Abstract
Bmp2 is essential for dentin formation. Bmp2 cKO mice exhibited similar phenotype to dentinogenesis imperfecta, showing dental pulp exposure, hypomineralized dentin, and delayed odontoblast differentiation. As it is relatively difficult to obtain lot of primary Bmp2 cKO dental papilla mesenchymal cells and to maintain a long-term culture of these primary cells, availability of immortalized deleted Bmp2 dental papilla mesenchymal cells is critical for studying the underlying mechanism of Bmp2 signal in odontogenesis. In this study, our goal was to generate an immortalized deleted Bmp2 dental papilla mesenchymal (iBmp2ko/ko dp) cell line by introducing Cre fluorescent protein (GFP) into the immortalized mouse floxed Bmp2 dental papilla mesenchymal (iBmp2fx/fx dp) cells. iBmp2ko/ko dp cells were confirmed by GFP and PCR. The deleted Bmp2 cells exhibited slow cell proliferation rate and cell growth was arrested in G2 phase. Expression of tooth-related marker genes and cell differentiation were decreased in the deleted cells. Importantly, extracellular matrix remodeling was impaired in the iBmp2ko/ko dp cells as reflected by the decreased Mmp-9 expression. In addition, with exogenous Bmp2 induction, these cell differentiation and mineralization were rescued as well as extracellular matrix remodeling was enhanced. Therefore, we for the first time described establishment of iBmpko/ko cells that are useful for study of mechanisms in regulating dental papilla mesenchymal cell lineages.
Dentin formation results from differentiation of dental papilla mesenchymal cells into odontoblasts occurring through a series of cytodifferentiation in a distinct spatial-temporal pattern during dentinogenesis (Ruch et al., 1995). Odontoblasts synthesize and secrete extracellular matrix proteins including collagenous and non-collagenous proteins (NCPs). These collagens and NCPs are required for dentin development and formation. Mutations of those genes are associated with dentinogenesis imperfecta (DGI) (MacDougall et al., 2006). Control of these gene expressions during dentinogeneis is a complex process and involved in many growth and transcription factor signaling pathways (Thesleff, 2003). Members of bone morphogenetic protein (Bmp) family have diverse biological functions during osteogenesis and embryonic development (Hogan, 1996; Ducy and Karsenty, 2000; Rosen, 2009). Among the Bmp family members, Bmp2 has been extensively studied for its various biological roles during chondrogenic and osteogenic differentiation as well as organ development (Zhang and Bradley, 1996; Ma et al., 2005; Lee et al., 2007; Singh et al., 2008). Bmp2 expression is observed in dental cells during tooth development (Aberg et al., 1997). Also, Bmp2 promotes dental pulp stem cell commitment to odontoblast lineages (Yang et al., 2009) and induces dental pulp cell differentiation (Chen et al., 2008; Cho et al., 2010). Bmp2 conditional knock-out (cKO) mice display abnormal tooth phenotypes with delayed odontoblast differentiation, abnormal dentin tubules, and decrease tooth-related gene expression (Feng et al., 2011; Yang et al., 2012; Guo et al., 2014).
However, roles of Bmp2 during odontogenesis have not been completely understood. Unlike bone and other tissues, it is relatively difficult to collect enough amounts of primary dental papilla mesenchymal cells from a single tooth. In addition, Bmp2 cKO in the mouse uterus results in female infertility due to the inability of the uterus to support post-implantation embryo development (Lee et al., 2007). Therefore, generation of a Bmp2 ablation dental papilla mesenchymal cell line would be a valuable tool for studying effects of Bmp2 on dental cell lineages and relevant molecular events involved in matrix mineralization and dentin regeneration. Previously, we generated an immortalized mouse Bmp2fx/fx dental papilla mesenchymal cell line (Wu et al., 2010). These cells display a stable capability for expansion as well as the identical gene expression profile to their primary dental papilla mesenchymal cells.
Here, we aimed to establish an immortalized mouse deleted Bmp2 dental papilla mesenchymal cell line and observed these cell behaviors. We further investigated cell growth as well as their genotypic and phenotypic characteristics as compared to that of the Bmp2fx/fx cells. Finally, we tested whether biological functions of these Bmp2 knock-out cells were rescued by exogenous Bmp2
Materials and Methods
Generation of immortalized deleted Bmp2 dental papilla mesenchymal cells
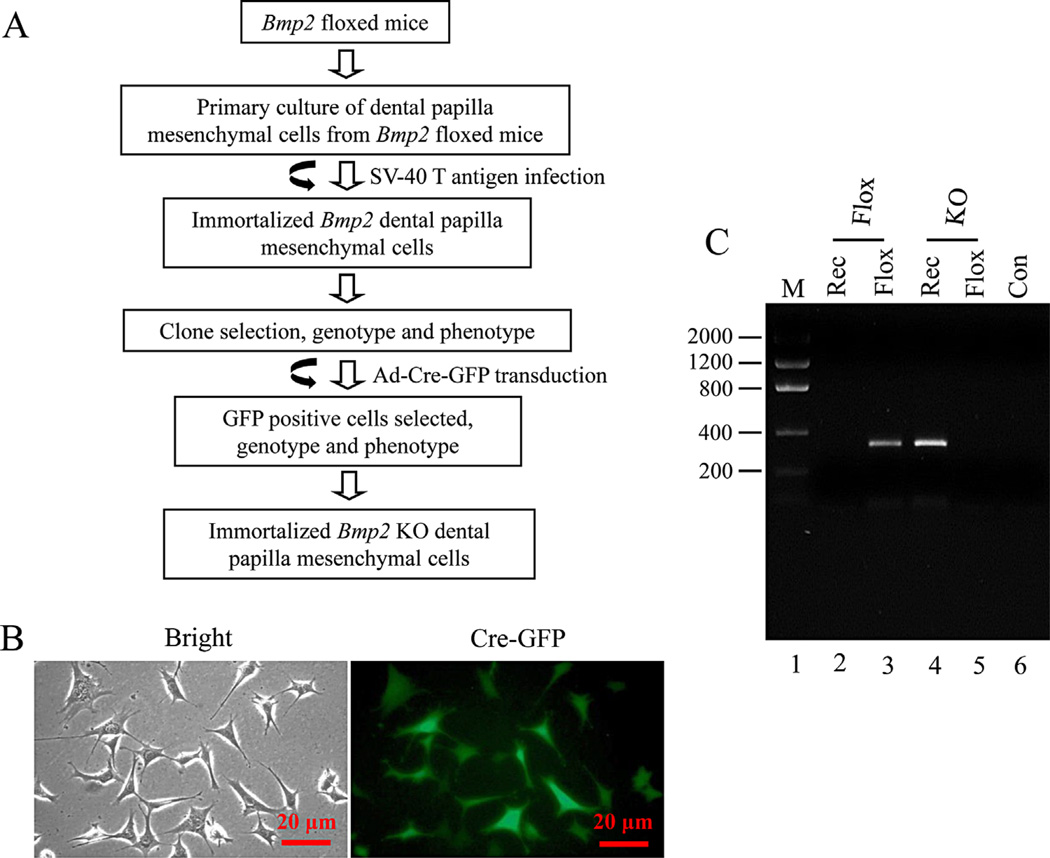
The immortalized mouse floxed Bmp2 dental papilla mesenchymal (iBmp2fx/fx dp) cells were maintained in alpha minimum essential medium (a-MEM, Invitrogen, San Diego, CA) containing 10% fetal calf serum (FCS) plus penicillin (100 U/ml) and streptomycin (100 mg/ml) and cultivated in 5% CO2 atmosphere under 37°C. Detail generation of the iBmp2fx/fx dp cells was described by our previous study (Wu et al., 2010) (Fig. 1A). For Bmp2 knock out, adenovirus with Cre recombinase and green fluorescent protein (Ad-Cre-GFP, Vector Biolabs, Malvern, PA) was added to the cells and the cells were transduced overnight for 14 h and then recovered in cultured medium. GFP positive cells were observed under a Nikon inverted fluorescent microscope. The positive cells were selectively picked up and re-plated at low densities to obtain further cell growth. Genomic DNAs were isolated from the iBmp2fx/fx dp and immortalized mouse Bmp2 knock-out dental papilla mesenchymal (iBmp2ko.ko dp) cells using DNA purification kit (Promega, Madison, WI). PCR genotyping was performed by amplification of the floxed/floxed (Bmp2fx/fx) and recombinant (Bmp2ko/ko) alleles using two pair primers: Bmp2fx/fx, forward 5′-GATGATGAGGTTCTTGGCGG-3′; reversed 5′-AGGGTTTCAGGTCAGTTTCCG-3′; Bmp2ko/ko, forward: 5′-GATGATGAGGTTCTTGGCGG-3′; reversed: 5′-AGCATGAACCCTCATGTGTTGG-3′. PCR conditions: 4 min at 94°, 35 cycles of 1 min at 94°C, 1 min at 62°C, and 2 min at 72°C, followed by 10 min at 72°C. The amplified products were run on a 1% agarose gel.
Fig. 1.
Generation of immortalized mouse Bmp2 knock-out dental papilla mesenchymal cells. A: Strategy for generation of immortalized Bmp2 knock out dental papilla mesenchymal cells. B: The iBmp2fx/fx dp cells were infected with Ad-Cre-GFP for 14 h. The GFP positive cells were seen under a Nikon inverted fluorescent microscope. C: Genomic DNA in the iBmp2fx/fx and iBmp2ko/ko dp cells was isolated and amplified by the floxed and recombinant Bmp2 specific primers. The amplified PCR products were run on an agarose gel and stained with ethidium bromide. Lane 1, lower molecular DNA marker; lane 2, iBmp2ko/ko; lane 3, iBmp2fx/fx; lane 4, iBmp2ko/ko; lane 5, iBmp2fx/fx; lane 6, Con, control; Rec, recombinant; KO, Bmp2 knock out.
Cell proliferation
Cell proliferation was identified by 5-bromo-2′-deoxyuridine (BrdU) incorporation and MTT method. Briefly, cells were plated into 6-well glass slides and incubated with 30 µM BrdU (Sigma–Aldrich, St. Louis, MO) in culture medium for 4 h. The cells were then treated with a mouse monoclonal anti-BrdU antibody (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), followed by a 1:1,000 dilution of the secondary antibody with Alexa Fluo® 488 green (Molecular Probes, Eugene, OR). For nucleus staining, the cells were incubated with a 1:5,000 dilution of Hoechst (Sigma–Aldrich). Images were obtained in a Nikon inverted microscope. Cell proliferation was expressed as a percentage of the number of BrdU positive cells relative to total number of Hoechst positive nuclei. For MTT assay, cells were seeded into 96-well plates with 1.0 × 103 cells/well and detected at days 1–6, respectively, using MTT cell proliferation assay kit (ATCC, Manassas, VA).
Cell cycle and migration assays
The iBmp2fx/fx and iBmp2ko/ko dp cells were grown with a-MEM containing with or without 10% FCS plus penicillin (100 U/ml) and streptomycin (100 mg/ml), and cultivated in 5% CO2 atmosphere under 37°C. The cells were harvested, washed twice with PBS, fixed, and permeabilized with 70% ethanol. For detection of DNA, the cells were incubated for 5 min at room temperature in citrate/phosphate buffer (pH 7.8) and then with 50 mg/ml of propidium iodide (PI) containing RNase A in Vindelov’s solution for 60 min at 37°C. The cell cycle was analyzed by dual laser BD FACSCalibur equipped with BD FACS-Flow Supply System (BD Biosciences, San Jose, CA). For cell migration, the iBmp2fx/fx and iBmp2ko/ko dp cells were maintained in α-MEM medium with supplemented with 10% FCS, 100 units/ml penicillin, and 100 µg/ml streptomycin. The cell migration assay was used BD Falcon cell culture inserts incorporating polyethylene terephthalate (PET) track-etched membranes with 8 µM perforations (BD Biosciences, San Diego, CA). The cell culture inserts were placed in 12-well plates. Cells (5 × 104 cells/ml) were added to the upper chamber in 250 µl of α-MEM containing 0.1% FCS. The lower chamber contained α-MEM with 0.1% FCS. After 12-h incubation of 5% CO2 at 37°C, the cells on the upper side of the membrane were carefully scraped off using cotton swabs and the cells on the lower side of the membrane were fixed in methanol and stained with HemaDiff eosin and thiazine (Statlab, Lewisville, TX). The number of cells that had migrated through the filters was quantified by counting 10 fields/membrane at a 200-fold magnification. Experiments were performed in triplicate of three separate studies.
RNA preparation and quantitative real time polymerase chain reaction
Total RNA was extracted from the iBmp2fx/fx and iBmp2ko/ko dp cells using RNA STAT-60 kit (Tel-Test, Inc., Friendswood, TX), treated with DNase I (Promega), and purified with RNeasy Mini Kit (Qiagen, Inc., Valencia, CA). RNA concentration was determined at an optical density of OD260. The RNA was transcribed into cDNA by SuperScript II reverse transcriptase (Invitrogen). Specific primers for the qRT-PCR were shown in Table 1. qRT-PCR amplification reaction was analyzed in real time on an ABI 7500 (Applied Biosystems, Foster City, CA) using SYBR Green chemistry, and threshold values were calculated using SDS2 software (Applied Biosystems). TheΔΔ Ct method was used to calculate gene expression levels normalized to cyclophilin A value. The results were performed in triplicate of three separate experiments and expressed as a relative fold change in gene expression compared to the control.
TABLE 1.
Primer sequences used for real time polymerase chain reactions
| Gene | Primer sequences | Tm (°C) |
|---|---|---|
| Bsp | Forward: 5′-AAAGTGAAGGAAAGCGACGA-3′ | 52 |
| Reversed: 5′-GTTCCTTCTGCACCTGCTTC-3′ | ||
| Col1a1 | Forward: 5′-CCTGACGCATGGCCAAGAAGA-3′ | 60 |
| Reversed: 5′-GCATTGCACGTCATCGCACA-3′ | ||
| Cyclo A | Forward: 5′-GAGCTCTGAGCACTGGAGAGA-3′ | 64 |
| Reversed: 5′-GATGCCAGGACCTGTATGCT-3′ | ||
| Dlx3 | Forward: 5′-GCGACACTCAGGAATCATTG-3′ | 50 |
| Reversed: 5′-CGGTCCATGCATTTGTTATC-3′ | ||
| Dmp1 | Forward: 5′-CAGTGAGGATGAGGCAGACA-3′ | 54 |
| Reversed: 5′-TCGATCGCTCCTGGTACTCT-3′ | ||
| Dspp | Forward: 5′-AACTCTGTGGCTGTGCCTCT-3′ | 59 |
| Reversed: 5′-TATTGACTCGGAGCCATTCC-3′ | ||
| Mmp-2 | Forward: 5′-CATCGCCCATCATCAAGTTCC-3′ | 55 |
| Reversed: 5′-CCGAGCAAAAGCATCATCCAC-3′ | ||
| Mmp-9 | Forward: 5′-TGGTGTGCCCTGGAACTCA-3′ | 64 |
| Reversed: 5′-TGGAAACTCACACGCCAGAAG-3′ | ||
| Osx | Forward: 5′-ACTCATCCCTATGGCTCGTG-3′ | 55 |
| Reversed: 5′-GGTAGGGAGCTGGGTTAAGG-3′ | ||
| Runx2 | Forward: 5′-TACAAACCATACCCAGTCCCTGTTT-3′ | 55 |
| Reversed: 5′-AGTGCTCTAACCACAGTCCATGCA-3′ |
Bsp, bone sialoprotein; Col1a1, a 1 collagen type; Cyclo A, cyclophilin A; Dlx3, distal-less 3; Dmp1, dentin matrix protein 1; Dspp, dentin sialophosphoprotein; Mmp-2, matrix metalloproteinase-2; Mmp-9, matrix metalloproteinase-9; Osx, Osterix.
Western blot analysis
Cells were maintained in α-MEM medium with 5% FCS, 100 U/ml of penicillin/streptomycin, 50 µg/ml ascorbic acid, 10 nM dexamethasone, and 10 mM sodium β-glycerophosphate, and were then washed with 1× cold PBS and lysed with RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/ml phenylmethylsulfonyl fluoride (PMSF), 50 KIU/ml aprotinin, 100 mM sodium orthovanadate; Santa Cruz Biotechnology, Inc.). Whole cell lysate was resolved by 7% SDS–PAGE gels and transferred to Trans-Blot membranes (Bio-Rad, Hercules, CA). Antibodies directed against mouse Bsp and Dmp1 (gifts from Dr. Larry Fisher, NIDCR), Col1α1, Dsp, Mmp-2, Mmp-9, Osx, Runx2 (Santa Cruz Biotechnology, Inc.) and Dlx3 (Abcam, Cambridge, MA) were used as primary antibodies. The membranes were blocked with 5% non-fat milk in TBST buffer (10 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% Tween-20) for 60 min at room temperature. After washing, the membranes were incubated with primary antibodies against those proteins with appropriate dilution (1:500–1,000) overnight at 4°C, respectively. The secondary antibody (horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG) was diluted to 1:5,000–10,000 at room temperature for 60 min. Immunoreactivity was determined using ECL chemiluminescence reagent (Thermo Scientific, Pittsburgh, PA). As a control, goat polyclonal anti-mouse β-actin antibody was used (Santa Cruz Biotechnology, Inc.). The band intensity was measured using ImageJ software (ImageJ, NIH/gov/iJ). Protein expression level of each sample was normalized to β-actin value. The iBmp2fx/fx protein was used as control and acts as onefold increase. The fold change in the iBmp2ko/ko protein was calculated by dividing the control group.
Analysis of Mmps by zymography
The supernatant harvested from the iBmp2fx/fx and iBmp2ko/ko dp cells was used as a crude enzyme. Gelatinolytic activities of Mmps were analyzed using 10% SDS–PAGE gels co-polymerized with 150 µg/ml gelatin (Bio-Rad Laboratories, Inc. Hercules, CA). Briefly, after separation of samples, electrophoresis gels were washed in 5% Triton X-100, equilibrated with collagenase assay buffer (50 mM Tris–HCl, pH 7.5, 5 mM CaCl2, 100 mM NaCl, 0.01% Triton X-100, 0.1 mM ZnCl2, 0.2% Brij 35 non-ionic detergent, and 2 mM Na3N) and incubated at 37°C overnight. Counterstaining with Coomassie brilliant blue R-250 revealed gelatin degradation.
In situ DQ-FITC-collagen IV and -gelatin degradation assays
Glass slides were pre-coated with DQ-FITC-collagen IV and DQ-FITC-gelatin (Life technologies, Grand Island, NY) at a final concentration of 40 ng/µl for 2 h at 37°C, respectively. After washing with PBS, the slides were air dried and fixed with 2% formaldehyde. After washing with PBS, the slides coated with the DQ-FITC-collagen IV and DQ-FITC-gelatin were equilibrated with a-MEM without serum. The iBmp2fx/fx and iBmp2ko/ko dp cells were added to the plates containing the DQ-FITC-collagen IV- or DQ-FITC-gelatin coated slides and cultured for 12 h, respectively. The cells were fixed with 4% formaldehyde for 15 min and washed with PBS. Then, the cells were mounted using Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA). Images were taken using a Nikon inverted fluorescent microscope coupled to cool CCD camera and NIS-GIEMENTS software.
Alkaline phosphatase (ALP) and mineralization assays
For detection of ALP activity, culture of the iBmp2fx/fx and iBmp2ko/ko dp cells was fixed with 70% ethanol for 5 min and washed in the buffer (100 mM Tris–HCl, pH 9.5; 100 mM NaCl; 50 mM MgCl2). In situ ALP staining was performed according to the supplier’s instructions (Bio-Rad Laboratories). For mineralization assay, these cells were plated in 6-well plates and cultured in calcifying medium (a-MEM supplemented with 5% FCS, penicillin [100 U/ml] and streptomycin [100 µg/ml], 50 µg/ml ascorbic acid, 10 nM dexamethasone, and 10 mM sodium β-glycerophosphate) at 37°C on given time periods. The cells were fixed in 10% formaldehyde neutral buffer and then stained with alizarin red S dye (Sigma–Aldrich).
Statistical analysis
Quantitative data were presented as means ± SD from three independent experiments and compared with the results of one-way ANOVA using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). The differences between groups were statistically significant at *P < 0.05 and **P < 0.01.
Results
Generation of immortalized mouse Bmp2 knock out dental papilla mesenchymal cells
To establish a Bmp2 knock-out dental papilla mesenchymal (iBmp2ko/ko dp) cell line, the immortalized mouse floxed Bmp2 dental papilla mesenchymal cells (iBmp2fx/fx) were infected with Ad-Cre-GFP and then selected. The transduced cells showed a high efficiency of infection observed under a Nikon fluorescent microscope (Fig. 1B). Several GFP positive cells were picked and re-grown. Knock out of Bmp2 gene by Cre recombinase in the iBmp2fx/fx dp cells was confirmed by PCR (Fig. 1C). This result showed that Cre recombinase deletes Bmp2 gene in the iBmp2fx/fx dp cells.
Knock out of Bmp2 in the iBmp2fx/fx dental papilla mesenchymal cells leads to change of cell proliferation rate
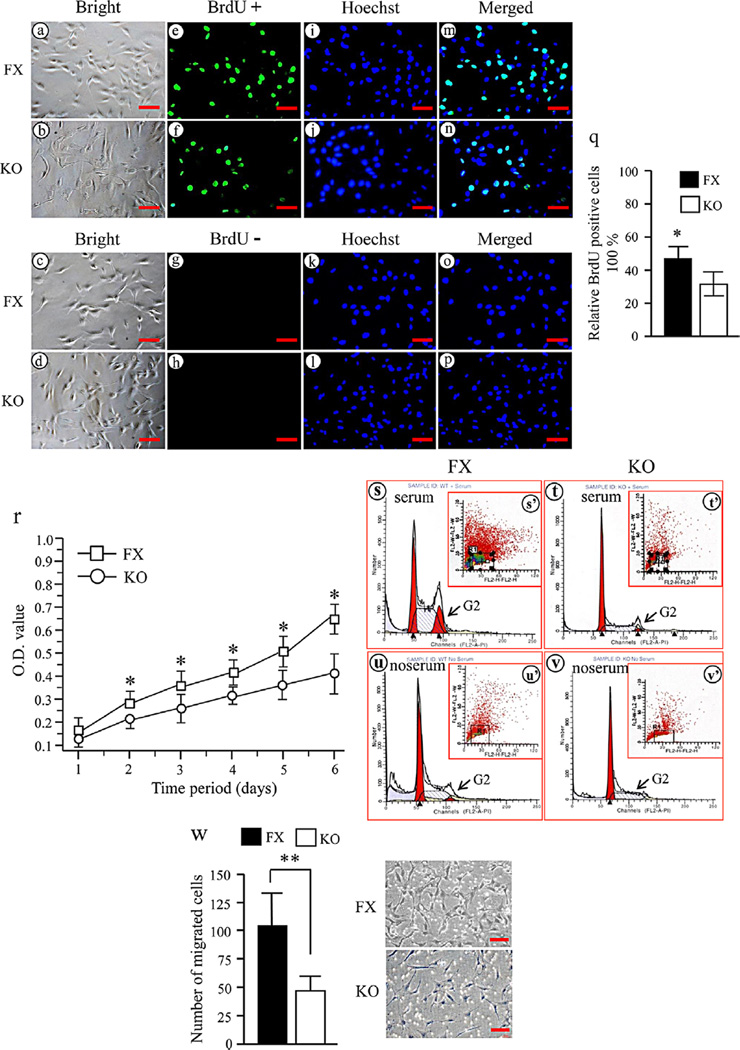
To explore the effect of Bmp2 on mouse dental papilla mesenchymal cell proliferation, the iBmp2fx/fx and iBmp2ko/ko dp cell proliferations were analyzed using BrdU and MTT assays. This result showed that the iBmp2ko/ko dp cells exhibit a slow growth rate compared to that of the iBmp2fx/fx dp cells (Fig. 2e–f, q–r). To investigate which mechanisms of Bmp2 regulate cell proliferation, we analyzed the cell cycle distribution of the iBmp2fx/fx and iBmp2ko/ko dp cells. The data showed that the iBmp2k/ko dp cell growth is inhibited in the G2 phase (Fig. 2s–v). These results suggested that the slow growth of the iBmp2ko/ko dp cells is partially due to G2 phase arrest. We next investigated whether Bmp2 modifies cell migration. When both of the iBmp2fx/fx and iBmp2ko/ko dp cells were cultured in the cell migration chamber at 12 h, the iBmp2ko/ko dp cell migration was impaired compared to that of the iBmp2fx/fx dp cells (P < 0.01) (Fig. 2w). These results indicate that Bmp2 promotes cell growth and migration.
Fig. 2.
Deletion of Bmp2 in the Bmp2fx/fx dp cells disrupts cell proliferation and migration. Proliferation of the iBmp2fx/fx and iBmp2ko/ko dp cells was immunostained using BrdU antibody after a 4-h BrdU incorporation (30 mM). The iBmp2fx/fx dp cells showed a higher proliferation rate (e) than that of the iBmp2ko/ko dp cells (f). BrdUþ (e, f) and BrdU- (g, h) show cells stained with and without BrdU antibody. a–d: The cells were photographed under a light microscope using a Nikon camera. i–l: The cells were stained with Hoechst for the nuclei. m–p: Images were merged. Scale bar, 20 mM. q: A percentage of the number of BrdU positive cells relative to the total number of Hoechst positive nuclei from the iBmp2fx/fx and iBmp2ko/ko dp cells was calculated (*P < 0.05). r: Proliferation data of the iBmp2fx/fx and iBmp2ko/ko dp cells by MTT assay. The iBmp2fx/fx dp cells showed higher proliferation rate than the Bmp2ko/ko dp cells from 1- to 6-day culture. Asterisk shows significant differences between the iBmp2fx/fx and iBmp2ko/ko dp cells (*P < 0.05). s–v: Cell cycle distributions were measured using BD FACSCalibur cytofluorometer detection of DNA content in the iBmp2fx/fx and iBMp2ko/ko dp cells with or without 10% fetal calf serum. Significant decrease of number of cells at G2 phase could be seen in the iBmp2ko/ko dp cells. w: The iBmp2fx/fx and iBmp2ko/ko dp cells that migrated through the porous membrane were stained and counted. Photographs represent the migrated iBmp2fx/fx and iBmp2ko/ko dp cells. Bar represents the mean ± SD (n = 3). **P < 0.01. FX, floxed; KO, Bmp2 knock out.
Knock out of Bmp2 in the iBmp2fx/fx dp cells down-regulates its downstream gene expression
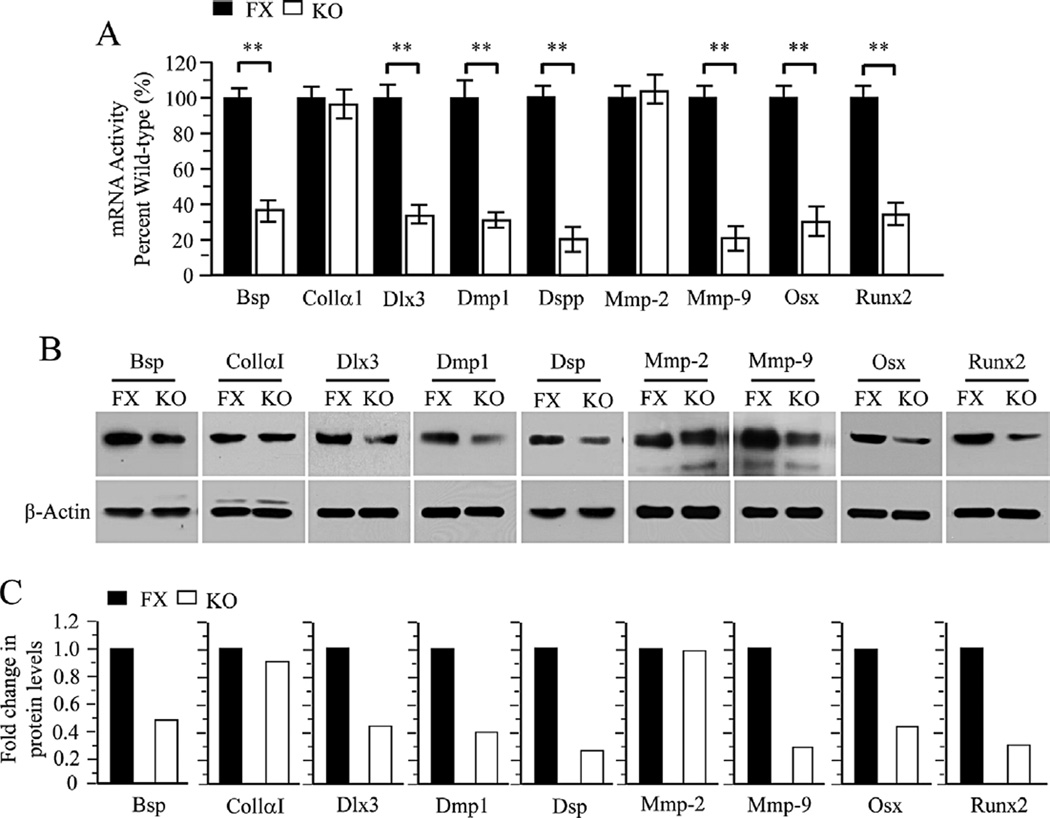
Bmp2 is involved in tooth and other tissue development and formation. We further investigated whether Bmp2 knock out down-regulates its downstream gene expression in the iBmp2ko/ko dp cells. Figure 3A shows that expression of several tooth-related genes was decreased in the iBmp2ko/ko dp cells as detected by qRT-PCR. It includes Bsp, Dlx3, Dmp1, Dspp, Mmp-9, Osx, and Runx2. These results were further confirmed using Western blot analysis (Fig. 3C and D).
Fig. 3.
Altered expression of tooth-related and processing protein genes in the iBmp2 dp cells. A: Total RNA was isolated from the iBmp2fx/fx and iBmp2ko/ko dp cells for the measurement of Bsp, Colla1, Dlx3, Dmp1, Dspp, Mmp-2, Mmp-9, Osx, and Runx2 transcripts by qRT-PCR. Cyclophilin A was used as an internal control. Expression of these mRNAs in the Bmp2fx/fx dp cells acts as a 1.0-fold increase. The bar graphs show mean ± SD from three independent experiments with triplicate samples for each transcript measurement. *P < 0.05, **P < 0.01. B: The iBmp2fx/fx and iBmp2ko/ko dp cells were lysed and protein expressional levels were detected by Western blot assay using antibodies specific to Bsp, Colla1, Dlx3, Dmp1, Dsp, Mmp-2, Mmp-9, Osx, and Runx2, respectively. b-actin was used as an internal control. C: The protein band intensity was quantitated by Image J software. The proteins from the iBmp2fx/fx dp cells were normalized to b-actin protein as control. The fold change in the Bmp2ko/ko protein expression levels was calculated by dividing the Bmp2fx/fx dp protein expression levels. This result demonstrates that several protein expression levels were decreased in the iBmp2ko/ko dp cells. FX, floxed; KO, Bmp2 knock out.
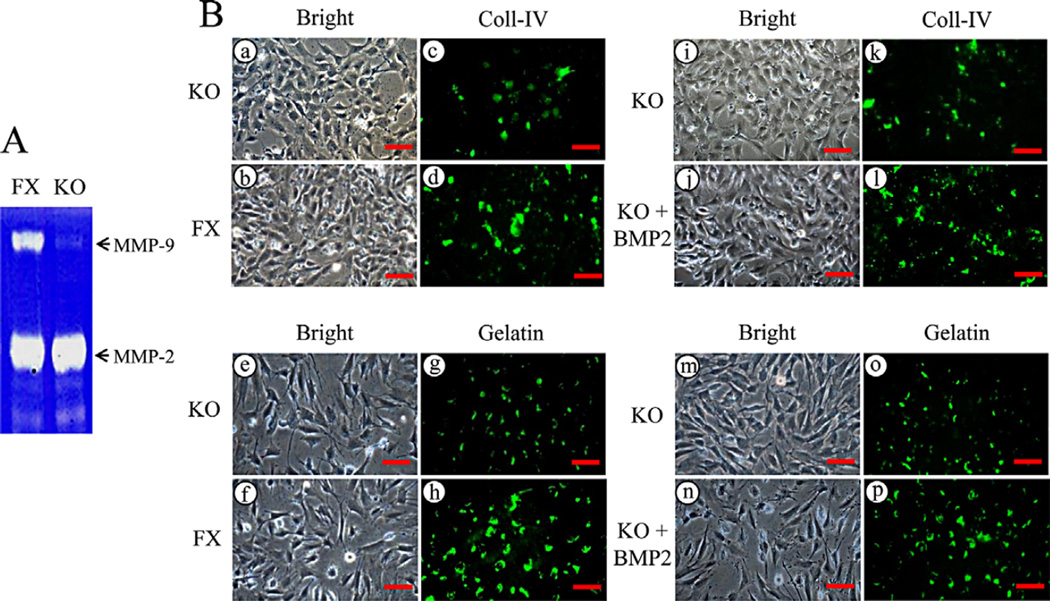
Bmp2 deletion is interfered to extracellular matrix remodeling via Mmp-9
Expression of Mmp-9 is reduced in the iBmp2ko/ko dp cells at RNA and protein levels as described above. As Mmp-9 is involved in numerous physiological and pathological roles including extracellular matrix remodeling (Lund et al., 2011), we further examined Mmp-9 secretion using zymorgraphy assay in both the dental papilla mesenchymal cells. The results showed that secretion of Mmp-9 in the iBmp2ko/ko dp cells is much less compared to that of the iBmp2fx/fx dp cells, whereas there was no significant change of Mmp-2 between the iBmp2fx/fx and iBmp2ko/ko dp cells (Fig. 4A). To assess Mmp-9 role in extracellular matrix remodeling mediated by Bmp2, in situ collagen IV and gelatin degradations were investigated as collagen IV and gelatin are substrates of Mmp-9. As shown in Figure 4Bc and d, g and h, faint fluorescent spots of collagen IV and gelatin degradations were observed in the iBmp2ko/ko dp cells compared to that of the iBmp2fx/fx dp cells. In contrast, when the iBmp2ko/ko dp cells were treated with exogenous Bmp2, number and intensity of collagen IV and gelatin degradation products in the iBmp2ko/ko dp cells were increased (Fig. 4Bk and l, o and p), indicating that Bmp2 is involved in extracellular matrix remodeling through Mmp-9 signal.
Fig. 4.
Bmp2 deletion is interfered to extracellular matrix remodeling via Mmp-9. A: The supernatant harvested from the iBmp2fx/fx and iBmp2ko/ko dp cells was analyzed using gelatinolytic activities. Expression of Mmp-9 in the iBmp2ko/ko dp cells was decreased. B: The iBmp2fx/fx and iBmp2ko/ko dp cells were grown on the DQ-FITC-collagen IV and DQ-FITC-gelatin-coated slides for 14 h. The cells were fixed and degradation spots of the DQ-FITC-collagen IV and DQ-FITC-gelatin were observed using a Nikon inverted fluorescent microscope (c-d, g-h, k, o). The iBmp2ko/ko dp cells were added to the DQ-FITC-collagen IV and DQ-FITC-gelatin-coated slides and then treated with or without 100 ng/ml of exogenous Bmp2 for 14 h. The cells were fixed and degraded spots of the DQ-FITC-collagen IV and DQ-FITC-gelatin were imaged by the fluorescent microscope (l, p). a–b, e–f, i–j, m–n: The cells were photographed under a light inverted microscope. Scale bars, 20 mM. FX, floxed; KO, Bmp2 knock out.
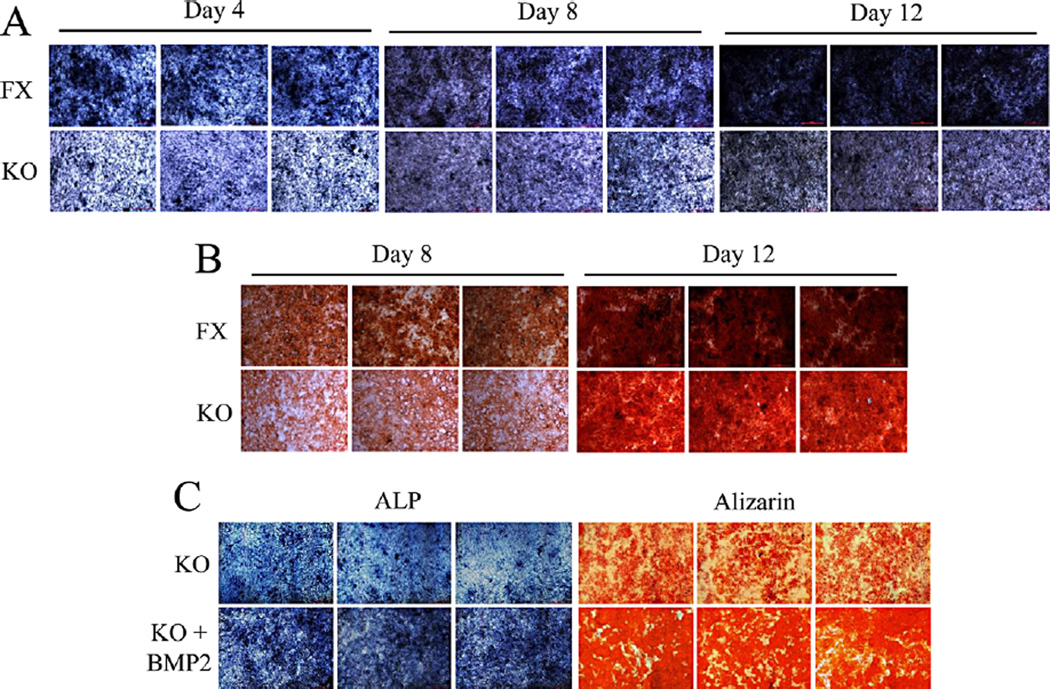
Deletion of Bmp2 causes delay of dental papilla mesenchymal cell differentiation and mineralization
To determine the effect of Bmp2 on cell differentiation and mineralization activities, we measured ALP activity by in situ ALP histochemistry as ALP is a marker of dental cell differentiation. Both cells were cultured in calcifying medium in given time periods. This result showed that delayed cell differentiation was seen in the iBmp2ko/ko dp cells (Fig. 5A). Also, deletion of Bmp2 gene led to low activity of cell mineralization by using alizarin red S staining (Fig. 5B). Furthermore, when exogenous Bmp2 protein was added to the iBmp2ko/ko dp cells, Bmp2 was able to rescue these cell differentiation and mineralization compared to these cells without Bmp2 induction (Fig. 5C).
Fig. 5.
Deletion of Bmp2 delays cell differentiation and mineralization. A: The iBmp2fx/fx and iBmp2ko/ko dp cells were cultured in the calcifying medium for 4, 8, and 12 days. ALP activity was analyzed using in situ ALP staining. B: For cell mineralization assay, both of iBmp2fx/fx and iBmp2ko/ko dp cells were treated with calcifying medium for 8 and 12 days. Mineralized nodules were visualized with alizarin red S staining. C: The Bmp2ko/ko dp cells were treated either with or without recombinant Bmp2 (10 ng/ml) in calcifying medium for 7 and 14 days, respectively. The 7-day-inducced cells were used for ALP assay while 14-day induced cells were assayed by alizarin red S staining. Exogenous Bmp2 rescued the iBmp2ko/ko dp cell differentiation and mineralization. FX, floxed; KO, Bmp2 knock out.
Discussion
Bmp2 is a multiple-functional growth factor and involved in many organ development and bone fracture healing (Zhang and Bradley, 1996; Ma et al., 2005; Lee et al., 2007; Rosen, 2009). Bmp2 cKO mice display retardation of tooth growth, abnormal dentin structure with wide predentin, thin dentin, decrease of dentin mineral density (Feng et al., 2011; Yang et al., 2012). The quality of dentin is altered with unmineralized areas, dysmorphic dentinal tubules, and delay odontoblast differentiation. Molecular mechanisms of Bmp2 during dentinogenesis have not been completely understood. Studies of physiology and pathology of differentiation of dental papilla mesenchymal cells to odontoblasts have been hampered as it is relatively hard to obtain lot of primary dental papilla mesenchymal cells (Wu et al., 2010). In the present study, we established the immortalized mouse Bmp2 KO dental papilla mesenchymal cells. The strategy for this study was to use the transduction of Ad-Cre-GFP into immortalized mouse floxed Bmp2 dental papilla mesenchymal cells as the iBmp2fx/fx dp cells show similar genotypic and phenotypic characteristics to that of the primary wild-type mouse dental papilla mesenchymal cells (Wu et al., 2010). The Ad-Cre-GFP showed a high rate of infection with visible marker and had highly efficiency of Bmp2 gene knock out.
In Bmp2 cKO mice, bone fracture healing was impaired (Tsuji et al., 2006) and teeth exhibited small sizes compared to that of the wild type (normal and Bmp2fx/fx) mice (Feng et al., 2011; Guo et al., 2014). We noted that the iBmp2ko/ko dp cell growth is slow compared to that of the iBmp2fx/fx dp cells. Further study indicated that cell cycle is arrested in the G2 phase of the Bmp2ko/ko dp cells, indicating that Bmp2 is involved in not only cell differentiation, but also cell growth. However, it needs to be further investigated which signal of Bmp2 is involved in cell cycle and growth.
We also observed that in the iBmp2ko/ko dp cells gene expression of several transcriptional factors, extracellular matrix proteins, and proteinases is reduced. It includes Bsp, Dlx3, Dmp1, Dspp, Mmp-9, Osx, and Runx2. Both of Dmp1 and Dspp are important markers of odontoblast differentiation and mineralization (D’Souza et al., 1997). Mutations of Dmp1 and Dspp genes cause DGI (Xiao et al., 2001; Sreenath et al., 2003; Ye et al., 2004). Bmp2 up-regulates expression of Dmp1 and Dspp genes in dental cells (Chen et al., 2008; Casagrande et al., 2010; Cho et al., 2010). Phenotype of Bmp2 cKO mice shows similar to that of Dmp1 and Dspp gene mutations in humans and mice, exhibiting thin dentin, dentin hypomineralization, dental pulp exposure, and delay of odontoblast differentiation (Sreenath et al., 2003; Ye et al., 2004; Feng et al., 2011). Using the iBmp2 dp cells, we will further elucidate the molecular mechanisms of how Bmp2 signal regulates tooth-related gene expression and dentinogenesis
Also, Runx2 and Osx are important transcription factors not only for osteogenesis and chondrogenesis, but also dentinogenesis. Mutations of Runx2 and Osx result in abnormal tooth and bone development and formation (Lee et al., 1997; D’Souza et al., 1999; Nakashima et al., 2002). Dlx3 is a transcription factor necessary for tooth development, and Dlx3 gene mutations cause autosomal dominant genetic disorder called tricho-dento-osseous syndrome (Price et al., 1999). Furthermore, expression of these genes is regulated by Bmp2 (Park and Morasso, 2002; Javed et al., 2008; Matsubara et al., 2008). Our study demonstrated that exogenous Bmp2 is capable of rescuing the cell differentiation and mineralization in the iBmp2ko/ko dp cells. It indicates that Bmp2 controls dental papilla mesenchymal cell growth, differentiation, and odontogenesis via a complex signal pathway.
More noticeably, Mmp-9 expression was dramatically reduced in the iBmp2ko/ko dp cells. The extracellular matrix remodeling was decreased in the iBmp2ko/ko dp cells, showing weak and low fluorescent spots of collagen IV and gelatin degradations, which are Mmp-9 substrates. Exogenous Bmp2 enhanced the degraded products, indicating that Bmp2 signal controls extracellular matrix remodeling via Mmp-9. Mechanism of Bmp2 in regulation of Mmp-9 expression might be involved in Runx2 signal pathway (Pratap et al., 2005).
Although many Bmp molecules besides Bmp2 including Bmp4 are expressed during the dentinogenesis (Aberg et al., 1997), our data suggest that Bmp molecules cannot compensate for Bmp2 loss in dentin formation. In Bmp2-cKO mice, Bmp4 expression is not changed (Yang et al., 2012), suggesting that each of them plays a unique role during odontogenesis through different receptor combinations (Cheifetz, 1999; Little and Mullins, 2009).
Conclusively, we established an immortalized mouse deleted Bmp2 dental papilla mesenchymal cell line. The deleted Bmp2 cell line decreases cell growth capability and cell cycle is arrested in the G2 phase. Expression of tooth-related genes is reduced in these Bmp2 ablation cells, resulting in retardation of the cell differentiation and mineralization. Finally, the Bmp2 deletion causes impairment of extracellular matrix remodeling viaMmp-9 signal. Therefore, the generated cell line would provide a useful tool for studies of the molecular mechanism involved in regulating the functions of dental papilla mesenchymal cell proliferation, differentiation, and extracellular matrix remodeling during dentin development and formation.
Acknowledgments
We are grateful to core facility center at The University of Texas Health Center at San Antonio, Texas performed cell cycle experiments. This research was supported by the National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research (NIDCR, DE19892) and partially by the Natural Science Foundation of China (81170929).
Contract grant sponsor: National Institute of Health (NIH)-USA.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Literature Cited
- Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nor JE. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- Cheifetz S. BMP receptors in limb and tooth formation. Crit Rev Oral Biol Med. 1999;10:182–198. doi: 10.1177/10454411990100020501. [DOI] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang HH, Chen L, Dong J, Gay I, MacDougall M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem. 2008;283:19359–19370. doi: 10.1074/jbc.M709492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YD, Yoon WJ, Woo KM, Baek JH, Park JC, Ryoo HM. The canonical BMP signaling pathway plays a crucial part in stimulation of dentin sialophosphoprotein expression by BMP-2. J Biol Chem. 2010;285:36369–36376. doi: 10.1074/jbc.M110.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, Thesleff I. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Feng J, Yang G, Yuan G, Gluhak-Heinrich J, Yang W, Wang L, Chen Z, Schulze McDaniel J, Donly KJ, Harris SE, Macdougall M, Chen S. Abnormalities in the enamel in bmp2-deficient mice. Cells Tissues Organs. 2011;194:216–221. doi: 10.1159/000324644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Feng J, Wang F, Li W, Gao Q, Chen Z, Shoff L, Donly KJ, Gluhak-Heinrich J, Chun YH, Harris SE, MacDougall M, Chen S. Bmp2 deletion causes an amelogenesis imperfecta phenotype via regulating enamel gene expression. J Cell Physiol. 2014;230:1871–1882. doi: 10.1002/jcp.24915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IK, Nielsen BS, Almholt K, Rono B, Hald A, Illemann M, Green KA, Christensen IJ, Romer J, Lund LR. Concomitant lack of MMP9 and uPA disturbs physiological tissue remodeling. Dev Biol. 2011;358:56–67. doi: 10.1016/j.ydbio.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Dong J, Acevedo AC. Molecular basis of human dentin diseases. Am J Med Genet Part A. 2006;140:2536–2546. doi: 10.1002/ajmg.a.31359. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Park GT, Morasso MI. Bone morphogenetic protein-2 (BMP-2) transactivates Dlx3 through Smad1 and Smad4: Alternative mode for Dlx3 induction in mouse keratinocytes. Nucleic Acids Res. 2002;30:515–522. doi: 10.1093/nar/30.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JA, Wright JT, Walker SJ, Crawford PJ, Aldred MJ, Hart TC. Tricho-dento-osseous syndrome and amelogenesis imperfecta with taurodontism are genetically distinct conditions. Clin Genet. 1999;56:35–40. doi: 10.1034/j.1399-0004.1999.550105.x. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Ruch JV, Lesot H, Begue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev. 2008;2:134–141. doi: 10.1159/000143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Wu LA, Feng J, Wang L, Mu YD, Baker A, Donly KJ, Gluhak-Heinrich J, Harris SE, MacDougall M, Chen S. Immortalized mouse floxed Bmp2 dental papilla mesenchymal cell lines preserve odontoblastic phenotype and respond to BMP2. J Cell Physiol. 2010;225:132–139. doi: 10.1002/jcp.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- Yang W, Harris MA, Cui Y, Mishina Y, Harris SE, Gluhak-Heinrich J. Bmp2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Res. 2012;91:58–64. doi: 10.1177/0022034511424409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, van der Kraan PM, Bian Z, Fan M, Walboomers XF, Jansen JA. Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res. 2009;88:1020–1025. doi: 10.1177/0022034509346258. [DOI] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/ chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]