Summary
In comparison to human T cells, efficient retroviral gene transfer and subsequent expansion of murine primary T cells is more difficult to achieve. Herein, we describe an optimized gene transfer protocol utilizing an ecotropic viral vector to transduce primary murine T cells activated with magnetic beads coated with agonistic anti-CD3 and CD28 antibodies. Activated T cells are subsequently centrifuged (spinoculated) on RetroNectin-coated tissue culture plates in the context of retroviral supernatant. Variables found to be critical to high gene transfer and subsequent efficient T cell expansion included CD3/CD28 magnetic bead to cell ratio, time from T cell activation to initial spinoculation, frequency of T cell spinoculation, interleukin-2 concentration in the medium, and the initial purity of the T cell preparation.
Keywords: Retroviral gene transfer, Murine primary T cells, Mouse T lymphocytes, Phoenix-eco packaging cells, Spinoculation, RetroNectin, Interleukin-2 (IL-2), Nylon wool column, CD3/CD28-activating magnetic beads
1. Introduction
Retroviral gene modification of human T cells provides a novel and potentially powerful approach to the treatment of a wide array of diseases including inherited or acquired immunodeficiencies, infection, autoimmune disorders, and cancer (1– 5). Similarly, retroviral-mediated gene transfer in murine T cells may enable investigators to better understand in vivo T cell biology as well as study the potential of genetically modified T cells in murine models of disease. However, in contrast to human T cells, which are readily transduced and expandable in the ex vivo setting, retroviral-mediated gene transfer into murine T cells is compromised both by poor gene transfer and inadequate subsequent T cell expansion and survival. Herein, we describe an optimized retroviral gene transfer protocol that allows for the generation of efficiently transduced and highly viable murine T cell populations.
In order to enhance gene transfer as well as T cell viability, we addressed several critical parameters in optimizing our protocol. First, we utilized the Pheonix-eco (6) ecotropic packaging cell line, due to the ubiquitous expression of ecotropic receptors on murine cells, including T cells (7, 8). Second, we use the high-titer, Moloney murine leukemia virus-derived vector SFG, a variant of the MFG gamma-retroviral vector (9). Third, we further explored T cell activation conditions and timing of retroviral exposure, which are critical for efficient retroviral integration and T cell survival (10). Fourth, we found that the purity of isolated murine T cells significantly impacted on transduction efficiency. Fifth, we found that repeated transduction through the centrifugation of retroviral supernatant and T cells on RetroNectin-coated nontissue culture plates (spinoculation), which enhances the colocalization of target cells and viral particles, (11), markedly improved gene transfer. Finally, we demonstrate the role of T cell culture conditions, specifically the concentration of IL-2, in the viability and expansion of transduced murine T cells. By optimizing these parameters we were able to achieve consistent and efficient gene transfer with expression of the target gene by highly viable murine T cells. The 19z1 SFG retroviral vector, encoding a chimeric antigen receptor (CAR) that targets the CD19 antigen on normal and malignant B cells (12), was utilized to illustrate the murine T cell transduction protocol described here.
2. Materials
1× sterile PBS.
Sterile PBS with 0.5% heat-inactivated fetal calf serum (FCS).
Complete RPMI-1640 medium with 10% heat-inactivated fetal calf serum (FCS), 20 mM HEPES (Gibco-BRL, Grand Island, NY), 1 mM sodium pyruvate (Gibco-BRL), 0.05 mM 2-ME (Gibco-BRL), 2 mM L-Glutamine (Gibco-BRL), 100 U/mL penicillin (Gibco-BRL), and 100 μg/mL streptomycin (Gibco-BRL).
Dulbeccos modified Eagles Medium (DMEM) with 10% FCS, 2 mM l -Glutamine (Gibco-BRL), 100 U/mL penicillin (Gibco-BRL), and 100 μg/mL streptomycin (Gibco-BRL).
Poly- l -lysine solution – 4 parts 0.1% gelatin (Fisher Scientific Cat. No. G8-500), 1 part 0.01% poly-l-lysine buffer (Sigma Cat. No. P4832).
Six-well flat bottom tissue culture plates with lids (BD Falcon).
Six-well flat bottom nontissue culture plates with lids (BD Falcon).
100-mm tissue culture plates (Corning).
12 × 75 mm Polystyrene Round-Bottom Test Tube, 5 mL, snap cap (BD Falcon, Cat. No. 352054).
Sterile Nylon Wool Fiber Column (Polysciences Cat. No. 21759).
10 mL Syringe (BD).
0.45 μm filter for syringes (Whatman Cat. No. 6780–2504).
ACK Lysing Buffer (BioWhittaker Cat. No. 10-548E).
Recombinant Mouse Interleukin-2 (rmIL-2) (Roche Cat. No. 11271164001).
Dynabeads Mouse CD3/CD28 T Cell Expander (Invitrogen Cat. No. 114-11D).
Dynal MPC-50 Magnet (Invitrogen Cat. No. 120–24).
RetroNectin (Takara Biomedicals, Otsu, Japan Cat. No. T100A).
Blue Max 50 mL Polypropylene Conical Tube (BD Falcon).
40 μm Nylon Cell Strainer (BD Falcon).
5 mL Syringe (BD).
PE-conjugated anti-19z1 antibody (Monoclonal Antibody Facility, MSKCC).
FITC-conjugated anti-mouse CD3 antibody (eBioscience).
FITC-conjugated anti-mouse CD4 antibody (eBioscience).
C57BL/6 mice for T cells.
Phoenix Eco Cell Line (ATCC product# SD 3444).
HEPES (Sigma Cat. No. H-7006).
CaCl2 (Mallinkrodt Cat. No. 4160).
Na2HPO4 (Sigma).
dd H2 O.
19z1 SFG retroviral plasmid ( see ref. 12).
3. Methods
The protocol described later outline (1) the transfection of Phoenix-eco retroviral producer cells (6), (2) the preparation of murine T cells for retroviral transduction, and (3) the transduction of murine T cells with Phoenix-eco retroviral supernatant.
3.1. Transfection of Phoenix-Eco Cells
The protocol describes the transfection of Phoenix-eco cells with the 19z1 SFG retroviral plasmid. It includes (a) preparation of the Phoenix-eco producer cells for transfection, (b) transfection of the Phoenix-eco cells, and (c) collection of the retroviral supernatant for gene transfer of murine T cells.
3.1.1. Preparation of Phoenix-Eco Producer Cells for Transfection (Day –1)
Before transfection, determine the amount of viral supernatant required. Generally 2 mL of viral supernatant (1 mL for each spinoculation) is sufficient for the transduction of 3 × 106 isolated mouse T cells ( see Subheading 3.3 ). Each 100-mm plate yields approximately 7 mL of viral supernatant.
One day prior to transfection, plate Phoenix-eco cells at 50% confluence per 100-mm plate in 10 mL DMEM + 10% FCS. Make sure to evenly distribute cells by rocking plates forward and backward, then side to side, 3–4 times. Leave the plate in a humidified 37°C, 5% CO2 incubator and do not disturb the cells as they attach to the plate.
On the day of transfection (day 0), the Phoenix-eco plate should be 90–100% confluent.
3.1.2. Calcium Phosphate Transfection (Day 0)
Coat 100-mm tissue culture plates with 10 mL Poly-L-lysine solution ( see Note 1 ). Immediately remove the solution which may be reused to coat further plates.
Resuspend confluent Phoenix-eco cells from day 1 plates by pipetting (these cells adhere very lightly to the plate and therefore do not require trypsin to generate a cell suspension). Add one-third of the resulting cell suspension to each Poly-l-lysine-coated plate in a final volume of 10 mL DMEM + 10% FCS. Ensure even distribution of cells by gently tilting plates forward and backward, then side to side, 3–4 times.
Store plates undisturbed at 37°C with 5% CO2 for 8 h.
When the Phoenix-eco cells are attached and evenly distributed at 30–50% confluence, they are ready for transfection. Prior to transfection, aspirate medium and very carefully replace with 10 mL of fresh DMEM + 10% FCS being sure not to dislodge adherent cells.
Allow 20 min to thaw all transfection reagents. Make 2x HEPES-buffered saline (HBS) by mixing 8.0 g of NaCl, 6.5 g of HEPES, in 10 mL of Na2HPO4 stock solution (5.25 g Na2HPO4 in 500 mL of water). Adjust pH to exactly 7.0 using NaOH or HCl and bring final volume of 2× HBS solution to 500 mL.
Prepare the transfection cocktail in 12 × 75-mm polystyrene round-bottom test tubes. Each cocktail will provide enough reagent to transfect 3,100 mm Phoenix-eco Poly-l-lysine-coated plates. To each tube, add 30 μg of 19z1-SFG DNA plasmid, dd H2O followed by 186 μL of calcium chloride for a final volume of 1,500 μL ( see Note 2 ).
- Next, while gently but continuously vortexing the cocktail prepared, slowly add 1,500 μL of 2× HBS dropwise for DNA precipitation ( see Note 3 ).
- Each tube now contains:
DNA 30 μg 2 M CaCl2 186 μL dd H2O 1,314 μL – volume of DNA 2× HBS 1,500 μL Total volume: 3,000 μL
Immediately add 1 mL of the resulting transfection mix dropwise onto each of the 100-mm Phoenix-eco Poly-l-lysine-coated plates distributing the cocktail evenly over the cells. Rock plates side to side/back and forth a few times to evenly distribute DNA/CaPO4 particles.
Incubate plates at 37°C with 5% CO2 for 16 h.
3.1.3. Generation of Retroviral Supernatant of Murine T Cell Transduction (Days 1–2)
Aspirate DNA/CaPO4 particles and medium, and carefully replace with 10 mL of fresh DMEM + 10% FCS along side of the plates so as not to dislodge adherent Phoenix-eco cells.
Incubate plates at 37°C with 5% CO2 overnight.
Aspirate and carefully replace the 10 mL of DMEM + 10% FCS with 7 mL complete RPMI ( see Note 4 ).
Incubate plates at 37°C with 5% CO2 over night.
3.1.4. Harvesting Retroviral Supernatant for Murine T Cell Transduction (Day 3)
Using a 10-mL syringe, draw up the day 3 retroviral supernatant from transfected Phoenix cells ( see Note 5 ). Filter supernatant through a 0.45-um filter attached to the end of the syringe to remove cellular debris.
Day 3 supernatants can be used either directly on activated T cells ( see Subheading 3.2 ) or stored at −80°C for later experiments ( see Note 6 ).
3.2. Preparation of Murine T Cells for Transduction
The protocol describes the preparation of murine T cells for retroviral transduction. Steps for murine T cell isolation include (a) harvesting of mouse splenocytes, (b) isolation of the CD3+ T cell fraction, and (c) activation of the resulting purified CD3+ T cell population.
3.2.1. Harvesting Mouse Splenocytes
Euthanize C57BL/6 mice by CO2 asphyxiation. Soak mouse briefly in 70% EtOH for sterility and surgically remove spleen.
To make a single cell suspension of splenocytes from mouse spleen, first place 40-μm nylon cell strainer on 50-mL polypropylene conical tube and wet the cell strainer surface with complete RPMI. Macerate fresh spleen on the cell strainer with a 5-mL syringe plunger.
When spleen is completely macerated on cell strainer, rinse cell strainer with 10 mL of complete RPMI to flush splenocytes into 50-mL conical tube.
Wash splenocytes with sterile PBS, pellet cells by centrifugation at 400 g for 5 min, repeat wash.
To lyse erythrocytes, resuspend washed cell pellet in 1 mL of ACK Lysing Buffer and incubate at room temperature for 7 min. Add 10 mL PBS and pellet cells by centrifugation.
Wash twice with PBS.
Resuspend splenocytes in complete RPMI to a concentration of 0.5–1 × 108 viable cells/mL. Each spleen will yield approximately 1 × 108 splenocytes of which 35–45% are CD3+ T cells (13).
3.2.2. Enrichment of CD3+ T Cells from Splenocyte Pool
The purity of the T cell population enhances retroviral transduction efficiency ( see Note 7 ). To this end, we utilize nylon wool columns to further enrich for the CD3+ T cell population as described (modified from the manufacturers protocol) (14).
Load nylon wool column with 5 mL complete RPMI. Tap gently to remove air bubbles.
Incubate prepared column for 1 h at 37°C.
Drain medium from the column.
Add 1–2 × 108 viable cells in a volume of 2 mL of complete RPMI. Drain to allow entire cell volume to enter into the nylon wool.
Add another 2 mL of complete RPMI to the top of the column. Drain column to allow the additional 2 mL to enter into the nylon wool.
Add another 4 mL of medium to the column wool to ensure top of the wool is covered.
Incubate splenocyte-loaded column for 1 h at 37°C in 5% CO2. Cover the top of the column with parafilm to maintain sterility.
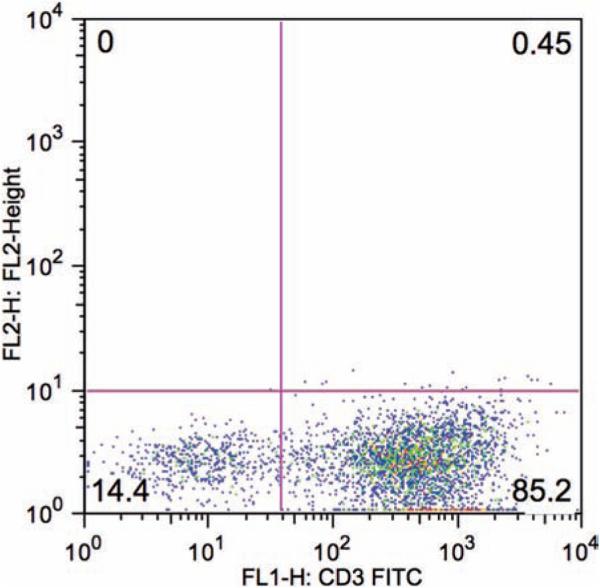
Drain the nylon wool column by gravity into a 50-mL polypropylene conical tube to collect the nonadherent CD3+ T cell fraction, washing the column once with 5 mL of complete RPMI to collect residual nonadherent cells. The resulting cell population is routinely 80–90% CD3+ (Fig. 1 ).
Pellet recovered cells by centrifugation and resuspend in complete RPMI to a concentration of 1 × 106 cells/mL.
Fig. 1.
FACS analysis of splenocytes eluted from a nylon wool column and stained with mouse anti-CD3 FITC-conjugated antibody. As shown here, the CD3+ T cell fraction of nonadherent cells accounts for 85% of the total recovered cell population.
3.2.3. Activation of CD3+ Murine T Cells. (Day 0)
CD3+ T cells are activated using Dynabeads Mouse CD3/CD28 T Cell Expander magnetic beads coated with agonistic mouse CD3 and CD28 antibodies.
Calculate the amount of CD3/CD28 beads required to obtain a 1:1 CD3/CD28 bead to cell ratio.
To wash beads prior to activation, dilute the required number of beads in 3 mL of PBS with 0.5% FCS in a FACS tube, and place suspended beads into the MPC-50 magnet and allow for beads to collect on the side of the tube (1 min).
Aspirate PBS/FCS solution, remove tube from magnet, resuspend beads in fresh PBS/FCS, and repeat magnetic isolation and aspiration of PBS/FCS for a total of three wash cycles.
Resuspend beads in 1 mL complete RPMI.
Add washed, resuspended beads to mouse T cells at a final concentration 1 × 106 cells/mL at a bead to cell ratio of 1:1 in complete RPMI supplemented with 20 IU/mL IL-2 ( see Note 8 ).
Plate the cells in 6-well tissue culture plates at a volume of 3 mL per well at a concentration of 1 × 106 cells/mL. Incubate cells at 37°C and 5% CO2 overnight.
3.3. Transduction of Murine T Cells with Viral Supernatant by Spinoculation (Day 1)
Optimal gene transfer into murine T cells is achieved when the first cycle of spinoculation is performed at 24 h following initial T cell activation ( see Note 9 ).
Coat 6-well nontissue culture plates with RetroNectin at 15 μg/mL in PBS. Incubate at room temperature for 3 h.
Aspirate RetroNectin/PBS solution and coat plates with PBS with 0.5% FCS. Incubate plates at room temperature for 30 min.
Just prior to spinoculation, aspirate PBS/FCS solution from RetroNectin-coated plates. The plates are now ready for transduction.
Harvest activated CD3+ T cells from day 0, pellet cells and resuspend cells at a concentration of 3 × 106 cells/mL in complete RPMI supplemented with 80 units of IL-2 ( see Note 10 ).
Add 1 mL of previously prepared fresh or thawed viral supernatant per well.
Immediately after adding the viral supernatant, add 1 mL of CD3+ T cells per well. Each well now has a total volume of 2 mL containing 3 × 106 T cells mixed with viral supernatant at a 1:1 vol/vol ratio.
Immediately centrifuge the plate(s) at 2,000 × g and 30°C, for 1 h (first spinoculation)
Incubate at 37°C with 5% CO2 overnight.
(Day 2) Being careful not to disturb T cells at the bottom of the wells, carefully aspirate 1 mL of medium from each well ( see Note 11 ).
Replenish with 1 mL of fresh or thawed viral supernatant.
Centrifuge plate(s) at 2,000 g and 30°C, for 1 h (second spinoculation)
Incubate at 37°C with 5% CO2 overnight.
(Day 3) Add 1 mL of fresh complete RPMI supplemented with 20 units of IL-2 to each well in 6-well plate. Subsequently, obtain T cell counts daily and maintain a T cell concentration of 1–2 × 106 cells/mL for optimal expansion and viability ( see Note 12 ).
(Day 5) Analyze transduction efficiency of T cells by FACS ( see Fig. 2 ).
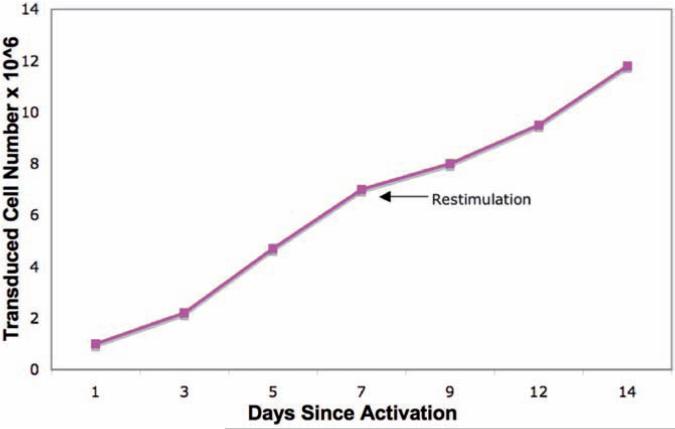
(Days 7) The transduced mouse T cells may be further expanded by restimulation with CD3/CD28 beads at 1:5 bead to cell ratio ( Fig. 3 ) making sure to maintain a T cell concentration of 1–2 × 106 cells/mL for optimal expansion and viability (see Note 12 ).
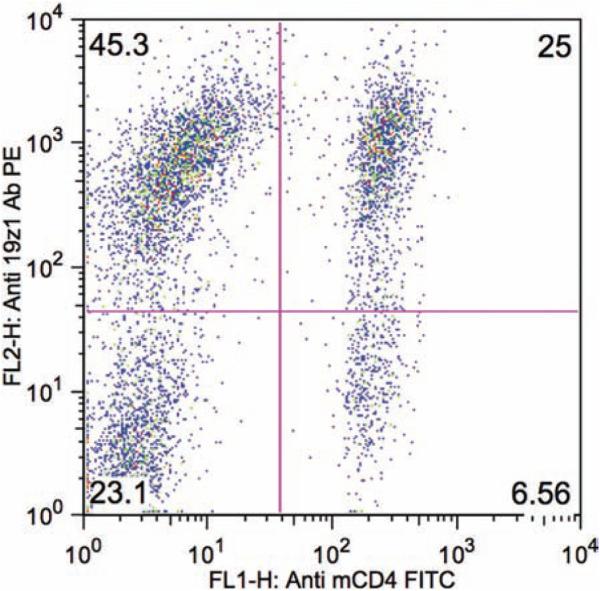
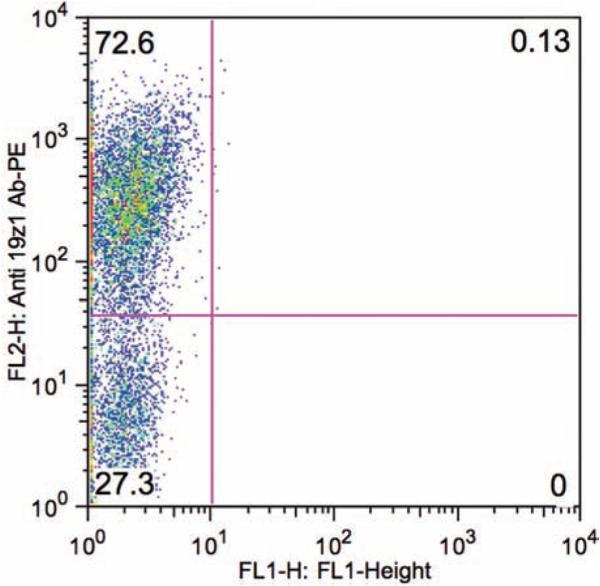
Fig. 2.
FACS analysis of transduced mouse T cells stained with PE-conjugated anti-19z1 antibody and FITC-conjugated anti-mouse CD4 antibody demonstrating 70% 19z1 + T cells at day 5 following T cell isolation.
Fig. 3.
Murine T cells may be further expanded by restimulation with CD3/CD28 magnetic beads following initial activation. T cells were activated with 1:1 beads to cell ratio on day 0, transduced, and restimulated ( arrow ) on day 7 with beads at a 1:5 bead to T cell ratio. Viable cells were counted via trypan blue exclusion with a hemocytometer and assessed by flow cytometry for 19z1 transduction. The viability of cells was consistently greater than 90% during data collection. Cell number represents the expansion of the transduced fraction over 2 weeks.
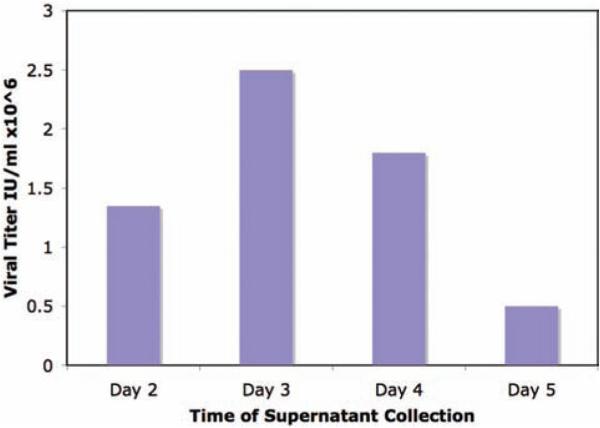
Fig. 4.
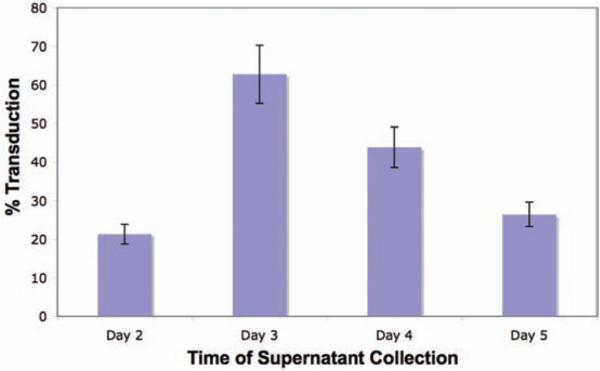
Optimal viral titer, as assessed by infection of NIH 3T3 cells with serial dilution of viral supernatant, ( a ) obtained from day 3 retroviral supernatant resulted in optimal murine T cell transduction ( b ) as assessed by FACS analysis of T cells at day 7 following T cell transduction.
Fig. 5.
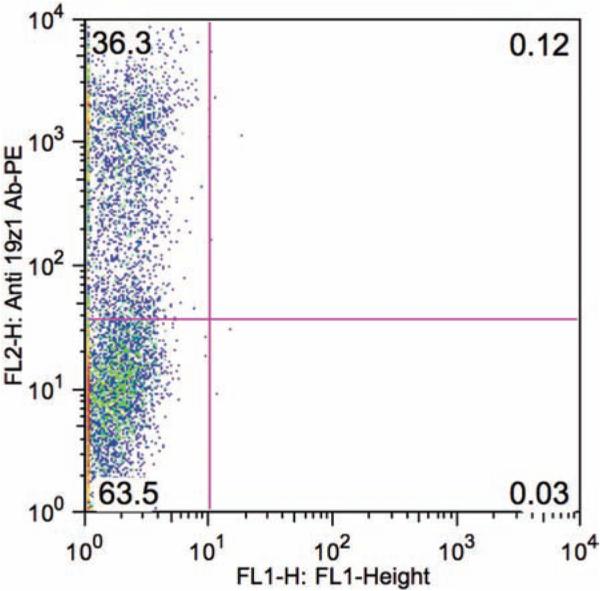
FACS analysis of transduction efficiencies of ( a) purified CD3+ T cells and ( b ) whole mouse splenocytes.
Fig. 6.
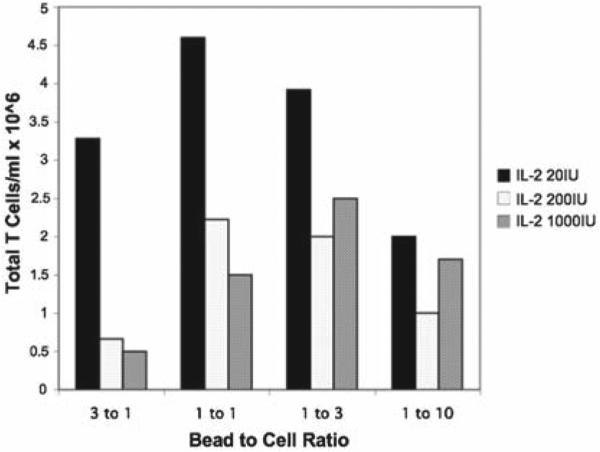
Representative result of five independent experiments demonstrating comparative T cell count with various CD3/CD28 bead to cell ratios and IL-2 concentrations. The starting concentration of T cells was 1 × 106 cells/ml and cells were counted at day 5 postactivation. Optimal expansion was obtained using a 1:1 bead to T cell ratio in the context of low dose (20 IU/ml) IL-2.
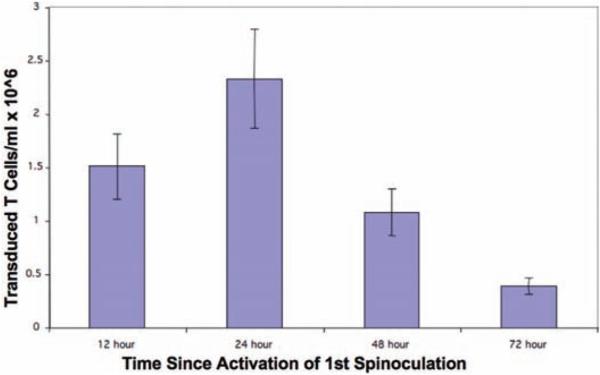
Fig. 7.
The number of transduced T cells at day 5 postactivation when the two spinoculation cycles were initiated at different time points following CD3/CD28 bead activation. These data demonstrate optimal gene transfer when mouse T cells were spinoculated 24 h following initial activation.
Acknowledgments
We thank Dr. Isabelle Riviere for reviewing the manuscript. Our work is supported by the US National Cancer Institute (grants CA95152, CA59350), The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, William H. Goodwin and Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC, and the Bocina Cancer Research Fund.
Footnotes
Poly-l-lysine is used to enhance adherence of Phoenix-eco cells to the tissue culture plates. Phoenix cells are otherwise loosely attached to plates and are often easily dislodged.
The mechanism of calcium phosphate coprecipitation based transfection is as follows: A controlled mixing of DNA, calcium chloride, and HBS generates a DNA precipitate that is subsequently dispersed onto the cultured cells. The precipitate is then taken up by the cells via endocytosis or phagocytosis. The positively charged calcium ions neutralize negatively charged phosphate backbones of DNA, so they enter into cells through a negatively charged membrane (15).
The vortex speed needs to be fast enough to thoroughly agitate the mixture for calcium phosphate coprecipitation, but not spill the contents within the tube. A setting at 60% of full power of the vortexer is usually adequate.
Retroviral supernatant in complete RPMI significantly enhances the transduction efficiency and the survival of transduced mouse T cells when compared to retroviral supernatant generated in DMEM with 10% FCS (data not shown).
Optimal retroviral titer is obtained at day 3 following Phoenix-eco cell transfection, resulting in the highest gene transfer of murine T cells ( Fig. 4 ).
The retroviral supernatant can be frozen at −80°C for later transduction and stored for up to 3 months, although its titer drops by one-half with each freeze-thaw cycle. The difference in the transduction efficiency of T cells between fresh and frozen supernatants, thawed once, however, is not significant when using this protocol.
CD3/CD28 bead activation of enriched CD3+ T cells allows for better transduction efficiency when compared to bead stimulation of unsorted splenocytes ( Fig. 5 ).
Optimal activation of mouse T cells, as assessed by total T cell number at day 5 following activation, is attained using a CD3/CD28 bead to cell ratio of 1:1 in medium supplemented with low dose IL-2 (20 IU/mL) ( Fig. 6 ).
Optimal gene transfer is obtained when initial spinoculation occurs at 24 h following CD3/CD28 bead activation ( Fig. 7 ).
A higher concentration of IL-2 is added here in anticipation of IL-2 dilution due to the addition of IL-2 free viral supernatant. Starting with 80 units/mL, the concentration of IL-2 returns to 20 units/mL following two cycles of spinoculation.
A quick spin of the 6-well plates at 100 g for 3 min could be used to settle the T cells to the bottom of the well if the plates are disrupted prior to medium aspiration.
Since mouse T cells exhibit density-dependent proliferation and survival preference, be careful to maintain a T cell concentration of 1–2 × 106 T cells/mL by either supplementing the T cell culture with complete RPMI with 20 IU IL-2/mL or splitting the culture as needed to maintain this T cell concentration.
References
- 1.Onodera M, Ariga T, Kawamura N, Kobayashi I, Ohtsu M, Yamada M, Tame A, Furuta H, Okano M, Matsumoto S, Kotani H, McGarrity GJ, Blaese RM, Sakiyama Y. Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood. 1998;91:30–6. [PubMed] [Google Scholar]
- 2.Palù G, Pira GLi, Gennari F, Fenoglio D, Parolin C, Manca F. Genetically modified immunocompetent cells in HIV infection. Gene Therapy. 2001;21:1593–1600. doi: 10.1038/sj.gt.3301569. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima A. Application of cellular gene therapy for rheumatoid arthritis. Mod Rheumatol. 2006;5:269–275. doi: 10.1007/s10165-006-0501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwu P, Yannelli J, Kriegler M, Anderson WF, Perez C, Chiang Y, Schwarz S, Cowherd R, Delgado C, Mule J, Rosenberg SA. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with tumor necrosis factor-alpha cDNA for the gene therapy of cancer in humans. J Immunol. 1993;150:4104–4115. [PubMed] [Google Scholar]
- 6.Pear WS, Scott ML, Nolan GP. Generation of high titre, helper-free retroviruses by transient transfection, in Robbins PD (ed). Methods in Molecular Medicine: Gene Therapy Protocols. Humana, Totowa, NJ. 1996:41–57. doi: 10.1385/0-89603-484-4:41. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Kavanaugh MP, North RA, Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- 8.Sadelain M. Methods for Retrovirus-Mediated Gene Transfer into Primary T-Lym phocytes. In: Robbins PD, editor. Methods in Molecular Medicine: Gene Therapy Protocols. Humana; Totowa, NJ: 1996. pp. 241–248. [DOI] [PubMed] [Google Scholar]
- 9.Riviere I, Brose K, Mulligan RC. Effects of retroviral design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagani AB, Riviere I, Tan C, Krause A, Sadelain M. Activation conditions determine susceptibility of murine primary T-lymphocytes to retroviral infection. J Gene Med. 1999;1:341–351. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<341::AID-JGM58>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Pollok KE, Hanenberg H, Noblitt TW, Schroeder WL, Kato I, Emanuel D, Williams DA. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol. 1998;72:4882–4892. doi: 10.1128/jvi.72.6.4882-4892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 13.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 14.Coligan JE, Kruisbeer AM, Marguilies DH, Shevach EM, Strober W. Current Protocols in Immunology. Wiley; NY: 1993. T cell enrichment by nonadherence to nylon; p. 3.2.1. [Google Scholar]
- 15.Loyter A, Scangos GA, Ruddle FH. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci USA. 1982;79:422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]