Table). The large majority of centers had participated previously in research about acute cerebral disorders. Fifty-one (72%) centers were involved in more than five neurotrauma research applications over the past five years (see Table 2).
Table 2. General structural characteristics of the participating centers (n = 71).
| Characteristic | N completed | N (%)* |
|---|---|---|
| Academic hospital (vs. non-Academic) | 71 | 65 (92%) |
| Trauma center designation | 71 | |
| - Level I | 48 (68%) | |
| - Level II | 4 (6%) | |
| - Level III | 1 (1%) | |
| - No designation / NA | 18 (25%) | |
| Urban location (vs. suburban and rural location) | 71 | 70 (99%) |
| Helicopter platform | 71 | 57 (80%) |
| Acute trauma team | 71 | 63 (89%) |
| The availability of a dedicated neuro ICU | 70 | 40 (57%) |
| Number of ICUs (median, IQR) | 69 | 3 (2–5) |
| The availability of an in-hospital rehabilitation unit | 70 | 36 (51%) |
| Neurotrauma research applications in the past 5 y | 71 | |
| - > 5 | 51 (72%) | |
| - 3–5 | 13 (18%) | |
| - 1–2 | 4 (6%) | |
| - 0 or unknown | 3 (4%) | |
| Distance nearest trauma center that receives patients with severe TBI (km, median, IQR) | 52 | 56 (17–100) |
Note. ICU = Intensive care unit; IQR = Interquartile Range
* Table presents number and percentage of centers unless otherwise specified
The median number of beds in the participating centers was 1000 (IQR 682–1395) of which 31 (IQR 22–44) were ICU beds (see Table 3 and S1 Fig). Centers had a median of 3 (IQR 2–6) resuscitation rooms at the ED and 24 (IQR 16–39) operating rooms. Three (IQR 2–4) of these were potentially available for TBI patients. The median number of annual ED visits was 53,428 (IQR 30,002–90,268). The median number of annual ICU admission was 1240 (IRQ 560–2019), of which 91 (IQR 52–160) were TBI patients.
Table 3. Volume characteristics of the participating centers (n = 71).
| Characteristic | N completed | Median (IQR) |
|---|---|---|
| Number of beds | ||
| Number of ED observational beds | 69 | 16 (7–32) |
| Number of hospital beds | 69 | 1000 (682–1395) |
| Number of ICU beds | 71 | 31 (22–44) |
| Number of resuscitation and operating rooms | ||
| Number of resuscitating rooms | 69 | 3 (2–6) |
| Number of operating rooms | 70 | 24 (16–39) |
| Number of operating rooms potentially available for TBI patientsA | 69 | 3 (2–4) |
| Number of patients | ||
| Annual ED visits | 63 | 53,428 (30,002–90,268) |
| Annual ICU admissions | 65 | 1240 (560–2019) |
| Number of TBI patients | ||
| Annual number of TBI patients at the ICU | 63 | 91 (52–160) |
| Annual neurosurgical procedures to evacuate contusion | 59 | 9 (4–21) |
| Annual decompressive craniectomies | 56 | 13 (8–22) |
Note. IQR = interquartile range; ED = emergency department; ICU = intensive care unit; TBI = traumatic brain injury; SAH = subarachnoid hemorrhage
A Operating rooms potentially available for TBI patients are the operating rooms that can be used for emergency and non-emergency TBI patients (e.g. trauma operating rooms, neurosurgical operating rooms etc). Rooms that are used for non-TBI surgery in TBI patients (e.g. orthopedic surgery in patients with multiple trauma) should be excluded here.
Seventy-five per cent (n = 53) of the centers had separate 24/7 emergency operation rooms. The majority of centers indicated that they had an electronic patient system at the ward (n = 57, 80%) and the ICU (n = 56, 79%). There was variation in the organization of the ICU in the participating centers; i.e. 45 (64%) centers had a closed ICU organization, 3 (4%) an open ICU organization and the remainder (n = 22, 32%) a mixed ICU organization. Centers from relatively high- and middle-income countries more often reported that they had a closed ICU structure (n = 40, 70%) compared to centers from relatively lower-income countries (n = 5, 39%). Step down beds were available in 71% (n = 50) of the centers. Centers from North and West Europe more often reported that they had a step down bed facility than centers from South and East Europe and Israel (n = 36, 80% vs. n = 14, 56%, p = .03, S1 Table). Maximum laboratorium turnaround times, the possibility for in-hospital coma stimulation and the location of TBI relevant facilities also varied widely among the included centers (see Table 4).
Table 4. Hospital facilities of the participating centers (n = 71).
| Characteristic | N completed | N (%) |
|---|---|---|
| General | ||
| Separate 24/7 emergency operation rooms | 71 | 53 (75%) |
| Electronic patient system | ||
| - Ward | 71 | 57 (80%) |
| - ICU | 71 | 56 (79%) |
| Facility for overnight observation | 69 | 54 (78%) |
| Lab turnaround time A | 68 | |
| - 0-30minutes | 25 (36%) | |
| - >30 minutes | 26 (38%) | |
| - NA. No lab SOP at the ED | 17 (25%) | |
| Organization of the ICU | 70 | |
| - Closed | 45 (64%) | |
| - Open | 3 (4%) | |
| - Mixed | 22 (32%) | |
| Step down beds | 70 | 50 (71%) |
| In-hospital coma stimulation | 70 | 34 (49%) |
| TBI related | ||
| Location TBI facilities | 71 | |
| - Different buildings | 20 (28%) | |
| - Same building, different floors | 45 (63%) | |
| - Same building, same floors | 6 (9%) |
Note. ICU = intensive care unit; NA = not applicable; SOP = Standard Operating Procedures; TBI = traumatic brain injury
A The laboratory turnaround times that are record in the lab Standard Operating Procedures (SOP) at the emergency department for severely injured patients
On average 14 neurologists, 10 neurosurgeons, 17 intensivists, 4 trauma surgeons and 10 ED physicians were working in the centers (see Table 5). Nearly all centers (n = 69, 97%) had at least one residency program for trainees towards becoming a specialist. The specialist most often in charge of TBI patients at respectively the ED, ward and ICU were predominately ED physicians, neurosurgeons and intensivists. Most centers had 24/7 in-house availability of OR personnel (n = 62, 87%) and CT technicians (n = 66, 93%). Median intensivist-to-patient ratio, and ICU nurse-to-patient ratio were 1: 5 (IQR 1:3 to 1:8) and 1:2 (IQR 1:1 to 1:3). Night coverage at the ICU was performed by a certified intensivist in two-third of the centers (n = 44, 65%) and by a trainee or fellow in the remainder of centers. Almost all centers from the relatively lower-income countries (n = 12, 92%) reported that night coverage was performed by a certified intensivist, in comparison to 58% of the centers from the relatively high- and middle-income countries. Also, more centers from South and East Europe (n = 22, 88%) had night coverage by a certified intensivist, compared to centers from North and West Europe (n = 22, 51%, S1 Table).
Table 5. Staffing characteristics of the participating centers (n = 71).
| Characteristic | N completed | N (%)* |
|---|---|---|
| Number of specialists (median, IQR) A | ||
| - Neurologist | 71 | 14 (8–21) |
| - Neurosurgeon | 68 | 10 (7–13) |
| - Intensivist | 68 | 17 (10–28) |
| - Trauma surgeon | 68 | 4 (0–10) |
| - ED physician | 69 | 10 (3–19) |
| Residency programs | ||
| - Neurologist | 70 | 65 (93%) |
| - Neurosurgeon | 71 | 67 (94%) |
| - Intensivist | 71 | 64 (90%) |
| - Trauma surgeon | 71 | 36 (51%) |
| Availability OR personnel | 71 | |
| - 24/7 in-house availability | 62 (87%) | |
| - On call within 30 minutes | 9 (13%) | |
| Availability CT technicians | 71 | |
| - 24/7 in-house availability | 66 (93%) | |
| - On call within 30 minutes | 5 (7%) | |
| Intensivist-to-patient ratio (median, IQR) | 69 | 1: 5 (1: 3–1: 8) |
| ICU nurse-to-patient ratio (median, IQR) | 69 | 1: 2 (1: 1–1: 3) |
| Night coverage ICU | 68 | |
| - Certified intensivist/ ICU physician | 44 (65%) | |
| - Trainee (in residency training) | 20 (29%) | |
| - Fellow in training for ICU | 4 (6%) |
Note. IQR = interquartile range; ED = emergency department; OR = operating rooms; CT = computed tomography
* Table presents number and percentage of centers unless otherwise specified
A Number of specialists is displayed per 40-hour workweek.
General process characteristics
With regard to computed tomography (CT) scanning in patients with mild TBI at the ED, 79% of the centers (n = 54) indicated to use CT guidelines (see Table 6). In addition, seven centers (10%) from Austria, Denmark, France, Spain and Sweden routinely determine S100B as a prognostic biomarker for neurological deterioration at the ED. There was variation among centers in their ICU admission policy; i.e. 44 (64%) centers generally admit patients with moderate TBI (Glasgow Coma Scale (GCS) 9–12) and CT abnormalities to the ICU, while 25 (36%) centers only admit these patients to the ICU in the presence of other risk factors. This variation was also shown for moderate TBI patients without CT abnormalities and patients with mild TBI on anti-coagulant therapy. There was a trend towards a higher ICU admission rate in centers from relatively high- and middle-income countries than in centers from relatively lower-income countries (S2 Table).
Table 6. General process information of the participating centers (n = 71).
| Characteristic | N Completed | N (%) |
|---|---|---|
| Emergency department | ||
| Use of CT scan guidelines at the ED | 68 | 54 (79%) |
| Routine use of S100B as prognostic biomarker at the ED | 71 | 7 (10%) |
| ICU admission policy | ||
| Patients with moderate TBI (GCS 9–12) without CT abnormalities are admitted to the ICU | 69 | |
| - No or only in the presence of other risk factors | 50 (72%) | |
| - General policy | 19 (28%) | |
| Patients with moderate TBI (GCS 9–12) with CT abnormalities are admitted to the ICU | 69 | |
| - No or only in the presence of other risk factors | 25 (36%) | |
| - General policy | 44 (64%) | |
| Patients with mild TBI (GCS 13–15) using anti-coagulant therapy are admitted to the ICU | 69 | |
| - No or only in the presence of other risk factors | 53 (77%) | |
| - General policy | 16 (23%) | |
| ICP monitoring | ||
| ICP monitoring is performed in patients with GCS<9 and CT abnormalities | 67 | |
| - No or only in the presence of other risk factors | 6 (9%) | |
| - General policy | 61 (91%) | |
| ICP monitoring is performed in patients with GCS<9 without CT abnormalities | 67 | |
| - No or only in the presence of other risk factors | 52 (78%) | |
| - General policy | 15 (22%) | |
| ICP monitoring is performed in patients with intraventricular hemorrhages | 67 | |
| - No or only in the presence of other risk factors | 46 (69%) | |
| - General policy | 21 (31%) | |
| ICP sensors that are used at the ICU: | 67 | |
| - Parenchymal | 21 (31%) | |
| - Ventricular | 6 (9%) | |
| - Both | 40 (60%) | |
| Management of elevated ICP | ||
| Threshold for medical management of elevated ICP | 66 | |
| - >15mmHg | 3 (5%) | |
| - >20mmHg | 57 (86%) | |
| - >25mmHg | 6 (9%) | |
| Threshold for decompressive craniotomy in elevated ICP | 61 | |
| - >20mmHg | 7 (12%) | |
| - >25mmHg | 35 (57%) | |
| - >30mmHg | 19 (31%) | |
| ICU policies | ||
| Structural variation between (neuro)surgeons with regard to their decision to place an ICP sensor | 69 | 33 (48%) |
| General policy with regard to the management of extremity fractures in patients with sTBI | 68 | |
| - Damage control | 58 (85%) | |
| - Definitive care | 10 (15%) |
Note. CT = computed tomography; ED = emergency department; ICU = intensive care unit; ICP = intracranial pressure; BTF = Brain Trauma Foundation; GCS = Glasgow Coma Scale; sTBI = severe traumatic brain injury
The large majority of participants (n = 61, 91%) indicated that their general policy is to insert intracranial pressure (ICP) monitors in patients with GCS <9 and CT abnormalities. However, centers vary in whether they would place an ICP monitor in patients with GCS <9 without CT abnormalities and patients with intraventricular haemorrhages. Variation in ICP monitoring is also reported within the centers, since half of the centers indicated that there is structural variation between (neuro)surgeons in their center with regard to the decision to place an ICP monitor. The threshold for medical management of elevated ICP was 20 mmHg in the large majority of centers (n = 57, 87%). However, centers varied widely in their threshold for decompressive craniotomy; i.e. in 12% (n = 7) the threshold was 20 mmHg, in 57% (n = 35) the threshold was 25 mmHg and in 31% (n = 19) the threshold was 30 mmHg.
Insurance and payment systems
In the majority of countries (n = 16, 80%), a health care insurance was compulsory for all inhabitants. In 45% of the countries (n = 9), patients nevertheless had to pay a part of the delivered care themselves via either a co-payment (5 countries) or a deductible (4 countries). Most centers were funded by the government (n = 60; 85%). Centers typically got reimbursed by all-in amounts per patient rather than by payment for individual interventions. Most doctors received a fixed monthly salary (n = 58, 82%). In 11% (n = 8) of the centers, doctors received an additional fee for services. Twenty-three (32%) centers received additional payment for the treatment of privately insured patients.
Discussion
We found considerable variation in general structure and process characteristics among 71 specialized neurotrauma centers participating in the CENTER-TBI study. Most of these centers were high-volume academic level I trauma centers situated in an urban location. Centers varied widely in their ICU organization, hospital facilities and admission- and treatment policies. The effectiveness of these structures and interventions can therefore adequately be studied with CER.
Our provider profiling questionnaires have strengths and limitations. One of the strengths is the comprehensive development process, which consisted of several stages and involved many experts. As a consequence, the questionnaires address all aspects relevant to TBI care. Secondly, local investigators were extensively informed about the aim, procedures and practical issues during presentations, workshops and emails. This might explain the 100% response rate. The length of our questionnaires can be regarded as a limitation. Long questionnaires have been associated with lower data quality [14, 15], an effect that is often due to fatigue and boredom [15]. Since the questionnaires could be spread over time and over different persons, the negative effect of length was however confined.
Another limitation of our study concerns the generalizability of our findings. The included centers comprise a group of neurotrauma centers participating in a European multicenter study. Our findings therefore cannot be generalized to all centers caring for neurotrauma patients in Europe. Furthermore, our study provides information on what centers reported rather than characteristics that were directly observed. Therefore, we cannot exclude that some of our findings provide a too optimistic picture. For example, almost all centers indicated that they would insert an ICP monitor in patients with severe TBI and CT abnormalities, which is recommended by Brain Trauma Foundation guidelines. However, a systematic review about guideline adherence reported that ICP monitoring guidelines were only followed in one-third of the patients [5]. Later, results from the ongoing CENTER-TBI study will provide insight into discrepancies between reported and actual policies in the participating centers.
The concordance rate between duplicate questions (median: 0.85), indicates a certain degree of subjectivity in the responses. The concordance rate was especially low for process questions, which indicates that there might be differences in policy among wards and doctors, no clear policy at all or difficulties in understanding and interpreting the questions. It might also indicate that some of the doctors that completed the questionnaire might not be representative of their department or center. Although our concordance rate was very similar to a 2001 survey study among European countries [11], results on process characteristics should be interpreted with caution. The reported concordance rate does not account for chance concordance since no statistical measures are available that do account for chance and can also provide one figure for different outcomes (dichotomous, categorical and continuous) that we had in our questionnaire. When interpreting the concordance rate, it should however be acknowledged that some answers might be similar by chance.
Finally, there were only 13 centers from a relatively lower-income country and 25 centers from South and East Europe (including Israel). We therefore had limited power to detect differences between centers from relatively high-and middle-income countries versus centers from relatively lower-income countries and centers from different geographic locations.
Although we studied a sample of highly specialized centers, we found substantial differences in important structural and process characteristics. Largest differences were seen in the specialization and organization of the ICU, i.e. half of the centers indicated to have a dedicated neuro ICU and 64% indicated to have a closed ICU organization. Additionally, rehabilitation facilities varied widely, with half of the centers having an in-hospital rehabilitation unit and the possibility for coma stimulation. We also found large differences in the reported policies regarding ICU admission and ICP monitoring across centers. The variation in structure and process among specialized neurotrauma centers was in line with previous survey studies [11, 12]. Enblad and associates [11] included European centers with a particular interest in neuro ICU and brain monitoring in their survey study. They also found large between-center differences in structures of care (e.g. 76% had a separate NICU, 50% had a neurosurgeon as ICU director). Checkley and associates [12] reported similar findings. They conducted a survey in 69 centers participating in the United States critical illness and injury outcome study. The majority of their centers were teaching hospitals with critical care training. However, 58% of their centers had a closed ICU organization and their annual hospital admission rate ranged from 1,170 to 56,330, indicating large between-center differences in volume. Also there were large differences in the protocols available at their surveyed ICUs.
Although in this study we only reported on general structure and process characteristics, it is clear that the between-center variation is substantial and provides an opportunity for CER. Variation among centers and countries comprises an important prerequisite for CER and enables between-center and between-country comparisons of effective structures and processes of care. We can for example study the influence of a dedicated neuro ICU on outcome in severe TBI patients by studying patients’ outcome in the 40 centers with a dedicated neuro ICU and in the 30 centers without a dedicated neuro ICU. This requires outcome data on patient level, which are currently collected in the CENTER-TBI study. In such a comparison it is important to correct for differences in other structural and process characteristics between these centers, which can potentially be accomplished with advanced statistical modelling. Other potential interesting topics for CER based on the current study include the effectiveness of an in-hospital rehabilitation unit, the effectiveness of high-volume vs. low-volume hospitals, the effectiveness of closed vs. mixed ICU organization, and the effectiveness of admission- and ICP monitoring policies.
Conclusion
Even among high-volume, specialized neurotrauma centers there is substantial variation in structures and processes of TBI care. This variation provides an opportunity to study effectiveness of specific aspects of TBI care and to identify best practices with CER approaches.
Supporting Information
(PDF)
(PDF)
Note. Table presents all definitions used in the paper in the order that they are used in the results section of the paper. TBI = traumatic brain injury; ICU = intensive care unit
(PDF)
A P-value for the difference between high/middle and low income countries B P-value for the difference between North-West and South-East Europe and Israel
(PDF)
A P-value for the difference between high/middle and low income countries B P-value for the difference between North-West and South-East Europe and Israel
(PDF)
Acknowledgments
The authors would like to thank all CENTER-TBI investigators and their staff, who are listed below, for completing the provider profiling questionnaires. Authors would further like to thank Nada Andelic, Sasha Brazinova, Ruben van der Brande, Peter Cameron, Guiseppe Citerio, Ari Ercole, Thomas van Essen, Mathieu van der Jagt, Erwin Kompanje, Fiona Lecky, Joukje van der Naalt, David Nelson, Wilco Peul, Jukka Ranta, Cecilia Roe, Gerard Ribbers, Nino Stochetti, Olli Tenovuo and Lindsay Wilson for their help with the development of the provider profiling questionnaires.
CENTER-TBI investigators and participants
Principal Investigators and contact information:
Professor A.I. Maas: Andrew.Maas@uza.be
Professor D. Menon: dkm13@wbic.cam.ac.uk
Adams Hadie 1, Alessandro Masala 2, Allanson Judith 3, Amrein Krisztina 4, Andaluz Norberto 5, Andelic Nada 6, Andrea Nanni 2, Andreassen Lasse 7, Anke Audny 8, Antoni Anna 9, Ardon Hilko 10, Audibert Gérard 11, Auslands Kaspars 12, Azouvi Philippe 13, Baciu Camelia 14, Bacon Andrew 15, Badenes Rafael 16, Baglin Trevor 17, Bartels Ronald 18, Barzó Pál 19, Bauerfeind Ursula 20, Beer Ronny 21, Belda Francisco Javier 16, Bellander Bo-Michael 22, Belli Antonio 23, Bellier Rémy 24, Benali Habib 25, Benard Thierry 24, Berardino Maurizio 26, Beretta Luigi 27, Beynon Christopher 28, Bilotta Federico 16, Binder Harald 9, Biqiri Erta 14, Blaabjerg Morten 29, Borgen Lund Stine 30, Bouzat Pierre 31, Bragge Peter 32, Brazinova Alexandra 33, Brehar Felix 34, Brorsson Camilla 35, Buki Andras 36, Bullinger Monika 37, Bučková Veronika 33, Calappi Emiliana 38, Cameron Peter 39, Carbayo Lozano Guillermo 40, Carise Elsa 24, Carpenter K. 41, Castaño-León Ana M. 42, Causin Francesco 43, Chevallard Giorgio 14, Chieregato Arturo 14, Citerio Giuseppe 44, 45, Cnossen Maryse 46, Coburn Mark Coburn 47, Coles Jonathan 48, Cooper Jamie D. 49, Correia Marta 50, Covic Amra 51, Curry Nicola 52, Czeiter Endre 53, Czosnyka Marek 54, Dahyot-Fizelier Claire 24, Damas François 55, Damas Pierre 56, Dawes Helen 57, De Keyser Véronique 58, Della Corte Francesco 59, Depreitere Bart 60, Ding Shenghao 61, Dippel Diederik 62, Dizdarevic Kemal 63, Dulière Guy-Loup 55, Dzeko Adelaida 64, Eapen George 15, Engemann Heiko 51, Ercole Ari 65, Esser Patrick 57, Ezer Erzsébet 66, Fabricius Martin 67, Feigin Valery L. 68, Feng Junfeng 61, Foks Kelly 62, Fossi Francesca 14, Francony Gilles 31, Frantzén Janek 69, Freo Ulderico 70, Frisvold Shirin 71, Furmanov Alex 72, Gagliardo Pablo 73, Galanaud Damien 25, Gao Guoyi 74, Geleijns Karin 41, Ghuysen Alexandre 75, Giraud Benoit 24, Glocker Ben 76, Gomez Pedro A. 42, Grossi Francesca 59, Gruen Russell L. 77, Gupta Deepak 78, Haagsma Juanita A. 46, Hadzic Ermin 64, Haitsma Iain 79, Hartings Jed A. 80, Helbok Raimund 21, Helseth Eirik 81, Hertle Daniel 28, Hill Sean 82, Hoedemaekers Astrid 83, Hoefer Stefan 51, Hutchinson Peter J. 1, Håberg Asta Kristine 84, Jacobs Bram 85, Janciak Ivan 86, Janssens Koen 58, Jiang Ji-yao 74, Jones Kelly 87, Kalala Jean-Pierre 88, Kamnitsas Konstantinos 76, Karan Mladen 89, Karau Jana 20, Katila Ari 69, Kaukonen Maija 90, Keeling David 52, Kerforne Thomas 24, Ketharanathan Naomi 41, Kettunen Johannes 91, Kivisaari Riku 90, Kolias Angelos G. 1, Kolumbán Bálint 92, Kompanje Erwin 93, Kondziella Daniel 67, Koskinen Lars-Owe 35, Kovács Noémi 92, Kálovits Ferenc 94, Lagares Alfonso 42, Lanyon Linda 82, Laureys Steven 95, Lauritzen Martin 67, Lecky Fiona 96, Ledig Christian 76, Lefering Rolf 97, Legrand Valerie 98, Lei Jin 61, Levi Leon 99, Lightfoot Roger 100, Lingsma Hester 46, Loeckx Dirk 101, Lozano Angels 16, Luddington Roger 17, Luijten-Arts Chantal 83, Maas Andrew I.R. 58, MacDonald Stephen 17, MacFayden Charles 65, Maegele Marc 102, Majdan Marek 33, Major Sebastian 103, Manara Alex 104, Manhes Pauline 31, Manley Geoffrey 105, Martin Didier 106, Martino Costanza 2, Maruenda Armando 16, Maréchal Hugues 55, Mastelova Dagmara 86, Mattern Julia 28, McMahon Catherine 107, Melegh Béla 108, Menon David 65, Menovsky Tomas 58, Morganti-Kossmann Cristina 109, Mulazzi Davide 38, Mutschler Manuel 102, Mühlan Holger 110, Negru Ancuta 111, Nelson David 82, Neugebauer Eddy 102, Newcombe Virginia 65, Noirhomme Quentin 95, Nyirádi József 4, Oddo Mauro 112, Oldenbeuving Annemarie 113, Oresic Matej 114, Ortolano Fabrizio 38, Palotie Aarno 91, 115, 116, Parizel Paul M. 117, Patruno Adriana 118, Payen Jean-François 31, Perera Natascha 119, Perlbarg Vincent 25, Persona Paolo 120, Peul Wilco 121, Pichon Nicolas 122, Piilgaard Henning 67, Piippo Anna 90, Pili Floury Sébastien 123, Pirinen Matti 91, Ples Horia 111, Polinder Suzanne 46, Pomposo Inigo 40, Psota Marek 33, Pullens Pim 117, Puybasset Louis 124, Ragauskas Arminas 125, Raj Rahul 90, Rambadagalla Malinka 126, Rehorčíková Veronika 33, Rhodes Jonathan 127, Richardson Sylvia 128, Ripatti Samuli 91, Rocka Saulius 125, Rodier Nicolas 122, Roe Cecilie 129, Roise Olav 130, Roks Gerwin 131, Romegoux Pauline 31, Rosand Jonathan 132, Rosenfeld Jeffrey 109, Rosenlund Christina 133, Rosenthal Guy 72, Rossaint Rolf 47, Rossi Sandra 120, Rostalski Tim 110, Rueckert Daniel 76, Ruiz de Arcaute Felix 101, Rusnák Martin 86, Sacchi Marco 14, Sahakian Barbara 65, Sahuquillo Juan 134, Sakowitz Oliver 135, 136, Sala Francesca 118, Sanchez-Pena Paola 25, Sanchez-Porras Renan 28, 135, Sandor Janos 137, Santos Edgar 28, Sasse Nadine 51, Sasu Luminita 59, Savo Davide 118, Schipper Inger 138, Schlößer Barbara 20, Schmidt Silke 110, Schneider Annette 97, Schoechl Herbert 139, Schoonman Guus 131, Schou Rico Frederik 140, Schwendenwein Elisabeth 9, Schöll Michael 28, Sir Özcan 141, Skandsen Toril 142, Smakman Lidwien 143, Smeets Dirk 101, Smielewski Peter 54, Sorinola Abayomi 144, Stamatakis Emmanuel 65, Stanworth Simon 52, Stegemann Katrin 110, Steinbüchel Nicole 145, Stevens Robert 146, Stewart William 147, Steyerberg Ewout W. 46, Stocchetti Nino 148, Sundström Nina 35, Synnot Anneliese 149, 150, Szabó József 94, Söderberg Jeannette 82, Taccone Fabio Silvio 16, Tamás Viktória 144, Tanskanen Päivi 90, Tascu Alexandru 34, Taylor Mark Steven 33, Te Ao Braden 68, Tenovuo Olli 69, Teodorani Guido 151, Theadom Alice 68, Thomas Matt 104, Tibboel Dick 41, Tolias Christos 152, Tshibanda Jean-Flory Luaba 153, Tudora Cristina Maria 111, Vajkoczy Peter 154, Valeinis Egils 155, Van Hecke Wim 101, Van Praag Dominique 58, Van Roost Dirk 88, Van Vlierberghe Eline 101, Vande Vyvere Thijs 101, Vanhaudenhuyse Audrey 25, 95, Vargiolu Alessia 118, Vega Emmanuel 156, Verheyden Jan 101, Vespa Paul M. 157, Vik Anne 158, Vilcinis Rimantas 159, Vizzino Giacinta 14, Vleggeert-Lankamp Carmen 143, Volovici Victor 79, Vulekovic Peter 89, Vámos Zoltán 66, Wade Derick 57, Wang Kevin K.W. 160, Wang Lei 61, Wildschut Eno 41, Williams Guy 65, Willumsen Lisette 67, Wilson Adam 5, Wilson Lindsay 161, Winkler Maren K.L. 103, Ylén Peter 162, Younsi Alexander 28, Zaaroor Menashe 99, Zhang Zhiqun 163, Zheng Zelong 28, Zumbo Fabrizio 2, de Lange Stefanie 97, de Ruiter Godard C.W. 143, den Boogert Hugo 18, van Dijck Jeroen 164, van Essen Thomas A. 121, van Heugten Caroline 57, van der Jagt Mathieu 165, van der Naalt Joukje 85
1 Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital & University of Cambridge, Cambridge, UK
2 Department of Anesthesia & Intensive Care,M. Bufalini Hospital, Cesena, Italy
3 Department of Clinical Neurosciences, Addenbrooke’s Hospital & University of Cambridge, Cambridge, UK
4 János Szentágothai Research Centre, University of Pécs, Pécs, Hungary
5 University of Cincinnati, Cincinnati, Ohio, United States
6 Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway
7 Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway
8 Department of Physical Medicine and Rehabilitation, University hospital Northern Norway
9 Trauma Surgery, Medical University Vienna, Vienna, Austria
10 Department of Neurosurgery, Elisabeth-Tweesteden Ziekenhuis, Tilburg, the Netherlands
11 Department of Anesthesiology & Intensive Care, University Hospital Nancy, Nancy, France
12 Riga Eastern Clinical University Hospital, Riga, Latvia
13 Raymond Poincare hospital, Assistance Publique–Hopitaux de Paris, Paris, France
14 NeuroIntensive Care, Niguarda Hospital
15 Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
16 Department Anesthesiology and Surgical-Trauma Intensive Care, Hospital Clinic Universitari de Valencia, Spain
17 Cambridge University Hospitals, Cambridge, UK
18 Department of Neurosurgery, Radboud University Medical Center
19 Department of Neurosurgery, University of Szeged, Szeged, Hungary
20 Institute for Transfusion Medicine (ITM), Witten/Herdecke University, Cologne, Germany
21 Department of Neurocritical care, Innsbruck Medical University, Innsbruck, Austria
22 Deparment of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden
23 NIHR Surgical Reconstruction and Microbiology Research Centre, Birmingham, UK
24 Intensive care Unit, CHU Poitiers, Poitiers, France
25 Anesthesie-Réanimation, Assistance Publique–Hopitaux de Paris, Paris, France
26 Department of Anesthesia & ICU, AOU Città della Salute e della Scienza di Torino—Orthopedic and Trauma Center, Torino, Italy
27 Department of Anesthesiology & Intensive Care, S Raffaele University Hospital, Milan, Italy
28 Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany
29 Department of Neurology, Odense University Hospital, Odense, denmark
30 Departments of Neuroscience and Nursing Science, Norwegian University of Science and Technology, Trondheim, Norway
31 Department of Anesthesiology & Intensive Care, University Hospital of Grenoble, Grenoble, France
32 BehaviourWorks Australia, Monash Sustainability Institute, Monash University, Victoria, Australia
33 Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia
34 Department of Neurosurgery, Bagdasar-Arseni Emergency Clinical Hospital, Bucharest, Romania
35 Department of Neurosurgery, Umea University Hospital, Umea, Sweden
36 Department of Neurosurgery, University of Pecs and MTA-PTE Clinical Neuroscience MR Research Group and Janos Szentagothai Research Centre, University of Pecs, Hungarian Brain Research Program, Pecs, Hungary
37 Department of Medical Psychology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany
38 Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
39 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
40 Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain
41 Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, The Netherlands
42 Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain
43 Department of Neuroscience, Azienda Ospedaliera Università di Padova, Padova, Italy
44 NeuroIntensive Care, Azienda Ospedaliera San Gerardo di Monza, Monza, Italy
45 School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy
46 Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands
47 Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany
48 Department of Anesthesia & Neurointensive Care, Cambridge Universiyt Hospital NHS Foundation Trust, Cambridge, UK
49 School of Public Health & PM, Monash University and The Alfred Hospital, Melbourne, Victoria, Australia
50 Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, UK
51 Institute of Medical Psycholology and Medical Sociology, Universitätsmedizin Göttingen, Göttingen, Germany
52 Oxford University Hospitals NHS Trust, Oxford, UK
53 Department of Neurosurgery, University of Pecs and MTA-PTE Clinical Neuroscience MR Research Group and Janos Szentagothai Research Centre, University of Pecs, Hungarian Brain Research Program (Grant No. KTIA 13 NAP-A-II/8), Pecs, Hungary
54 Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
55 Intensive Care Unit, CHR Citadelle, Liège, Belgium
56 Intensive Care Unit, CHU, Liège, Belgium
57 Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK
58 Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium
59 Department of Anesthesia & Intensive Care, Maggiore Della Carità Hospital, Novara, Italy
60 Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium
61 Department of Neurosurgery, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
62 Department of Neurology, Erasmus MC, Rotterdam, the Netherlands
63 Department of Neurosurgery, Medical Faculty and clinical center University of Sarajevo, Sarajevo, Bosnia Herzegovina
64 Department of Neurosurgery, Regional Medical Center dr Safet Mujić, Mostar, Bosnia Herzegovina
65 Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
66 Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary
67 Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark
68 National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand
69 Rehabilitation and Brain Trauma, Turku University Central Hospital and University of Turku, Turku, Finland
70 Department of Medicine, Azienda Ospedaliera Università di Padova, Padova, Italy
71 Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway
72 Department of Neurosurgery, Hadassah-hebrew University Medical center, Jerusalem, Israel
73 Fundación Instituto Valenciano de Neurorrehabilitación (FIVAN), Valencia, Spain
74 Department of Neurosurgery, Shanghai Renji hospital, Shanghai Jiaotong University/school of medicine, Shanghai, China
75 Emergency Department, CHU, Liège, Belgium
76 Department of Computing, Imperial College London, London, UK
77 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore; and Monash University, Australia
78 Department of Neurosurgery, Neurosciences Centre & JPN Apex trauma centre, All India Institute of Medical Sciences, New Delhi-110029, India
79 Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands
80 Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, USA
81 Department of Neurosurgery, Oslo University Hospital, Oslo, Norway
82 Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden
83 Department of Intensive Care Medicine, Radboud University Medical Center
84 Department of Medical Imaging, St. Olavs Hospital and Department of Neuroscience, Norwegian University of Science and Technology, Trondheim, Norway
85 Department of Neurology, University Medical Center Groningen, Groningen, Netherlands
86 International Neurotrauma Research Organisation, Vienna, Austria
87 National Institute for Stroke & Applied Neurosciences of the AUT University, Auckland, New Zealand
88 Department of Neurosurgery, UZ Gent, Gent, Belgium
89 Department of Neurosurgery, Clinical centre of Vojvodina, Novi Sad, Serbia
90 Helsinki University Central Hospital
91 Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland
92 Hungarian Brain Research Program—Grant No. KTIA 13 NAP-A-II/8, University of Pécs, Pécs, Hungary
93 Department of Intensive Care and Department of Ethics and Philosophy of Medicine, Erasmus Medical Center, Rotterdam, The Netherlands
94 Department of Neurological & Spinal Surgery, Markusovszky University Teaching Hospital, Szombathely, Hungary
95 Cyclotron Research Center, University of Liège, Liège, Belgium
96 Emergency Medicine Research in Sheffield, Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
97 Institute of Research in Operative Medicine (IFOM), Witten/Herdecke University, Cologne, Germany
98 VP Global Project Management CNS, ICON, Paris, France
99 Department of Neurosurgery, Rambam Medical Center, Haifa, Israel
100 Department of Anesthesiology & Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, UK
101 icoMetrix NV, Leuven, Belgium
102 Cologne-Merheim Medical Center (CMMC), Department of Traumatology, Orthopedic Surgery and Sportmedicine, Witten/Herdecke University, Cologne, Germany
103 Centrum für Schlaganfallforschung, Charité–Universitätsmedizin Berlin, Berlin, Germany
104 Intensive Care Unit, Southmead Hospital, Bristol, Bristol, UK
105 Department of Neurological Surgery, University of California, San Francisco, California, USA
106 Department of Neurosurgery, CHU, Liège, Belgium
107 Department of Neurosurgery, The Walton centre NHS Foundation Trust, Liverpool, UK
108 Department of Medical Genetics, University of Pécs, Pécs, Hungary
109 National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, Victoria, Australia
110 Department Health and Prevention, University Greifswald, Greifswald, Germany
111 Department of Neurosurgery, Emergency County Hospital Timisoara, Timisoara, Romania
112 Centre Hospitalier Universitaire Vaudois
113 Department of Intensive Care, Elisabeth-Tweesteden Ziekenhuis, Tilburg, the Netherlands
114 Department of Systems Medicine, Steno Diabetes Center, Gentofte, Denmark
115 Analytic and Translational Genetics Unit, Department of Medicine; Psychiatric & Neurodevelopmental Genetics Unit, Department of Psychiatry; Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
116 Program in Medical and Population Genetics; The Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, Cambridge, MA, USA
117 Department of Radiology, Antwerp University Hospital and University of Antwerp, Edegem, Belgium
118 NeuroIntenisve Care Unit, Department of Anesthesia & Intensive Care Azienda Ospedaliera San Gerardo di Monza, Monza, Italy
119 International Projects Management, ARTTIC, Munchen, Germany
120 Department of Anesthesia & Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy
121 Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands
122 Intensive Care Unit, CHU Dupuytren, Limoges, France
123 Intensive Care Unit, CHRU de Besançon, Besançon, France
124 Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France
125 Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania
126 Rezekne Hospital, Latvia
127 Department of Anaesthesia, Critical Care & Pain MedicineNHS Lothian & University of Edinburg, Edinburgh, UK
128 Director, MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, UK
129 Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway
130 Division of Surgery and Clinical Neuroscience, Oslo University Hospital, Oslo, Norway
131 Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, the Netherlands
132 Broad Institute, Cambridge MA Harvard Medical School, Boston MA, Massachusetts General Hospital, Boston MA, USA
133 Department of Neurosurgery, Odense University Hospital, Odense, Denmark
134 Department of Neurosurgery, Vall d'Hebron University Hospital, Barcelona, Spain
135 Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany
136 University Hospital Heidelberg, Heidelberg, Germany
137 Division of Biostatistics and Epidemiology, Department of Preventive Medicine, University of Debrecen, Debrecen, Hungary
138 Department of Traumasurgery, Leiden University Medical Center, Leiden, The Netherlands
139 Department of Anaesthesiology and Intensive Care, AUVA Trauma Hospital, Salzburg, Austria
140 Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark
141 Department of Emergency Care Medicine, Radboud University Medical Center
142 Department of Physical Medicine and Rehabilitation, St.Olavs Hospital and and Department of Neuroscience, Norwegian University of Science and Technology, Trondheim, Norway
143 Neurosurgical Cooperative Holland, Department of Neurosurgery, Leiden University Medical Center and Medical Center Haaglanden, Leiden and The Hague, The Netherlands
144 Department of Neurosurgery, University of Pécs, Pécs, Hungary
145 Universitätsmedizin Göttingen, Göttingen, Germany
146 Division of Neuroscience Critical Care, John Hopkins University School of Medicine, Baltimore, USA
147 Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, UK
148 Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy
149 Australian & New Zealand Intensive Care Research Centre, Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
150 Cochrane Consumers and Communication Review Group, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia
151 Department of Reahabilitation, M. Bufalini Hospital, Cesena, Italy
152 Department of Neurosurgery, Kings college London, London, UK
153 Radiology/MRI Department, CHU, Liège, Belgium
154 Neurologie, Neurochirurgie und Psychiatrie, Charité–Universitätsmedizin Berlin, Berlin, Germany
155 Pauls Stradins Clinical University Hospital, Riga, Latvia
156 Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France
157 Director of Neurocritical Care, University of California, Los Angeles, USA
158 Department of Neurosurgery, St.Olavs Hospital and Department of Neuroscience, Norwegian University of Science and Technology, Trondheim, Norway
159 Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania
160 Department of Psychiatry, University of Florida, Gainesville, Florida, USA
161 Division of Psychology, University of Stirling, Stirling, UK
162 VTT Technical Research Centre, Tampere, Finland
163 University of Florida, Gainesville, Florida, USA
164 Department of Neurosurgery, The HAGA Hospital, The Hague, The Netherlands
165 Department of Intensive Care, Erasmus MC, Rotterdam, the Netherlands
Data Availability
There are however legal constraints that prohibit us from making the data available. Since there are only a limited number of centers per country included in this study (for 2 countries only 1 center), data will be identifiable. Readers may contact Dr. Hester Lingsma (h.lingsma@erasmusmc.nl) for requests for the data.
Funding Statement
Data used in preparation of this manuscript were obtained in the context of CENTER-TBI, a large collaborative project with the support of the European Commission 7th Framework program (602150). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015;157(10):1683–96. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, et al. Epidemiology of traumatic brain injury in Europe: a living systematic review. J Neurotrauma. 2015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas AI, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A Prospective Longitudinal Observational Study. Neurosurgery. 2015;76(1):67–80. 10.1227/NEU.0000000000000575 [DOI] [PubMed] [Google Scholar]
- 4.Ad Hoc Committee on Health Research Relating to Future Intervention Options. Investing in health research and development. Geneva: World Health Organization, 1996 TDR/Gen/96.1.
- 5.Cnossen MC, Scholten AC, Lingsma H, Synnot A, Tavender E, Gantner D, et al. Adherence to guidelines in adult patients with traumatic brain injury: A living systematic review. J Neurotrauma. 2015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AI, Menon DK, Lingsma HF, Pineda JA, Sandel ME, Manley GT. Re-orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J Neurotrauma. 2012;29(1):32–46. 10.1089/neu.2010.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Io Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 8.Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30(20):1737–46. 10.1089/neu.2012.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulger EM, Nathens AB, Rivara FP, Moore M, MacKenzie EJ, Jurkovich GJ, et al. Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med. 2002;30(8):1870–6. . [DOI] [PubMed] [Google Scholar]
- 10.European Brain Injury Consortium. EBIC Center Survey [cited 2015 October, 12th]. Available from: http://www.ebic.nl/survey.
- 11.Enblad P, Nilsson P, Chambers I, Citerio G, Fiddes H, Howells T, et al. R3-survey of traumatic brain injury management in European Brain IT centres year 2001. Intensive Care Med. 2004;30(6):1058–65. . [DOI] [PubMed] [Google Scholar]
- 12.Checkley W, Martin GS, Brown SM, Chang SY, Dabbagh O, Fremont RD, et al. Structure, process, and annual ICU mortality across 69 centers: United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2014;42(2):344–56. 10.1097/CCM.0b013e3182a275d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Comission. Remuneration of researchers in the public and private sectors Brussels: European Communities; 2007. [Google Scholar]
- 14.Kalantar JS, Talley NJ. The effects of lottery incentive and length of questionnaire on health survey response rates: a randomized study. J Clin Epidemiol. 1999;52(11):1117–22. . [DOI] [PubMed] [Google Scholar]
- 15.Galesic M, & Bosnjak M. Effects of Questionnaire Length on Participation and Indicatiors of Response Quality in a Web Survey. Oxford Journals. 2009;73(2):349–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Note. Table presents all definitions used in the paper in the order that they are used in the results section of the paper. TBI = traumatic brain injury; ICU = intensive care unit
(PDF)
A P-value for the difference between high/middle and low income countries B P-value for the difference between North-West and South-East Europe and Israel
(PDF)
A P-value for the difference between high/middle and low income countries B P-value for the difference between North-West and South-East Europe and Israel
(PDF)
Data Availability Statement
There are however legal constraints that prohibit us from making the data available. Since there are only a limited number of centers per country included in this study (for 2 countries only 1 center), data will be identifiable. Readers may contact Dr. Hester Lingsma (h.lingsma@erasmusmc.nl) for requests for the data.