Abstract
Background
Ingenol mebutate gel is a topical field treatment for actinic keratosis (AK). The treatment elicits application-site reactions in most patients. This analysis evaluated the relationship between the severity of reactions and the speed of their resolution.
Methods
Patients in Phase III studies were treated for AKs on the face (n=218), scalp (n=56), and trunk and extremities (n=209). All of the patients were treated with either ingenol mebutate gel 0.015% once daily for three consecutive days (face/scalp) or ingenol mebutate gel 0.05% once daily for two consecutive days (trunk/extremities). Local skin reactions (LSRs) were assessed on a 5-point scale from 0 to 4 in six categories, yielding composite scores in the range of 0 to 24.
Results
The composite LSR score on the day after the last application of ingenol mebutate gel was an important predictor of the speed of resolution of LSRs. The rate of resolution was greatest for AKs treated on the face, followed by the scalp, and then the trunk and extremities. All patients were expected to have minimal LSR scores for the face and scalp at 2 weeks, and for the trunk and extremities at 4 weeks.
Conclusion
The absolute reduction in LSR scores was proportional to the composite LSR score on the day after the last application of ingenol mebutate gel treatment. The rate of resolution for LSRs was dependent on the anatomic site treated as well as the day 4 composite score.
Keywords: ingenol mebutate, local skin reaction, actinic keratosis
Introduction
Actinic keratosis (AK) is a common precursor to sun-related squamous cell carcinoma. Common types of lesion-directed therapies for AK include cryotherapy and curettage.1,2 Patient-applied topical field therapies, including fluorouracil, imiquimod, and diclofenac, require adherence to a prolonged treatment regimen to achieve effective lesion clearance. Adherence and tolerability may be compromised by the adverse cosmetic effects and persistent local skin reactions (LSRs) associated with these treatments.3 Office-based field treatments, such as photodynamic therapy and ablative laser treatment, may be accomplished in one or more office visits, although LSRs may nonetheless be persistent,4,5 and delayed wound healing may occur with laser resurfacing.5
Four recently concluded Phase III studies have demonstrated the effectiveness and safety of ingenol mebutate gel applied as a topical field treatment of AK for two or three consecutive days.6,7 This treatment produced significant short-term clearance of AK versus placebo, clinically relevant sustained clearance, and long-term lesion reduction.6,7 Rates of complete clearance (primary end point) and partial clearance (secondary end point), defined as ≥75% reduction from baseline in the number of visible AKs, were assessed on day 57. Safety end points included adverse events and LSRs, assessed according to a prespecified scale. In most patients, the treatment elicited application-site reactions. The LSRs typically occurred within 1 day of treatment initiation, peaked in severity up to 1 week after completion of treatment, and resolved within 2 weeks for areas treated on the face and scalp, or within 4 weeks for areas treated on the trunk and extremities.6
In this analysis, we evaluated the relationship between the severity of the composite LSRs and the speed of their resolution in patients who were treated for AKs of the face, the scalp, or the trunk and extremities.
Methods
Data were collected from four multicenter, randomized, parallel-group, double-blind studies.6 For the face and scalp locations, results from two pivotal Phase III trials (NCT00916006 and NCT00915551) were included in the analysis. A total of 220 patients were treated for AKs on the face, and 56 patients were treated for AKs on the scalp. Two patients were withdrawn from the facial analysis. For trunk and extremity locations, results from two pivotal Phase III studies (NCT00742391 and NCT00942604) were included in the analysis. A total of 209 patients were treated for AKs on the trunk and extremities. All patients provided written consent prior to enrollment. The protocols for all studies were submitted to Institutional Review Boards (IRBs) and Independent Ethics Committees (IECs), in the United States and Australia, respectively. Approval from the IRBs/IECs was obtained before the start of the studies.
All patients had 4–8 AKs within a 25 cm2 area and were treated with ingenol mebutate gel, 0.015%, once daily for three consecutive days (face/scalp) or ingenol mebutate gel 0.05% once daily for two consecutive days (trunk/extremities). LSRs were assessed on days 3 or 4 (1 day after the last application), day 8 (week 1), day 15 (week 2), day 29 (week 4), and day 57 (week 8). LSRs, which included erythema, flaking/scaling, crusting, swelling, pustulation/vesiculation, and erosion/ulceration, were assessed on a 5-point scale from 0 to 4 (with higher numbers indicating greater severity), yielding composite scores in the range of 0 to 24. The composite score is the sum of the six individual scores that were recorded at each study visit for each patient. A simple regression model was used to predict the week 1, 2, 4, and 8 composite LSR scores from the composite LSR score on the day after the last application. The percentage reduction in composite LSR scores from day 3 or 4 to week 1, 2, 4, and 8 could be assumed to be the same across all three groups (low, medium, and high composite LSR score at day 4) but specific for each of the three anatomic locations.
Results
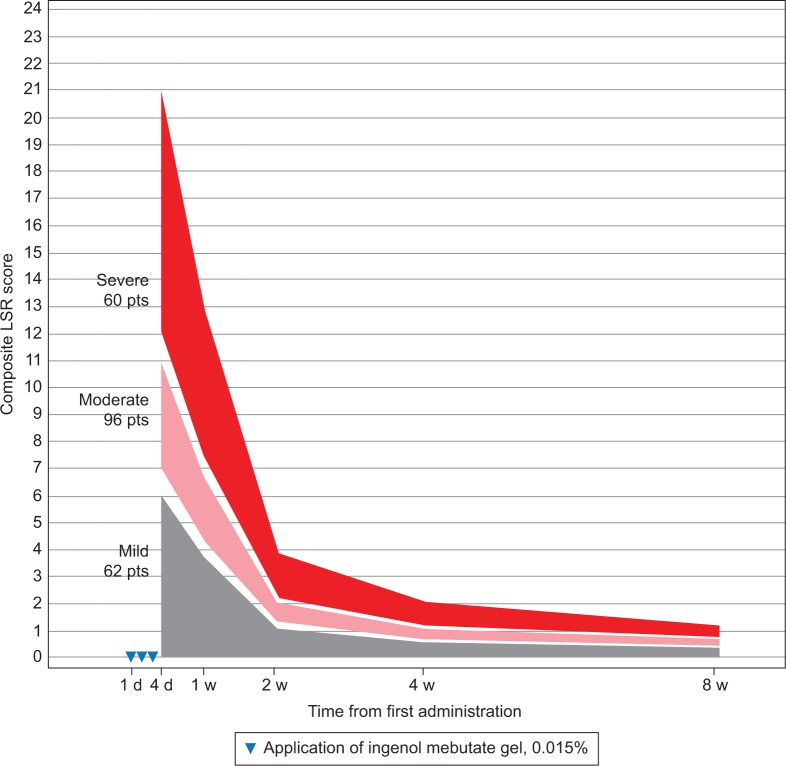
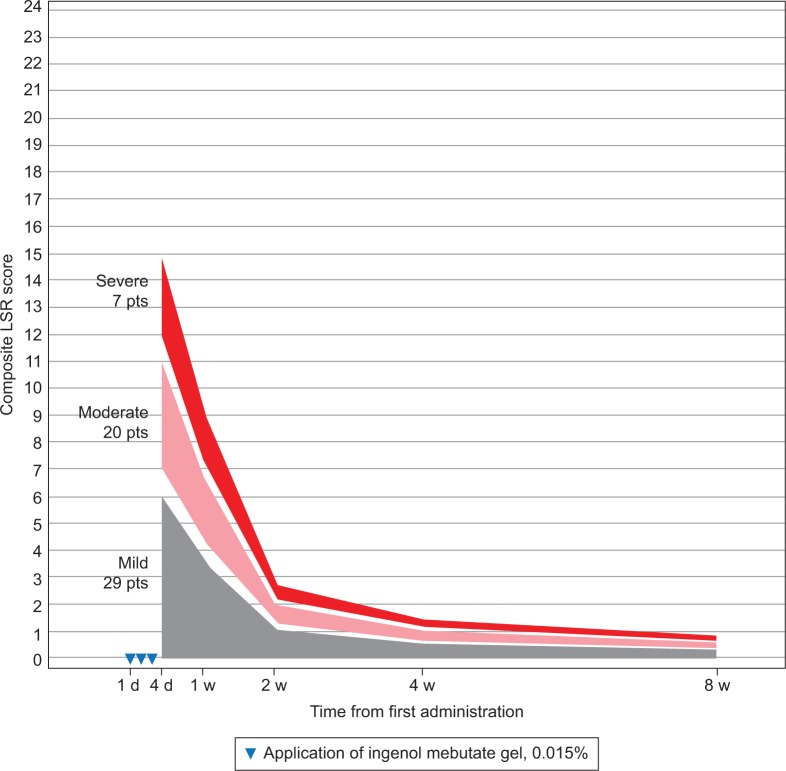
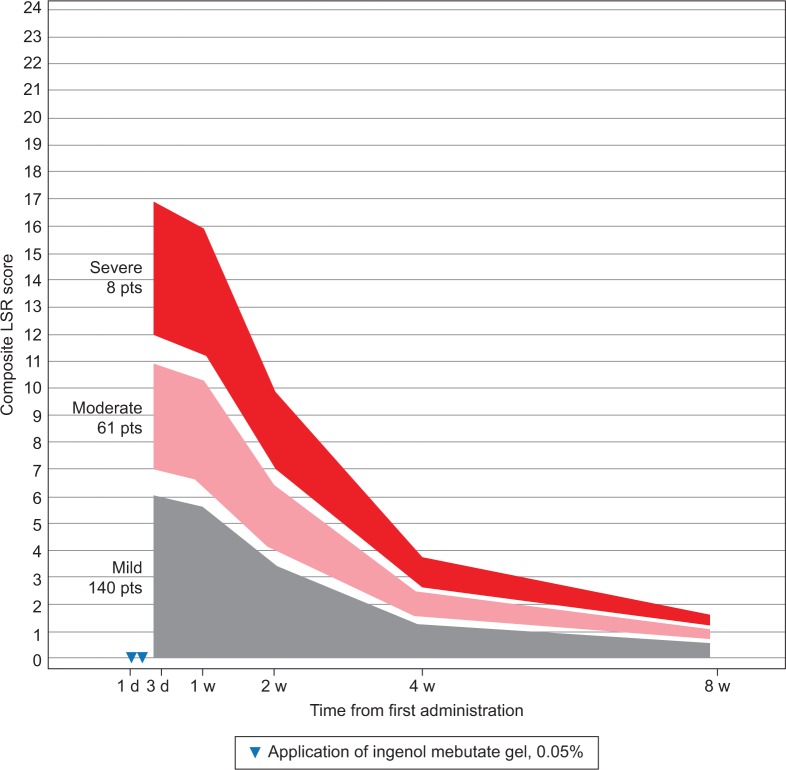
Patients were grouped by LSR severity according to their composite LSR score at 1 day after the last application of ingenol mebutate gel (Table 1). LSRs peaked on the day after the last application for the majority of patients – face, 88%; scalp, 68%; and trunk and extremities, 58% – or by 1 week after – face, 12%; scalp, 23%; and trunk and extremities, 33%. Regression analysis using composite LSR scores from all posttreatment assessments is graphically represented for patients who were treated with ingenol mebutate gel for AKs on the face (Figure 1), scalp (Figure 2), and trunk and extremities (Figure 3).
Table 1.
Classification of patients by range of LSR composite score at 1 day after the last application of ingenol mebutate gel
| LSR Composite score | Mild | Moderate | Severe |
|---|---|---|---|
| Face | |||
| Score range | 0–6 | 7–11 | 12–21 |
| Patients, n | 62 | 96 | 60 |
| Scalp | |||
| Score range | 0–6 | 7–11 | 12–15 |
| Patients, n | 29 | 20 | 7 |
| Trunk/extremities | |||
| Score range | 0–6 | 7–11 | 12–16 |
| Patients, n | 140 | 61 | 8 |
Abbreviation: LSR, local skin reaction.
Figure 1.
Face: expected composite LSR score at week 1–8 in three groups, based on the composite score at day 4 for patients treated with ingenol mebutate gel, 0.015%, for AKs on the face (N=218)
Abbreviations: AK, actinic keratosis; d, day; LSR, local skin reaction; pts, patients; w, week.
Figure 2.
Scalp: expected composite LSR score at week 1–8 in three groups, based on the composite score at day 4 for patients treated with ingenol mebutate gel 0.015% for AKs on the scalp (N=56).
Abbreviations: AK, actinic keratosis; d, day; LSR, local skin reaction; pts, patients; w, week.
Figure 3.
Trunk and extremities: expected composite LSR score at week 1–8 in three groups, based on the composite score at day 3 for patients treated with ingenol mebutate gel 0.015% for AKs on the trunk and extremities (N=209).
Abbreviations: AK, actinic keratosis; d, day; LSR, local skin reaction; pts, patients; w, week.
The model calculated the expected reduction in LSRs as a percentage of the day 4 score (Table 2). The composite LSR score on the day after the last application of ingenol mebutate gel was found to be an important predictor of the resolution of LSRs. Among patients treated on the face, a high initial composite LSR score of 21 at day 4 was predicted to decrease to 13.0 at 1 week, to 3.9 at 2 weeks, to 2.1 at 4 weeks, and to 1.2 at 8 weeks (Table 3). An intermediate initial LSR score of 10 was predicted to decline to 6.2, 1.8, 1.0, and 0.6, respectively, and a low initial LSR score of 5 was predicted to drop to 3.1, 0.9, 0.5, and 0.3, respectively. Similar patterns were seen in patients treated on the scalp (Table 4) and the trunk and extremities (Table 5).
Table 2.
Percentage of day 4 composite score at weeks 1–8 based on the model expectation of resolution (95% CI), as a percentage of day 4 score (%)
| Model expectation of resolution (95% CI), percentage of day 4 score (%)
| |||
|---|---|---|---|
| Face (%) | Scalp (%) | Trunk and extremities (%) | |
| Week 1 | 62 (58–66) | 77 (66–87) | 94 (88–100) |
| Week 2 | 18 (17–20 | 33 (25–40) | 58 (54–63) |
| Week 4 | 10 (8–11) | 16 (11–22) | 22 (19–25) |
| Week 8 | 6 (4–7) | 10 (6–14) | 10 (8–12) |
Abbreviation: CI, confidence interval.
Table 3.
Face: expected composite score based on a sample of ascending day 4 composite LSR scores
| LSR score at day 4 | Expected LSR (90% prediction limits)
|
|||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | Week 8 | |
| 5 | 3.1 (0.0–7.9) | 0.9 (0.0–3.2) | 0.5 (0.0–2.4) | 0.3 (0.0–1.9) |
| 10 | 6.2 (1.4–10.9) | 1.8 (0.0–4.2) | 1.0 (0.0–2.9) | 0.6 (0.0–2.1) |
| 15 | 9.3 (4.5–14.1) | 2.8 (0.4–5.1) | 1.5 (0.0–3.4) | 0.8 (0.0–2.4) |
| 21 | 13.0 (8.2–17.8) | 3.9 (1.5–6.2) | 2.1 (0.2–4.0) | 1.2 (0.0–2.8) |
Abbreviation: LSR, local skin reaction.
Table 4.
Scalp: expected composite score based on a sample of ascending day 4 composite LSR scores
| LSR score at day 4 | Expected LSR (90% prediction limits)
|
|||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | Week 8 | |
| 5 | 3.8 (0.0–9.0) | 1.6 (0.0–5.4) | 0.8 (0.0–3.5) | (0.0–2.4) |
| 10 | 7.7 (2.4–12.9) | 3.3 (0.0–7.0) | 1.6 (0.0–4.3) | 1.0 (0.0–29) |
| 15 | 11.5 (6.1–16.8) | 4.9 (1.1–8.7) | 2.5 (0.0–5.2) | 1.5 (0.0–34) |
Abbreviation: LSR, local skin reaction.
Table 5.
Trunk and extremities: expected composite score based on a sample of ascending day 4 composite LSR scores
| LSR score at day 3 | Expected LSR (90% prediction limits)
|
|||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | Week 8 | |
| 5 | 4.7 (0.2–9.2) | 2.9 (0.0–6.4) | 1.1 (0.0–3.4) | 0.5 (0.0–2.1) |
| 10 | 9.4 (4.9–13.9) | 5.8 (2.3–9.4) | 2.2 (0.0–4.5) | 1.0 (0.0–2.6) |
| 17 | 15.9 (11.4–20.5) | 9.9 (6.3–13.5) | 3.8 (1.4–6.1) | 1.7 (0.1–3.3) |
Abbreviation: LSR, local skin reaction.
The expected percentage reduction in composite LSR scores from day 3 or 4 to weeks 1, 2, 4, and 8 was similar across all the three groups (low, medium, and high composite LSR score at day 4) and specific for each of the three anatomic locations. However, the absolute reduction in LSRs was dependent on the day 4 composite score as well as the anatomic site. The rate of resolution was directly proportional to the composite score on day 4 across all anatomic locations, with the rate of resolution greatest for the face, followed by the scalp, and finally, by the trunk and extremities. No serious adverse events were reported during the study phase.
Discussion
Ideal topical treatment for AK must result in sustained clearance. The ability to accomplish this is one of the primary benefits of field therapy, as seen with the application of ingenol mebutate gel for two or three consecutive daily doses.7 The efficacy of ingenol mebutate gel might be attributed to its dual mechanism of action, which combines rapid direct cell death and specific activation of protein kinase Cδ, including a neutrophil-mediated oxidative burst, making ingenol mebutate gel a pleiotropic effector.8 Although several therapies are approved for the treatment of AK, a major advantage of ingenol mebutate gel therapy over others is the reduced number of doses required, yielding similar efficacy as 60 days of diclofenac gel (3.0%) or 16 weeks of imiquimod (5%).9,10 Some patients experience a brisk initial reaction. We showed that this is typically followed by rapid healing, whereby all patients treating the face and scalp are expected to have minimal LSR scores at 2 weeks, and, for the trunk and extremities, at 4 weeks.6
The observed differences in the rate of reduction of LSRs between the face and scalp, and the trunk and extremities, may be due to factors affecting absorption. For example, the percutaneous absorption rate is higher in the face versus the scalp, which may be partly due to the thicker epidermis on the scalp than on the face. In addition, both the face and scalp have higher absorption rates, compared with other parts of the body.11,12
Conclusion
We found that the absolute reduction in LSR score is proportional to the composite LSR score on the day after the last application of ingenol mebutate gel and is dependent on the anatomic location. The rate of LSR reduction was proportional to the LSR score on day 4 and was highest for the face, followed by the scalp, and then the trunk and extremities.
This information may be useful for the treatment of AK using ingenol mebutate gel, as clinicians who are aware of the time course of resolution of LSRs can better manage the expectations and concerns of their patients.
Acknowledgments
The abstract of this paper was presented at the American Academy of Dermatology 74th Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the Journal of the American Academy of Dermatology. Writing assistance was provided by Kavin G Shah, MD, MPH, from p-value communications and funded by LEO Pharma Inc.
Footnotes
Disclosure
SCJO has nothing to disclose. KMK and TS report being employees of LEO Pharma. ML is an employee of the Mount Sinai Medical Center, which receives research funds from Amgen, Anacor, Boehringer Ingleheim, Celgene, Lilly, Janssen Biotech, Kadmon, LEO Pharmaceuticals, Medimmune, Novartis, Pfizer, Sun Pharmaceuticals, and Valeant.
References
- 1.Stockfleth E, Peris K, Guillen C, et al. A consensus approach to improving patient adherence and persistence with topical treatment for actinic keratosis. Int J Dermatol. 2015;54(5):509–515. doi: 10.1111/ijd.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern AC, Hanson LJ. Awareness of, knowledge of and attitudes to nonmelanoma skin cancer (NMSC) and actinic keratosis (AK) among physicians. Int J Dermatol. 2004;43(9):638–642. doi: 10.1111/j.1365-4632.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- 3.Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 1: Overview and investigational topical agents. Cutis. 2012;89(5):241–250. [PubMed] [Google Scholar]
- 4.Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140(1):41–46. doi: 10.1001/archderm.140.1.41. [DOI] [PubMed] [Google Scholar]
- 5.de Vries K, Prens EP. Laser treatment and its implications for photodamaged skin and actinic keratosis. Curr Probl Dermatol. 2015;46:129–135. doi: 10.1159/000367958. [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl M, Shumack S, Stein Gold L, Melgaard A, Larsson T, Tyring SK. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149(6):666–670. doi: 10.1001/jamadermatol.2013.2766. [DOI] [PubMed] [Google Scholar]
- 8.Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66(3):486–493. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Rivers JK, Arlette J, Shear N, Guenther L, Carey W, Poulin Y. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146(1):94–100. doi: 10.1046/j.1365-2133.2002.04561.x. [DOI] [PubMed] [Google Scholar]
- 10.Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50(5):714–721. doi: 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Stoughton RB. Percutaneous absorption of drugs. Annu Rev Pharmacol Toxicol. 1989;29:55–69. doi: 10.1146/annurev.pa.29.040189.000415. [DOI] [PubMed] [Google Scholar]
- 12.Lev-Tov H, Maibach H. Regional variations in percutaneous absorption. J Drugs Dermatol. 2012;11(10):e48–e51. [PubMed] [Google Scholar]