Abstract
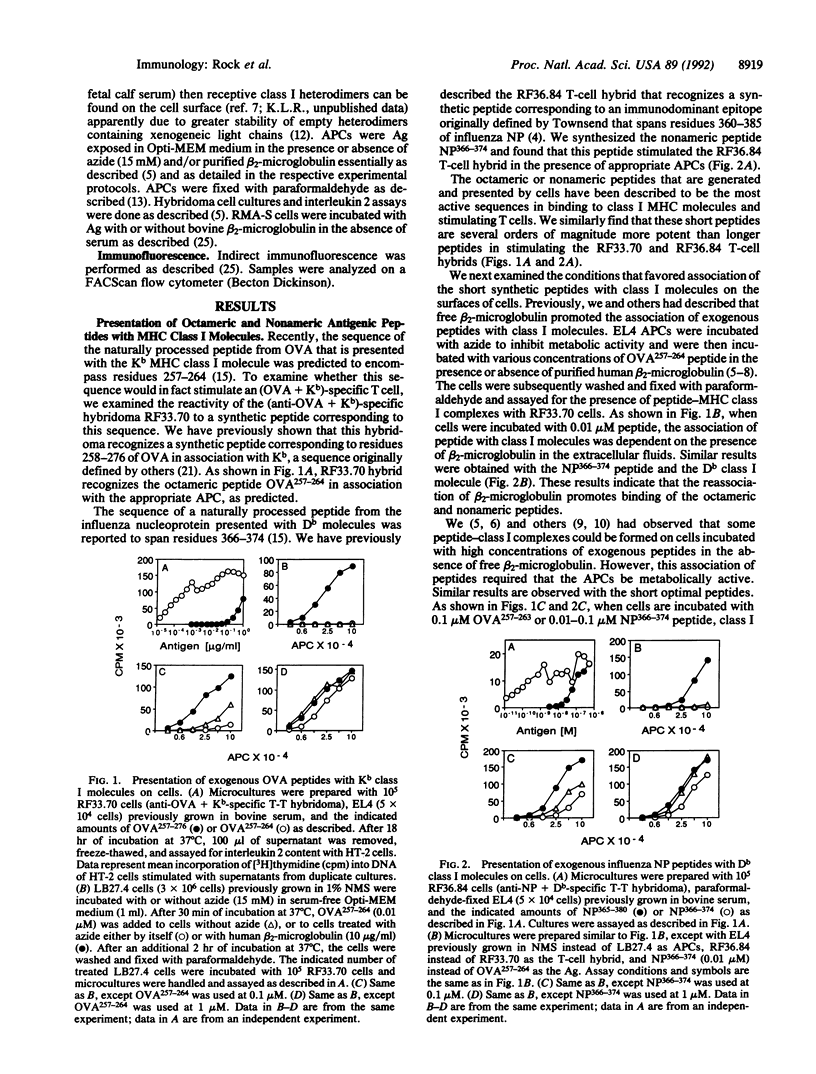
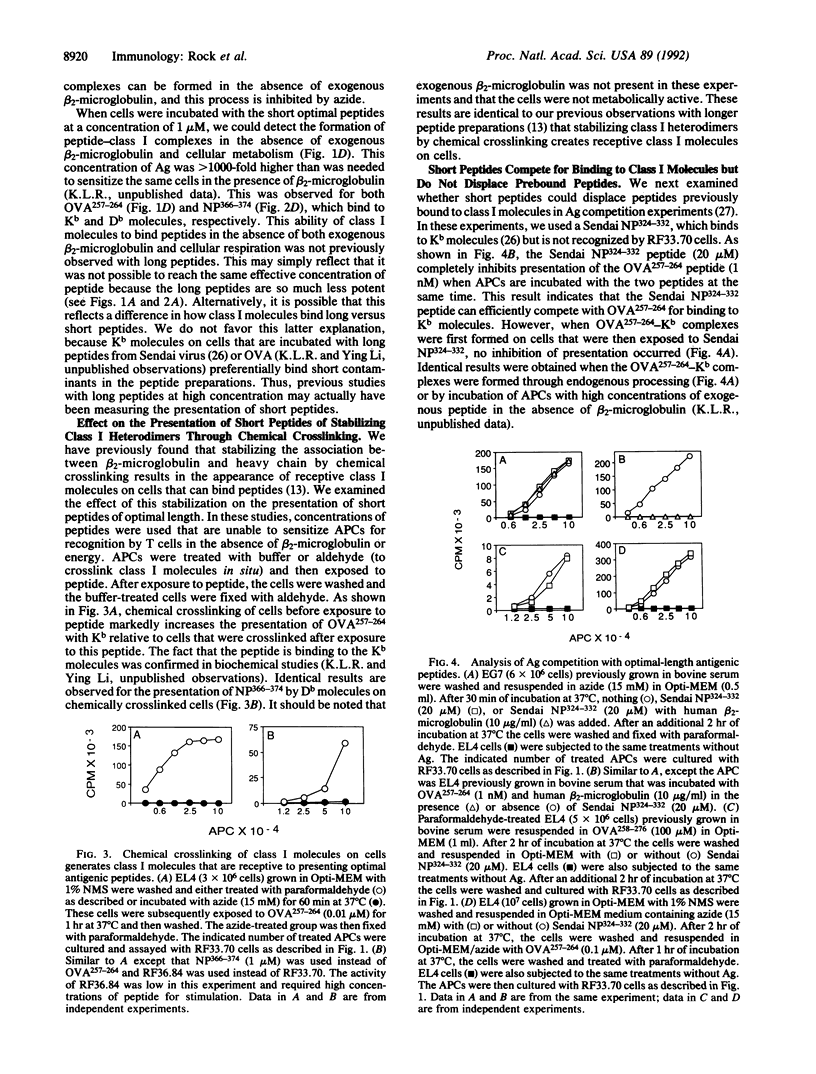
The association of major histocompatibility complex (MHC) class I molecules on the surface of cells with synthetic antigenic peptides of eight or nine amino acid residues was examined. Peptides were synthesized that correspond to the antigenic sequences from ovalbumin and influenza nucleoprotein believed to be naturally processed and presented by cells with Kb and Db MHC class I molecules, respectively. Consistent with the results of others, these peptides were 10(3)-10(5) times more active in stimulating specific T cells as compared to peptides of longer sequences. When cells are incubated with these peptides at less than 0.01-0.1 microM, the association of the peptides with class I molecules is dependent on (i) the reassociation of free beta 2-microglobulin from the extracellular fluids, (ii) a process that requires cells to be metabolically active, or (iii) stabilization of class I heterodimers by chemical crosslinking. In contrast, when cells are incubated with these peptides at greater than 0.1-1.0 microM, the peptides associate with class I molecules in the absence of exogenous beta 2-microglobulin, energy, or chemical crosslinking. Antigen competition experiments suggest that the class I molecules that bind peptides offered at high concentration become only transiently receptive to binding peptide. The concentration of peptides required for presentation to T cells under these conditions corresponds to those that stabilize Kb molecules on the surface of RMA-S mutant cells in the absence of exogenous beta 2-microglobulin. These results support the concept that the receptivity of class I molecules on cells is determined by the dissociation of beta 2-microglobulin from MHC class I that lacks bound peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attaya M., Jameson S., Martinez C. K., Hermel E., Aldrich C., Forman J., Lindahl K. F., Bevan M. J., Monaco J. J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992 Feb 13;355(6361):647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- Benjamin R. J., Madrigal J. A., Parham P. Peptide binding to empty HLA-B27 molecules of viable human cells. Nature. 1991 May 2;351(6321):74–77. doi: 10.1038/351074a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Elliott T., Cerundolo V., Elvin J., Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991 May 30;351(6325):402–406. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Falo L. D., Jr, Colarusso L. J., Benacerraf B., Rock K. L. Serum proteases alter the antigenicity of peptides presented by class I major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8347–8350. doi: 10.1073/pnas.89.17.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S., Corr M., Takeshita T., Boyd L. F., Pendleton C. D., Germain R. N., Berzofsky J. A., Margulies D. H. Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J Exp Med. 1992 Jun 1;175(6):1417–1422. doi: 10.1084/jem.175.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S., Takeshita T., Boehncke W. H., Takahashi H., Boyd L. F., Germain R. N., Berzofsky J. A., Margulies D. H. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991 Jan 3;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Luescher I. F., Romero P., Cerottini J. C., Maryanski J. L. Specific binding of antigenic peptides to cell-associated MHC class I molecules. Nature. 1991 May 2;351(6321):72–74. doi: 10.1038/351072a0. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. Evidence of specific competition for accessory cell antigen presentation. J Exp Med. 1983 May 1;157(5):1618–1634. doi: 10.1084/jem.157.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Db class I major histocompatibility complex binding of an exogenous influenza peptide. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):301–304. doi: 10.1073/pnas.88.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Gramm C., Benacerraf B. Dissociation of beta 2-microglobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell. 1991 May 17;65(4):611–620. doi: 10.1016/0092-8674(91)90093-e. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Benacerraf B. Low temperature and peptides favor the formation of class I heterodimers on RMA-S cells at the cell surface. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4200–4204. doi: 10.1073/pnas.88.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L. E., Gamble S. R., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Kb class I major histocompatibility complex binding of exogenous peptides. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7517–7521. doi: 10.1073/pnas.87.19.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L., Gamble S. Generation of class I MHC-restricted T-T hybridomas. J Immunol. 1990 Aug 1;145(3):804–811. [PubMed] [Google Scholar]
- Rock K. L., Rothstein L., Gamble S., Gramm C., Benacerraf B. Chemical cross-linking of class I molecules on cells creates receptive peptide binding sites. J Immunol. 1992 Mar 1;148(5):1451–1457. [PubMed] [Google Scholar]
- Schumacher T. N., De Bruijn M. L., Vernie L. N., Kast W. M., Melief C. J., Neefjes J. J., Ploegh H. L. Peptide selection by MHC class I molecules. Nature. 1991 Apr 25;350(6320):703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Burke T. A., Biggs J. A. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med. 1992 May 1;175(5):1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. H., Barber B. H. The conformational flexibility of class I H-2 molecules as revealed by anti-peptide antibodies specific for intracytoplasmic determinants: differential reactivity of beta 2-microglobulin "bound" and "free" H-2Kb heavy chains. Mol Immunol. 1990 Feb;27(2):169–180. doi: 10.1016/0161-5890(90)90112-d. [DOI] [PubMed] [Google Scholar]
- Tada N., Kimura S., Hatzfeld A., Hämmerling U. Ly-m11: the H-3 region of mouse chromosome 2 controls a new surface alloantigen. Immunogenetics. 1980;11(5):441–449. doi: 10.1007/BF01567813. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Potter T. A., Sherman L. A. The role of beta 2-microglobulin in peptide binding by class I molecules. Science. 1990 Dec 7;250(4986):1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- Widmann C., Maryanski J. L., Romero P., Corradin G. Differential stability of antigenic MHC class I-restricted synthetic peptides. J Immunol. 1991 Dec 1;147(11):3745–3751. [PubMed] [Google Scholar]



