Abstract
Made up of millions of enteric neurons and glial cells, the enteric nervous system (ENS) is in a key position to modulate the secretomotor function and visceral pain of the gastrointestinal tract. The early life developmental period, through which most of the ENS development occurs, is highly susceptible to microenvironmental perturbation. Over the past decade, accumulating evidence has shown the impact of stress and early life adversity (ELA) on host gastrointestinal pathophysiology. While most of the focus has been on alterations in brain structure and function, limited experimental work in rodents suggest that the enteric nervous system can also be directly affected, as shown by changes in the number, phenotype and reactivity of enteric nerves. The work of Medland et al in the current issue of this Journal demonstrate that such alterations also occur in pigs, a larger mammalian species with high translational value to human, This work also highlights a sex-differential susceptibility of the ENS to the effect of ELA, which could contribute to the higher prevalence of GI disorders in women. In this mini-review we will discuss the development and composition of the ENS and related gastrointestinal sensory-motor and secretory functions. We will then focus on the influence of stress on the enteric nervous system, with a particular emphasis on neurodevelopmental changes. Finally, we will discuss the influence of sex on those parameters.
Keywords: enteric nervous system, development, sex differences, early life adversity, stress
Introduction
Since the early description of the general adaptation syndrome by Hans Selye (1), the physiological adaptations following acute and chronic stress responses have been well characterized. Evidence show that stress stimulus triggers rapid, short, delayed or long lasting responses through multiple interacting mechanisms to maintain homeostasis (2). Failure to engage the appropriate response to stress has been implicated as an important factor in the onset and exacerbation of a wide range of disorders, including depression (3) and functional gut diseases such as irritable bowel syndrome (IBS)(4). One of the vulnerable periods where stress could lead to long lasting maladaptive responses is the early life developmental period (5, 6). Given the brain is the central organ orchestrating the stress response, studies on mechanisms of early life adversity (ELA)-induced maladaptation focused on the brain structural and functional changes. There is now strong evidence that ELA causes modulates dendritic sprouting in distinct brain regions (5). Whether comparable changes occur in peripheral tissues namely in the gastrointestinal tract (GIT) that is endowed with the enteric nervous system (ENS) exhibiting a strikingly similar repertoire of neurons, nerve fibers, glia, neurotransmitters and modulators to that in the brain, is not known. In this issue of Neurogastroenterology & Motility, Medland et al. (7) using an ELA model of early weaning stress (EWS) in pigs, where newborns are weaned on day 15 instead of day 28 (normal weaning)(8), demonstrate, for the first time in a large mammalian species, a sustained modulation of ileal enteric neuron number, phenotype, neuronal enzyme activity and mucosal epithelial cell neurosecretory functions. They also highlight a key contribution of sex to those alterations. In this mini-review, we will discuss the development and composition of the ENS and related gastrointestinal sensory-motor and secretory functions. We will then focus on the influence of stress on the enteric nervous system, with a particular emphasis on neurodevelopmental changes. Finally, we will discuss the influence of sex on those parameters.
The enteric nervous system
The mammalian GIT, in addition to its complex digestive functions, has several unique features. It is the largest single cell layer interface (mucosal epithelium) providing barrier functions; the largest immune response reservoir of the body and; is the seat of a unique and complex intrinsic neural network. The later, the ENS dubbed also as “Little Brain” is embedded in the gut wall and is the most complex division of the peripheral nervous system (9). Key component of the gut, it coordinates inordinate functions including mixing and propagation of gastrointestinal luminal contents, supply of digestive enzymes, absorption, fluid exchanges, storage and excretion, secretions of enteric neuroendocrine and epithelial cells, barrier function, immune responses and blood flow (9, 10).
Composition, embryonic origin and organization of ENS
In mammals, the ENS is composed of a considerable number of neurons (over 80–100 million in rodents and 400–600 million in humans)(11) that can be grouped into about 20 identifiable neuronal types (12), enteric glia (up to 4–7 times more numerous than neurons) (13) and a network of nerve fibers communicating and projecting to effector tissues (11).
The enteric neurons and glial cells that form the ENS originate from the neural crest - one of the three types of cells of ectoderm germ line - and migrate cranio-caudally to populate the entire digestive tract (14). Migration, organization and maturation of these cells within the gut tissue continues during gestation and even after birth in some species (14) suggesting that factors that affect migration and organization during the perinatal period could have long term effects on the development of ENS.
The ENS is organized roughly into two major ganglionated plexuses and several small aganglionated plexus (9, 11). The neurons are clustered in the myenteric plexus (Auerbach) located between the longitudinal and circular muscle layers and the submucosal plexus (Meissner) as a single layer (small animals) or two to three layers (large animals and humans) sandwiched between the submucosal matrix and external circular smooth muscle layer tunica muscularis (9, 11). The enteric glia populations are distributed around all classes of neurons in the ENS plexi and in the mucosa beneath the epithelium (13).
Functionally, ENS neurons can be identified as motor neurons, intrinsic sensory or intrinsic primary afferent neurons (IPANs) and interneurons, although different classes of interneurons are reported to have sensory functions (15), while mechanosensitive neurites are shown to have afferent and efferent functions (16). The ENS neurons code and use over 30 neurotransmitters/mediators that are similar to those present in the central nervous system (17). Cholinergic and nitrergic neurotransmissions are among the most abundant in the ENS and play key roles in the secretomotor, mucosal barrier and immune responses of the human gut (17).
ENS Role: motility, secretion, barrier function
ENS neurons interact with each other as well as with enteric smooth muscle cells, enteric glial cells (EGC), enterocytes, neuroendocrine cells (enterochromaffin cells or ECC), immune cells, interstitial cells of Cajal (ICCs) and extrinsic nerves to control almost all gut functions (11).
The submucosal plexus regulates the movement of water and electrolytes across the gut wall through intrinsic reflex circuits involving secretomotor neurons innervating the mucosa. On the other hand the myenteric plexus plays role in coordinating the contractility of the circular and longitudinal smooth muscle cells throughout the digestive tract (9).
Over the past decade, evidence has accumulated showing the role of EGC in the control of motility (13), mucosal barrier function (18) and as the cellular and molecular bridge between nerves, epithelial cells and ‘neuropods’ (13).
Developmental changes and plasticity of ENS
For the ENS to accomplish the above tasks, in an environment under constant threat from within and outside, requires not only a functional microcircuit but also a considerable capacity to adapt to developmental changes (age, sex) and environmental challenges (metabolic, circadian, noxious stimuli, trauma, inflammation, stress, etc…). During the neonatal period, the ENS goes through a stage of “pruning” (19), which removes excess neurons generated during development. Postnatally, microbial colonization, immune system development and aging continue to shape the ENS (20, 21). Plasticity following microenvironment changes within the gut tissue such as during inflammation are also well documented (22). The fact that neural crest derived enteric stem cells are present in the pre-, postnatal, and adult gut suggests also these cells enable ENS to adapt to developmental or environmental challenges (23). Equally relevant is the plasticity of enteric glia in response to systemic or local challenges (24).
Enteric nervous system and stress
Plethora of preclinical work show that various types of stressors (exteroceptive, interoceptive), applied at different stages of life (early life, adolescence, adulthood) can significantly affect the GIT, leading to alterations in secretomotor and visceral pain functions in rodents and pigs (25, 26). An increasing number of reports, to which the work of Medland et al. (7) adds up, suggest these effects may be partly due to changes in ENS plasticity and phenotype, especially when stress is applied during the sensitive perinatal period.
Stress signaling system in the ENS
Stress effects on the body and the GIT are mediated by corticotropin-releasing factor (CRF) via activation of two types of G-protein coupled receptors: CRF type 1 (CRF1) and CRF type 2 (CRF2) (27). Both CRF1 and CRF2 are expressed throughout the GIT of mammals. In rodents, CRF1 and its ligands (CRF and Ucn1) are present in the colon and ileum at the gene and protein levels in various cell types including neuronal (ENS), endocrine (ECC) and immune cells (mast cells, eosinophils, T-helper lymphocytes in the lamina propria). The CRF2 ligands (Ucn2 and Ucn3) and CRF2 are also expressed in the colon of rodent. CRF2 are localized at the gene and protein levels in cells of the rodent colonic ENS although less prominently than that of CRF1 (27). Transcripts of urocortins and CRF2b, the most common isoform in the periphery, are detected in myenteric neurons(28).
In humans, CRF1 and CRF2 are present in the colonic ENS (3). CRF1 and CRF2 are also localized in human colonic lamina propria mononuclear cells (macrophages), subepithelial mast cells and epithelial cells supporting a local action to influence neuronal and immune responses (27). CRF and Ucn1 are present in the mucosa, macrophages and ECC, while Ucn 3 is located in ECC, EGC and the ENS (3).
Influence of stress on ENS structure, development and function
Extensive works support a role for stress in alterations of epithelial secretion in rodents and show a major influence on cholinergic neurons. Previous work demonstrated that stress in the form of neonatal maternal separation stress (NMS) increases colonic mucosal nerve fiber density (29) and cholinergic neurons in the colon of weanling rats (30). This results in abnormal cholinergic regulation of epithelial permeability an effect mediated by CRF via activation of CRF2 on cholinergic neurons (30). Alterations in the structure and composition of the ENS have also been found in adult male rats exposed to 28 days of heterotypic chronic and acute stress which exhibit an increase in cholinergic and VIPergic neurons in ileal submucosal plexus, while in the myenteric plexus, the number of NOS-positive neurons is decreased (31). In this issue, Medland et al. (7) add to the existing literature by demonstrating for the first time using EWS that this effect is reproducible and long lasting in a larger mammalian species (pigs) which has translational relevance for humans (8). Additionally, they show that there is a sex-specific hypersensitivity of secretomotor neuron function and upregulation of the cholinergic ENS which occurs in females exclusively (7). The mechanisms behind this sex difference are not fully understood yet and further work is needed to uncover them, but these data suggest a higher susceptibility of females to the effects of ELA, which could explain the preponderance of functional GI disorders in women. Whether ELA also affects enteric glia function and plasticity is unknown and will be important to address in future studies.
Enteric nervous system and sex
Sex differences of phenotype include direct effects of gonadal hormones - at either organizational or activational levels - and of genes represented unequally in the genome because of their X- or Y-linkage (32). The influence of sex on the ENS has been poorly studied so far and remains an attractive area of investigation.
Sex hormones and ENS
Sex hormones (estradiol, testosterone) secretion in mammals is under the control of the hypothalamo-pituitary-gonadal (HPG) axis which encompasses the hypothalamic secretion of gonadotropin-releasing hormone (GnRH), activation of the pituitary GnRH type I receptor (GnRH-R) with subsequent secretion of gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH)(33). In turn, FSH via FSH receptor (FSH-R) stimulates the ovarian follicle and estrogen production in women and spermatogenesis in men (34). LH via LH receptor (LH-R) stimulates ovulation and secretion of progesterone in women and androgen secretion in men (34). Estradiol genomic effects are mediated through two estrogen receptors ERα and ERβ while non-genomic effects are linked to GPR30 activation. Progesterone exerts its effects via activation of receptors PR-A and PR-B, while testosterone targets the androgen receptor, AR. Most of these receptors are present in the GIT of mammals (35, 36), however their expression in the ENS has not been systemically evaluated.
GnRH, LH and FSH
GnRH and GnRH-R
Mammals possess two types of GnRH: GnRH1 and GnRH2. Both have neuroendocrine, paracrine, and autocrine functions in a wide range of organs, as well as neurotransmitter/neuromodulatory roles in the central and peripheral nervous systems (37). There is only one type of GnRH receptor (GnRH-R) expressed in mammals which binds both GnRH types (37). No GnRH is secreted from the hypothalamus to the peripheral circulation; hence the endogenous effect of GnRH on non-pituitary tissue depends on autocrine/paracrine effects.
GnRH1 and GnRH2 mRNAs are expressed throughout the GIT of humans, although GnRH2 expression is lowest and mainly found in the small intestine (37). In rats, GnRH mRNA is present in gastric glands, small and large intestine epithelium, and the pancreas.(38) GnRH is expressed in neurons of the human ENS, in the cytoplasm of approximately 50% of the myenteric neurons in ileum and colon and in some submucosal neurons, although the exact percentage is unknown (39). In rats, GnRH is expressed in parasympathetic ganglion cells of the myenteric plexus (38). GnRH mRNA is present in myenteric neurons culture from rat small intestine (40).
GnRH-R expression in the GIT is controversial, with some reports suggesting no expression (both mRNA and protein) in both man and rat small and large intestine (41), while others support the presence of m-GnRH-R like on murine enteric neurons (42).
The role of GnRH in the gut is not completely elucidated (36) and its influence on enteric nerves is controversial, with both continuous stimulation by the GnRH analog buserelin found to enhance survival of female rat small intestine myenteric neurons in culture (43) or to have no effect (single or multiple applications)(41). On the contrary, GnRH analogs administered in vivo lead to the loss of enteric neurons (44).
LH and LH-R
To date, there is no evidence of LH protein or mRNA expression in the GIT of human or rat (45). In contrast, LH-R is expressed in submucosal and myenteric neurons as well as in EGC, mast cells and endothelial cells of human ileum and colon (46). In female rats, LH-R protein is located in enteric nerve cell bodies, with immunoreactive fibers found in the smooth muscle layers of fundus, ileum, and colon (45). In the rat fundus, ileum and colon, submucous (n.d., 4% and 13%) and myenteric (8%, 9% and 21%) neurons are immunoreactive to LH-R, respectively (41, 45).
LH exposure causes reduced neuronal survival of primary enteric neuron cultures in vitro (41), a pathway likely involved in the intestinal dysfunction and loss of enteric neuron leading to constipation in humans following repeated GnRH exposure (44).
FSH and FSH-R
FSH and FSH-R have not been detected in human or rat GIT (45).
Estrogen receptors: ERα, ERβ and GPR30
ERα and ERβ are found throughout the length of the GIT in mammals, while there is very little information available for GPR30 distribution (see (35) for review). In rats and mice, ERα and ERβ proteins are present in enteric neurons, within the nucleus and the cytoplasm, with some ERα staining reaching the nerve fibers (47, 48). In human, ERβ proteins are found in enteric neurons of both myenteric and submucosal plexus (49). Evidence also suggests the presence of ERβ on enteric neurons, EGC and ICC, while ERα is only present on ICC. There are no data available regarding GPR30 in ENS.
Despite the presence of estrogen receptors on enteric nerves, only very few studies have addressed how it directly affect the ENS. A neuroprotective effect of estradiol has been established by showing that in vivo administration of estrogen (estradiol or the GPR30 receptor agonist, G1) protects against MPTP-induced dopaminergic neuron loss by inhibiting macrophages infiltration (50). In female rats, estradiol (endogenous or exogenous) stimulates myenteric neurons to produce nNOS and causes the recruitment of additional neurons to the active nNOS pool in the stomach and colon (51), which may have implications in the delay gastric emptying and increased colonic transit time observed in pregnancy.
Progesterone receptor
In rodents and bovine, progesterone receptors are only detected in the upper gut and mainly located in smooth muscle cells (see (35) for review). In human, PR mRNA expression is found in the musculature of normal colon in women (see (35) for review), and on ICC, being absent on enteric neurons and EGC, in rectal samples from healthy controls and patients with obstruction defecation (52).
Androgen receptor
In human and bovine GI tract, AR-B is the major isoform compared to AR-A with putative overexpression of AR-B proteins in the small gut compared to distal regions, mainly in muscles (see (35) for review). There is no evidence of AR expression on enteric neurons.
Sex chromosomes modulation of the ENS
To date, only one study has assessed the role of sex chromosomes in the function or structure of the ENS. Done in the context of Hirschprung’s disease, it supports a role for SRY, the testis-determinant gene, in the sexual dimorphism of the disease (53).
ELA, sex and the HPG axis
In humans, GnRH is detectable in the hypothalamus by 10 weeks gestational age with FSH and LH produced by 10–13 weeks. Gonadotropin (FSH and LH) levels peak at mid-gestation and then decline towards term due to negative feedback at both the hypothalamus and pituitary by the high levels of placental steroids. With the withdrawal of placental steroids at birth, gonadotropins rise and remain elevated for the first 1–2 years in girls and first 6 months in boys, a period referred to as mini-puberty, with a subsequent decrease for the remainder of childhood until puberty (54). This suggests that the HPG axis is functionally active and plays a biological role in a sex-dependent manner during early childhood (55). Sex differences in HPG axis function are also seen in young rodents (56), suggesting a conserved mechanism among species.
In mammals, stressors can alter the HPG axis function, resulting in reduced gonadal steroids and, thereby, altered reproductive function (57). Animal studies have indicated that HPA hormones down-regulate the HPG axis at all levels, and act primarily on the intermediaries (GnRH, LH, FSH)(58). In rodents, CRF limits LH synthesis (59). The literature and studies above suggest that alterations in the HPG axis function by stress in the early life development period affect the physiology in a sex-dependent manner. In their manuscript, Medland et al. (7) argue that sex hormones may not account for the sex differential effect of ELA on the ENS because the changes detected in animals were seen before puberty. As discussed above, the HPG axis is already fully functional in that developmental period. Additionally, because the male pigs used in the study were castrated shortly after birth (9 days old), the effect of stress on the HPG axis may have been lost in males while conserved in females (7). Using intact males could help clarify some of the underlying mechanisms involved in this sex difference.
Clinical relevance to IBS
Whether the alterations in ENS induced by stress in animal models also occur in humans is currently unknown. Nevertheless, there is evidence that mucosal biopsies from IBS patients exhibit neural outgrowth and that the supernatants collected from these biopsies can increase neurogenesis in enteric neurons primary cultures (60). Further investigations are warranted to understand mechanisms through which stress affects the ENS, whether there is a key period to produce those changes and more importantly, whether these alterations are reversible once established. As shown by Medland et al.(7) in this issue and as discussed in this mini-review, the inclusion of sex as a biological variable and addressing its action at all levels (gonadal, chromosomal) in these studies will be key to deepen our understanding and develop potential therapies that will benefit patients.
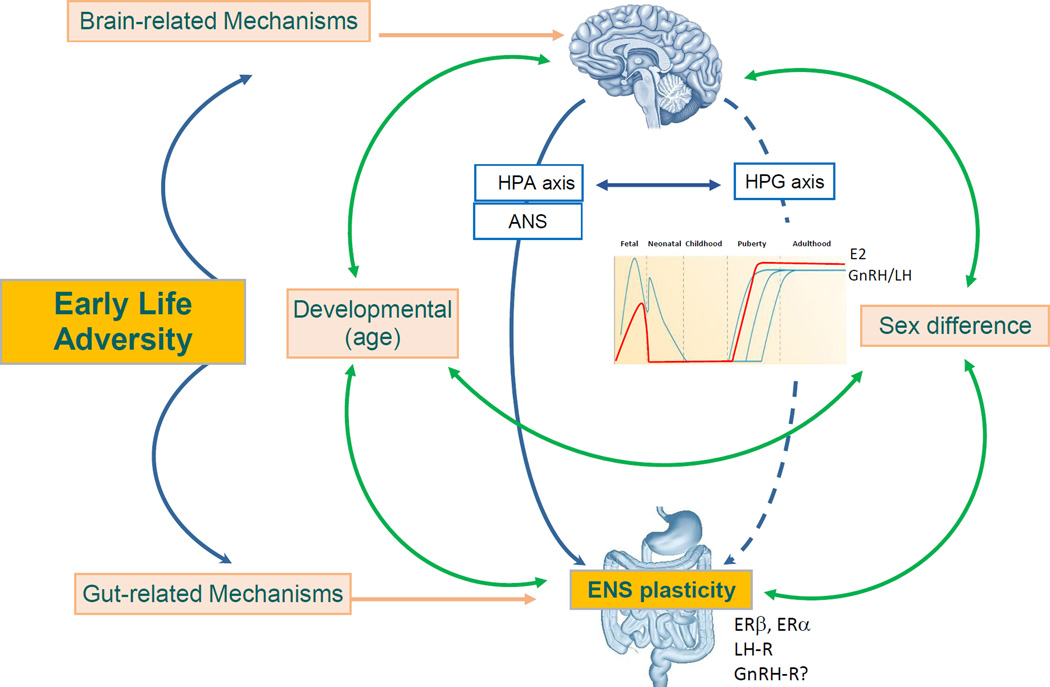
Figure 1. Pathways involved in the influence of early life adversity (ELA) on enteric nervous system plasticity (phenotype, number, chemical codes, activity).
Both the brain and the gut are affected by ELA. Centrally, ELA can modulate the HPA axis and the ANS which can in turn impact on the ENS plasticity. ELA also affects the HPG axis. Age and sex affect brain and gut development, as well as the HPA axis, HPG axis and ANS. While sex hormones (estradiol, progesterone, testosterone) are absent from the body from birth to puberty, the HPG axis is active in neonates (GNRH/LH secretion) in a sex-dependent manner. The ENS expresses sex hormones and gonadotropin hormones receptors supporting its direct modulation by the HPG axis.
Key Points.
-
-
The enteric nervous system (ENS) orchestrates various gastrointestinal functions: digestion, motility, secretion, permeability, immune and nociception.
-
-
The differentiation, organization and development of the ENS occur throughout the perinatal period which is highly susceptible to microenvironmental changes.
-
-
Early life adversity (ELA) affects the enteric nervous system development, phenotype and function in a sex dependent manner, with females being more sensitive, which may have implications for the higher prevalence of functional gastrointestinal disorders in women.
Acknowledgments
Funding
Supported by NIH NIDDK K01 DK088937 R01 DK078676, P50 DK064539 and P30 DK41301 (Model Core).
Abbreviations
- CRF
corticotropin-releasing factor
- CRF1
CRF receptor type 1
- CRF2
CRF receptor type 2
- ECC
enterochromaffin cells
- EGC
enteric glial cells
- ELA
early life adversity
- ENS
enteric nervous system
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- EWS
early weaning stress
- FSH
follicular stimulating hormone
- FSH-R
FSH receptor
- GIT
gastrointestinal tract
- GnRH
gonadotropin-releasing hormone
- GnRH-R
GnRH receptor
- HPG
hypothalamo-pituitary-gonadal
- IBS
irritable bowel syndrome
- ICC
interstitial cells of Cajal
- IPANs
intrinsic primary afferent neurons
- ISP
inner submucosal plexus
- LH
luteinizing hormone
- LH-R
LH receptor
- NMS
neonatal maternal separation stress
- NOS
nitric oxide synthase
- OSP
outer submucosal plexus
- VIP
vasoactive intestinal peptide
References
- 1.Selye H. Syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nature neuroscience. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Baram TZ. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res. 2007;62:240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- 7.Medland JE, Pohl CS, Edwards LL, et al. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016 doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. American journal of physiology Gastrointestinal and liver physiology. 2015;309:G927–G941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershon MD, Erde SM. The nervous system of the gut. Gastroenterology. 1981;80:1571–1594. [PubMed] [Google Scholar]
- 10.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Current opinion in gastroenterology. 2008;24:149–158. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- 11.Furness JB. The enteric nervous system and neurogastroenterology. Nature reviews Gastroenterology & hepatology. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 12.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. The Anatomical record. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. The Journal of clinical investigation. 2015;125:918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao MM, Foong JP, Bornstein JC, Li ZL, Vanden Berghe P, Boesmans W. Enteric nervous system assembly: Functional integration within the developing gut. Developmental biology. 2016 doi: 10.1016/j.ydbio.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2007;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Kugler EM, Michel K, Zeller F, et al. Mechanical stress activates neurites and somata of myenteric neurons. Frontiers in cellular neuroscience. 2015;9:342. doi: 10.3389/fncel.2015.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen MB. The enteric nervous system I: organisation and classification. Pharmacology & toxicology. 2003;92:105–113. doi: 10.1034/j.1600-0773.2003.t01-1-920301.x. [DOI] [PubMed] [Google Scholar]
- 18.Neunlist M, Rolli-Derkinderen M, Latorre R, et al. Enteric glial cells: recent developments and future directions. Gastroenterology. 2014;147:1230–1237. doi: 10.1053/j.gastro.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Chalazonitis A, Gershon MD, Greene LA. Cell death and the developing enteric nervous system. Neurochemistry international. 2012;61:839–847. doi: 10.1016/j.neuint.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Salhy M, Sandstrom O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mechanisms of ageing and development. 1999;107:93–103. doi: 10.1016/s0047-6374(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 21.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. The Journal of clinical investigation. 2015;125:956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nature reviews Gastroenterology & hepatology. 2014;11:611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 23.Schafer KH, Van Ginneken C, Copray S. Plasticity and neural stem cells in the enteric nervous system. Anatomical record (Hoboken, NJ: 2007) 2009;292:1940–1952. doi: 10.1002/ar.21033. [DOI] [PubMed] [Google Scholar]
- 24.von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- 27.Tache Y, Million M. Role of Corticotropin-releasing Factor Signaling in Stress-related Alterations of Colonic Motility and Hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan PQ, Wu SV, Pothoulakis C, Tache Y. Urocortins and CRF Receptor Type 2 Variants in the Male Rat Colon: Gene Expression and Regulation by Endotoxin and Anti-inflammatory Effect. American journal of physiology Gastrointestinal and liver physiology. 2016 doi: 10.1152/ajpgi.00337.2015. ajpgi.00337.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 30.Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Fei G, Fang X, et al. Changes in Enteric Neurons of Small Intestine in a Rat Model of Irritable Bowel Syndrome with Diarrhea. J Neurogastroenterol Motil. 2016;22:310–320. doi: 10.5056/jnm15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold AP, Chen X, Itoh Y. What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation. Handb Exp Pharmacol. 2012:67–88. doi: 10.1007/978-3-642-30726-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roch GJ, Busby ER, Sherwood NM. GnRH receptors and peptides: skating backward. Gen Comp Endocrinol. 2014;209:118–134. doi: 10.1016/j.ygcen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Yen SS. Clinical applications of gonadotropin-releasing hormone and gonadotropin-releasing hormone analogs. Fertil Steril. 1983;39:257–266. [PubMed] [Google Scholar]
- 35.Houdeau E. Sex Differences in Gastrointestinal Physiology and Diseases: From Endogenous Sex Hormones to Environmental Endocrine Disruptor Agents. In: Neigh G, Mitzelfelt M, editors. Sex Differences in Physiology. Elsevier; [Google Scholar]
- 36.Ohlsson B. Gonadotropin-Releasing Hormone and Its Physiological and Pathophysiological Roles in Relation to the Structure and Function of the Gastrointestinal Tract. Eur Surg Res. 2016;57:22–33. doi: 10.1159/000445717. [DOI] [PubMed] [Google Scholar]
- 37.Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Ohlsson B, Veress B, Janciauskiene S, Montgomery A, Haglund M, Wallmark A. Chronic intestinal pseudo-obstruction due to buserelin-induced formation of anti-GnRH antibodies. Gastroenterology. 2007;132:45–51. doi: 10.1053/j.gastro.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Hammar O, Ohlsson B, Veress B, Alm R, Fredrikson GN, Montgomery A. Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol. 2012;47:1165–1173. doi: 10.3109/00365521.2012.706826. [DOI] [PubMed] [Google Scholar]
- 40.Ho JS, Nagle GT, Mathias JR, et al. Presence of gonadotropin-releasing hormone (GnRH) receptor mRNA in rat myenteric plexus cells. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:817–821. doi: 10.1016/0305-0491(95)02114-0. [DOI] [PubMed] [Google Scholar]
- 41.Sand E, Voss U, Ohlsson B, Ekblad E. Luteinizing hormone receptors are expressed in rat myenteric neurons and mediate neuronal loss. Auton Neurosci. 2015;193:104–107. doi: 10.1016/j.autneu.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Mathias JR, Clench MH. Relationship of reproductive hormones and neuromuscular disease of the gastrointestinal tract. Dig Dis. 1998;16:3–13. doi: 10.1159/000016844. [DOI] [PubMed] [Google Scholar]
- 43.Ohlsson B, Ekblad E, Veress B, Montgomery A, Janciauskiene S. Antibodies against gonadotropin-releasing hormone (GnRH) and destruction of enteric neurons in 3 patients suffering from gastrointestinal dysfunction. BMC Gastroenterol. 2010;10:48. doi: 10.1186/1471-230X-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sand E, Voss U, Hammar O, et al. Gonadotropin-releasing hormone analog buserelin causes neuronal loss in rat gastrointestinal tract. Cell Tissue Res. 2013;351:521–534. doi: 10.1007/s00441-012-1534-1. [DOI] [PubMed] [Google Scholar]
- 45.Sand E, Bergvall M, Ekblad E, D'Amato M, Ohlsson B. Expression and distribution of GnRH, LH, and FSH and their receptors in gastrointestinal tract of man and rat. Regul Pept. 2013;187:24–28. doi: 10.1016/j.regpep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Hammar O, Veress B, Montgomery A, Ohlsson B. Expression of Luteinizing Hormone Receptor in the Gastrointestinal Tract in Patients with and without Dysmotility. Drug Target Insights. 2012;6:13–18. doi: 10.4137/DTI.S9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell-Thompson M, Reyher KK, Wilkinson LB. Immunolocalization of estrogen receptor alpha and beta in gastric epithelium and enteric neurons. The Journal of endocrinology. 2001;171:65–73. doi: 10.1677/joe.0.1710065. [DOI] [PubMed] [Google Scholar]
- 48.Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochemistry and cell biology. 2004;121:399–405. doi: 10.1007/s00418-004-0644-6. [DOI] [PubMed] [Google Scholar]
- 49.Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. European journal of cancer (Oxford, England : 1990) 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 50.Cote M, Bourque M, Poirier AA, et al. GPER1-mediated immunomodulation and neuroprotection in the myenteric plexus of a mouse model of Parkinson's disease. Neurobiology of disease. 2015;82:99–113. doi: 10.1016/j.nbd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280:R1546–R1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 52.Bassotti G, Villanacci V, Bellomi A, et al. An assessment of enteric nervous system and estroprogestinic receptors in obstructed defecation associated with rectal intussusception. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:e155–e161. doi: 10.1111/j.1365-2982.2011.01850.x. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Kido T, Garcia-Barcelo MM, Tam PK, Tabatabai ZL, Lau YF. SRY interference of normal regulation of the RET gene suggests a potential role of the Y-chromosome gene in sexual dimorphism in Hirschsprung disease. Human molecular genetics. 2015;24:685–697. doi: 10.1093/hmg/ddu488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehlers K, Halvorson L. Gonadotropin-releasing Hormone (GnRH) and the GnRH Receptor (GnRHR) Glob libr women's med. 2013 [Google Scholar]
- 55.Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson AM. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Human reproduction update. 2006;12:341–349. doi: 10.1093/humupd/dml018. [DOI] [PubMed] [Google Scholar]
- 56.Bjelobaba I, Janjic MM, Kucka M, Stojilkovic SS. Cell Type-Specific Sexual Dimorphism in Rat Pituitary Gene Expression During Maturation. Biology of reproduction. 2015;93:21. doi: 10.1095/biolreprod.115.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. Journal of neuroendocrinology. 2014;26:573–586. doi: 10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biology of reproduction. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 59.Naylor AM, Porter DW, Lincoln DW. Central administration of corticotrophin-releasing factor in the sheep: effects on secretion of gonadotrophins, prolactin and cortisol. The Journal of endocrinology. 1990;124:117–125. doi: 10.1677/joe.0.1240117. [DOI] [PubMed] [Google Scholar]
- 60.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e1004. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]