SUMMARY
Different individuals exhibit distinct behaviors, but studying the neuronal basis of individuality is a daunting challenge. Here, we considered this question in the vomeronasal organ, a pheromone-detecting epithelium containing hundreds of distinct neuronal types. Using light-sheet microscopy, we characterized in each animal the abundance of 17 physiologically-defined types, altogether recording from a half-million sensory neurons. Inter-animal differences were much larger than predicted by chance, and different physiological cell types showed distinct patterns of variability. One neuronal type was present in males and nearly absent in females. Surprisingly, this apparent sexual dimorphism was generated by plasticity, as exposure to female scents or single ligands led to both the elimination of this cell type and alterations in olfactory behavior. That an all-or-none apparent sex difference in neuronal types is controlled by experience—even in a sensory system devoted to “innate” behaviors—highlights the extraordinary role of “nurture” in neural individuality.
eTOC Blurb
To address the roles of “nature” and “nurture” in sculpting the brain, Xu et al. imaged more than a half-million sensory neurons from animals of different sexes and experiences. Dramatic individual differences arose from experience and affected supposedly-innate behaviors.
INTRODUCTION
Much of what distinguishes the abilities and behavior of individual persons or animals lies within the nervous system. While recent advances in genomics have permitted detailed mapping of individual differences on a genome-wide scale, relating such differences to neural structure and function remains challenging. At a macroscopic level, inter-individual variability of the brain, for example in terms of cortical thickness/surface area (Schwarzkopf et al. 2011) and grey/white matter structure, are correlated with performance in basic and higher cognitive functions (Mueller et al. 2013). The emerging connectome (Lu et al. 2009) as well as single neuron innervation pattern analysis (Chou et al. 2010) also suggest diverse neural wiring across individual organisms. However, the complexity of neuronal circuitry has made it extremely difficult to study the functional basis for individual difference at the single-neuron level. Addressing this challenge requires sampling at cellular resolution on a large scale.
Neuronal individuality is generated by stochastic gene expression, biological factors (“nature”), and experience (“nurture”) (Gimelbrant et al. 2007; Yagi 2013). In wild-type mice, one prominent example of “nature”-induced individual variability is sexual dimorphism. Previous work has identified both sexually-dimorphic behaviors and a small number of dimorphic nuclei or neuronal populations (Morris et al. 2004; Shah et al. 2004; Yang et al. 2013; Young 1982). However, at present there are few cases in which the physiological properties and circuit function of such neurons are known (Bergan et al. 2014). On the other hand, it is widely recognized that the environment has far-reaching influence on individual differences. An extensive literature on activity-dependent plasticity of cells (Flavell and Greenberg 2008; Santoro and Dulac 2012; Zhao and Reed 2001) provides a backdrop for the role of “nurture” in generating neuronal individuality.
To study the interplay between nature and nurture in generating individual differences, here we focused on the vomeronasal organ (VNO), a chemosensory structure devoted to the detection of social cues often called pheromones. Vomeronasal sensory neurons (VSNs) express single receptor types from families comprising approximately 300 genes (Touhara and Vosshall 2009), endowing this system with extensive diversity and the potential for individual differences to be manifest at the level of cell types. Most of the prominently dimorphic nuclei in the mouse brain lie downstream of the VNO. However, whether these sensory neurons themselves are dimorphic is unknown: in several species, the male and female VNO are of different sizes (Dawley and Crowder 1995; Segovia and Guillamón 1982), yet studies of dimorphism at the level of VSN receptor-gene expression have not found evidence for functional dimorphism (Herrada and Dulac 1997; Ibarra-Soria et al. 2014; Matsunami and Buck 1997; Zhang et al. 2010). Conversely, experience has been documented to affect gene expression in VSNs (vomeronasal receptors and otherwise) (Broad and Keverne 2012; Zhang et al. 2010) and neuronal survival in the main olfactory epithelium (Santoro and Dulac 2012; Zhao and Reed 2001). But how plasticity is organized on the scale of cell types, circuits and behavior in chemosensation remains largely mysterious.
We performed large-scale recording of VSN activity to analyze the functional cell types present in mice with different sex and experiences. We performed calcium imaging by objective-coupled planar illumination (OCPI) microscopy (Holekamp et al. 2008) to simultaneously monitor the activity of approximately 10,000 neurons (Figure S1 A&B, Movie S1, and Experimental Procedures) in each preparation we studied. Exhaustive recording of activity by OCPI microscopy enabled an unprecedented cellular-resolution functional comparison of neural differences among individual animals. Combined with the ability to biologically and environmentally manipulate the olfactory system in laboratory-housed mice, we assessed the roles of sex, hormones and sensory experience in establishing individual differences. We demonstrated that dramatic individual differences can be detected in the abundance of specific functional VSN types, and unexpectedly found that differences between male and female cell types were a result of a novel form of experience-dependent plasticity. Finally, we found that individual differences in sensory neuron types were closely coupled to voluntary exploratory behavior, and that narrowly-targeted plasticity induced striking changes in males’ investigation of female scents.
RESULTS
Functional Classification of Cell Types
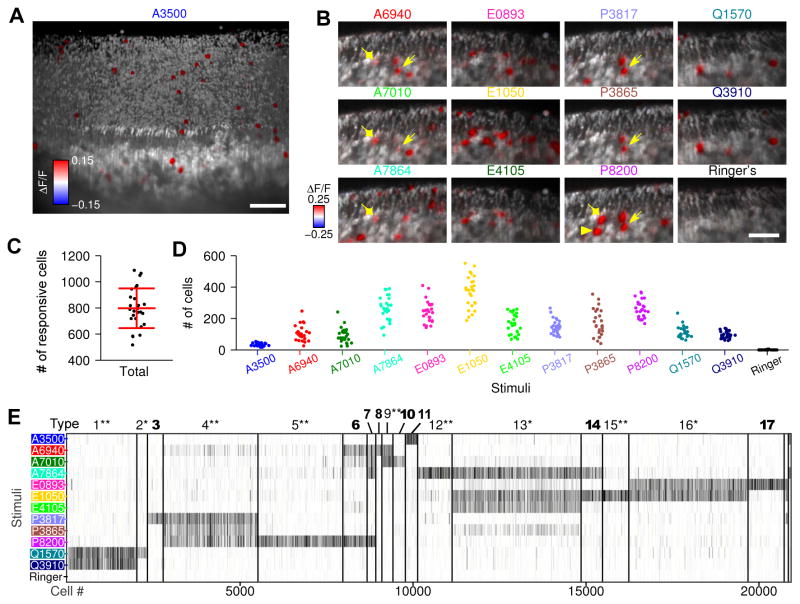
We performed calcium imaging of VNOs while interrogating neuronal responses to 12 sulfated steroids (Movie S1, Figure 1A&B), spanning the androgens, estrogens, pregnanolones, and glucocorticoids, a class of social cues originally isolated from mouse urine (Nodari et al. 2008). Using OCPI microscopy (Holekamp et al. 2008; Turaga and Holy 2012; Xu and Holy 2013), we collected image stacks (volumes) with frame size 710 μm×710 μm, scanning 282 μm along the z axis (Figure S1 A&B). Each volume typically contains approximately 10,000 neurons (see Experimental Procedures), each with signals at both dendritic knob and cell body (Figure S1 C). We measured the somata responses, and found that in such a volume, there were typically 700–1000 neurons responsive to at least one of the stimuli (Figure 1C). The numbers of responsive neurons varied by stimulus and individual (Figure 1D), ranging from a minimum average of 19 neurons (for the androgen ketoetiocholanolone sulfate, A3500) to a maximum of 552 neurons (for the estrogen 17β-estradiol disulfate, E1050). These cell numbers correspond to ~0.2% to 5.5% of the VSNs in the imaging volume.
Figure 1. Large-scale recording of mouse vomeronasal sensory responses to sulfated steroids and classification of VSN physiological types.
(A) Three-dimensional rendering from an imaging volume of the whole-mount VNO. Gray scale is the raw fluorescence intensity of GCaMP2, and red/blue color scale represents the GCaMP2 fluorescence intensity change (ΔF/F) caused by stimuli, here exemplified by the androgen ketoetiocholanolone sulfate (A3500). Cells that responded to A3500 are visualized by the red cell bodies inside the tissue and the red dendritic knobs on the tissue surface (see comparison in Figure S1 C).
(B) Two-dimensional slices of VNO imaging volumes show cellular responses to the other 11 sulfated steroids but not Ringer’s control. VSNs responsive to single steroids (illustrated by epipregnanolone sulfate, P8200) were heterogeneous: arrowheads indicate a cell responding exclusively to P8200; diamonds indicate a cell responding to P8200 and three androgens (A6940, A7010 and A7864); arrows indicate a cell responding to all pregnanolones (P8200, P3865 and P3817) and two androgens. Scale bar, 50 μm.
(C &D) Cell counts in each VNO imaging volume, in terms of total number of steroid-responsive cells (C) and number of cells responding to each stimulus (D). Each dot represents a single imaging volume. The label “# of cells” always means “# of responsive cells” in all the figures.
(E) Cluster organization of cells (from 26 imaging volumes) revealed at least 17 reproducibly-identified physiological types of VSN. *: previously-reported VSN physiological types (Meeks et al. 2010; Turaga and Holy 2012); **: corresponding to observed mitral cell responses in the accessory olfactory bulb (Meeks et al., 2010); bold: newly-discovered types. Each column represents a single cell. For each stimulus, gray intensity represents the average induced fluorescence change ΔF/F across 4 trials.
Consistent with previous reports (Haga-Yamanaka et al. 2014; Isogai et al. 2011; Meeks et al. 2010; Nodari et al. 2008; Turaga and Holy 2012), VSNs activated by single compounds were functionally heterogeneous and could be classified into different types on the basis of stimulus responsiveness (Meeks et al. 2010; Turaga and Holy 2012) (Figure S1 E–I). We reasoned that a much larger dataset might offer greater statistical power to detect rare types. By combining 26 VNO imaging volumes—comprising over 250,000 vomeronasal neurons—we identified 20,853 reproducibly steroid-responsive cells, nearly 10% of the total population and sufficient to expect many instances of each distinct receptor type expressed by these neurons. Based on the similarities of cell physiological responses, we clustered all steroid-responsive VSNs into 17 different types reproducible across multiple preparations (Figure 1E, type-18 and-19 cells were detected in only a small subset of preparations). Among these 17 types, 9 types matched previously reported physiological types of VSNs studied by multi-electrode array and/or calcium imaging (Meeks et al. 2010; Turaga and Holy 2012), and many were also reflected in the responses of downstream neurons such as accessory olfactory bulb (AOB) mitral cells (Meeks et al. 2010).
Here we also discovered 8 new VSN physiological types. Most of the newly identified types were narrowly tuned to single sulfated steroids: type 3 to allopregnanolone sulfate (P3817), type 8 to epitestosterone sulfate (A6940, Figure S1 E–I), type 10 to testosterone sulfate (A7010), type 11 to ketoetiocholanolone sulfate (A3500), and type 17 to 17α-estradiol sulfate (E0893). These narrowly tuned cell types, typically with a cell count as low as 10 per imaging volume, each accounted for ~0.1% of the neuronal population, 10-fold lower than that of the previously reported cell types. Such rare cell types could be reproducibly identified due to the exhaustive sampling and large numbers of neurons assembled in 26 imaging volumes.
Individual Variability is Cell-type Specific
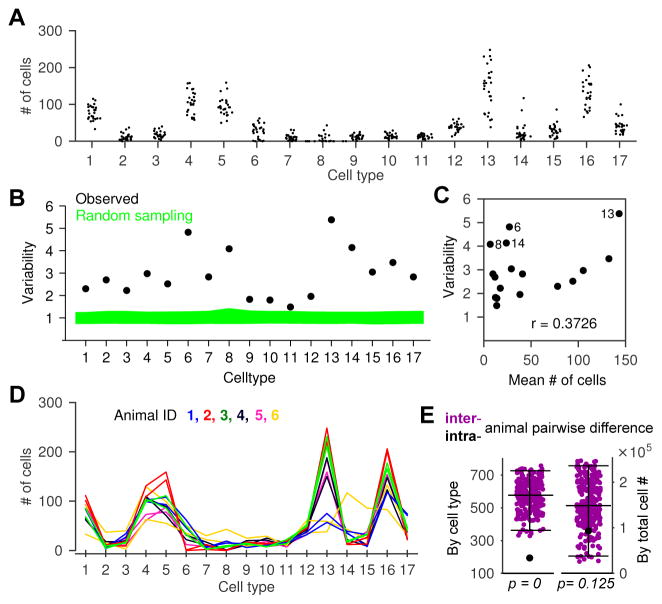
We first asked whether the variability in the numbers of each neuronal type from one animal to the next (Figure 2A) merely reflected random chance or indeed captured systematic differences among individual animals. If each VSN chooses its developmental fate independently but with the same “rules” from animal to animal, random sampling would predict a multinomial distribution for the counts of each cell type (see Figure S2 A for a comparison of multiple models). Using this observation we simulated random sampling (see Experimental Procedures) and compared the expected variability to our experimental observation in all the animals we recorded.
Figure 2. Individual mice exhibited non-stochastic variability in VSN functional types.
(A) Cell counts of each VSN functional type in each imaging volume.
(B) Cell count variability (see Experimental Procedures) of each type in all imaging volumes. Green represents the 95% confidence interval if cell numbers are drawn from a multinomial distribution.
(C) Variability was not correlated with cell abundance, r: Pearson’s correlation coefficient (p=0.14).
(D) Pairs of imaging volumes recorded from the same animals. Each color represents one animal, and the two lines with the same color are the two non-overlapping imaging volumes from the same animal.
(E) Left (by cell type): summed difference of intra-animal pairs (●) was smaller than that of any combination of inter-animal pairs (●, all 265 possible permutations of pairs in E are shown). Right (by total cell #): intra-animal difference (●) is not significantly smaller than all possible inter-animal differences ●.
Error bars represent 95% confidence interval.
See also Figure S2.
The observed variability exceeded expectations by as much as 5-fold (Figure 2B) with striking differences among the different types. For all 17 cell types, the excess variability was statistically significant (above the 95% confidence interval of random sampling). The degree of excess variability did not obviously correlate with factors such as abundance (Figure 2C): the four most variable were types 6, 8, 14 (among the rarest types) and 13 (the most common type). Neither did the variability obviously correlate with tuning width, as the more variable types included those (e.g., type 8 and 15) sensitive to a single ligand as well as those (e.g., type 14, 6 and 13) responding to multiple ligands (Figure 2E).
To determine whether variability in one type might be related to variability in another, we examined the pairwise correlations in cell-type abundance across animals. The correlation matrix revealed that certain functional types were positively correlated or anti-correlated (Figure S2 C), regardless the similarity between cell response profiles (Figure S2 D), indicating that response similarity is uncorrelated with mechanisms that determine fate across cell types and/or individuals. In order to capture the core “axes” that generate correlations between certain types, we performed a Principal Component Analysis (PCA) of the scaled cell counts (see Experimental Procedures Variability Analyses). The largest component showed a strong positive correlation among type 6, 8 and 14, and anticorrelation with type 13 (and more mildly with types 5 and 16). This “axis” of variation was therefore extremely selective for particular cell types, and indicates that some cell types grow in abundance at the expense of specific other types.
To determine whether the observed variability reflected features specific to individual animals, in 6 preparations we imaged two non-overlapping volumes (Figure 2D) and compared the intra-individual differences in cell-type counts against the inter-individual differences. Intra- and inter-individual differences were not correlated to each other (Figure S2 E). We found that, on average, the intra-individual difference was dramatically lower than the inter-individual difference (Figure 2E, left). With those 12 recordings, no way of pairing them across animals produced lower discrepancy than the actual pairing within the same animal (p=0.0038, permutation test, see Experimental Procedures). Intra-animal variability might be lower in part due to consistency in the health of the preparation. However, when we ignored cell type classifications and compared the pairwise differences in total number of responsive cells, there was not a significant difference between inter- and intra-animal recordings (Figure 2E, right, permutation test, p=0.125).
We conclude that the variability in functional cell types is significantly greater than expected by chance, involves tradeoffs among specific cell types, and is a signature of the individual animal.
Sex Differences in Cell Types
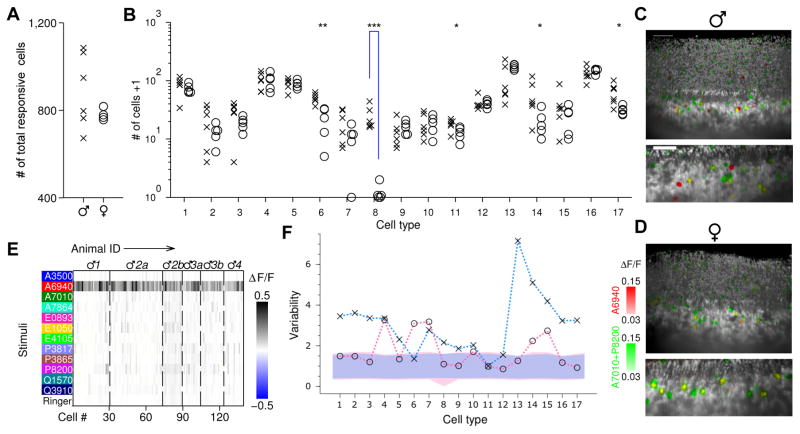
To determine whether individual differences in the abundance of neuronal types were influenced by sex, we analyzed cell counts separately for 6 male and 5 female data sets from the previous pool (the other 15 data sets in the pool were not included here because those mice not only differed in sex but also in other aspects described below). Both males and females possessed neurons responding to each ligand. Neither the total number (Figure 3A) nor the number of neurons responding to each ligand (Figure S3 A) varied significantly between males and females, although males had a non-significant tendency towards more cells responding to epitestosterone sulfate (A6940, p=0.10), testosterone sulfate (A7010, p=0.24), and epipregnanolone sulfate (P8200, p=0.017, not significant in the test with Ŝidák correction for multiple independent comparisons, see Experimental Procedures). This indicates that males and females can detect all of these sulfated steroids, and that even the numbers of cells responsive to each ligand do not show dramatic sex differences. Likewise, at the level of cell types, we found that the large majority of VSN types existed in both sexes (Figure 3A, Figure S3 B): for 15 out of 17 types, we found that the cell counts were comparable between males and females, indicating that the majority of steroid-responsive VSN types were equally expressed in vomeronasal organs from mice of either sex.
Figure 3. Functional neuronal types in VNOs from male and female mice.
(A &B) Cell counts in each male (×) and female (○) VNO imaging volume, in terms of total number of steroid-responsive cells (a) and number of cells of each VSN functional type (B). Type 8 were more abundant in male than in female mice (p=3.1×10−5, Students’ t-test, significance tested with Ŝidák correction for multiple comparisons). ***: p<0.001; **: p<0.01; *: p<0.05. Note type 6 and 11 were not significant when corrected for multiple comparisons. Throughout the figures involves multiple cell types, y axes are in logarithmic scale, and “# of cells +1” were plotted to avoid displaying log0=−∞.
(C &D) Three-dimensional images (upper) and two-dimensional slices (lower) of male (C) and female (D) VNOs. Red indicates the fluorescence change caused by epitestosterone sulfate (A6940) (ΔF/FA6940), and green colorizes max(ΔF/FA7010, ΔF/FP8200). Type-8 cells, exclusively responsive to A6940, are therefore red; note these were present only in the male VNO but not in the female VNO. Responses induced by P8200 (green) were particularly intense and spread to neighboring pixels, producing a green halo around the yellow cells. Scale bar, 50 μm.
(E) Type-8 VSNs were found in all 6 male VNO imaging volumes (from 4 individual male mice). Dashed lines separate cells from different imaging volumes. Each imaging volume is labeled with the animal’s identity; multiple imaging volumes collected from the same animal are differentiated by letter.
(F) Variability of male (×) and female (○) mice. For each sex, cell count variability of each type, and 95% confidence interval for multinomial random sampling, are shown as in Figure 2B. The confidence intervals are shown in pink and blue for the female and male groups, respectively. Within-sex variabilities were not distinguishable from random sampling in 3 (male) and 11 (female) cell types.
See also Figure S3 & S4, and Movie S2–4.
However, this analysis also revealed a dramatic example of apparent sex difference. VSN type 8 (Movie S2), responding selectively to epitestosterone sulfate, was found almost exclusively in male mice (Figure 3B–E, Movie S3&4). In all 6 male VNO imaging volumes, an average of 23 (23.2±4.8, mean±s.e.m.) type-8 cells were found in each male imaging volume, yielding a total of 139 type-8 cells across all 6 imaging volumes (Figure 3B &E). In contrast, only one plausible example of a type-8 cell was found in 5 imaging volumes from females (Figure S3 C&D). Across preparations, this male/female difference, at least one-hundred fold, was highly significant even when corrected for multiple independent comparisons (p=3.1×10−5). In addition to type 8, we found that type-6 neurons, responding strongly to epipregnanolone sulfate and more weakly to certain androgen sulfates, also differed (p=0.0038, at borderline significance p=0.0030 after correcting for multiple independent comparisons) between males and females, by a ratio of approximately two-fold (male 49.0±3.8, and female 20.2±5.4, mean±s.e.m.)(Figure 3B).
To test whether the apparent absence of type-8 neurons from females might be an artifact of clustering, we performed a second analysis in which neurons most similar to type 8 were examined regardless of their clustering classification. This analysis did not reveal any additional type-8 candidates among the female imaging volumes (Figure S3 C &D). We also wondered whether females might have type-8 neurons that were less sensitive than those in males. We therefore performed a subset of experiments using mice expressing the more sensitive GCaMP3 in VSNs, and interrogated the neuronal responses to a range of concentrations, up to 100μM, of sulfated steroids (Figure S4). This approach detected 1–2 fold more total epitestosterone sulfate-responsive cells (GCaMP3 vs. GCaMP2: 200.0 vs. 162.8 for males, and 268.0 vs. 104.4 for females); in males, type-8 neurons were present at levels comparable to the GCaMP2 experiments (21.0±4.0 vs. 23.7±4.8, mean±s.e.m)(Figure S4 A &B), while in females no clear examples of type-8 neurons were found (Figure S4 E &F).
The essentially “all-or-none” sexually-specific presence of a cell type is rare in the mammalian nervous system (Morris et al. 2004; Yang et al. 2013), particularly for cells with physiologically-defined properties (Bergan et al. 2014). In addition to the difference in cell counts of functional types between males and females, we also measured the individual variability of cell counts within the same sex, and noted that males were substantially more variable than females: in females, the variability of 11/17 types was not distinguishable from random sampling, but this was true for only 3/17 in males (Figure 3F); the rest were more variable than predicted if fate were determined cell-autonomously.
Thus it appears that sex differences contribute to the large variability of VSN cells among animals, particularly for certain functional types which show strong sex differences in abundance. However, even with the same sex (and particularly among males), individual mice exhibited variability well above chance levels.
Differential experiences of males and females change VSN cell types
To explain sex differences in animals and humans, three intertwined mechanisms are typically invoked: a difference of genes (X and Y chromosomes), a difference of hormones (androgens and estrogens), and a difference of experience (e.g., expectations and conditions of rearing) (McCarthy and Arnold 2011; Morris et al. 2004). For vomeronasal sensory neurons, the first two biological explanations might initially appear to be most plausible (Alekseyenko et al. 2006; Gatewood et al. 2006; Segovia and Guillamón 1982). However, in testing these explanations, the corresponding biological manipulation would change the animal’s hormones and metabolites thereof (Mucignat-Caretta et al. 2004). Because vomeronasal neurons detect steroid metabolites (Nodari et al. 2008), such manipulations would also substantially change the animal’s sensory experience of its own scents. Thus, we decided to first test whether a mouse’s long-term olfactory experience affects the composition of sensory neuron types in males and females.
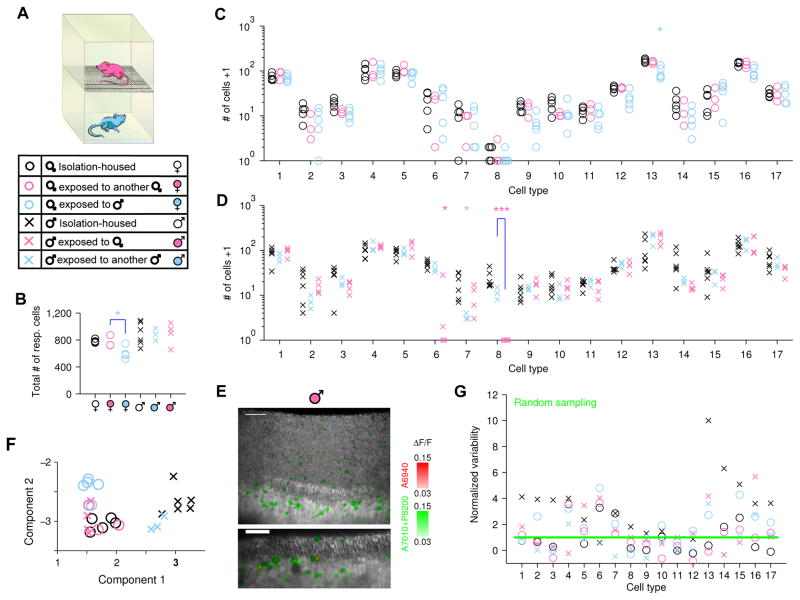
In total, we examined six different categories of individuals: isolation-housed males and females (as shown in Figure 3), females exposed to either a male or a female, and males exposed to either a male or a female. To provide long-term exposure to chemical cues from other animals without allowing sexual behavior or intense social contact, we designed two-layer stacking cages to house mice (Figure 4A) that permit chemical cues from the upper animal to automatically fall down to the animal in the lower cage. In some experiments we alternatively collected fresh bedding from one sex and supplied it to the other (see Experimental Procedures). After 9–13 weeks (matching the duration of isolation-housing in the previous experiments), we removed the vomeronasal organ from the lower animal and imaged tissue responses to these same sulfated steroids.
Figure 4. Exposure to female scents triggers the disappearance of the male-specific VSN type.
(A) Stacked cages provide chronic exposure to chemical social cues without permitting aggressive encounters or mating. Six groups of animals with varied sex and social cue experience are represented by different markers throughout the figure.
(B–D) Number of cells in VNO imaging volume from mice with different olfactory experiences (see 6 groups in A). B, total number of cells responding to sulfated steroids; C &D, number of cells in each specific VSN functional type. Type-8 cells showed a dramatic decrease in abundance in males exposed to females compared to males exposed to other males (p=7.0×10−5, Students’ t-test, significance tested with Ŝidák correction for multiple comparison). Statistical tests were performed for all conditions against isolation-housed animals and also between groups with same- and opposite-sex experience. ***: p<0.001; *: p<0.05 (the latter not significant when corrected for multiple comparisons).
(E) Three-dimensional rendering (upper) and two-dimensional slice (lower) of a VNO imaging volume from a male mouse exposed to a female. Coloration is identical to Figure 3C&D. The absence of red cells indicates the absence of type-8 VSNs, in contrast with isolation-housed males in Figure 3C and male-exposed males (see also Movie S3, 5 & 6). Scale bar, 50 μm.
(F) Whole-animal view of VNO cell-type composition from mice with different sexes and experiences. A linear-discriminant projection onto the two components with largest eigenvalues are shown. Isolation-housed males (×) and females (○) were well separated; males exposed to female cues (×) would be grouped with females (note overlap with circles).
(G) Normalized variability for each group of mice with the same sex and experience. For the 6 groups of animals, the numbers of cell types with variability consistent with random sampling (below the 95% confidence level marked by the green line) were 3 (×), 11 (○), 5 (°), 8 (°), 12 (×) and 9 (×) out of 17 types.
For vomeronasal organs from female mice, exposure to male chemical cues caused the total number of steroid-responsive neurons to decrease slightly (Figure 4B; p=0.035). While it appeared that some cell types responding to sulfated androgens and estrogens (types 9, 12, and 13) were somewhat rarer (Figure 4C, Figure S5 A), no individual case was significant when corrected for multiple comparisons (p=0.060, 0.039, 0.018). Crucially, for these females exposed to the males, we still did not detect evident type-8 VSNs: only a single clear type-8 cell was found in all 5 image volumes. Consequently, females with long-term exposure to male cues were virtually indistinguishable from isolation-housed females. Likewise, we found that females exposed to the chemical cues of other females were similarly unchanged (Figure 4C, Figure S5 A).
For vomeronasal organs from male mice, exposure to female chemical cues did not change the total number of steroid-responsive VSNs (Figure 4B). Such exposure also did not cause statistically-significant changes in the number responsive to each individual steroid (Figure S5 A), although there appeared to be trend-level effects for epitestosterone (A6940, p=0.068) and testosterone (A7010, p=0.070) sulfate in exposed animals, even when exposed to the same sex. Consequently, socially-experienced males were able to detect the same cues as isolation-housed animals.
However, in males exposed to females, type-8 neurons were virtually absent (Figure 4D–E, Figure S5 B&C, and Movie S5). In 4 imaging volumes, we did not observe any type-8 VSNs, a marked contrast with isolation-housed males (139 such neurons in 6 imaging volumes, p=7.0×10−5). This difference was not simply due to generic social experience, as males exposed to other males did not have a statistically-significant reduction in type-8 neurons (p=0.059, Figure 4D, and Movie S6). We do not exclude the possibility that exposure to males caused fewer type 8 and, more likely, type 7 (p=0.019), but neither of these effects were significant once corrected for multiple comparisons, and were much milder than the effects of exposure to females on type 8 neurons. To test whether this change affected strength of response specifically to A6940, rather than abundance of particular functional types, we analyzed the response amplitude of types 6, 7, 8, and 9 cells to both A6940 and any second effective ligand (A7010 or P8200), and found that responses of the remaining cells were unaltered for all of their ligands (Figure S5 D). We also confirmed the disappearance of type-8 cells from female-exposed males with a clustering-free analysis (Figure S5 B&C). Consequently, this change represents a difference in the abundance of a particular cell type rather than decreased responsiveness to a particular ligand.
To provide an unbiased view of the distribution of VSN types at the level of whole animals, we performed a linear discriminant analysis of the features that best distinguished among animals with different sexes and history of sensory experiences. Consistent with the result for types 8 and 6, we found that exposure to female cues “converted” male VNOs into a pattern of responsiveness indistinguishable from that of females (Figure 4F), suggesting that experience, not sex, determines the composition of VSN types. Besides the clear segregation among groups with different sex and experiences, this analysis also showed the pattern of variability among individual animals within each group. In order to see whether the scatter reflects randomness or individual difference, we examined the individual variability within six groups, and found that animals with the same sex and experience exhibited variability consistent with random sampling in approximately half of the type/experience conditions (48/102, Figure 4G), remarkably different from the result of 0/17 cases when combining animals from different groups (Figure 2B). Thus, much (but not all) of the individual variability we observed stems from the different explicit sensory experiences we provided to these animals. The remaining excess variability in the data captured the individual differences among animals with the same sex and the same controlled sensory experience (see Discussion).
Apparent Sex Differences are Primarily Controlled by Experience
Functional types 8 and 6 differed by sex and, more intriguingly, by animal’s experience with male/female cues. Because of other evidence for female hormones modulating VSN types (Dey et al. 2015), we tested whether these cell types might also be modulated by hormones.
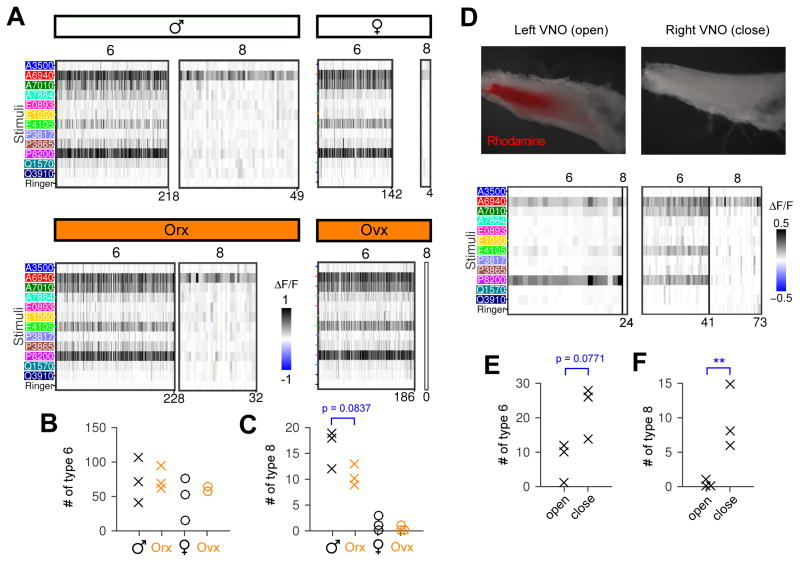
We first manipulated hormone production by surgical removal of the ovaries (ovx) and testes (orx) and analyzed VSN profiles in these animals (Figure 5A). Compared to the intact female control, ovx females possessed similar numbers of type-6 cells (p=0.42, Figure 5B) and no type-8 cells were found (Figure 5A, right; all 17 cell types are shown in Figure S6 A–E). Compared with the females, both type 8 and type 6 are more abundant in males (Figure 5A, left). Neither type 6 nor type 8 were significantly changed by orchiectomy (p=0.82 and p=0.084, Figure 5B &C), and orx males resembled intact males rather than females.
Figure 5. Sensory experience, rather than internal hormones, causes the main differences between male and female VSNs.
(A) Type 6 and 8 cells in gonadectomized mice. Type 8 cells existed in both normal and orchiectomized males (Orx), but were rarely detected in females even after ovariectomy (Ovx). One of the intact female controls had 3 type-8 cells, the largest number we observed in all 21 females we recorded (1 had three, 1 had two, 3 had one, and the remaining 16/21 females had none). Cells for each group were pooled from 3 GCaMP3 mice. See Figure S6 A–E for all 17 cell types in example mice.
(B &C) Number of type-6 (B) and type-8 cells (C) in individual animals. Orx males and Ovx females were not significantly different from normal male and female controls. Note a trend (p=0.084, Students’ t-test) of decreasing abundance of type 8 in males after orchiectomy, but these animals were not statistically distinguishable from control males.
(D) Upper: two VNO tissues dissected from the same male mouse. The right naris was permanently closed prior to exposure to the female. Chemical access was limited to the open side, as shown by Rhodamine 6G uptake from dye-soaked nestlets. During physiological recording, stimuli accessed the VNO by superfusion, no matter whether the tissue was from the closed or open side. Lower: type-6 and type-8 neurons from the open and closed VNO. Note type 8 were robustly detected on the closed side, in contract to the open side (only a single cell). See a naris closed female VNO in Figure S6.
(E &F) Number of type 6 (E) and type 8 (F) detected in multiple open and closed male VNOs. **: p<0.01, Students’ t-test.
See also Figure S6.
An alternative hormonal mechanism—one that could be conflated with experience—is an alteration in the male hormonal milieu triggered by exposure to females. To test this possibility, we occluded one naris in each male throughout the exposure period, thereby giving both sides the same exposure to circulating hormones but differing sensory experience. Dye-tracing demonstrated the effectiveness of unilateral naris occlusion in limiting exposure of the VNO to chemical cues (Figure 5D, top), consistent with a previous report (Jang et al. 1999). When the two sides were isolated and tested physiologically, VNOs from the open side, like all the female-exposed males in Figure 4, had almost no type-8 neurons. In contrast, VNOs from the closed side possessed type-8 neurons as observed in unexposed males (Figure 5 D bottom, and Figure 5 F). Likewise, the closed side appeared to have more (yet not significant) type-6 neurons than the open side (p=0.078, Figure 5E). Finally, if the lack of type-8 cells was due to presence of female odor cues, naris occlusion in female mice might cause a recovery of type 8 in the female VNO. However, this manipulation cannot entirely distinguish sensory exposure from an internal hormonal effect: both hormones and their VSN-active metabolites circulate (Axelson et al. 1984; Corpéchot et al. 1997), and the proximity of VSN dendritic knobs to a sizable blood vessel implies that “internal exposure” might suffice to cause sensory activation and negate the effect of naris occlusion. We detected 4 type-8 cells from the closed side in a female (Figure S6 F), perhaps suggesting a small increase compared to normal females, but not a change that crossed statistical significance or produced numbers analogous to males.
Altogether, gonadectomy and naris occlusion indicate that the dominant factor in this form of plasticity is sensory experience.
Female Urine Activates VSNs and Causes Experience-Dependent Plasticity
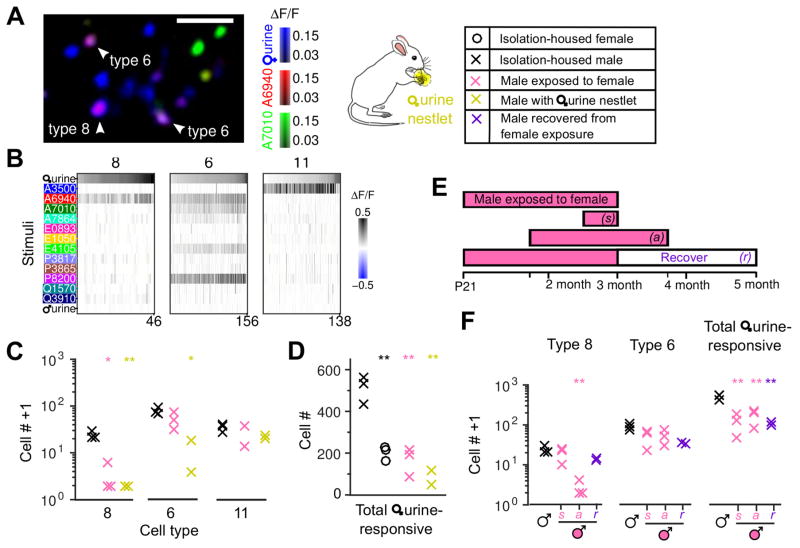
Sensory experience with female cues altered VSN types, and specifically type 8 and more modestly type 6. To further learn about why these two types, and not others, showed evident experience-dependent plasticity, we tested how the different neuronal classes responded to female mouse urine, a dominant source of chemosensory cues during the exposure period. Urine perceptibly activated just 3 of the 17 types, which were type 8, type 6, and (most weakly) type 11 (Figure 6A&B).
Figure 6. Female urine activated specific cell types and caused experience-dependent loss of cell responses.
(A) Overlap of type-8 and 6 neurons with responses to female mouse urine (1:50 dilution). A two-dimensional slice is shown; arrowheads represent examples of type-8 and type-6 cells that responded to female urine extracts. Scale bar, 50 μm.
(B) Female urine extracts activated type-8, 6 and 11 VSNs, with decreasing strength. All urine-responsive neurons that also responded to at least one sulfated steroid are shown.
(C) The number of type-8, 6, and 11 in isolation-housed males (×), males exposed to females (×), and males directly exposed to raw female urine soaked in nestlets (×). Chronic exposure to female mice or to female urine nestlets reduced (to different extents) the abundance of each urine-responsive type compared to non-exposed male control (×).
(D) The total number of female urine (1:50 dilution) responsive cells (including type 8 and 6) in different groups of mice.
(E) The time window for experience-dependent plasticity. Male mice were exposed to females at different developmental stages for varying exposure durations (pink bars). Besides the regular long-term exposure (~ 2 months) starting from postnatal day 21, exposure starting from adult (after 7-week-old), short-term exposure (1–2 weeks), and recovered (for another 2 months after removing exposure) were tested.
(F) Cell counts from mice listed in (E). Note type-8 neurons significantly decreased after long-term exposure even during adulthood, but were not affected by short-term exposure. Type 8 (but not the overall female urine-responsive) neurons recovered significantly from long-term exposure p=0.039, compared to long-term exposed male group. ** p<0.01, * p<0.05, Students’ t-test, each compared to non-exposed male control.
This result provides a natural explanation for selective plasticity: neuronal types showing the greatest plasticity were those that responded most strongly to the stimulus. Type-8 neurons, the type exhibiting strongest plasticity, were also absent in isolation-housed males presented with nestlets soaked with female urine (Figure 6C), further demonstrating that activation of these particular neurons led to the observed plasticity. Direct exposure to female urine nestlets also reduced the number of type-6 cells (p=0.011), but not type 11 that responds weakly to female urine (p=0.070).
To test whether this plasticity phenomenon is restricted to just types 6 and 8, we also examined many other cells that were activated by female mouse urine but not among the 17 classified functional types. For isolation-housed animals, the average number of neurons responsive to female urine was 509±38 in males and 200±20 in females; males exposed to females or female urine-soaked nestlets had an average of 170±43 or 84±27 (mean±s.e.m.), respectively, a decline of 3–5 fold (Figure 6D). Consequently, this plasticity phenomenon is widespread.
Finally, we identified the developmental factors controlling this plasticity. To determine whether this plasticity was confined to the early post-weaning period, instead of beginning exposure around the weaning age (P21), we began with isolation-housed mature males (7-week-old) and commenced a two-month exposure to females (Figure 6E, labeled as adult); this experience nearly eliminated type 8 neurons (Figure 6F). We also found that a two-week exposure did not suffice (labeled as short). Finally, female-exposed males that were then re-isolated from females for an additional two months exhibited a partial return of type-8 neurons (labeled as recover). These results suggest that this plasticity requires long-term exposure, is not restricted to an early-life critical period, and is at least partially reversible.
Male Attraction to Female Urine Requires Type-8 and Type-6 VSNs
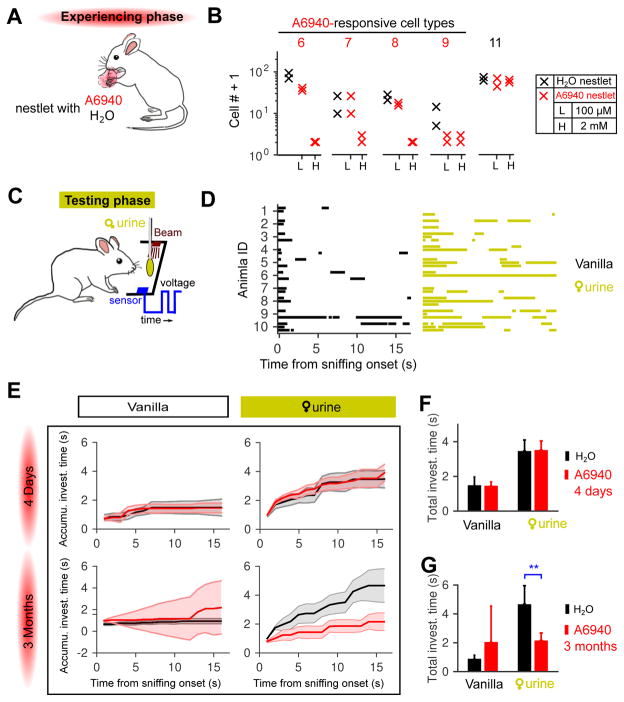
To examine the consequences of a more restrictive sensory experience, we chronically exposed male mice to nestlets soaked with just A6940 or water (Figure 7A) and examined the cell plasticity of selectively targeted VSN types. A modest dose of epitestosterone sulfate (100 μM A6940) induced little or no change in type 6 and type 8 (Figure 7B, & S7 A&B), perhaps consistent with a previously-reported 100-fold dilution of stimuli entering the VNO (He et al. 2010). Upon increasing the concentration, after 3 months of continuous exposure no A6940-responsive neurons (types 6, 7, 8, and 9) were observed in the exposed animals. Type 7 and type 9 were not responsive to female urine, so their activity-dependent loss further indicates the generality of this plasticity mechanism.
Figure 7. Long-term exposure eliminated responses to epitestosterone sulfate (A6940), and reduced male investigation of female mouse urine.
(A) Males were exposed to nestlets soaked with water or A6940 daily during the 4-day or 3-month experience phase.
(B) Cell counts of A6940-responsive cell types after long-term exposure to low (100 μM) or high dosage (2 mM) of A6940.
(C) Schematic of apparatus used to record episodes of olfactory exploration in freely-behaving animals. The infrared beam was broken (see the blue voltage trace) when the mouse contacted the cotton swab with 20μL of the tested stimulus.
(D) Investigatory episodes of 10 male mice to control (1:10000 vanilla only, left) and 1:1 female urine solid-phase extracts (right). Each animal participated in 3 trials, each of which was aligned (t=0) to the time of the first sniff of the cotton swab. Blocks represent continuous periods of investigation. Blank trials were “failure trails” in which the animals never investigated the cotton swab. See Figure S7 for more animals and stimuli tested.
(E) Duration of investigation of female urine extracts by male mice with short-term (4 days) and long-term (3 months) stimulus exposure. Cumulative time of ddH2O-exposed (black trace) mice and A6940-exposed mice (red trace) are shown. Data are presented as median±standard error in the median (computed by bootstrap).
(F &G) The total investigation time of each group in E. The total investigation time to female urine extracts was not significantly different between ddH2O-exposed and males with short-term A6940 exposure (F), but dramatically decreased by long-term A6940 exposure (G) (**: p<0.0152, Wilcoxon rank-sum test).
See individual sniffing patterns and breakdown of investigation time in Figure S7.
This result establishes long-term exposure to a particular chemical cue as a tool for eliminating a specific subset of responses from the vomeronasal organ. We used this tool to examine the behavioral contributions of a subset of female urine responsive cells, type-8 and type-6 VSNs, when males investigated female urine. We depleted volatile (main olfactory) cues from urine by making extracts (see Experimental Procedures), and doped samples with an independent odorous cue, vanilla, to attract attention and ensure initial investigation. We presented control stimuli and urine extracts on a cotton swab, and used an optical beam-break detector (Guo and Holy 2007) to measure the timing and duration of investigation periods in freely-behaving animals (Figure 7C).
From initial contact, males exposed to water investigated female urine extracts for periods more than twice as long as their investigation of the vehicle control (Figure 7D&E). In contrast, males exposed to epitestosterone sulfate exhibited a striking decrease in their investigation of female urine, to levels that were indistinguishable from the vehicle control (Figure 7E&G, & S7 E&F). To control for a possible direct hormonal effect of possible ingestion of epitestosterone sulfate, we also examined males exposed for 4 days (as frequently used for hormonal priming) (Melmed et al. 2015) rather than 3 months (Figure 7E&F, Figure S7 C&D). These short-exposure animals exhibited behavior equivalent to that of water-exposed mice, suggesting that the behavioral change was conditional on the elimination of responses to epitestosterone sulfate by long-term plasticity.
Besides total investigation time, we quantified investigation time in the 1st visit and re-visit(s), as well as the number of visits to female urine extracts; these measures further substantiated the robust effects of long-term exposure (Figure S7 G–I). We also measured investigation time to pure sulfated steroids. No significant differences emerged, although control male mice, but not sulfated epitestosterone-exposed males, exhibited a slight trend to sniff epitestosterone sulfate more than vehicle control, especially in the re-visit(s) (Figure S7 E & I (bottom); p=0.098, Wilcoxon rank-sum test).
Thus, we find that the innate interest of males in exploring female urine requires type-8 and type-6 VSNs for detection. Although these two response types account for <20% of all cells responsive to female urine extracts, their loss reduced male investigatory behavior to levels seen with socially-irrelevant odors.
DISCUSSION
The vomeronasal system is widely believed to drive “innate” responses to social odors. Many of these behaviors are strongly dimorphic—aggression or mating—and consequently males and females are expected to differ in their underlying neuronal circuitry (Dulac and Kimchi 2007; Stowers and Logan 2010). Here, we showed that isolation-housed males have at least one type of sensory neuron that is nearly absent in females. It would seem reasonable to expect this difference to be inherited, determined by the genes or hormones specific to each sex. Instead, we find that this extreme example of a sex difference is entirely due to sensory experience. Our finding that neuronal differences are a consequence of plasticity, in a sensory system long held to subserve the innate differences between males and females, brings new mechanistic appreciation of the extraordinary importance of experience in the development of neuronal circuits and behavior. The successful detection of individual differences at the circuit level with cellular resolution also demonstrates the promise of using large-scale functional imaging to dissect the neural basis for individuality.
A Clean System for Studying the Effect of Environment on Individual Differences
It is widely recognized that the environment has far-reaching influence on self-concept and individual behavior of humans, yet empirically testing the effects of nature vs. nurture in humans is a formidable challenge because of the number of variables to control. Such control is easier to achieve in rodent models, yet the effect of environment on individual differences, even sex differences, is poorly studied in rodents (McCarthy and Arnold 2011). The mouse accessory olfactory system (AOS) exhibits sexual dimorphism at multiple circuit levels, which from hormonal manipulations like gonadectomy combined with implantation of sex steroids (Alekseyenko et al. 2006; Guillamón et al. 1988; Segovia and Guillamón 1982) has been thought to be a consequence of circulating gonadal hormones. Because the AOS is well-documented to control of innate social behavior, it is not unreasonable to speculate that innate biological factors, such as internal hormones, should determine its role in male sexual behavior (Del Punta et al. 2002; Ferrero et al. 2013; Haga et al. 2010; Stowers et al. 2002), male aggression (Chamero et al. 2007; Norlin et al. 2003; Stowers et al. 2002; Tachikawa et al. 2013), interspecies defensive behaviors (Papes et al. 2010), maternal aggression (Bean and Wysocki 1989; Del Punta et al. 2002; Kimchi et al. 2007; Norlin et al. 2003), female sexual behavior (Dey et al. 2015; Kimchi et al. 2007), and lactating behavior (Kimchi et al. 2007). Here, we identified one potential confound of such studies: hormonal manipulations may change the metabolites in the animal’s urine, saliva, tears, etc. (Clissold et al. 1984; Mucignat-Caretta et al. 2004), thereby altering the olfactory environment for the animal. Therefore, the relative roles of internal hormones and/or sensory experience in generating AOS circuit dimorphism remained to be addressed.
In this study, we observed that isolation-housed male and female mice possess functionally different sensory cells. By co-housing the two sexes in a two-layer cage without close physical contact, we provided the animals with exposure to both male and female scents and found that sex differences diminished. To directly differentiate hormonal and sensory effects, we exploited the bilaterality of the VNO, with both sides experiencing the same levels of circulating hormones but with just one side receiving sensory exposure. All these procedures took full advantages of powerful physiological tools and the amenability of the mouse vomeronasal system to comprehensively assess the roles of sex, hormones, and experience. Previously studies about the role of environment on sexually different neural structures or behavioral performance (Dalla and Shors 2009; Lenz and Sengelaub 2006) have not differentiated the role of environment and innate factors. Here, with the mouse model we discovered a remarkable role of environment in generating sex differences in neuronal circuitry, not only serving as an important cautionary insight that researchers should pay closer attention to environmental confounds prior to attributing differences to other factors, but also with interesting implications even for the discussion of human activities such as school performance and the workplace.
Beyond sex differences, the mouse housing manipulations also enabled study of the neural differences among animals with the same sex but different experiences. A male or female chemical environment explained much of the variability within sex (Figure 3F). However, we also found that isolation-housed males were more variable than males with access to other males (Figure 4G), despite both being exposed to the same general male chemical environment. This indicates that additional experience-dependent phenomena contribute to individual differences, and suggests that increased stimulus exposure leads to a stabilization of neuronal types (see further discussion below). Even in animals with the same sex and housing condition, a subset of cell types, typified by the type 13 in isolation-housed males, exhibited dramatic animal-to-animal variability (Figure 3F, & 4 G). The origin of the excess variability in type 13, as well as the anti-correlation of its abundance to type 6 and 8, is largely unknown. It is possible that type 13, as a large cluster exhibiting more cellular heterogeneity than some other types, is an aggregate of neurons expressing receptors with similar tuning profiles. If so, further subdivision (using receptor tagging or an expanded stimulus set) might shed light on the origin of this type’s variability.
Plasticity: “Use it and Lose it”
In the nervous system, many known mechanisms of plasticity contribute to the maintenance and strengthening of oft-excited connections; this positive contribution of activity to circuit development and stabilization is often summarized as “use it or lose it.” In contrast, the plasticity we discovered in the vomeronasal organ can be better summarized as “use it and lose it”: the most strongly-activated neuronal types (type 8 and 6) were most dramatically reduced after chronic female exposure. Type-11 neurons, which exhibited much lower responsive strength (Figure 6B), exhibited a slightly-decreasing trend, and cell types not responding to female urine were not affected by female exposure. Besides female exposure, activating these neurons via nestlets soaked with female urine or a sulfated steroid (epitestosterone sulfate, A6940) also caused reductions in their number (Figure 6C&D, & 7 B).
This plasticity required prolonged exposure: while it could be triggered by a two-month exposure, one or two weeks were insufficient (Figure 6E&F). Thus, this plasticity appears to represent an entirely different phenomenon from light/dark adaptation in the retina, a process occurring on timescales of seconds or minutes. Although “adaptation” has been proposed to explain the lack of c-fos activation in the BALB/c male VNO after ESP-1 exposure (Haga et al. 2010) as well as down-regulation of pup pheromone-induced c-fos activation (Tachikawa et al. 2013), the decrease in c-fos signals may alternatively reflect reduced investigation of familiar chemicals (Woodley et al. 2004) instead of a change in sensory capacity. In behaving animals with continuous ESP-1 exposure, distinguishing short-term and long-term effects, or behaviorally-induced vs. neurally-induced phenomena, remains difficult without direct physiological investigation.
One possible mechanism for changes in cellular function is altered gene expression, particularly of the vomeronasal receptors themselves. Studies of the influence of experience on gene expression in VSNs (vomeronasal receptors and otherwise) (Broad and Keverne 2012; Zhang et al. 2010) showed maximal changes of approximately 2-fold, insufficient to account for the all-or-none phenomenon we observed in type-8 neurons. It is not known whether the reported changes corresponded to different numbers of cells or changes of expression level within cells. Recently Weid and colleagues also found that odorant exposure induced a 25–70% decrease in the transcription of corresponding receptors in the main olfactory epithelium (von der Weid et al. 2015). One potential confound in whole-tissue expression studies is that even a small amount of leaky receptor expression (Hanchate et al. 2015) can mask effects that have strong impact on the single-cell level—due to their rarity of type-8 neurons (0.2% of the total population), even a small leak in the expression of the type-8 receptor by other VSNs could mask the dramatic functional changes that occur from the elimination of this type from the VNO.
The 2-month period for this plasticity is long enough for sensory neuron turnover in VNO epithelium (Wilson and Raisman 1980). The plasticity of type-8 VSNs was observed at both developmental stages and in adulthood. Moreover, we found this plasticity was bidirectional, as re-isolating male mice from females led to the reappearance of type-8 neurons (Figure 6E&F). These results are consistent with possibility that the observed plasticity of type-8 cell responsiveness may involve type-8 cell death and rebirth. Odor-mediated changes in neuronal survival in the main olfactory epithelium have been documented previously (Santoro and Dulac 2012; Zhao and Reed 2001). However, the direction of the effect—with stimulation prolonging survival of sensory neurons—appears to be opposite to the plasticity identified in this study.
What advantage might such plasticity confer to animals? We suspect that the strength of this phenomenon is partly due to the controlled sensory environment of the laboratory, and that under natural circumstances with diverse stimuli, all neuronal types may be under a certain amount of sensory-induced pressure; rather than total loss, the abundance of cell types may be modulated in a more graded fashion by the differences in such pressures. This plasticity might therefore be a reflection of cell-survival or receptor-choice mechanisms that ensure balance in the expression of neuronal types and the ability of organisms to detect a wide variety of scents, in essence a long-term form of “decorrelation” (or “gain control” in the abundance of particular cell types) in sensory input. This proposed role is consistent with the decreased animal-to-animal variability of females compared to males (Figure 3F) given that females produce a substantially-larger number and intensity of vomeronasal cues than males (He et al. 2008; Nodari et al. 2008; Stowers et al. 2002; Tolokh et al. 2013). Altogether, the mechanism of plasticity remains unknown, and an important topic for future studies.
Sulfated Steroids & Behavior
Sulfated steroids, as active ligands for VSNs, were purified from female mouse urine; at present they constitute by far the most active class of known ligands for VSNs (Nodari et al. 2008; Turaga and Holy 2012). The overlap of epitestosterone sulfate with urine responses in type-8 neurons (Figure 6A&B) might reflect the presence of epitestosterone sulfate in female urine; however, we were unable to detect this compound in female mouse urine (not shown). The fact that many VSN types respond to multiple ligands (Figure 1B&E) makes it plausible that another, possibly related, ligand in C57BL/6 female mouse urine activates type-8 neurons.
Here, we used sulfated steroids to “tag” individual functional types of VSNs. We found that investigatory behavior was directly predicted by the observed influence of experience on VSN types: animals possessing type-8 neurons exhibited more investigation of female mouse urine (and possibly A6940) than those that did not (Figure 7C&E). These results were independent of whether the experience was with living female mice or with a single VSN ligand, consistent with the interpretation that these behavioral alterations were a consequence of modulating the abundance of specific VSN types. While female mouse urine excites many non-type-8 VSNs, these results also suggest that enhanced investigation of this stimulus may be dependent upon this specific neuronal type.
Studying Individual Differences by Exhaustive Imaging
Individual differences in neuronal circuitry underlie individuality in behavior, but studying these differences at a cellular level in neural assemblies remains a major challenge: circuits contain tens or hundreds of different cell types—and many thousand individual neurons—distributed throughout cubic millimeters (or more) of tissue. Large-scale recording by light sheet/OCPI microscopy (Holekamp et al. 2008; Keller and Ahrens 2015) provides one of the few viable strategies for studying systematic functional differences between individuals. Here, by recording from approximately 10,000 neurons per VNO preparation, OCPI microscopy enabled detection of individual differences in terms of specific functional cell types in a local circuit. While fast volumetric imaging allows one to collect data sets on the scale needed to study individual differences, analysis of these data sets presents another layer of challenge. We segmented ~30,000 stimulus-responsive neurons from a half million VSNs in the imaging volumes, and identified 17 specific functional VSN types, including previously-unrecognized types across multiple animals. This exhaustive recording and detailed analysis of neuronal response profiles were enabling technologies for detection individual differences at the single-neuron level. With the rapid advance in large-scale recording techniques and analysis tools, we believe the current work will herald a new era of studying individual differences in brain circuits.
SUMMARY of EXPERIMENTAL PROCEDURES
Whole-mount VNO epithelia from GCaMP mice were imaged with a custom light sheet microscope OCPI (Holekamp et al. 2008). 3D imaging volumes were registered to correct tissue movement, and individual cells were segmented to obtain single cell responses. Pooled cells from multiple data sets were clustered into specific physiological cell types. Animals were isolation housed, unless exposed to chemical cues from another animal in custom two-layer stacked cages starting on postnatal day 21 (P21) for over 2 months. For direct chemical exposures, experimental mice were presented with soaked nestlets on a daily basis. Unilateral sensory deprivation was achieved by permanent surgical enclosure of one naris on P21. Olfactory investigatory behavior was assessed with a custom optical detector (Guo and Holy 2007). Data acquisition and analysis was carried out using custom software written in C++, Matlab, and Julia. Full EXPERIMENTAL PROCEDURES are described in the Supplemental information.
Supplementary Material
Highlights.
Individual differences in cell types are not random
Sex differences in pheromone-sensing neurons are controlled by experience
Changes in specific cell types are governed via “use it and lose it” plasticity
Targeting plasticity to specific cell types changes animal behavior
Acknowledgments
We thank Kara Janiszak, Jack Chong and Mark Xu, who helped collect a portion of the behavioral data. We thank members of our lab for support, especially Ningdong Kang and Xiaoyan Fu. Thanks to Terra Barnes, Adish Dani, Cody Greer, Gary Hammen, Daniel Kerschensteiner, Maiko Kume, Karen O’Malley, Richard Roberts, Diwakar Turaga, Yue Yang, Massimo Scanziani and Jeffry Isaacson for comments on the manuscript. This work was supported by grants from NIH/NIDCD R01 DC005964, NIH/NIDCD R01 DC010381, NIH/NINDS/NIAAA R01 NS068409, and NIH Director’s Pioneer award DP1 OD006437 (TEH).
Footnotes
AUTHOR CONTRIBUTIONS
PSX and TEH designed the study. PSX and DL performed the experiments. All authors performed analyses. PSX and TEH wrote software and the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alekseyenko OV, Baum MJ, Cherry JA. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140:1349–57. doi: 10.1016/j.neuroscience.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Axelson M, Graham CE, Sjövall J. Identification and quantitation of steroids in sulfate fractions from plasma of pregnant chimpanzee, orangutan, and rhesus monkey. Endocrinology. 1984;114:337–44. doi: 10.1210/endo-114-2-337. [DOI] [PubMed] [Google Scholar]
- Bean NJ, Wysocki CJ. Vomeronasal organ removal and female mouse aggression: the role of experience. Physiol Behav. 1989;45:875–82. doi: 10.1016/0031-9384(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. Elife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Keverne EB. The post-natal chemosensory environment induces epigenetic changes in vomeronasal receptor gene expression and a bias in olfactory preference. Behav Genet. 2012;42:461–71. doi: 10.1007/s10519-011-9523-9. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JCS, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–49. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold PM, Hainey S, Bishop JO. Messenger RNAs coding for mouse major urinary proteins are differentially induced by testosterone. Biochem Genet. 1984;22:379–87. doi: 10.1007/BF00484236. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–80. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawley EM, Crowder J. Sexual and seasonal differences in the vomeronasal epithelium of the red-backed salamander (Plethodon cinereus) J Comp Neurol. 1995;359:382–90. doi: 10.1002/cne.903590303. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–4. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell. 2015;161:1334–1344. doi: 10.1016/j.cell.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17:675–83. doi: 10.1016/j.conb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K, Liberles SD. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502 doi: 10.1038/nature12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–90. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimelbrant A, Hutchinson JN, Thompson BR, Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–40. doi: 10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- Guillamón A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44:281–90. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chem Senses. 2007;32:463–73. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–22. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife. 2014;3:e03025. doi: 10.7554/eLife.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchate NK, Kondoh K, Lu Z, Kuang D, Ye X, Qiu X, Pachter L, Trapnell C, Buck LB. Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science. 2015;350:1251–1255. doi: 10.1126/science.aad2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–8. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Schwartz J, Santilli M, Wood C, Durnin MH, Yu CR. Distinct signals conveyed by pheromone concentrations to the mouse vomeronasal organ. J Neurosci. 2010;30:7473–83. doi: 10.1523/JNEUROSCI.0825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–73. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Holekamp TF, Turaga D, Holy TE. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron. 2008;57:661–72. doi: 10.1016/j.neuron.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Ibarra-Soria X, Levitin MO, Saraiva LR, Logan DW. The olfactory transcriptomes of mice. PLoS Genet. 2014;10:e1004593. doi: 10.1371/journal.pgen.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–5. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, David H, Maruniak JA, Walters E. Advances in Chemical Signals in Vertebrates chapter Fos-Like Immunoreactivity in the Vomeronasal Receptor Neurons of Mice. Boston, MA: Springer US; 1999. pp. 535–547. [Google Scholar]
- Keller PJ, Ahrens MB. Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy. Neuron. 2015;85:462–483. doi: 10.1016/j.neuron.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–14. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Lu J, Tapia JC, White OL, Lichtman JW. The interscutularis muscle connectome. PLoS Biol. 2009;7:e32. doi: 10.1371/journal.pbio.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–84. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–83. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci. 2010;13:723–30. doi: 10.1038/nn.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams textbook of endocrinology. Elsevier Health Sciences; 2015. [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Bondì M, Caretta A. Endocrine status affects bladder size and postvoid residual urinary volume in mice. Horm Behav. 2004;46:11–8. doi: 10.1016/j.yhbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–95. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–18. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlin EM, Gussing F, Berghard A. Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr Biol. 2003;13:1214–9. doi: 10.1016/s0960-9822(03)00452-4. [DOI] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SW, Dulac C. The activity-dependent histone variant H2BE modulates the life span of olfactory neurons. Elife. 2012;1:e00070. doi: 10.7554/eLife.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Song C, Rees G. The surface area of human V1 predicts the subjective experience of object size. Nat Neurosci. 2011;14:28–30. doi: 10.1038/nn.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia S, Guillamón A. Effects of sex steroids on the development of the vomeronasal organ in the rat. Brain Res. 1982;281:209–12. doi: 10.1016/0165-3806(82)90160-2. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–9. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Stowers L, Logan DW. Sexual dimorphism in olfactory signaling. Curr Opin Neurobiol. 2010;20:770–5. doi: 10.1016/j.conb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 2013;33:5120–6. doi: 10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolokh II, Fu X, Holy TE. Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb. J Neurosci. 2013;33:13903–13. doi: 10.1523/JNEUROSCI.0037-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–32. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Turaga D, Holy TE. Organization of vomeronasal sensory coding revealed by fast volumetric calcium imaging. J Neurosci. 2012;32:1612–21. doi: 10.1523/JNEUROSCI.5339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid B, Rossier D, Lindup M, Tuberosa J, Widmer A, Col JD, Kan C, Carleton A, Rodriguez I. Large-scale transcriptional profiling of chemosensory neurons identifies receptor-ligand pairs in vivo. Nat Neurosci. 2015;18:1455–63. doi: 10.1038/nn.4100. [DOI] [PubMed] [Google Scholar]
- Wilson KC, Raisman G. Age-related changes in the neurosensory epithelium of the mouse vomeronasal organ: extended period of postnatal growth in size and evidence for rapid cell turnover in the adult. Brain Res. 1980;185:103–13. doi: 10.1016/0006-8993(80)90675-7. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29:659–69. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PS, Holy TE. Whole-mount imaging of responses in mouse vomeronasal neurons. Methods Mol Biol. 2013;1068:201–10. doi: 10.1007/978-1-62703-619-1_14. [DOI] [PubMed] [Google Scholar]
- Yagi T. Genetic basis of neuronal individuality in the mammalian brain. J Neurogenet. 2013;27:97–105. doi: 10.3109/01677063.2013.801969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK. A comparison of hypothalami of rats and mice: lack of gross sexual dimorphism in the mouse. Brain Res. 1982;239:233–9. doi: 10.1016/0006-8993(82)90844-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Marcucci F, Firestein S. High-throughput microarray detection of vomeronasal receptor gene expression in rodents. Front Neurosci. 2010;4:164. doi: 10.3389/fnins.2010.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Reed RR. X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system. Cell. 2001;104:651–60. doi: 10.1016/s0092-8674(01)00262-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.