Abstract
Introduction
Exemestane, the irreversible steroidal aromatase inhibitor, and fulvestrant, the pure estrogen antagonist, are active as single drugs in postmenopausal women with advanced hormone-responsive breast cancer. We designed a phase II study with the purpose of determining whether combining these 2 drugs with different and potentially complementary mechanisms of action will improve the clinical benefit.
Patients and Methods
Forty postmenopausal women with hormone-responsive advanced breast cancer received intramuscular injection of fulvestrant 250 mg every 28 days in combination with daily exemestane 25 mg until disease progression. We examined the influence of fulvestrant on exemestane pharmacokinetics and the effect of exemestane and fulvestrant on serum IGF-1 (insulin-like growth factor 1) and IGFBP-3 (IGF-binding protein 3) levels.
Results
The observed proportion of patients free of progressive disease at 6 months after the initiation of treatment with exemestane and fulvestrant was 50%, a rate similar to that achieved with single-agent exemestane or fulvestrant in the first- or second-line setting. Pharmacokinetics parameters showed that coadministration of fulvestrant did not result in clinically relevant changes in exemestane plasma concentrations. A comparison of IGF-1 and IGFBP-3 levels demonstrated the increase of 35% and 12%, respectively, in mean levels from baseline to day 120.
Conclusions
The combination of exemestane and fulvestrant did not improve clinical benefit. The observed lack of improved efficacy was not related to altered drug exposure.
Keywords: Endocrine therapy, Exemestane, Fulvestrant, IGF-binding protein 3, Insulin-like growth factor, Metastatic breast cancer
Introduction
Adjuvant endocrine therapy reduces the risk of breast cancer recurrence by approximately 50% and mortality by approximately 30% in hormone-responsive early-stage breast cancer.1–6 Postmenopausal women with hormone receptor–positive metastatic breast cancer have a number of endocrine therapies available, including the selective estrogen receptor (ER) modulators tamoxifen and toremifene, aromatase inhibitors (AI), the pure estrogen antagonist fulvestrant, and progestational agents megestrol acetate and estradiol.7–13 Invariably, resistance to the endocrine therapy develops. Several mechanisms of endocrine resistance have been proposed, and different strategies have been developed to reverse that resistance.14–16
The concept of combining the irreversible, steroidal AI exemestane with fulvestrant to delay the development of endocrine resistance and to prolong the duration of clinical benefit was based on the preclinical models that showed that the efficacy of fulvestrant depends on the background estrogen environment in the breast tumor.17 Xenograft models suggested that the combination of anastrozole and fulvestrant resulted in longer growth inhibition and was more effective in delaying development of endocrine resistance than either treatment alone.18
In this study, we tested the hypothesis that a combination of fulvestrant and exemestane will improve the proportion of patients free of progressive disease at 6 months after initiation of treatment. In addition, we examined whether fulvestrant influences exemestane pharmacokinetics and what effect a combination of exemestane and fulvestrant had on serum insulin-like growth factor 1 (IGF-1) and IGF-binding protein 3 (IGFBP-3) levels, because different patterns of IGF-1 and IGFBP-3 response to various endocrine therapies have been reported previously.19,20
Patients and Methods
Patient Population
All patients were postmenopausal women with histologically proven locally advanced or metastatic breast cancer. Hormonally responsive disease was defined as ER and/or progesterone receptor positive (>10% by immunohistochemistry). Postmenopausal status was defined as either age older than 55 years with no menses for ≥6 months or prior bilateral oophorectomy or ovarian irradiation, or age ≤55 years with no menses for >12 months and postmenopausal follicular stimulating hormone levels. Measurable disease was not required. However, in patients with measurable disease, the Response Evaluation Criteria in Solid Tumors (RECIST) criteria were followed to monitor and assess response.21
No more than 1 prior chemotherapy regimen for stage IV meta-static breast cancer was allowed. Prior treatment with tamoxifen, anastrazole, or letrozole in the neoadjuvant or adjuvant setting was permitted. One prior endocrine therapy for advanced or metastatic disease was allowed, however, prior treatment with exemestane or fulvestrant was the exclusion criterion. The patients were allowed to receive concomitant bisphosphonates. ECOG (Eastern Cooperative Oncology Group) performance status was required to be 0–2. Participants were required to have adequate bone marrow, hepatic and renal function defined as absolute neutrophil count ≥ 1000/μL, platelets ≥ 100,000/μL, serum creatinine ≤ 1.5 X institutional upper limit of normal (ULN), total bilirubin ≤1.5 X ULN, and aspartate aminotransferase/alanine aminotransferase ≤2.5 X ULN unless the patient had liver metastases. In the presence of liver metastases, transaminases had to be ≤5 X ULN and alkaline phosphatase ≤2.5 X ULN. The patients with lymphangitic disease, carcinomatous meningitis, bone marrow only metastases, and a rising tumor marker without any other sites of metastatic disease or the presence of bleeding diathesis were excluded. All the participants signed informed written consent before treatment.
Treatment Plan
Exemestane 25 mg was administered orally with food once daily, starting on day 1. On day 8, fulvestrant 250 mg was administered intramuscularly and thereafter every 28 ± 5 days. A cycle consisted of approximately 4 weeks starting from day 8. No dose reduction or dose escalation was allowed for either exemestane or fulvestrant. Treatment was continued until disease progression, toxicity, or voluntary withdrawal. The patients were assessed for response after every 2 cycles by physical examination and imaging studies. If a patient had stable disease after 12 treatment cycles, then the frequency of imaging studies was reduced to every third treatment cycle (12 weeks) at the discretion of the investigator. Response and progression was evaluated by using the international RECIST criteria. The participants were assessed for toxicity at each study visit. Toxicity was assigned by using the National Cancer Institute Common Toxicity Criteria version 2.0.
Statistical Design
The primary endpoint of this study was to determine the proportion of patients free of progressive disease at 6 months after the initiation of exemestane and fulvestrant. Based on historic data, this combined therapy would be of clinical value if at least 70% of patients with advanced breast cancer were progression free at 6 months.7 The combination would be deemed uninteresting if the progression-free survival at 6 months was <50% (ie, a median time to progression was <6 months). A Fleming single-stage phase II trial design (with P0 =0.5, P1 =0.7; α =0.10, β =0.10) indicated that a sample size of 40 patients was needed to demonstrate this difference. An intent-to-treat analysis was conducted. Kaplan-Meier curves generated the median time to progression.
Exemestane Pharmacokinetics
A secondary objective of the trial was to determine the pharmacokinetics of exemestane when administered alone and again in combination with fulvestrant in the first 9 patients. Blood samples were collected for 24 hours after dosing of exemestane on day 7 (ie, at the end of the 1-week single-agent exemestane treatment period) and again after approximately 120 ± 7 days of combination treatment with exemestane and fulvestrant. The collection days were selected based on published data that demonstrated steady-state concentrations 7 days after exemestane therapy and approximately 120 days for fulvestrant given as single agents at the doses used in this study.17,18 Approximately 5 mL of venous whole blood was obtained before dosing and then at 1, 2, 4, 6, 8, and 24 hours after exemestane ingestion on each of the 2 time points. Blood was collected in prechilled heparinized tubes, immediately placed in an ice-water mixture, and centrifuged at 1000g to 1200g for 10 to 15 minutes at 4°C to reduce the risk of exemestane degradation. Duplicate plasma aliquots were frozen at approximately −70°C until the time of analysis. Plasma concentrations of exemestane were measured by using a validated, sensitive, and specific high-performance liquid chromatography method with tandem mass spectrometric detection (PRA International Early Development Services, Zuidlaren, the Netherlands). The lower limit of quantitation of this assay was 0.1 ng/mL when using 0.50-mL aliquots. The selectivity of the method was demonstrated in the presence of fulvestrant at 25.0 ng/mL in human plasma. Assay results were reported in ng/mL.
Exemestane plasma concentration data were analyzed by noncompartmental methods via Win Nonlin (version 1.5; Pharsight, Cary, NC). Peak plasma concentrations (Cmax) and the time at which they occurred (Tmax.) were determined by inspection of individual patient concentration-time curves. The area under the concentration-time curve was estimated by using linear trapezoidal rule. The apparent terminal elimination rate constants (λz) were determined by linear least-squares regression of plasma concentration-time points that were determined to lie in the terminal log-linear region of the plasma concentration-time profiles. The apparent elimination half-life (T1/2) was calculated as 0.693/λz.
Serum IGF-1 and IGFBP-3 Measurements
To assess the effect of exemestane in combination with fulvestrant on serum IGF-1 and IGFBP-3 levels, blood samples were collected at baseline before therapy, on day 7 (ie, at the end of 1-week single-agent exemestane treatment period), and on day 120 (± 7 days). Approximately 5 mL of venous blood was collected into serum separator tubes, allowed to clot for at least 30 minutes, and then centrifuged at 3000 rpm for 10 minutes. Serum was frozen at −70°C until the time of analysis. Serum levels of IGF-1 were determined by using a FreeIgF-1 ELISA kit (Beckman-Coulter/Diagnostic Systems Laboratories, Webster, TX). The intra-assay variation was 4.0%, interday assay variation was 9.1%, and a theoretic sensitivity of 0.15 ng/mL.
Results
Patients
Patient and disease characteristics are presented in Table 1. A total of 40 patients were enrolled between November 2005 and December 2009. The median age was 58 years (range, 43–84 years). The median time from the initial breast cancer diagnosis to development of metastatic disease was 5 years (range, 0–21 years). Eight (20%) patients presented with de novo metastatic disease, 14 (35%) developed distant disease recurrence while still receiving adjuvant endocrine therapy, and 9 (23%) developed disease recurrence after completion of 5-year adjuvant hormonal therapy. Nine (23%) patients received adjuvant hormonal therapy for less than 2 to 3 months either due to adverse effects or refusal to proceed with further treatment. Five (13%) patients received prior chemotherapy for metastatic disease, and 8 (20%) received prior hormonal therapy with nonsteroidal AI for metastatic disease. The dominant sites of metastases were bones, in 28 (70%); followed by lungs, in 12 (30%); and liver, in 4 (10%) patients.
Table 1.
Main Patient and Tumor Characteristics
| Characteristic | |
|---|---|
| Median Age (Range), y | 58 (43–84) |
| Race, n (%) | |
| White | 36 (90) |
| African-American | 4 (10) |
| Receptor Status, n (%) | |
| ER positive | 40 (100) |
| PR positive | 30 (75) |
| PR negative | 10 (25) |
| HER2-neu positive | 1 (3) |
| Metastatic Organ Involvement, n (%) | |
| Lungs | 12 (30) |
| Lymph nodes | 17 (42) |
| Bones | 28 (70) |
| Liver | 4 (10) |
| Chest wall | 4 (10) |
| Time From Primary Diagnosis to Metastatic Disease, Median (Range), y | 5 (0–21) |
| Relapse and/or Progression, n (%) | |
| During adjuvant endocrine therapy | 14 (35) |
| >12 mo after completion of adjuvant endocrine therapy | 9 (23) |
| Presenting with de novo metastatic disease | 8 (20) |
| Never on adjuvant hormonal therapya | 9 (23) |
Abbreviations: ER =estrogen receptor (ER); HER =human epidermal growth factor receptor; PR =progesterone receptor (PR).
Patients refused or stopped adjuvant hormonal therapy after taking it for <2–3 mo.
Toxicity
Treatment-related adverse events of grade ≥2 are described in Table 2. Most adverse events were grade 2. The most-frequent toxicities were grade 2 fatigue in 10 (25%) patients, followed by grade 2 bone pain and arthralgias reported by 8 (20%) and 6 (15%) patients, respectively. Removal from the study due to toxicity occurred in 1 patient with persistent grade 4 nausea and vomiting. The only other grade 4 adverse events were thromboembolism, chest pain, and hypercalcemia seen in 2 (5%), 1 (3%), and 1 (3%) patients, respectively. No grade 5 toxicity was observed.
Table 2.
Toxicity Per Patient
| Toxicity | Grade 2 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 1 (3) | 0 | 0 |
| Anemia | 2 (5) | 2 (5) | 0 |
| Thrombocytopenia | 2 (5) | 0 | 0 |
| Nonhematologic | |||
| Fatigue | 10 (25) | 4 (10) | 0 |
| Chest pain | 0 | 0 | 1 (3) |
| Arthralgia | 6 (15) | 0 | 0 |
| Thromboembolism | 0 | 0 | 2 (5) |
| Nausea | 3 (8) | 1 (3) | 1 (3) |
| Vomiting | 3 (8) | 1 (3) | 1 (3) |
| Anorexia | 4 (10) | 2 (5) | 0 |
| Dyspnea | 1 (3) | 1 (3) | 0 |
| Hypercalcemia | 0 | 0 | 1 (3) |
| Bone pain | 8 (20) | 2 (5) | 0 |
Efficacy
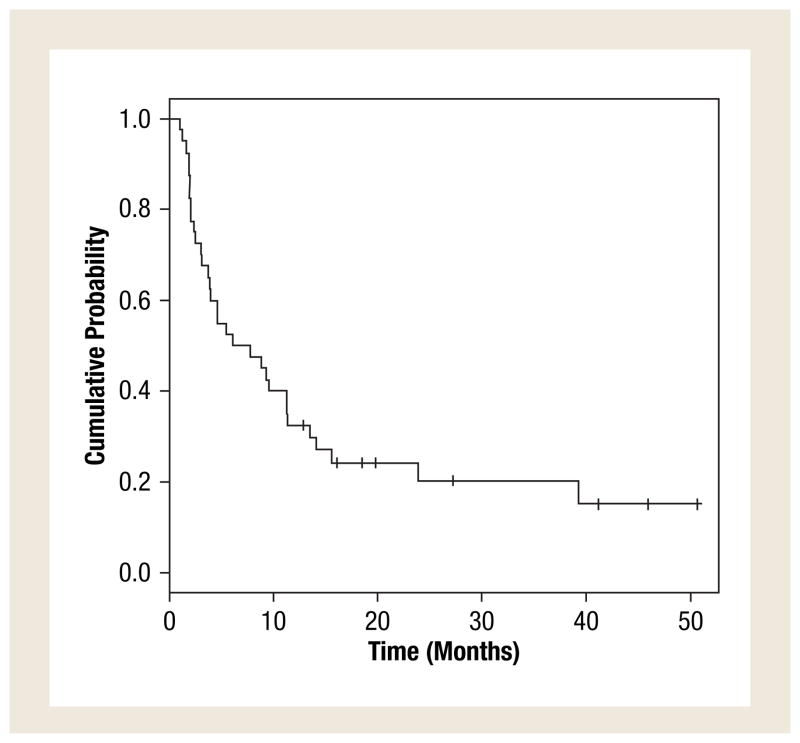
The treatment efficacy is described in Table 3. No patient had a complete response. Three (7.5%) patients had partial response that lasted for at least 6 months, and 17 (42.5%) had stable disease that lasted for at least 6 months. The overall clinical benefit defined as complete response plus partial response plus stable disease that lasted for 6 or more months was 50%. The median time to progression was 6.9 months (95% CI, 3.9–13.5 months). The progression-free survival is shown in Figure 1.
Table 3.
Objective Response Rates and Clinical Benefit Rates
| Response Category | n (%) |
|---|---|
| Complete Response | 0 (0) |
| Partial Response | 3 (8) |
| Overall Response Rate | 3 (8) |
| Stable Disease ≥ 6 Mo | 17 (42) |
| Overall Clinical Benefit | 20 (50) |
| Stable Disease < 6 Mo | 8 (20) |
| Progressive Disease | 12 (30) |
Figure 1.
Kaplan-Meier Plot of Progression-Free Survival
Exemestane Pharmacokinetics
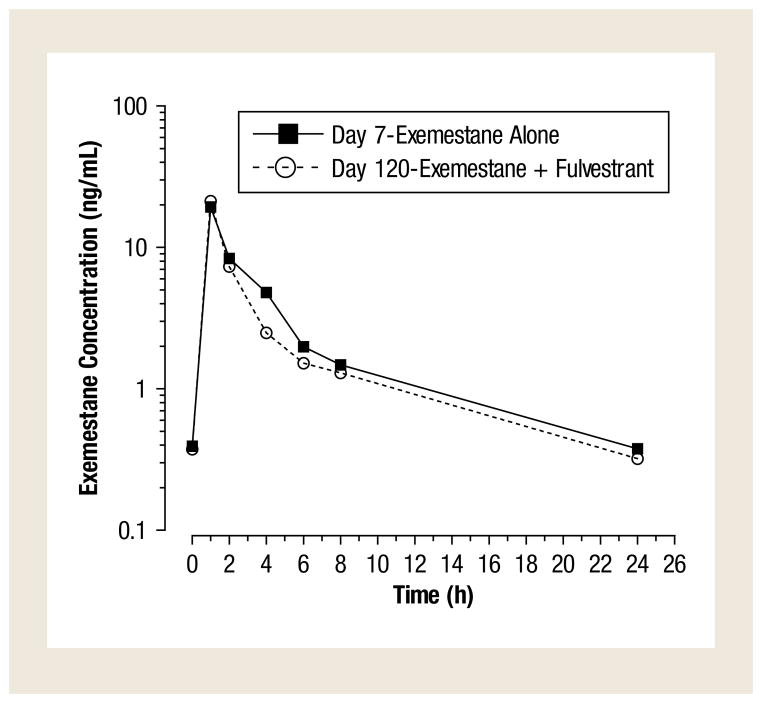
Exemestane plasma concentration–time profiles when administered alone (day 7), and again in combination with fulvestrant (day 120), were determined in 9 patients. Mean steady-state plasma concentration–time profiles of exemestane when administered alone were similar to those observed when exemestane was combined with fulvestrant (Figure 2). The pharmacokinetic parameters of exemestane when given alone and in combination with fulvestrant are summarized in Table 4. Exemestane Cmax, area under the curve of 0–24 and half-life when combined with fulvestrant were within 12% of values when given alone. These results indicate that coadministration of fulvestrant does not result in clinically relevant changes in exemestane plasma concentrations.
Figure 2.
Mean (SD) Steady-State Plasma Concentration-Time Profiles of Exemestane Given Alone (Day 7; Closed Squares) or in Combination With Fulvestrant (Day 120; Open Circles)
Table 4.
Summary of Mean ± SD Exemestane Pharmacokinetic Parameters When Given Alone (Day 7) and in Combination With Fulvestrant (Day 120)
| Parameter | Exemestane Alone (Day 7) | Exemestane + Fulvestrant (Day 120) | % Changea | P Valueb |
|---|---|---|---|---|
| Cmax, ng/mL | 20.1 ± 7.7 | 21.2 ± 11.1 | 5.3 | .9102 |
| AUC 0–24, ng · h/mL | 61.7 ± 17.1 | 54.7 ± 22.9 | −11.3 | .2031 |
| T1/2, h | 7.94 ± 1.32 | 8.44 ± 2.16 | 6.3 | .5469 |
Abbreviation: AUC =area under the curve.
Relative to day 7.
Wilcoxon signed rank test.
Serum IGF-1 and IGFBP-3 Levels
IGF-1 and IGFBP-3 serum levels on day 1 (baseline), day 7 (exemestane alone), and day 120 (exemestane and fulvestrant) for the 23 patients who had levels determined on all 3 occasions are summarized in Table 5. A repeated measure analysis of variance indicated a statistically significant difference across days for both IGF-1 (119 ± 41.1 ng/mL on day 1, 141 ± 55.1 ng/mL on day 7, and 161 ± 61.2 ng/mL on day 120; P =.0002) and IGFBP-3 (4946 ± 1188 ng/mL on day 1, 5273 ± 1372 ng/mL on day 7, and 5537 ± 1166 ng/mL on day 120; P =.0058). A comparison of IGF-1 levels among the 3 days by using a Student-Newman-Keuls multiple comparison test demonstrated IGF-1 levels increased with time on treatment (P =.0002). Similarly, IGFBP-3 levels also increased with time (P =.0058), but only the difference between day 1 and day 120 was statistically significant (P <.01). The increase in mean levels from baseline to day 120 was 35% for IGF-1 and only 12% for IGFBP-3.
Table 5.
Summary of Mean ± SD IGF-1 and IGFBP-3 Serum Levels
| Parameter | n | Baseline | Day 7 | Day 120 | P Valuea |
|---|---|---|---|---|---|
| IGF-1, ng/mL | 23 | 119 ± 41.1 | 141 ± 55.1 | 161 ± 61.2 | .0002b |
| IGFBP-3, ng/mL | 22 | 4946 ± 1188 | 5273 ± 1372 | 5537 ± 1166 | .0058c |
Abbreviations: IGF =insulin-like growth factor; IGFBP =insulin-like growth factor– binding protein.
Repeated measures analysis of variance.
Baseline vs. day 7 (P <.05), baseline vs. day 120 (P <.001), day 7 vs. day 120 (P <.05).
Baseline vs. day 7 (P >.05), baseline vs. day 120 (P <.01), day 7 vs. day 120 (P >.05).
Discussion
This phase II study did not achieve the primary endpoint because the observed proportion of patients free of progressive disease at 6 months after the initiation of treatment with the combination of exemestane and fulvestrant was 50%. This rate is similar to that observed in patients who received the single drug fulvestrant or exemestane as the first or second line of hormonal therapy for meta-static breast cancer.11,12,22–27
The observed lack of improved efficacy of a combination of fulvestrant and exemestane does not seem to be related to their pharmacokinetics. Steady-state exemestane pharmacokinetic parameters on day 7 (exemestane alone) and day 120 (exemestane plus fulvestrant) were similar, which suggests that coadministration of fulvestrant did not result in clinically relevant changes in exemestane plasma concentrations.
It is possible that the lower response rate seen in our study is related to the fact that the majority of participants had bone involvement, which makes the determination of objective response difficult. It also is possible that the 250 mg dose of fulvestrant given every month in this study could be a contributing factor to lower-than-expected progression-free survival at 6 months. The time-to-progression curves of studies when using the 28-day, 250-mg schedule indicated early progression of some patients treated with fulvestrant, which suggests that a loading dose of fulvestrant should be given.25,28 Our data (Figure 2) support this observation; one-third of our patients had disease progression after 2 cycles of therapy. Development of regimen with an loading dose of fulvestrant on days 0 and 14, and a high dose of fulvestrant (500 mg) did show that a steady-state concentration of fulvestrant was reached earlier with loading dose and high dose than with the approved dose.29,30
The concept of combining 2 hormonal agents with different and potentially complementary mechanism of action was evaluated in the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial. 2 This randomized trial was designed to compare the efficacy and safety of anastrazole and the combination of anastrazole plus tamoxifen with that of tamoxifen alone, as adjuvant treatment for postmenopausal women with breast cancer. It has been shown that the combination of tamoxifen and steroidal AI anastrazole was not better than tamoxifen alone.31 The observed lack of improved efficacy of the combination was thought to be related to the estrogen agonist activity of tamoxifen that predominates in a low estrogen environment created by an aromatase inhibitor. Results of our study showed that even pure estrogen inhibitor fulvestrant does not perform better in estrogen-deprived milieu. The combination of exemestane and fulvestrant was not more effective than treatment with either agent alone in delaying development of resistance to endocrine therapy.
Analysis of preliminary data suggests that IGF/IGF receptor pathway signaling may contribute to antiestrogen resistance through crosstalk with ER signaling.32 IGF-1 is a more potent mitogen for breast cancer cell lines than estradiol and epidermal growth factor.33 Because IGF-1 in the tumor microenvironment may be influenced either by local synthesis or by plasma levels, we examined the effect of exemestane alone (day 7) and combination of exemestane and fulvestrant (day 120) on serum IGF-1 and IGFBP-3 levels. Our results demonstrated that IGF-1 and IGFBP-3 levels increased just 7 days after starting exemestane therapy and increased with time on treatment with the combination of exemestane and fulvestrant. Whether raising levels of IGF-1 ligand led to elevated insulin-like growth factor 1 receptor signaling that caused antiestrogen resistance needs to be determined.
Conclusion
Results of our study showed that the combination of exemestane and fulvestrant was not more effective than either treatment alone and that it did not delay resistance to endocrine therapy. The observed lack of improved efficacy was not related to altered drug exposure. Increased plasma levels of IGF-1 and IGFBP-3 during therapy generate a hypothesis that IGF/IGF receptor pathway signaling may contribute to antiestrogen resistance.
Footnotes
Disclosure
Support in part provided by Pfizer Pharmaceuticals.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.The Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, et al. Effect of anastrazole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 3.Thürlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 4.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (intergroup exemestane study): a randomized controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. J Natl Cancer Inst. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Chapman J-AW, et al. Final analysis of NCIC CTG MA. 27: a randomized phase III trial of exemestane versus anastrozole in postmenopausal women with hormone receptor positive breast cancer. Cancer Res. 2010;70(suppl 2):75s. (abstract S1–1) [Google Scholar]
- 7.Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes DF, Van Zyl JA, Hacking A, et al. Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. J Clin Oncol. 1995;13:2556–66. doi: 10.1200/JCO.1995.13.10.2556. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar A, Douma J, Davidson N, et al. Phase II, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19:3357–66. doi: 10.1200/JCO.2001.19.14.3357. [DOI] [PubMed] [Google Scholar]
- 10.Nabholtz JM, Buzdar A, Pollack M, et al. Anastrazole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18:3758–67. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 11.Paridaens RJ, Therasse P, Dirix LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in post-menopausal women: the European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–90. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrazole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–38. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowsett M, Martin LA, Smith I, et al. Mechanisms of resistance to aromatase inhibitors. J Steroid Biochem Mol Biol. 2005;95:167–72. doi: 10.1016/j.jsbmb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Bedard PL, Freedman OC, Howell A, et al. Overcoming endocrine resistance in breast cancer: are signal transduction inhibitors the answer? Breast Cancer Res Treat. 2008;108:307–17. doi: 10.1007/s10549-007-9606-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SRD, Martin L-A, Head J, et al. Aromatase inhibitors: combinations with fulvestrant or signal transduction inhibitors as a strategy to overcome endocrine resistance. J Steroid Biochem Mol Biol. 2005;95:173–81. doi: 10.1016/j.jsbmb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Osipo C, Gajdos C, Liu H, et al. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 18.Macedo LF, Sabnis GJ, Goloubeva OG, et al. Combination of anastrazole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68:3516–22. doi: 10.1158/0008-5472.CAN-07-6807. [DOI] [PubMed] [Google Scholar]
- 19.Lien EA, Johannessen DC, Aakvaag A, et al. Influence of tamoxifen, aminoglutethimide and goserelin on human plasma IGF-I levels in breast cancer patients. J Steroid Biochem Mol Biol. 1992;41:541–3. doi: 10.1016/0960-0760(92)90380-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari L, Bajetta E, Martinetti A, et al. Could exemestane affect insulin-like growth factors, interleukin 6 and bone metabolism in postmenopausal advanced breast cancer patients after failure on aminoglutethimide, anastrazole or letrozole? Int J Oncol. 2003;22:1081–9. [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 23.Lønning PE, Bajetta E, Murray R, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: a phase II trial. J Clin Oncol. 2000;18:2234–44. doi: 10.1200/JCO.2000.18.11.2234. [DOI] [PubMed] [Google Scholar]
- 24.Howell A, Robertson JFR, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind randomized trial. J Clin Oncol. 2004;22:1605–13. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 25.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrazole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy; results of a North American trial. J Clin Oncol. 2002;20:3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JFR. Fulvestrant (Faslodex) — how to make a good drug better. Oncologist. 2007;12:774–84. doi: 10.1634/theoncologist.12-7-774. [DOI] [PubMed] [Google Scholar]
- 27.Buzdar AU, Robertson JFR, Eiermann W, et al. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrazole, letrozole and exemestane. Cancer. 2002;95:2006–16. doi: 10.1002/cncr.10908. [DOI] [PubMed] [Google Scholar]
- 28.Howell A, Bergh J. Insights into the place of fulvestrant for the treatment of advanced endocrine responsive breast cancer. J Clin Oncol. 2010;28:4548–50. doi: 10.1200/JCO.2010.30.6266. [DOI] [PubMed] [Google Scholar]
- 29.Ohno S, Rai Y, Iwata H, et al. Three dose regimens of fulvestrant in postmenopausal Japanese women with advanced breast cancer: results from a double-blind, phase II comparative study (FINDER1) Ann Oncol. 2010;21:2342–7. doi: 10.1093/annonc/mdq249. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2) Breast Cancer Res Treat. 2010;123:453–61. doi: 10.1007/s10549-010-1022-9. [DOI] [PubMed] [Google Scholar]
- 31.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–10. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Moerkens M, Ramaiaghari S, et al. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim YH, Yee D. Insulin-like growth factor-1 and breast cancer therapy. Clin Cancer Res. 2005;11:944–50. [PubMed] [Google Scholar]