Abstract
Thousand and twelve dementia-free elderly (60–98 years old) enrolled in the In Chianti Study (Italy) were evaluated at baseline (1998–2000) and at 3-year follow-up (2001–2003) with the aim of analyzing the association of lifetime socioeconomic status (SES) with prevalent and incident cognitive impairment no-dementia (CIND). SES was defined from information on formal education, longest held occupation, and financial conditions through life. CIND was defined as age-adjusted Mini-Mental State Examination score one standard deviation below the baseline mean score of participants without dementia. Logistic regression and Cox proportional-hazards models were used to estimate the association of SES with CIND. Demographics, occupation characteristics (i.e., job stress and physical demand), cardiovascular diseases, diabetes, apolipoprotein E (APOE) genotype, smoking, alcohol consumption, depressive symptoms, and C-reactive protein were considered potential confounders. Prevalence of CIND was 17.7%. In the fully adjusted model, low education (OR = 2.1; 95% confidence intervals, CI = 1.4 to 3.2) was associated with prevalent CIND. Incidence rate of CIND was 66.0 per 1000 person-years. Low education (HR = 1.7; 95% CI = 1.04 to 2.6) and manual occupation (HR = 1.9; 95% CI =1.0 to 3.6) were associated with incident CIND. Among covariates, high job-related physical demand was associated with both prevalent and incident CIND (OR = 1.6; 95% CI = 1.1 to 2.4 and HR = 1.5; 95% CI = 1.0 to 2.3). After stratification for education, manual occupation was still associated with CIND among participants with high education (HR = 2.2; 95% CI = 1.2 to 4.3 versus HR = 1.4; 95% CI = 0.2 to 10.4 among those with low education). Proxy markers of lifetime SES (low education, manual occupation and high physical demand) are cross-sectional correlates of CIND and predict incident CIND over a three-year follow-up.
Keywords: Cognitive impairment no-dementia, epidemiology, education, finances, occupation, socioeconomic status
INTRODUCTION
Cognitive impairment that does not meet the criteria for dementia is usually referred as mild cognitive impairment (MCI) or cognitive impairment, no-dementia (CIND) [1, 2]. Whereas MCI definition is based on specific consensual diagnostic criteria [3, 4], different operational criteria for CIND has been debated. The majority of studies identified cases of CIND according to the Mini-Mental State Examination (MMSE) with cut-offs of 1 [5], 1.5 [6], or 2 [7] standard deviations below the mean score derived from persons with no dementia. CIND definition is suitable and convenient when evaluating cognitive impairment in large cohort studies.
Findings from previous community-based studies suggested that CIND is a common condition in older persons, although prevalence and incidence vary widely according to the operational criteria. Estimated prevalence of CIND varies from 5 to 23% [6–8] and usually increases with increasing age [9]. In a population-based study of 75+ year-old persons, the incidence rate of CIND was reported to be 42 per 1000 person-years [10]. Understanding risk factors for CIND is important because individuals affected by this condition are at increased risk of developing dementia [11]. It has been projected that the number of persons affected by dementia will double in the next 20 years [12]. Thus, it is critical to put in place preventive intervention that may reduce the burden of this condition on individuals, their families, and society at large.
Findings from epidemiological and clinical studies have suggested that some demographic [13] and clinical characteristics, such as medical comorbidities, [14, 15] may increase the risk of developing CIND. In addition, proxy measures of socioeconomic status (SES), such as education and manual occupation , have been reported to be associated with CIND in different population-based studies [15–17], although the level of evidence for such association is still considered questionable [1]. Persons with low SES may have little awareness of healthy behaviors, and are likely to poorly manage their health, both in term of compliance with pharmacological and non-pharmacological treatments and use of preventive services. A low SES is often associated with negative health behaviors and high risk of death [18, 19]. Moreover, a low SES has been found to be associated with chronic multimorbidity [20] and poorer self-reported health specifically in the old population [21]. Thus, further studies are needed to strengthen the evidence of the correlation between SES indicators and CIND. The aim of the present study was to evaluate whether different lifetime’s SES indicators, such as early-life education, longest held occupation, and financial condition through lifetime are associated with the prevalence of CIND and/or predict the development of CIND in late life.
MATERIALS AND METHODS
Study design
The InCHIANTI study (“Invecchiare in Chianti”, i.e., Aging in the Chianti Area) [18] is a prospective population-based study designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA) in collaboration with the Laboratory of Epidemiology, Demography and Biometry at the National Institute on Aging (NIA). The baseline data were collected between September 1998 and March 2000. The InCHIANTI study design and main objectives have been extensively reported elsewhere [22]. The study protocol was approved by the Instituto Nazionale per la Ricerca e Cura dell’ Anziano (National Institute for Research and Care of the Elderly, INRCA) ethical committee.
Participants
In August 1998, 1270 individuals aged 65 years and older and 30 men and women in each decade of age between 20 and 60 years and in the age group of 61–64 were randomly selected from the population registry from the rural town of Greve in Chianti and the suburban town of Bagno a Ripoli, located in the Chianti region of Tuscany, Italy. All individuals were contacted by mail, received an extensive description of the purposes and known risks of the study procedures, and all gave their informed consent. Of the 1530 persons originally sampled, 1453 (94%) agreed to participate in the study. Of these, 1203 aged 60 or older at baseline were included in the present analyses. After the exclusion of participants with dementia at baseline (see Measure of cognitive status), 1012 subjects were available. During 3-year follow-up (mean 2.7 years, 95% CI = 2.68–2.76), 72 participants died, 89 refused to continue the study, and 11 moved or could not be located anymore. Thus, 840 individuals were re-examined at follow-up. All participants responded to a structured home interview, underwent full medical and functional examinations, and donated a blood sample. A proxy was interviewed when the subject was unable to provide the required information.
Socio-demographic characteristics
Information on demographic characteristics, such as age and gender was collected during the home interview.
Measures of cognitive status
Impaired cognitive function and dementia were ascertained using a two stage screening procedure. During the home interview, participants were first evaluated using the MMSE [23]. Additionally, participants who reported difficulty in performing activities of daily living were asked questions aimed at understanding whether the cause of abnormality was cognitive impairment. Those with a score >26 were considered non-demented, while those with a MMSE score between 22 and 26 and those with a MMSE score <22 received additional neuropsychological tests assessing memory (paired words test) [24], concentration/attention (digit test from the Weschler adult intelligence test) [25], and visuo-spatial ability (the Caltagirone drawings) [26]. If based on these additional tests the memory of the participant was considered normal, he or she was reattributed a full score on the MMSE memory items. Analogously, 5 points were reattributed to the item “subtract seven five times from 100” and 1 point to the “pentagon drawing” when the performance in additional tests assessing analogous neuropsychological functions was considered normal. After this procedure, we reanalyzed the MMSE score. The participants for whom the new score was >26 were considered “not demented”, while those for whom the newly calculated score remained between 22 and 26 and those with a score <22 were scheduled for the second stage screening. The second stage screening was performed by geriatricians and a psychologist with long standing clinical experience in the evaluation of older patients with cognitive impairment. A diagnosis of dementia independent of the etiology was established using a standard evaluation protocol based on the DSM IV criteria [27]. Subjects with a clinical diagnosis of dementia (n = 82) were excluded from the analyses as well as those with missing data on the presence or not of dementia (n = 13). Subjects without a diagnosis of dementia but with a MMSE score less than 21 (n = 96) were also excluded. Thus, the baseline analyses included data from 1012 participants. CIND was defined as a MMSE score 1 standard deviation below the mean in age-defined strata of participants at baseline without dementia [5, 11]. Seven strata were identified (<66; 66–70; 71–75; 76–80; 81–85; 86–90; >90 yrs) in order to minimize cohort effects and to allow sufficient figures in each of the strata. A cutoff of 1 SD was chosen for the screening test because it was found to be associated with a higher relative prognostic power in predicting the development of dementia compared with higher cut-offs [28]. It has been reported that around one third of persons classified as CIND with this definition progress to dementia during a 3-year follow-up [5]. Among the 840 subjects evaluated at follow-up, 136 with baseline CIND were excluded from the survey analyses. Of the 704 remaining subjects, 59 with incident diagnosis of dementia, 19 with a follow-up MMSE score less than 21 and 5 with missing follow-up MMSE score were also excluded, leaving 621 participants for follow-up analyses.
Measures of socioeconomic status (SES)
Education
Years of schooling completed were used as early-life indicator of SES. First, participants were classified in four groups, as having no education (illiterate) to 3 years of education, 4–5 years of education (primary school), 6–8 years of education (low-upper school), and 9+ years of education (high-upper and academic education). Second, participants were divided into two groups, those with 4 or less years of education and those with 5+ (completing primary school or more) years of education. One person had missing value for education.
Occupation
During the baseline interview, participants or relatives were questioned about jobs held during their lifetime. Information was collected on up to four main different occupations. The subjects’ longest held job was considered as the main occupation. Occupations were grouped according to the Italian Central Institute of Statistics (ISTAT) classification system [29]. Although the ISTAT system for categorizing occupations is closely associated with educational attainment, it is also influenced by other factors not directly reflecting the intellectual and knowledge requirements of the occupation (i.e., skill level and skill specialization). Following the criteria of the Professional Nomenclature and Classification of Occupations (NUP06) [30] developed by the ISTAT and the Italian Institute for the Development of the Professional Education of Workers (ISFOL) [31], participants were divided in two groups: manual workers [blue-collar workers (unskilled and semiskilled manual workers; codes: from 7.1 to 8.6), farmers (code 6.4) and craftsmen (skilled manual workers; codes: from 6.1 to 6.3 and from 6.5 to 6.6)] and non-manual workers [white-collar workers/technicians (skilled mainly non-manual workers; codes: from 3.1 to 5.5); and academic/self-employed (codes: from 1.1 to 2.6)]. Thirty-six women were homemakers during their entire life. This group of women was excluded from any analysis evaluating occupation.
Occupation-related factors
Job stress and physical demand
For each job listed, participants graded level of stress and physical demand on a Likert scale [32]. In particular, participants were asked to score on a scale from 0 to 10 how stressful (very pleasing to very stressful) and physically demanding (no physically demanding to extremely physically demanding) was each of the jobs mentioned. Both level of stress and physical demand were dichotomized as >5 versus ≤5 score.
Financial conditions
Participants were questioned about their financial situation and based on their response were dichotomized into “good” or “sufficient” versus “poor” financial conditions. In addition, participants were asked whether they had any financial crisis during life. Four subjects refused to provide information concerning previous financial crises and 28 refused to respond to questions concerning financial conditions.
Other covariates
As major possible confounders, we considered age, gender, history of stroke, hypertension, diabetes, APOE genotype, smoking and alcohol habits, depressive symptoms, and inflammation. Diseases were ascertained by an experienced physician according to pre-established criteria that combine information from self-reported physician diagnoses, current pharmacologic treatment, medical records, clinical examinations, and blood tests. Information on smoking and alcohol habits was collected during the home interview. Participants were classified according to smoking habits in two groups; never or former smokers (if they stop smoking at least three years before the interview) and current smokers. Participants were divided in two groups according to the number of wine glasses they were used to drink at the time of interview: >7 versus ≤7 glasses per week.
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) scale [33]. The CES-D is one of the most common screening tests for determining the presence of depressive feelings and behaviors during the past week. Possible range of scores is zero to 28, with the higher scores indicating the presence of more symptoms.
Blood samples were obtained from participants after 12-h fast and a 15-min rest period. Aliquots of serum and plasma were immediately stored in a deep freezer at –80°C and were subsequently used to analyze inflammation. Concentrations of C-reactive protein (CRP) were measured by using a high-sensitivity enzyme-linked immunosorbent assay, a competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies. CRP measurement was available for 925 participants. Genomic DNA was prepared from peripheral blood samples that were taken at baseline, and APOE allelic status was determined following a standard procedure. APOE status was available for 923 participants.
Statistical analysis
Logistic regression models were used to study the cross-sectional association between SES indicators and prevalent CIND. A first model evaluated the effects of SES indicators adjusting for gender only. A second model was adjusted for gender, occupation-related factors (job-related stress and physical demand), CRP, smoking, alcohol consumption, depressive symptoms, diseases, and APOE genotype. Prevalence per 100 of CIND was calculated in the whole population and according to factors emerged as significantly associated with prevalent CIND in multivariate analysis. Cox proportional-hazards models estimated the independent effect of different SES indicators on the risk of developing CIND over the three-year follow-up adjusting for gender and for multiple confounders. Hazard ratios (HR) and 95% confidence interval (CI) of developing CIND associated with one unit difference in the independent variable were calculated from the coefficient estimated by these models.
Incidence rates of CIND were calculated as the number of events that occurred during the 3-year follow-up period divided by person-years of follow-up in the whole population and according to factors significantly associated with incident CIND in multivariate analysis. Finally, since education and occupation are usually interrelated, we did stratified analyses to assess the effect of education on the relation between occupation and CIND.
RESULTS
Baseline analyses
Among the 1012 participants evaluated at baseline, 54.3% were women, and the mean age was 73.5 years (60–98 years). Almost thirty percent of the subjects had a low level of education (<5 years of schooling; 1.4% were illiterate); the majority (81.1%) were classified as manual workers. Women were more likely to have low education than men (39.2 versus 16.2% had <5 years of schooling, p < 0.001), but no difference was found in prevalence of manual occupation. Overall, 179 (17.7%, 95% CI = 15.3–20.0) baseline participants were classified as being affected by CIND. Table 1 describes the characteristics of the study population according to CIND status at baseline.
Table 1.
Baseline characteristics of the study population (n = 1012) by cognition
| Characteristics | Cognitive status |
||
|---|---|---|---|
| Cognitively intact (n = 833) |
CIND (n = 179) |
p | |
| Age, yrs, mean (SD) | 73.6 (6.9) | 73.5 (6.5) | 0.953 |
| Female | 432 (51.9) | 118 (65.9) | <0.001 |
| Educational level, yrs | |||
| 0–3 | 135 (16.2) | 60 (33.7) | <0.001 |
| 4–5 | 425 (51.0) | 92 (51.7) | 0.872 |
| 6–8 | 158 (19.0) | 21 (11.8) | <0.02 |
| 9+ | 115 (13.8) | 5 (2.8) | <0.001 |
| Occupation | |||
| Blue-collar workers | 156 (18.7) | 46 (25.7) | <0.03 |
| Farmers | 177 (21.2) | 47 (26.3) | 0.143 |
| Craftsmen | 256 (30.7) | 54 (30.2) | 0.882 |
| White collar workers/technicians | 179 (21.5) | 24 (13.4) | <0.01 |
| Academic professions/self-employed | 36 (4.3) | 1 (0.6) | <0.01 |
| Job stress (Likert scale) mean (SD) |
4.2 (3.1) | 3.9 (2.9) | 0.352 |
| % Likert >5 | 253 (31.6) | 44 (25.7) | 0.131 |
| Job physical demand (Likert scale) mean (SD) |
5.0 (3.1) | 5.7 (3.1) | <0.0091 |
| % Likert >5 | 336 (41.9) | 88 (51.5) | <0.02 |
| Economic conditions | |||
| Lifetime financial crisis | 335 (40.4) | 80 (44.7) | 0.285 |
| Late-life poor finances | 66 (8.1) | 22 (12.9) | <0.04 |
| Health status | |||
| Hypertension | 340 (44.4) | 70 (44.0) | 0.933 |
| Stroke | 30 (3.9) | 11 (6.9) | 0.09 |
| Diabetes mellitus | 86 (11.2) | 15 (9.4) | 0.513 |
| Depression (CES-D scale), mean (SD) | 11.1 (3.7) | 11.7 (4.2) | <0.03 |
| Smoking, current | 134 (16.1) | 27 (15.1) | 0.739 |
| Alcohol drinking >7 glasses/week | 381 (45.7) | 68 (38.0) | <0.05 |
| CRP (µg/mL), median (range) | 2.6 (1.2–5.3) | 2.6 (1.3–6.3) | 0.164 |
| APOEε4 | 123 (16.1) | 21 (13.1) | 0.342 |
Participants with CIND were more likely to be women and to have poor education, more likely to be manual workers and less likely to report employment as white collar workers/technicians or academic jobs. In addition, CIND compared to controls tended to report higher physically demanding jobs, poorer financial conditions, and higher mean number of depressive symptoms. There were no difference in job stress, prevalence of chronic diseases, smoking habits, and APOE genotype. The baseline mean MMSE score was 26.7 ± 1.9 in participants without CIND and 22.5 ± 1.1 in those with CIND. In gender adjusted models, less years of school education were associated with progressive increase in the odds of CIND, from 3.1 (95% CI = 1.1–8.4) in the group of persons with 6–8 years of schooling to 5.9 (95% CI = 1.9–17.6) in subjects with 0–3 years compared to the reference group with 9 years or more of formal education. When education was categorized in two groups, low education was still significantly associated with CIND (Table 2; Model 1). Among covariates, high job-related physical demand was also independently associated with CIND (Table 2; Model 2).
Table 2.
Odds ratio (OR) and 95% confidence intervals (CI) for prevalent CIND associated with SES indicators adjusted for gender (Model 1); SES indicators adjusted for gender, occupation-related factors (job stress and physical demand), alcohol consumption, smoking, hypertension, stroke, diabetes, CRP, depressive symptoms, and APOE genotype (Model 2)
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
|
|---|---|---|
| Gender (female vs. male) | 1.5 (1.04–2.2) | 1.5 (0.9–2.6) |
| Education (low vs. high) | 2.2 (1.5–3.3) | 2.1 (1.4–3.2) |
| Occupation (manual vs. non-manual) |
1.4 (0.9–2.4) | 1.3 (0.8–2.4) |
| Lifetime financial crisis (yes vs. no) |
1.1 (0.7–1.5) | 1.01 (0.7–1.5) |
| Late-life financial conditions (poor vs. good) |
1.3 (0.7–2.2) | 1.3 (0.7–2.4) |
| Job physical demand (high vs. low) |
– | 1.6 (1.1–2.4) |
| Job stress (high vs. low) | – | 0.7 (0.5–1.2) |
| Smoke (current vs. never/former) | – | 1.4 (0.8–2.3) |
| Alcohol (>7 vs. ≤7 glasses/week) | – | 0.9 (0.6–1.6) |
| Hypertension | – | 0.9 (0.6–1.3) |
| Diabetes | – | 0.8 (0.4–1.4) |
| Stroke | – | 2.3 (1.1–5.2) |
| CRP (µ/mL) | – | 1.01 (0.9–1.02) |
| CES-D scale | – | 1.01 (0.9–1.1) |
| APOE ε4 | – | 0.7 (0.4–1.2) |
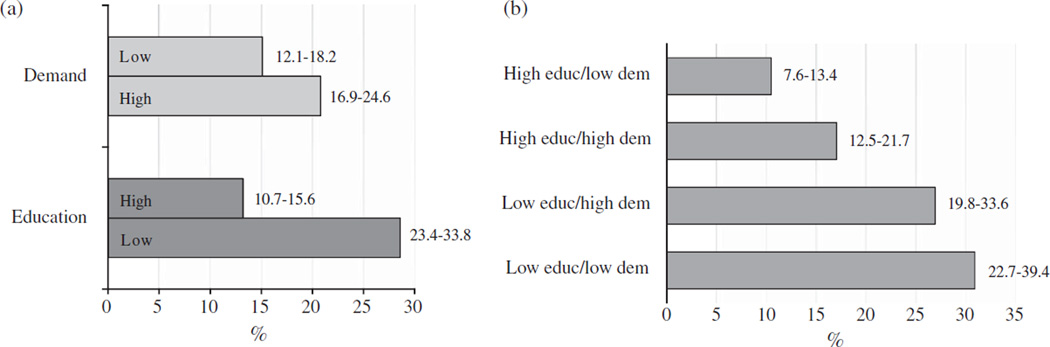
Figure 1 shows prevalence of CIND according to education and job physical demand and according to the combinations of these two SES factors. Prevalence of CIND varied from 10.5% in the group of subjects with high education and low physical demand to more than 20% in the groups of subjects with low education independently of high or low physical demand (Fig. 1a and b).
Fig. 1.
Prevalence (per 100) and 95% confidence intervals of CIND at baseline according to education (high/low) and job physical demand (low/high) (a) and their possible combinations (b). Educ = education; dem = physical demand.
Longitudinal analyses
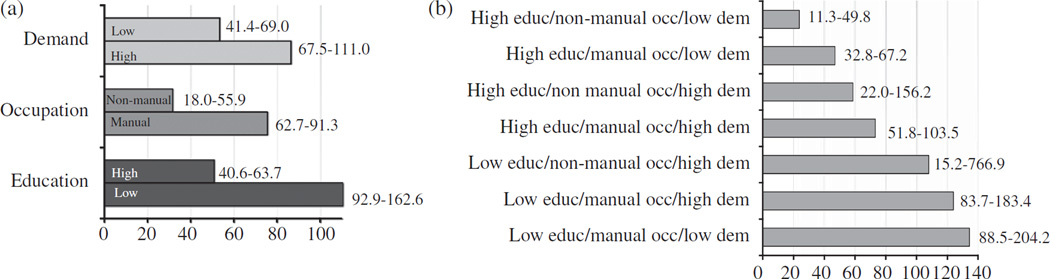
Incidence rate of CIND at follow-up was 66.0 (95% CI = 55.4–78.7) per 1000 person-years. Participants who developed CIND were more likely to be female (63.2 versus 49.6%, p < 0.007), to have low education (39.2 versus 16.3, p < 0.001), manual occupation (90.1 versus 76.5, p < 0.001), and jobs highly physically demanding (51.2 versus 36.3, p < 0.003). At baseline, they were also more likely to have a lower MMSE score (25.9 SD = 1.6 versus 27.3 SD = 1.6, p < 0.001), and a higher proportion of them had a history of stroke (6.1 versus 2.1%, p < 0.02). In gender adjusted models, compared to participant with 6 or more years of schooling, the HR for CIND was 2.2 (95% CI = 1.4–3.5) in the group of persons with 4–5 years of schooling and 3.2 (95% CI = 1.9–5.5) in those with 0–3 years. The independent association between low education and CIND was confirmed in a fully-adjusted model (Table 3; Model 1 and 2). Manual occupation and high job physical demand also emerged as risk factors for CIND (Table 3; Model 1 and 2). When either one of the two independent variables was deleted from Model 2 the association of the other one with CIND slightly increased (HR = 2.0; 95% CI = 1.03–3.9 for manual occupation and HR = 1.6; 95% CI = 1.02–2.4 for high physical demand). Figure 2 shows incidence rates of CIND according to education, occupation and job-related physical demand and according to the combinations of these three factors. Incidence rates of CIND varied from 23.7 per 1000 person-years in the group of subjects with high education/non manual occupation/low physical demand to more than 100 per 1000 person-years in the groups of subjects with low education independently of occupation and physical demand (Fig. 2a and b). Finally, in the education-stratified analysis, manual occupation was still associated with CIND (HR = 2.2; 95% CI = 1.2–4.3) among participants with high education, while among those with low education the effect of occupation on CIND was small and not significant (HR = 1.4; 95% CI = 0.2–10.4).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for incident CIND due to all SES indicators adjusted for gender (Model 1); SES indicators adjusted for gender, occupation-related factors (job stress and physical demand), alcohol consumption, smoking, hypertension, stroke, diabetes, CRP, depressive symptoms and APOE genotype (Model 2)
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|
|---|---|---|
| Gender (female vs. male) | 1.4 (0.93–1.9) | 1.4 (0.7–2.6) |
| Education (low vs. high) | 1.6 (1.04–2.3) | 1.7 (1.04–2.6) |
| Occupation (manual vs. non-manual) |
2.2 (1.2–4.0) | 1.9 (1.0–3.6) |
| Lifetime financial crisis (yes vs. no) |
1.0 (0.7–1.5) | 0.9 (0.6–1.3) |
| Late-life financial conditions (poor vs. good) |
0.8 (0.4–1.5) | 0.9 (0.4–1.7) |
| Job physical demand (high vs. low) |
– | 1.5 (1.0–2.3) |
| Job stress (high vs. low) | – | 0.8 (0.5–1.3) |
| Smoke (current vs. never/former) |
– | 1.2 (0.7–2.1) |
| Alcohol (>7 vs. ≥7 | – | 1.2 (0.6–2.1) |
| glasses/week) | ||
| Hypertension | – | 0.9 (0.6–1.4) |
| Diabetes | – | 0.9 (0.5–1.9) |
| Stroke | – | 0.8 (0.3–2.0) |
| CRP (µg/mL) | – | 0.9 (0.9–1.02) |
| CES-D scale | – | 1.0 (0.9–1.1) |
| APOEε4 | – | 1.3 (0.8–2.0) |
Fig. 2.
Incidence rates and 95% confidence intervals of CIND (per 1000 person-years) at follow-up according to education (high/low), occupation (non-manual/manual), and physical demand (low/high) (a) and their possible combinations (b). Educ= education; occ =occupation; dem = physical demand.
DISCUSSION
In this population-based study of Italian older persons living in semi-rural areas, prevalence and incidence rates of CIND widely varied according to lifespan SES and related factors. In multivariate analysis, early-life low education and adulthood high job physical demand emerged as independently associated with prevalent CIND, whereas low education, manual occupation and high job physical demand were associated with incident CIND. Moreover, results showed that the effect of manual occupation on CIND was significant only in highly educated persons.
Education
Previous population-based studies showed that low education is associated with a higher risk of dementia, AD, and cognitive impairment in the old age [34]. In addition, education has been found to be a risk factor of conversion of MCI to dementia [35]. In an Italian rural population, De Ronchi and colleagues showed that low education is strongly related to CIND [7]. The results of our cross-sectional and longitudinal analyses confirm previous findings; participants who had spent few years in school showed increasing odds and risk of CIND with a dose-effect response trend, similarly to what already reported for dementia [36]. Previous authors hypothesized that health risk behaviors or diseases which are more frequent in low-educated subjects might explain the association between education and cognitive impairment [37]. In our study, the extensive adjustment for diseases, smoking, alcohol drinking, depressive symptoms, and inflammation did not attenuate the odds of CIND associated with low education. More recently, the relationship between education and cognition has been attributed to the ‘cognitive reserve hypothesis’ [38], which refers to the ability of the brain to tolerate the pathology of age-and disease-related changes without obvious clinical evidence [39]. The reserve hypothesis was proposed at the beginning of the 90s, when observational studies showed a reduced dementia risk in persons with high education. More recently, it has also been suggested that in addition to education, other aspects of life experiences may increases resiliency against cognitive decline [40]. A previous study showed that high levels of work complexity may modulate the higher dementia risk due to low education [41]. Interestingly, in our study, participants with high education and manual occupation were still at higher risk to develop CIND. In our population, the presence of several participants with high education but manual occupation could have been useful in identifying the interaction between these two SES factors. This finding might be consistent with the cognitive reserve hypothesis, since different experiences during lifetime may not only supply, but also weaken the cognitive reserve.
Occupation and related factors
Few observations are available in the literature regarding the association between occupation and CIND [15, 17]. In our study, manual occupation was independently associated with incident CIND. Moreover, a job-related factor, high physical demand, was associated with both prevalent and incident CIND. Most studies have analyzed occupational categories and not occupation-related factors, such as physical demand. Indeed, by simply characterizing occupation as manual versus non-manual it may be difficult to disentangle the relative contributions of various occupational aspects, such as physical demand and stress [42]. Only one previous study evaluated physical demand and cognition, showing that high physical demands jobs were more likely to be found in subjects affected by AD than in controls [42]. An interesting hypothesis is that a high job physical demand as well as a manual occupation could be both associated with health risk behaviors, e.g., smoking or alcohol consumption [43], which are risk factors for cerebrovascular diseases, which in turn can cause cognitive impairment [44]. The fact that the adjustment for vascular risk factors and stroke did not affect our findings is against this theory. However, since it is very difficult to entirely control for lifespan health-related variables, we cannot completely rule out this hypothesis. Another hypothesis is that a manual occupation or a high physical demand job through life could have either caused depression and anxiety or limited participants’ social activities during lifetime which have been found related to higher dementia and cognitive impairment risk [40, 45]. A fascinating hypothesis is that physical demand might induce biological changes during life, such as increasing production of oxidative stress and free radicals [46], which may ultimately contribute to cognitive impairment through neurodegeneration. Indeed, participants with high job physical demand had higher levels of CRP at time of examination (6.3 versus 4.4 µg/mL, p < 0.006), but in multivariate analysis the adjustment for this marker of inflammation did not attenuate the strength of the association between physical demand and CIND.
The major strengths of our study include the evaluation of a large-scale community population and the comprehensive assessment of socioeconomic status. However, a few limitations are worthy of mention. Firstly, participants were from a semi-rural area and the large majority of the participants in our study were manual workers, even those who had a good educational attainment; therefore the results may not be generalizable to other populations living in urban areas. Secondly, the information on socioeconomic status is self-reported, thus misclassifications bias cannot be ruled out. However, potential misclassifications are unlikely to differ between those who were or not affected by CIND. The Likert scale, used for the evaluation of job-related stress and physical demand, may be subject to distortion from several causes. Respondents may avoid using extreme response categories (central tendency bias); agree with statements as presented (acquiescence bias); or try to portray themselves in a more favorable light (social desirability bias).
Finally, the information regarding all the confounders is related to baseline, and not to the time when the exposure was present. This might have affected the results.
Despite the above mentioned limitations, our findings might have relevant public health implications. First, they underline the importance of evaluating socioeconomic attainment when studying risk factors for cognitive impairment, even from a life course perspective; in fact, older adults going through different socioeconomic conditions during lifespan may differentially decline in terms of mental health. Second, as persons affected by CIND are at increased risk of developing dementia, studies on factors associated with CIND which are theoretically amenable of prevention, are critical in order to reduce the global burden of mental diseases in the elderly.
Acknowledgments
The InChianti study baseline (1998–2000) was supported as a ‘targeted project’ (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD9164 and 263 MD 821336); the InChianti follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111). Researchers’ work was independent from funders.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=718).
REFERENCES
- 1.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in late life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. International Psychogeriatric Association Expert Conference on mild cognitive impairment Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Palmer K, Wang H-X, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in non-demented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 6.Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, Emsley CL, Dickens J, Evans R, Musick B, Hall KS, Hui SL, Hendrie HC. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 7.De Ronchi D, Berardi D, Menchetti M, Ferrari G, Serretti A, DalMonte E, Fratiglioni L. Occurrence of cognitive impairment and dementia after the Age of 60: a population-based study from Northern Italy. Dement Geriatr Cogn Disord. 2005;19:97–105. doi: 10.1159/000082660. [DOI] [PubMed] [Google Scholar]
- 8.Graham JE, Rockwood K, Beattie LB, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 9.Choo IH, Lee DY, Lee JH, Kim KW, Jhoo JH, Ju YS, Yoon JC, Kim SG, Ha J, Woo JI. The prevalence of cognitive impairment with no dementiain older people: the Seoul study. Int J Geriatr Psychiatry. 2009;24:306–312. doi: 10.1002/gps.2107. [DOI] [PubMed] [Google Scholar]
- 10.Caracciolo B, Palmer K, Monastero R, Winblad B, Beckman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 11.Di Carlo A, Lamassa M, Baldereschi M, Inzitari M, Scafato E, Farchi G, Inzitari D. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 13.De Ronchi D, Palmer K, Pioggiosi P, Atti AR, Berardi D, Ferrari B, Dalmonte E, Fratiglioni L. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord. 2007;24:266–273. doi: 10.1159/000107102. [DOI] [PubMed] [Google Scholar]
- 14.Lyketsos CG, Toone L, Tschanz J, Rabins PV, Steinberg M, Onyike CU, Corcoran C, Norton M, Zandi P, Breitner JC, Welsh-Bohmer K, Anthony J, Østbye T, Bigler E, Pieper C, Burke J, Plassman B, Green RC, Steffens DC, Klein L, Leslie C, Townsend JJ, Wyse BW, Munger R, Williams M Cache County Study Group. Population-based study of medical comorbidity in early dementia and ‘cognitive impairment, no dementia (CIND)’: association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry. 2005;13:656–664. doi: 10.1176/appi.ajgp.13.8.656. [DOI] [PubMed] [Google Scholar]
- 15.Atti AR, Forlani C, De Ronchi D, Palmer K, Casadio P, Dalmonte E, Fratiglioni L. Cognitive impairment after age 60: clinical and social correlates in the ‘Faenza Project’. J Alzheimers Dis. 2010;21:1325–1334. doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo A, Baldereschi M, Amaducci L, Maggi S, Grigoletto F, Scarlato G, Inzitari D. Cognitive impairment without dementia in older people: prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48:775–782. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 17.Fei M, Qu YC, Wang T, Yin J, Bai JX, Ding QH. Prevalence and distribution of cognitive impairment no dementia (CIND) among the aged population and the analysis of sociodemographic characteristics: the community-based cross-sectional study. Alzheimer Dis Assoc Disord. 2009;23:130–138. doi: 10.1097/WAD.0b013e318190a59d. [DOI] [PubMed] [Google Scholar]
- 18.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329:103–109. doi: 10.1056/NEJM199307083290207. [DOI] [PubMed] [Google Scholar]
- 20.Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98:1198–1200. doi: 10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandola T, Ferrie J, Sacker A, Marmot M. Social inequalities in self reported health in early old age: follow-up of prospective cohort study. BMJ. 2007;334:990–997. doi: 10.1136/bmj.39167.439792.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InChianti study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. The Measurement and Appraisal of Adult Intelligence. Baltimore: Williams and Wilkins; 1958. [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 26.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. pp. 133–158. [Google Scholar]
- 28.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Subclassifications for mild cognitive impairment: prevalence and predictive validity. Psychol Med. 2003;33:1029–1038. doi: 10.1017/s0033291703007839. [DOI] [PubMed] [Google Scholar]
- 29.Italian National Institute of Statistics. [Updated December 13, 2010, Accessed on January 10, 2011];Classification of occupations. 2006 http://www.istat.it/strumenti/definizioni/professioni/
- 30.Italian National Institute of Statistics. [Accessed on January];Nomenclature and classification of professional units. 2010 10:2011. http://www.istat.it/strumenti/definizioni/professioni/nup/nup06/ [Google Scholar]
- 31. [Accessed on January 10, 2011];Istituto per lo Sviluppo della Formazione Professionale dei Lavoratori. http://www.isfol.it.
- 32.Likert R. A Technique for the Measurement of Attitudes. Arch Psychology. 1932;140:1–55. [Google Scholar]
- 33.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 34.Caamaño-Isorna F, Corral M, Montes-Martìnez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26:226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- 35.Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 36.Anntila T, Helkala E-L, Kivipelto M, Hallikainen M, Alhainen K, Heinonen H, Mannermaa A, Tuomilehto J, Soininen H, Nissinen A. Midlife income, occupation, APOE status, and dementia. A population-based study. Neurology. 2002;59:887–893. doi: 10.1212/wnl.59.6.887. [DOI] [PubMed] [Google Scholar]
- 37.Ngandu T, von Strauss E, Helkala E-L, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Education and dementia. What lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 38.Fratiglioni L, Wang H-X. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 39.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 40.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 41.Karp A, Andel R, Parker MG, Wang HX, Winblad B, Fratiglioni L. Mentally stimulating activities at work during midlife and dementia risk after age 75: follow-up study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2009;17:227–236. doi: 10.1097/JGP.0b013e318190b691. [DOI] [PubMed] [Google Scholar]
- 42.Smith KA, Fritsch T, Cook TB, McClendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63:598–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- 43.Radi S, Ostry A, LaMontagne AD. Job stress and other working conditions: relationships with smoking behaviors in a representative sample of working Australians. Am J Ind Med. 2007;50:584–596. doi: 10.1002/ajim.20492. [DOI] [PubMed] [Google Scholar]
- 44.Kivipelto M, Solomon A. Alzheimer’s disease - the ways of prevention. J Nutr Health Aging. 2008;12:89S–94S. doi: 10.1007/BF02982595. [DOI] [PubMed] [Google Scholar]
- 45.Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 46.Fielding RA, Meydani M. Exercise, free radical generation, and aging. Aging (Milano) 1997;9:12–18. doi: 10.1007/BF03340124. [DOI] [PubMed] [Google Scholar]