In a recent cutting-edge review,1 Kiecolt-Glaser underlined the need of further research aimed at understanding the relationship between depression and nutrition and their potential interactive influence on inflammation. Previous studies have shown that depression is associated with upregulated inflammatory response, characterized by increased levels of pro-inflammatory cytokines and other acute-phase proteins.2 Diet may also influence inflammation: a healthy dietary pattern rich in fruit, vegetables and olive oil, such as the Mediterranean diet, is associated with lower levels of inflammatory markers, perhaps because of the anti-inflammatory properties of antioxidants.3 We examined (1) whether older individuals with depressive symptoms are more likely to develop a pro-inflammatory state and (2) whether such an excess risk of developing a pro-inflammatory state is lower in those who have a healthy (Mediterranean-style) diet. We tested this hypothesis using data from the InCHIANTI study,4 a prospective population-based study of older persons in Tuscany (Italy). Participants were evaluated at enrollment (1998–1999) and again at 3- and 6-year follow-up visits. Depressive symptoms were assessed at baseline with the Center for Epidemiologic Studies-Depression scale (CES-D). Adherence to the Mediterranean diet was assessed at baseline by a well-validated dietary questionnaire and a Mediterranean Diet Score (0–9, higher score indicating better adherence) was computed according to Trichopoulou et al.5 Levels of interleukin-6 (IL-6) and C-reactive protein (CRP) were assessed at baseline, 3- and 6-year follow-up. The sample for the present analyses consisted of 793 participants aged ⩾65 years with baseline data on depressive symptoms and inflammatory markers and at least one follow-up measure for each inflammatory marker. At enrollment, depressed (CESD ⩾20) participants were older, more often women and sedentary, had lower level of physical functioning, took more medications and were less adherent to a Mediterranean-style diet. The longitudinal association between depressive symptoms and inflammatory markers was estimated using random coefficient analyses with random intercept and slopes. After adjustment for age, sex, physical activity, lower extremity function, number of medications, use of antidepressants and NSAIDs, CES-D scores (per s.d. increase) were associated with higher IL-6 increase over time, but not with CRP change. A significant depression*time effect (β = 0.09, s.e. = 0.02, P < 0.0001) indicated that higher depressive symptoms were associated with a steeper IL-6 increase over time. To examine the moderation effect of a healthy diet, we entered interaction terms depression*diet*time to the models including the interactions terms nested within this interaction. The interaction term was significant only in analysis focusing on IL-6 (P = 0.01). To further illustrate the interaction, the Mediterranean diet score was dichotomized around the median and the analyses were stratified by healthy diet status. Figure 1 shows that the unadjusted mean change in IL-6 levels after 6 years of follow-up differed significantly across depression and diet groups. Mean increase in IL-6 levels was higher among the depressed non-adherent to a healthy diet than in all other groups. Higher depressive symptoms were associated with a major increase in IL-6 levels over time in participants non-adherent to a healthy diet (β = 0.13, s.e. = 0.03, P < 0.0001), but not in those adherent (β = 0.04, s.e. = 0.03, P = 0.17), after adjustment for confounders. Similar results were obtained repeating all analyses using the clinical cutoff of 20 points on the CES-D. Taken together, these findings indicate that in older persons depressive symptoms are associated with increased inflammation over time, and that a healthy diet can buffer the effect of depression on inflammation. This interaction could be explained by shared biological pathways, such as the opposite modulation exerted by stress or antioxidants on transcription factor nuclear factor kappa B, which upregulate pro-inflammatory cytokines,6,7 or the accumulation promoted by depression and unhealthy diet of visceral fat, which in turn promotes inflammation directly or through other mechanisms such as hypothalamic–pituitary–adrenal axis dysregulation.8 Moreover, depression and diet likely have a bidirectional relationship: depression and stress may promote unhealthy dietary preference,9 whereas in turn a healthy diet may lower the risk of incident depression over time.10 The findings of our study provide empirical support to Kiecolt-Glaser’s hypothesis1 about the joint contribution of depression and diet to inflammation, and suggest that intervention aimed at improving the quality of diet may be especially effective in buffering the inflammatory process boosted by depression, which ultimately could result in various health benefits.
Figure 1.
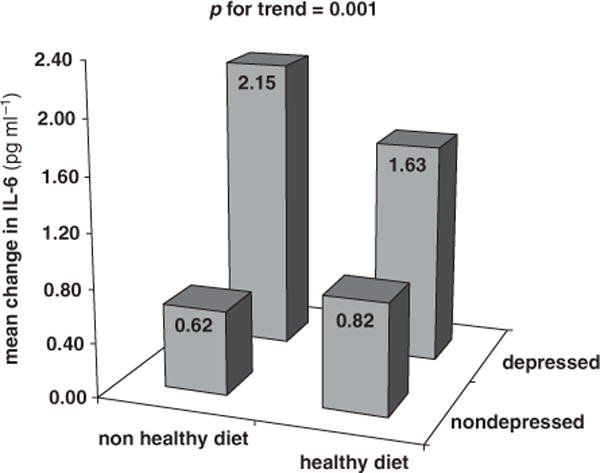
Unadjusted mean change in IL-6 levels after 6 years of follow-up across depression status and adherence to a healthy (Mediterranean-style) diet. Depressed mood: CES-D⩾20. Healthy diet: Mediterranean diet score⩾5. IL-6: at baseline, IL-6 was measured with an ultra-sensitive ELISA (CytoScreen Human IL-6, Biosource International Inc., Camarillo, CA, USA). Minimum detectable threshold was 0.10 pg ml−1 and the inter-assay CV was 7%. At 3- and 6-year follow-up, IL-6 was measured using a solid-phase high-sensitivity quantitative sandwich ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN, USA). Minimum detectable threshold was 0.10 pg ml−1 and the inter-assay CV was 7%. In order to make IL-6 measures obtained at baseline comparable with measures from both follow-ups, a pilot study based on 100 randomly selected serum baseline specimens was run using a solid-phase high-sensitivity quantitative sandwich ELISA as described for follow-ups. A regression equation (r-square = 0.79) was developed at the National Institute on Aging to predict the IL-6 sandwich ELISA results from the original ELISA results for the pilot subjects. This equation was then used to predict sandwich ELISA results for all subjects at baseline. The correlation between the estimated high-sensitivity measure of IL-6 and the original measure was high (Pearson’s R = 0.89). The estimated high-sensitivity baseline measure of IL-6 was used in this study.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kiecolt-Glaser JK. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howren MB, Lamkin DM, Suls J. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano D, Ceriello A, Esposito K. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Bandinelli S, Benvenuti E, et al. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 5.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 6.Semba RD, Lauretani F, Ferrucci L. Arch Biochem Biophys. 2007;458:141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AH, Maletic V, Raison CL. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 9.van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Age Ageing. 2003;32:81–87. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, Schlatter J, et al. Arch Gen Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]


