Abstract
A glomerulus is the network of capillaries that resides in the Bowman’s capsule that functions as a filtration unit of kidney. The glomerular function ensures that essential plasma proteins are retained in blood and the filtrate is passed on as urine. The glomerular filtration assembly is composed of three main cellular barriers that are critical for the ultrafiltration process, the fenestrated endothelium, glomerular basement membrane and highly specialized podocytes. The podocytes along with their specialized junctions “slit diaphragm” form the basic backbone of this filtration assembly. The presence of high amounts of protein in urine a condition commonly referred as proteinuria indicates a defective glomerular filtration barrier. Various glomerular disorders including Nephrotic syndrome are characterized by significant alteration in the structure of podocytes that is associated with prolonged increase in the glomerular permeability leading to heavy proteinuria. Recent identification of proteins that are specifically localized at the slit diaphragm whose mutations and knockouts are known to result in loss of renal function has significantly advanced our understanding of the molecular makeup of this filtration assembly. The present review is an effort to summarize the recent developments in this field and highlight our understanding of the glomerular filtration barrier assembly.
Keywords: Glomerulus, Endothelial cells, Glomerular basement membrane, podocytes
Introduction
The kidney glomerulus is a very specialized structure that functions in filtering blood and retaining essential plasma proteins. The glomerular filter is composed of three major cell types which perform a collective function of selective ultrafiltration of the blood plasma: the fenestrated endothelium, the intervening glomerular basement membrane and the epithelial podocytes (Brenner, et al., 1978, Rennke Venkatachalam. 1979). The glomerular basement membrane (GBM) provides the primary structural support for the glomerular tuft that contains glomerular capillary. Endothelial and smooth muscle-like mesangial cells providing capillary support and are located inside the GBM, whereas podocytes are attached on the outer surface of the GBM (Faul, et al., 2007). This three-layered structure (endothelium, GBM, and podocytes) facilitates the flow of plasma water and small solutes while restricts the flow of large plasma proteins such as albumin (Figure 1). The presence of high amounts of albumin in the urine indicates a defect in either one or all of the layers of glomerular filtration barrier.
Figure 1. Schematic presentation of glomerular filtration barrier.
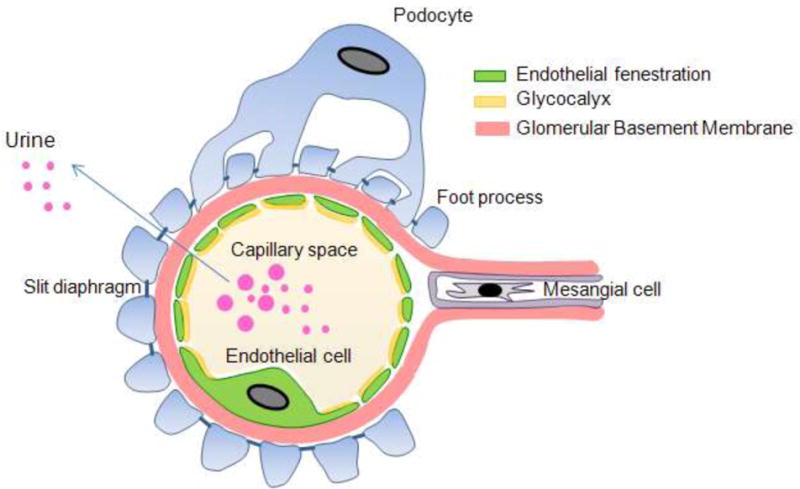
Glomerular filtration barrier showing three distinguished layers: Fenestrated endothelial cells, glomerular basement membrane and podocytes.
Glomerular Endothelial cells
Endothelial cells (EC) line the inner surface of entire vascular tree and form an anticoagulant barrier between blood and tissues. A major morphological characteristic of the glomerular endothelial cells (GEnC) are fenestrations that appear as transcytoplasmic holes (Satchell Braet. 2009). There are other endothelial cells that posses these tanscytoplasmic holes like glomerular endothelium that have a unique assemblage structural features such as absence of the diaphragm and retention of the basal lamina. These cells play an important physiological role involving filtration of blood in the glomerulus (Satchell Braet. 2009). The systemic endothelium forms a lining of all blood vessels and represents the principal regulator of vascular permeability. Three types of endothelium have been reported that include continuous (without fenestration), fenestrated and discontinuous (Risau. 1998). Fenestrations are observed as transcellular holes round or ovoid through the most attenuated part of the EnC cytoplasm. These cells are localized in the organ where high amounts of exchange are required between the intra and extra-vascular compartments. Endothelial fenestrations are categorized into three types according to type of endothelium and presence or absence of the diaphragm. The first and most common type is found in organs such as endocrine tissues, gastrointestinal mucosa and renal peritubular capillaries (Satchell Braet. 2009). These endothelial cells are characterized by the fenestration size of 60–70nm in diameter and presence of protein “plasmalemmal vesicle-associated protein-1” (PV-1 or PLVAP), a type II transmembrane glycoprotein that is an integral part of this diaphragm (Satchell Braet. 2009, Stan, et al., 2004, Stan, et al., 1999). The second type of fenestration in endothelial cells are discontinuous that lack the basal lamina and are found mainly in spleen, bone marrow and liver endothelial. These endothelial cells do not posses diaphragm and also lack PV-1 in in-vivo. Fenestration size in different cells varies from 100–170nm (Satchell Braet. 2009, Braet, et al., 1994). The third type of fenestrations (60–80nm) are similar to the first type, however, like the second type they also do not posses diaphragm and lack PV-1. The fenestrations are mainly concentrated toward the peripheral cytoplasm and arranged in a cluster of sieves separated by the ridges of cytoplasm. GEnC fenestrations are thus localized in the region of cytoplasm that is opposite to podocytes foot processes and filtration slit across the basement membrane (Satchell Braet. 2009, Vasmant, et al., 1984). These cells also posses gelatinous surface coat known as glycocalyx that is composed of proteoglycans and sialoproteins that, plays an important role in regulating permeability as well as modifying ligand-receptor and cellular interactions (Weinbaum, et al., 2007). The specific composition of the glycocalyx in the fenestration is important for the permeability properties (Singh, et al., 2007). Studies also suggest that the size of glomerular EC fenestrate is much too large to easily exclude albumin and other large proteins from the glomerular filtrate. It is also suggested that the glomerular capillary wall is not a perfect barrier for macromolecules and it allows the albumin to move across and tubular reuptake of albumin allowing urine to remain albumin free (Russo, et al., 2007, Obeidat Ballermann. 2012). The biophysical model indicates that glycocalyx contributes to about 50% of overall hydraulic resistance of glomerular filtration barrier and any change in the composition of glycocalyx possibly affects GFR (Drumond Deen. 1994). There are several disease-based and animal model studies demonstrating significant amount of damage to the glomerular endothelium (Chowdhury. 1996, Toyoda, et al., 2007). GEnC normal development and fenestration is highly dependent of VEGF and TGF β1 signaling and inhibition of these growth factors leads to glomerular endothelial re-differentiation (Satchell Braet. 2009). Although the glomerular endothelial cells have an important role in glomerular filtration, it has not received significant attention, primarily due to lack of a direct evidence of its involvement in inducing proteinuria or loss of glomerular filtration function (Satchell Braet. 2009).
Glomerular Basement Membrane
The glomerular basement membrane (GBM) represents the extracellular matrix component of selectively permeable glomerular filtration barrier that separates vasculature from urinary space. The components that make up this structure are actually synthesized by the glomerular endothelial cells and the podocytes cells (Miner. 2012). GBM like other basement membranes is a sheet of extracellular matrix that contains four major macromolecules:
Laminin is a ubiquitous basement membrane component found in different isoform and secreted as αβγ heterotrimers and its structure is stabilized by the intra-chain disulphide bonds. Nomenclature of the hetrodimers are based upon the specific αβγ chain composition such as laminin α2β2γ1 is referred as laminin 221 laminin, type IV collagen, nidogen, and heparin sulphate proteoglycan such as agrin. GBM has important physiological properties that restrict the passage of plasma proteins such as albumin into the urinary space. The fenestrated endothelium apparently allows the flow of plasma through capillaries to reach GBM, however, some evidences indicate that the fenestrations are plugged by a glycocalyx-like material that imparts barrier like properties (Haraldsson Jeansson. 2009).
(Miner. 2012). In mature GBM mainly LM-521 is the major constituent, whereas, the developmental transition is observed in the following fashion, LM-111 to LM-521 to LM-521. Importantly the mutation of laminin β2 (Lamb2) either in mice or humans results in the development of congenital nephrotic syndrome with variable ocular and neurological manifestations, called as Pierson syndrome (Matejas, et al., 2010, Noakes, et al., 1995). Studies suggest that reduced LM-521 leads to the improper permselective GBM that ultimately results in leakage of albumin across the glomerular filtration barrier (Jarad, et al., 2006). Recently Lamb2(−/−) mouse model of the Pierson syndrome, was rescued by overexpression of laminin β1 in podocytes and correlated with high amount of deposition of LM511 in the GBM (Suh, et al., 2011).
The second constituent of the GBM is type IV collagen, which like other collagens is a trimeric extracellular matrix protein that consists of Gly-X-Y amino acid triplet repeats. Three α chains wrap around one another to form the collagen triple helix. There are six different α chains that interact with each other in specific stoichiometries to forms the network forming blocks called protomers such as (α1)2α2, α3α4α5, and (α5)2α6 protomers. The (α1)2α2 is mainly expressed during the glomerular development. Mutations encoding the collagen IVα3,α4, orα5 chains leads to the defects in the GBM and the severity of phenotype depends on the specific mutation. The homozygous mutations in COL4A3 or COL4A4 genes, (which encode the α3 and α4 chains of type IV collagen respectively) causes severe autosomal recessive Alport syndrome, a basement membrane disease that leads to kidney failure associated with deafness and ocular abnormalities. The X-linked form of Alport syndrome which is caused by mutations in COL4A5 also shares similar glomerular histopathology (Miner. 2012, Gubler. 2008). Importantly, the complete absence of α3α4α5(IV) network due to total lack of one of these chains, is expected to cause more severe GBM abnormalities. It has been investigated that as compared to the (α1)2α2 network the α3α4α5(IV) network is more resistant to proteases and highly cross linked, providing greater stability for the GBM architecture (Miner. 2012, Gubler. 2008).
The third constituent of GBM is nidogen proteins, which exists in two forms nidogen-1 and nidogen-2. It is a dumbbell-shaped ubiquitous basement membrane proteins has been known to link the laminin and collagen IV and therefore plays an important role in the formation of GBM (Fox, et al., 1991). The individual mutants of nidogen-1 and nidgen-2 does not show any mortality and the structure and function of basement membranes also remain unaffected (Murshed, et al., 2000, Schymeinsky, et al., 2002), whereas, combined deletion of both the genes leads to prenatal death in mice but interestingly, the basement membrane remains normal (Bader, et al., 2005). Agrin (heparan sulfate proteoglycan) is the fourth GBM constituent, which has multiple splice forms, of which, one secreted form is present in the GBM. Agrin is highly negatively charged due to the presence of sulfated glycosaminoglycan (GAG) side chains that ultimately provide a net negative charge on GBM (Kanwar, et al., 2007). The net negative charge of GBM is crucial component of glomerular capillary wall filtration barrier to plasma albumin which is also a negatively charged and repelled by the GBM. Therefore the smaller size and positively charged molecules, cross the filtration barrier more rapidly than neutral molecules, that in turn cross faster than the negative ones (Bohrer, et al., 1978). Concept of charge selectivity has recently been investigated with conflicting findings. Podocytes specific knockdown of argin leads to reduction in GBM anionic charge with no effect on the glomerular filtration barrier (Harvey, et al., 2007). GBM anionic charge reduction in vivo by infusion of heparanase, which removes heparin sulfate side chains from proteoglycans, does not lead to proteinuria (van den Hoven, et al., 2008). Overall the structural composition of GBM plays important role in the development and maintenance of glomerular filtration barrier.
Podocytes
Podocytes are highly specialized epithelial cells of glomerulus and contain complex cellular organization that surrounds the outside of glomerular capillaries facing the Bowman’s capsule and the primary urine. Podocytes have a large central cell body from which primary processes are projected towards the glomerular capillaries and eventually divided into numerous foot processes which rest on the glomerular basement membrane (Haraldsson, et al., 2008, Marshall. 2007, Pavenstadt, et al., 2003). Podocytes are polarized epithelial cells with apical or luminal and a basal cell membrane domain. The basal membrane, which contains the soles of foot processes, is affixed to the GBM. The apical membrane consists of the sialoglycoproteins such as podoclyxin, podoendin etc that make the surface negatively charged (Huang Langlois. 1985). Both apical and basal membranes are heterogeneous in nature with respect to their lipid composition. (Schwarz, et al., 2001). Foot processes from the different cell bodies interdigitate and space between adjacent foot processes is connected via a thin membranous structure that is 40nm wide commonly known as the filtration slit or slit-diaphragm (Pavenstadt, et al., 2003, Tryggvason, et al., 2006). Over the last decade several proteins have been identified that are localized at the slit diaphragm and play a critical role in the maintenance of podocytes structure and function (Marshall. 2007, Pavenstadt, et al., 2003, Tryggvason, et al., 2006). Several of these proteins are transmembrane proteins that link the slit diaphragm with the actin cytoskeleton of podocytes and therefore participate in the signaling events that regulate the overall structure and function of podocytes (Figure 2) (Tryggvason, et al., 2006, Tryggvason, et al., 2006).
Figure 2. Schematic presentation of slit diaphragm proteins.
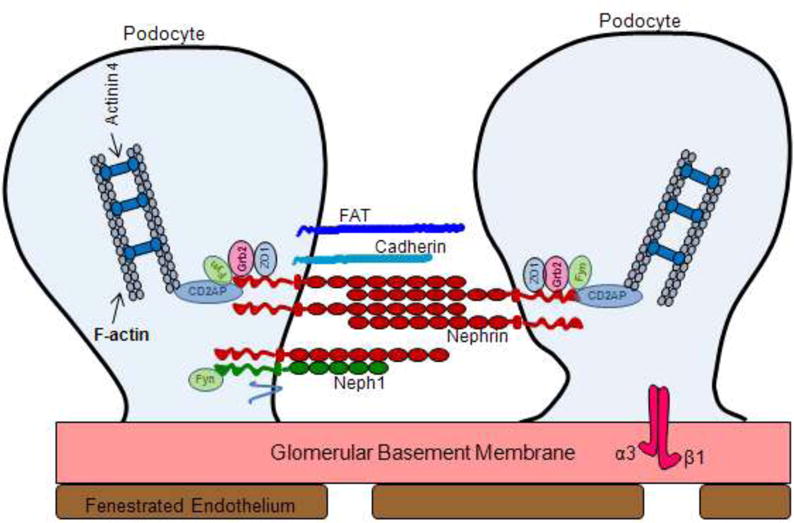
Structural component proteins involved in the formation of glomerular slit diaphragm.
Actin Cytoskeleton
The actin cytoskeleton forms the structural framework of podocytes and contributes towards the unique morphology and ultimately its function. It is categorized into two groups, the longitudinal actin microfilaments and the meshwork of actin filaments beneath the cell membrane. The podocytes contain three morphologically and functionally different segments: a cell body, major processes and foot processes (FPs). Major processes arise from the cell body and split into foot processes. In the major processes, the cytoskeleton is composed of mainly microtubules. These organizations of longitudinal microfilaments in the foot process and the microtubules in the primary process have several functions that include providing structural support to the cell, contraction and expansion ability of the cells, anchoring to the intracellular molecules. The foot processes also contain actin based cytoskeleton which is associated with GBM in focal contact. The foot processes are characterized by cortical network of short branched actin filaments and the presence of highly ordered parallel, contractile actin filament bundles, which are thought to modulate the permeability of the filtration barrier through changes in foot process morphology (Faul, et al., 2007, Marshall. 2007, Drenckhahn Franke. 1988). Beside that they are also involved in the cell signaling and transportation of the molecules from the foot processes to the cell body. The other part of the actin cytoskeleton is the sublemmenal actin meshwork. This provides anchorage for transmembrane proteins and is important for transmembrane signaling. Actin associated proteins such as actinin-4 and synaptopodin play important roles to regulate the dynamics of actin cytoskeleton. α-Actinin-4 is an actin filament cross linking protein that co-localizes with actin in podocytes. Mutations in α-actinin 4 leads to the development of proteinuria in humans (Smoyer, et al., 1997). In addition, knock down or over-expression of α-actinin 4 in mice leads to the proteinuria and foot process effacement (Kos, et al., 2003, Michaud, et al., 2003). Synaptopodin is another actin associated protein that modulates the expression of α-actinin, by elongating the α-actinin induced actin filaments. Recent study suggest the importance of synaptopodin in the maintenance of podocyte structure and function, where the podocyte effacement induced by protamin sulphate was rescued by heparin in wild-type mice, but not in the synpatopodin-null mice (Asanuma, et al., 2005).
Slit-diaphragm and transmembrane proteins
The slit diaphragm of a podocyte is extracellularly placed where it bridges the gap between two adjacent foot processes. It is freely permeable to water and small solutes but is believed to form a size selectivity barrier for the passage of larger molecules (Schurer, et al., 1980, Schnabel, et al., 1990). The podocyte proteins (nephrin, NEPH1, podocin, zona occludens-1 (ZO-1), CD2 adaptor protein (CD2AP), FAT and P-cadherin etc) that contribute towards the structural framework of slit diaphragm are essential for the maintenance of glomerular filtration function. These proteins form a signaling network that connects the diaphragm to intracellular actin cytoskeleton, and participate in signaling that regulates podocytes structure and function (Tryggvason, et al., 2006, Verma, et al., 2006, Shih, et al., 2001). Importantly, genetic mutations in humans and inactivation of these genes in mouse models have shown to result in massive proteinuria leading to renal failure and death (Haraldsson Jeansson. 2009, Pavenstadt, et al., 2003, Tryggvason, et al., 2006).
Nephrin is the central component of the glomerular ultra filter expressed primarily in podocytes as a transmembrane protein. It has a short intracellular domain, a transmembrane domain, an extracellular domain with eight distal IgG-like motifs and one proximal fibronectin type III–like motif (Tryggvason, et al., 2006, Verma, et al., 2006). It has been shown biochemically that nephrin molecules interact with one another in a homophilic fashion (Tryggvason, et al., 2006). The length of nephrin extracellular domain is about 35 nm and nephrin molecules from adjacent foot processes are thought to interact in the trans fashion to form the basic framework of slit diaphragm, thus forming a porous molecular sieve (Haraldsson, et al., 2008, Tryggvason, et al., 2006). The intracellular domain of nephrin has been shown to associate with actin cytoskeleton and linked with adapter proteins such as CD2-associated protein, Nck proteins etc. The intracellular domain of Nephrin has several potential tyrosine phosphorylation sites, some of which provide a docking site for SH2 domain-containing kinases and adaptor proteins (Putaala, et al., 2000). Recent investigation highlight that nephrin tyrosine phosphorylation regulates podocyte cell morphology via Nck adaptor proteins (Verma, et al., 2006, Jones, et al., 2006). The adaptor protein super family Nck has two members; Nck1 and Nck2 and both are associated with the regulation of actin dynamics. Both proteins have one SH2 and three SH3 domains. The SH2 domain interacts with phosphotyrosines and therefore, recruits Nck, whereas the SH3 domain recruits several other proteins involved in the regulation of the actin cytoskeleton (Patrakka Tryggvason. 2007). Nephrin was shown to transiently phosphorylate in a proteinuria model during foot process effacement, as well as during glomerular development when podocyte foot processes are formed. This suggests that nephrin phosphorylation increases during rapid actin polymerization and cytoskeletal reorganization events leading to the recruitment of Nck and its downstream effectors at the cytoplasmic region of nephrin (Verma, et al., 2006, Patrakka Tryggvason. 2007). Several biochemical and genetic experiments have confirmed the significance of Nephrin in maintaining the structural integrity of slit diaphragm and glomerular filtration function (Putaala, et al., 2000, Kestila, et al., 1998). Moreover, in humans mutations in nephrin has been shown to result in congenital nephrotic syndrome, a genetic condition characterized by massive proteinuria with loss of kidney function (Putaala, et al., 2000, Kestila, et al., 1998).
CD2-associated protein (CD2AP) is another intracellular protein that is localized in podocytes where it can interact with the C-terminal domain of nephrin (Shih, et al., 2001). CD2AP has been shown to act as a linker between cell-membrane receptors and actin-modifying proteins (Barletta, et al., 2003) and therefore, CD2AP may be involved in connecting nephrin to the actin cytoskeleton in podocytes. It is suggested that Nephrin interaction with CD2AP might be important in the steady state situation, where as Nck-Nephrin interaction may play a role in cytoplasmic reorganization that takes place during injury to the glomerulus (Patrakka Tryggvason. 2007). In addition, the N-terminal domain of CD2AP binds to an intermediary protein, p85 and facilitates nephrin-induced AKT signaling which protects podocytes from apoptosis (Huber, et al., 2003). Phosphorylation of tyrosine in the cytoplasmic tail of Nephrin by Src family kinase (Src is a tyrosine kinase with a critical role in cell signaling) initiates a signaling cascade that promotes antiapoptotic signals (Huber, et al., 2003). The significance of Fyn-dependent phosphorylation of nephrin (Fyn is a member of the Src family of protein tyrosine kinases) is highlighted by the fact that proteinuria and podocyte effacement develop in mice where Fyn kinase was genetically deleted (Verma, et al., 2003).
Neph1 is another transmembrane protein structurally related to Nephrin with five extracellular IgG-like motifs. The Neph family of transmembrane proteins contains three homologues (Neph1, Neph2, and Neph3, also termed filtrin that are widely expressed in mammalian tissues (Sellin, et al., 2003, Ihalmo, et al., 2003). Neph1 and Neph2 are located in the slit diaphragm and the in vitro data suggests that Nephrin can form heterodimers with Neph1 or Neph2 through their extracellular domains, but that Neph1 and Neph2 do not interact with each other (Gerke, et al., 2003). Neph1 has been widely studied for its role in podocyte maintenance (Garg, et al., 2007, Liu, et al., 2003, Wagner, et al., 2008). Similar to Nephrin, Neph1 and its interaction with nephrin has been investigated for their role in signaling (Garg, et al., 2007, Wagner, et al., 2008, Harita, et al., 2008). Mice deficient in Neph1 were shown to develop proteinuria (Donoviel, et al., 2001) and renal failure but the functional significance of Neph2 and Neph3 are not clear.
FAT1 and FAT2 are large transmembrane proteins containing 34 tandem cadherin-like repeats and are localized at the slit diaphragm in podocytes (Inoue, et al., 2001). Although the FAT1 and FAT2 deficient mice display proteinuria, their exact role in podocyte biology is not well understood (Tryggvason, et al., 2006, Ciani, et al., 2003). Podocin is a 42-kDa hairpin-like integral membrane protein encoded by NPHS2 gene and similar to nephrin and Neph1 is localized at the slit diaphragm in podocytes However, unlike nephrin and Neph1 its N and C-terminal ends are directed into the intracellular space, at the podocyte cell membrane (Boute, et al., 2000, Roselli, et al., 2002). It has been proposed to serve as a scaffolding protein and contributes towards the structural organization of slit diaphragm. Podocin interacts with the intracellular domains of nephrin and Neph1 and with CD2-associated protein (CD2AP) (Schwarz, et al., 2001, Sellin, et al., 2003). The podocin-knockout mice develop severe proteinuria and die within few days of birth (Roselli, et al., 2002).
Recent identification of the members of the myosin superfamily that bind actin and function primarily as molecular motors has also been implicated as key players in the maintenance of podocyte morphology and function. Among the unconventional myosin motors, Myo1e was the first myosin identified to play a role in the podocyte development. The analysis of genetic mutations in humans and Myo1e mice knockout models demonstrate the significance of Myo1e in podocyte biology, where loss of Myo1c specifically in podocytes induces podocyte effacement and proteinuria (Krendel, et al., 2009, Chase, et al., 2012). Studies also suggest that Myo1e plays a critical role in the endocytosis of slit diaphragm proteins; however, more detailed studies are required to investigate the mechanisms involved in the trafficking of podocyte proteins and how that regulates the development and function of podocytes (Mele, et al., 2011). Myh9 is another non-muscle myosin that has gained much attention due to multiple reports that identify a direct linkage of mutations in this gene with kidney disorders (Pecci, et al., 2008, Sekine, et al., 2010). Human studies as well as animal knockdown models of Myh9 genes demonstrated focal segment glomerulosclerosis like symptoms (Johnstone, et al., 2011, Zhang, et al., 2012). In addition, a recent knockdown of Myh9 gene was shown to induce podocyte effacement and loss of slit diaphragm in a Zebrafish model system (Muller, et al., 2011). Another unconventional myosin that was recently characterized as a slit diaphragm protein is Myo1c that belongs to the myosin I family and was identified as an interacting partner of slit-diaphragm proteins Nephrin and Neph1 (Arif, et al., 2011). Further biochemical studies suggest that Myo1c is involved in the translocation of Neph1 and Nephrin from the cytoplasmic region to the podocyte cell membrane (Arif, et al., 2011). The development of Myo1c mouse models where Myo1c is selectively deleted from podocytes will provide further understanding of the in-vivo significance of Myo1c in podocytes biology.
In addition to the above mentioned proteins, various other junctional adhesion and scaffolding proteins that are present in podocytes and contribute towards the structural integrity of slit diaphragm have been identified through affinity pull-down experiments using Nephrin as bait. These proteins include IQGAP1 (IQ motif-containing GTPase-activating protein 1), MAGI-2 (membrane-associated guanylate kinase inverted 2), CASK (calcium/calmodulin-dependent serine protein kinase), α-actinin, αII spectrin, βII spectrin, catenins, ZO-1, MAGI-1, densin and many more (Patrakka Tryggvason. 2007). There are several laboratories around the world that are actively involved in genetic analysis including high throughput genome sequencing that has discovered several gene candidates including Apolipoprotein L1 (APOL1) that was recently shown to associate with the development of FSGS (focal and segmental glomerulosclerosis) in humans (Fine, et al., 2012, Tzur, et al., 2010). Identification of novel genes as well understanding their role in the maintenance of glomerular filtration function will significantly progress our understanding of the glomerular diseases and provide a platform for the development of new therapeutic targets.
Conclusion
The kidney glomerulus functions by retaining the essential plasma proteins from blood and ensures selective ultrafiltration. It has three major components, a fenestrated endothelium, GBM and podocytes and collectively they form the glomerular filtration assembly. Recent studies have identified podocytes as the critical component of this assembly whose dysfunction is associated with proteinuria that is characterized by the leakage of albumin into urine leading to renal failure. Although a number of proteins are constantly being identified that are critical for podocyte function and hence critical for the glomerular filtration function, a lot need to be learned about the mechanisms that regulate the maintenance of podocytes and their specialized junctions “slit diaphragm”. Further understanding of the events that lead to podocyte damage during various glomerular disorders may provide novel therapeutic targets that will aid in the development of treatment for these deadly diseases.
Acknowledgments
NephCure Foundation (NCF), NephCure Postdoctoral Grants 2012-RFP-001 to E. A., National Institutes of Health, NIDDK, Grant RO1 1R01DK087956 to D. N. are duly acknowledged.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Contributor Information
Ehtesham Arif, Email: ehtesham@mail.med.upenn.edu.
Deepak Nihalani, Email: deepakn@mail.med.upenn.edu.
References
- 1.Brenner BM, Hostetter TH, Humes HD. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978;298:826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- 2.Rennke HG, Venkatachalam MA. Glomerular permeability of macromolecules. Effect of molecular configuration on the fractional clearance of uncharged dextran and neutral horseradish peroxidase in the rat. J Clin Invest. 1979;63:713–717. doi: 10.1172/JCI109354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faul C, Asanuma K, Yanagida-Asanuma E, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risau W. Development and differentiation of endothelium. Kidney Int Suppl. 1998;67:S3–6. doi: 10.1046/j.1523-1755.1998.06701.x. [DOI] [PubMed] [Google Scholar]
- 6.Stan RV, Tkachenko E, Niesman IR. PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol Biol Cell. 2004;15:3615–3630. doi: 10.1091/mbc.E03-08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braet F, De Zanger R, Sasaoki T, et al. Assessment of a method of isolation, purification, and cultivation of rat liver sinusoidal endothelial cells. Lab Invest. 1994;70:944–952. [PubMed] [Google Scholar]
- 9.Vasmant D, Maurice M, Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec. 1984;210:17–24. doi: 10.1002/ar.1092100104. [DOI] [PubMed] [Google Scholar]
- 10.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Satchell SC, Neal CR, et al. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 12.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 13.Obeidat M, Ballermann BJ. Glomerular endothelium: a porous sieve and formidable barrier. Exp Cell Res. 2012;318:964–972. doi: 10.1016/j.yexcr.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Drumond MC, Deen WM. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994;266:F1–12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury AK. Participation of endothelial cells in the development of glomerulosclerosis: a study on murine serum sickness nephritis with mitomycin C. Pathol Int. 1996;46:173–182. doi: 10.1111/j.1440-1827.1996.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda M, Najafian B, Kim Y, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 17.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318:973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:331–335. doi: 10.1097/MNH.0b013e32832c9dba. [DOI] [PubMed] [Google Scholar]
- 19.Matejas V, Hinkes B, Alkandari F, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noakes PG, Miner JH, Gautam M, et al. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 21.Jarad G, Cunningham J, Shaw AS, et al. Proteinuria precedes podocyte abnormalities inLamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh JH, Jarad G, VanDeVoorde RG, et al. Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome. Proc Natl Acad Sci U S A. 2011;108:15348–15353. doi: 10.1073/pnas.1108269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubler MC. Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephrol. 2008;4:24–37. doi: 10.1038/ncpneph0671. [DOI] [PubMed] [Google Scholar]
- 24.Fox JW, Mayer U, Nischt R, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murshed M, Smyth N, Miosge N, et al. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000;20:7007–7012. doi: 10.1128/mcb.20.18.7007-7012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schymeinsky J, Nedbal S, Miosge N, et al. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22:6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bader BL, Smyth N, Nedbal S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanwar YS, Danesh FR, Chugh SS. Contribution of proteoglycans towards the integrated functions of renal glomerular capillaries: a historical perspective. Am J Pathol. 2007;171:9–13. doi: 10.2353/ajpath.2007.070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohrer MP, Baylis C, Humes HD, et al. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest. 1978;61:72–78. doi: 10.1172/JCI108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey SJ, Jarad G, Cunningham J, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Hoven MJ, Wijnhoven TJ, Li JP, et al. Reduction of anionic sites in the glomerular basement membrane by heparanase does not lead to proteinuria. Kidney Int. 2008;73:278–287. doi: 10.1038/sj.ki.5002706. [DOI] [PubMed] [Google Scholar]
- 32.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 33.Marshall SM. The podocyte: a potential therapeutic target in diabetic nephropathy? Curr Pharm Des. 2007;13:2713–2720. doi: 10.2174/138161207781662957. [DOI] [PubMed] [Google Scholar]
- 34.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 35.Huang TW, Langlois JC. Podoendin. A new cell surface protein of the podocyte and endothelium. J Exp Med. 1985;162:245–267. doi: 10.1084/jem.162.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz K, Simons M, Reiser J, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 38.Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell. 2006;125:221–224. doi: 10.1016/j.cell.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- 40.Smoyer WE, Mundel P, Gupta A, et al. Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol. 1997;273:F150–157. doi: 10.1152/ajprenal.1997.273.1.F150. [DOI] [PubMed] [Google Scholar]
- 41.Kos CH, Le TC, Sinha S, et al. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest. 2003;111:1683–1690. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaud JL, Lemieux LI, Dube M, et al. Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol. 2003;14:1200–1211. doi: 10.1097/01.asn.0000059864.88610.5e. [DOI] [PubMed] [Google Scholar]
- 43.Asanuma K, Kim K, Oh J, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schurer W, Fleuren GJ, Hoedemaeker PJ, et al. A model for the glomerular filter. Ren Physiol. 1980;3:237–243. doi: 10.1159/000172766. [DOI] [PubMed] [Google Scholar]
- 45.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma R, Kovari I, Soofi A, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih NY, Li J, Cotran R, et al. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putaala H, Sainio K, Sariola H, et al. Primary structure of mouse and rat nephrin cDNA and structure and expression of the mouse gene. J Am Soc Nephrol. 2000;11:991–1001. doi: 10.1681/ASN.V116991. [DOI] [PubMed] [Google Scholar]
- 49.Jones N, Blasutig IM, Eremina V, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 50.Patrakka J, Tryggvason K. Nephrin–a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007;13:396–403. doi: 10.1016/j.molmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 52.Barletta GM, Kovari IA, Verma RK, et al. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- 53.Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma R, Wharram B, Kovari I, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 55.Sellin L, Huber TB, Gerke P, et al. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 2003;17:115–117. doi: 10.1096/fj.02-0242fje. [DOI] [PubMed] [Google Scholar]
- 56.Ihalmo P, Palmen T, Ahola H, et al. Filtrin is a novel member of nephrin-like proteins. Biochem Biophys Res Commun. 2003;300:364–370. doi: 10.1016/s0006-291x(02)02854-1. [DOI] [PubMed] [Google Scholar]
- 57.Gerke P, Huber TB, Sellin L, et al. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14:918–926. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- 58.Garg P, Verma R, Nihalani D, et al. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu G, Kaw B, Kurfis J, et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner MC, Rhodes G, Wang E, et al. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem. 2008;283:35579–35589. doi: 10.1074/jbc.M805507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harita Y, Kurihara H, Kosako H, et al. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem. 2008;283:9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donoviel DB, Freed DD, Vogel H, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inoue T, Yaoita E, Kurihara H, et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001;59:1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- 64.Ciani L, Patel A, Allen ND, et al. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 66.Roselli S, Gribouval O, Boute N, et al. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krendel M, Kim SV, Willinger T, et al. Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol. 2009;20:86–94. doi: 10.1681/ASN.2007111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chase SE, Encina CV, Stolzenburg LR, et al. Podocyte-specific knockout of myosin 1e disrupts glomerular filtration. Am J Physiol Renal Physiol. 2012;303:F1099–1106. doi: 10.1152/ajprenal.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mele C, Iatropoulos P, Donadelli R, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pecci A, Panza E, Pujol-Moix N, et al. Position of nonmuscle myosin heavy chain IIA (NMMHC-IIA) mutations predicts the natural history of MYH9-related disease. Hum Mutat. 2008;29:409–417. doi: 10.1002/humu.20661. [DOI] [PubMed] [Google Scholar]
- 71.Sekine T, Konno M, Sasaki S, et al. Patients with Epstein-Fechtner syndromes owing to MYH9 R702 mutations develop progressive proteinuric renal disease. Kidney Int. 2010;78:207–214. doi: 10.1038/ki.2010.21. [DOI] [PubMed] [Google Scholar]
- 72.Johnstone DB, Zhang J, George B, et al. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol. 2011;31:2162–2170. doi: 10.1128/MCB.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Conti MA, Malide D, et al. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood. 2012;119:238–250. doi: 10.1182/blood-2011-06-358853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller T, Rumpel E, Hradetzky S, et al. Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney Int. 2011;80:1055–1063. doi: 10.1038/ki.2011.256. [DOI] [PubMed] [Google Scholar]
- 75.Arif E, Wagner MC, Johnstone DB, et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol. 2011;31:2134–2150. doi: 10.1128/MCB.05051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fine DM, Wasser WG, Estrella MM, et al. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol. 2012;23:343–350. doi: 10.1681/ASN.2011060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


